Introduction:
Many studies purport that obesity, and specifically visceral fat, impact survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. However, these studies involve crude measures of obesity [eg, body mass index (BMI)] or visceral fat [eg, linear measurements on computed tomographic (CT) scans]. Some studies purport that weight loss and muscle wasting (ie, sarcopenia) presage poor survival in these patients. This study was undertaken to accurately measure and reexamine the impact of visceral fat, subcutaneous fat, and sarcopenia on pancreatic cancer.
Materials and methods:
CT scans of 100 patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma were reviewed using specialized software to precisely determine the cross-sectional area (CSA) of subcutaneous fat, visceral fat, and psoas muscles at the level of L5 vertebra. In addition, linear measurements of subcutaneous fat and visceral fat were undertaken. Measures of cancer progression included tumor (T) status, nodal (N) status, American Joint Committee on Cancer stage, and overall survival after resection. Regression analysis was utilized, with and without standardization of all measurements to body size. Median data are presented.
Results:
The median patient age was 67 years, with a BMI of 24 kg/m2. Cancer stage was IIB for 60% of patients. BMI, CSA of visceral fat, CSA for subcutaneous fat, CSA for psoas muscles, and linear measurements of visceral and subcutaneous fat were not significantly related to any measures of cancer progression or survival. Standardization to body size did not demonstrate any relationships with cancer progression or survival.
Conclusions:
Precise and reproducible measures of visceral fat, subcutaneous fat, and muscle mass, even when standardized to body size, do not predict cancer progression or survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma. Pancreatic cancer biology and behavior is too complex to predict with a CT scanner. The main focus of pancreatic cancer research should continue to be at the molecular, genetic, and immunologic levels.
Keywords: Pancreaticoduodenectomy, Pancreatic adenocarcinoma, Computed tomography, Obesity, Sarcopenia
Introduction
Despite significant improvements in perioperative outcomes and surgical technique, long-term survival in pancreatic cancer patients has not improved in the modern era of surgery. Twenty percent of patients diagnosed with pancreatic cancer are deemed operable and ultimately undergo resection; nonetheless, even these patients have a dismal prognosis with a median survival of <2 years1. Modern cancer treatment paradigms focus on identifying genetically distinct subsets of patients with unique molecular alterations that may be targeted by specific therapies. Although this approach has shown promise with other malignancies2–5, there have been no breakthrough advancements with regard to pancreatic cancer.
Given this limited and disappointing understanding of pancreatic cancer biology, there has been an increased focus on the theoretical ability to predict pancreatic tumor behavior and aggressiveness based on computed tomographic (CT) measurements of specific body tissues, for example, visceral fat and muscle mass. This paradigm is based upon numerous publications that have found potential relationships between obesity [increased body mass index (BMI) or visceral fat] or sarcopenia (decreased muscle mass) and oncologic outcome in some malignancies, such as colon and rectal cancer, liver cancer, and renal cell carcinoma6–11. One report hypothesized that this relation may be based on the known observation that visceral fat can increase levels of inflammatory cytokines and adipokines in human serum, thus increasing tumorogenesis12. However, this alleged relationship between visceral fat and cancer biology has been questioned in other reports13,14. Sarcopenia has also been associated with inferior outcomes following oncologic resection of colon cancer15, hepatocellular carcinoma16, and esophageal cancer.17 Interestingly, sarcopenia has recently been linked to poor outcomes following pancreaticoduodenectomy for pancreatic cancer18.
Nonetheless, when thoroughly examining the studies describing a relationship between pancreatic cancer and the aforementioned CT measurements, we can see that these studies generally lack standardization in regard to measurement techniques and definitions of obesity and sarcopenia. These differences in methodology may have led to different conclusions. For example, in comparing 2 published papers, one suggested that patients with pancreatic cancer who are in the lower quartile of muscle mass have worse survival, whereas the other paper suggested worse survival in association with obesity and concomitant sarcopenia18,19. Since these groups are different, as were the methodology and definitions, it is hard to draw any practical conclusions. Other studies, including some of ours, looking at the impact of visceral fat and BMI on pancreatic cancer outcomes came to totally different, and sometimes conflicting, conclusions20–24.
Therefore, in designing this study, we aimed to obtain accurate, objective, and reproducible volumetric measurements of visceral fat and muscle mass, using technologically advanced and dedicated CT software. After performing these precise measurements, we aimed to examine the impact of visceral fat and muscle mass on the behavior and progression of pancreatic cancer, and thus to determine whether prognosis can be predicted based on these measurements. This can have important clinical implications in the long and generally frustrating management of this disease.
Materials and methods
With Institutional Review Board approval, CT scans of 100 patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma were reviewed. OsiriX (32-bit software version 3.8.1; Pixmeo, Geneva, Switzerland) medical imaging software was utilized to measure 3 cross-sectional areas (CSA): subcutaneous fat area, visceral fat area (VFA), and total psoas muscle area at the L5 vertebral level (Fig. 1). In addition, to further define the implications of fat mass assessment, linear measurements of subcutaneous and visceral fat were obtained; for subcutaneous fat, the sum of the abdominal wall fat thickness and hip girdle fat thickness was determined (Fig. 2), and for visceral fat, the perinephric visceral fat thickness was measured (Fig. 3).
Figure 1.
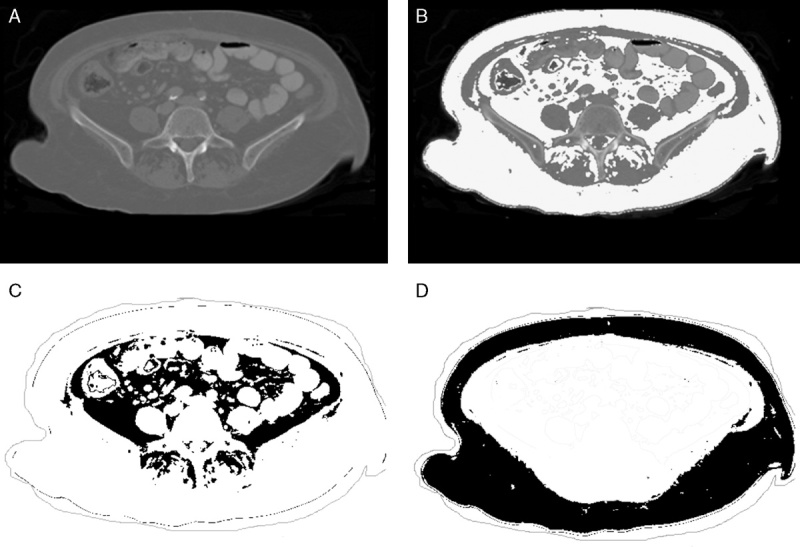
A, Original DICOM image. B, The white highlighted area represents visceral and subcutaneous fat set to a threshold value of −30 to −190 HU. C, The black highlighted area represent visceral fat. D, The black highlighted area represents subcutaneous fat, respectively.
Figure 2.
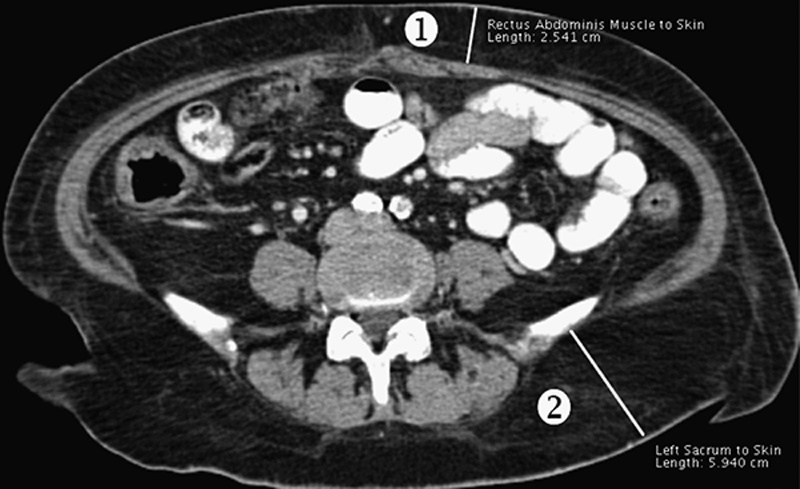
Abdominal wall fat thickness (1): paramedian vertical distance between the left rectus abdominus fascia and the skin at the level of the umbilicus. Hip girdle fat thickness (2): distance between the iliac plate and skin at the level of the posterior superior iliac spine.
Figure 3.
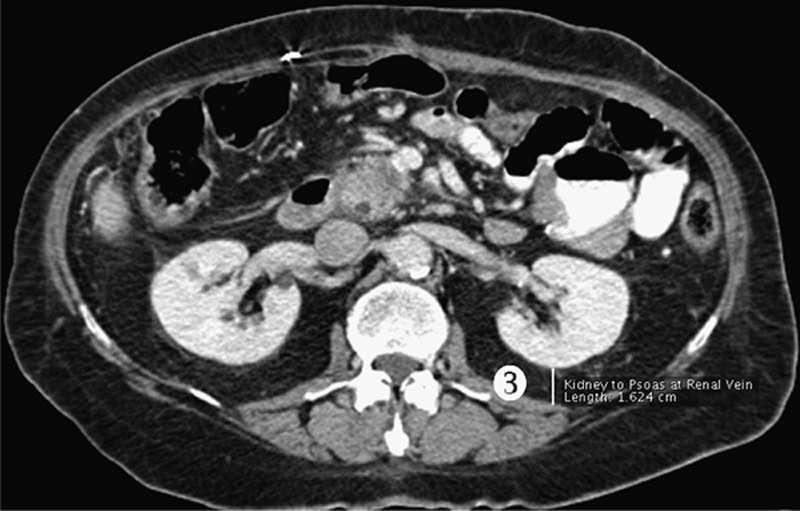
PNF thickness (3): vertical distance between the left posterior renal capsule and the junction of the abdominal wall and paraspinal musculature at the level of the left renal vein.
All patients were determined preoperatively to be free of metastases (M0) based on imaging. The progression of pancreatic cancer in each patient was determined by the tumor (T) status, nodal (N) status, overall American Joint Committee on Cancer (AJCC) stage, and overall patient survival after pancreaticoduodenectomy.
Data management and analysis
Patient data were stored in an institutional pancreatic cancer database. Statistical analysis utilized Graphpad Instat version 3.06 and Graphpad Prism 5 (Graphpad Software Inc., San Diego, CA). Significant relationships were determined using linear regression.
χ2 analysis or t tests were also used, where appropriate, and significance was accepted with 95% probability. Log-rank and Wilcoxon tests on the Kaplan-Meier survival curves were used. For illustrative purposes, data are reported as median (mean±SD). Survival data are presented as median predicted survival with 95% confidence intervals.
Results
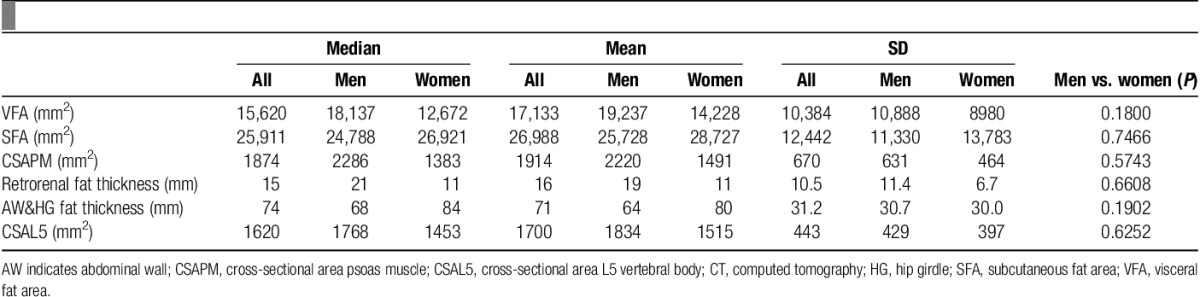
One hundred randomly selected patients, 58% men, with a median age of 67 years (66±10.6 y) underwent pancreaticoduodenectomy for pancreatic adenocarcinoma between 2004 and 2012. Baseline characteristics of these patients are displayed in Table 1. Seventy five percent of patients had an R0 margin status and 60% had an AJCC stage of IIB. Median survival for all patients was 17 months, (19±16.5 mo). Median operative duration was 284 minutes (300±84.6 min), and intraoperative blood loss was 400 mL (535±423.4 mL).
Table 1.
Baseline characteristics of patients undergoing pancreaticoduodenectomy.
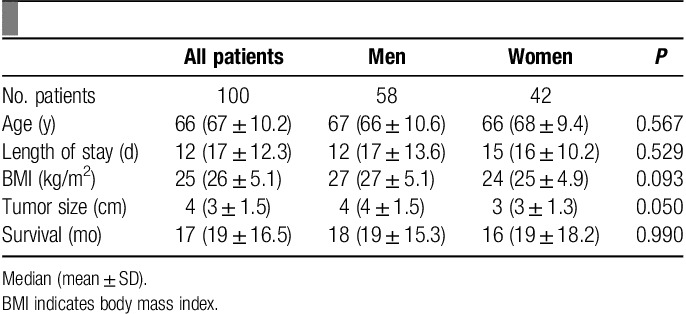
Table 2 summarizes the results of the measurements of subcutaneous fat, visceral fat, and muscle mass.
Table 2.
CT measures including cross-sectional areas of VFA, SFA, CSAPM, CSAL5, and linear measurements of retrorenal fat and AW and HG fat.
Comprehensive linear regression analyses failed to demonstrate any significant correlations between cancer progression and any of the CSA measurements or linear measurements performed, or with BMI (Fig. 4 and Table 3).
Figure 4.
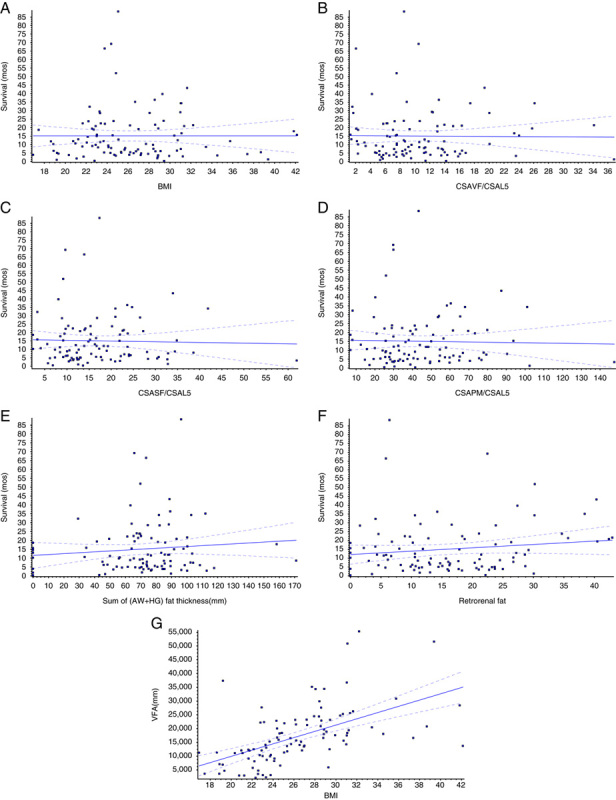
Summary of regression analyses comparing computed tomography–measured variables and body mass index (BMI) with survival. A, BMI versus survival (P=0.9984). B, CSAVF/CSAL5 versus survival (P=0.9214). C, Cross-sectional area of subcutaneous fat (CSASF)/CSAL5 versus survival (P=0.7894); *CSASF standardized to cross-sectional area at the fifth lumbar vertebrate (CSAL5). D, CSAPM/CSAL5 versus survival (P=0.8066). E, Abdominal wall (AW)+hip girdle (HG) fat thickness (mm) versus survival (P=0.3010). F, Retrorenal fat thickness (mm) versus survival (P=0.1856). G, BMI versus visceral fat area (VFA) (P<0.0001).
Table 3.
P-values from regression analyses comparing quantitative CT measured variables to measures of pancreatic cancer progression.
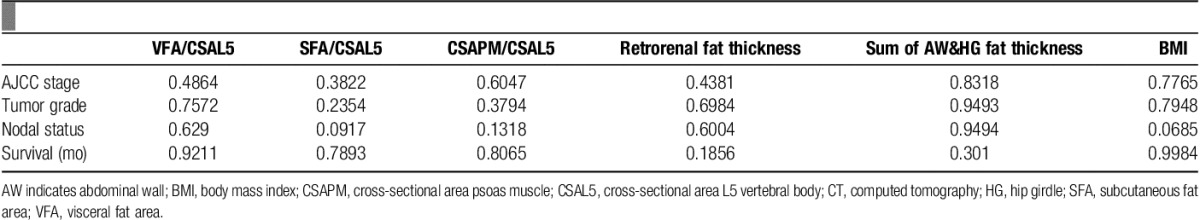
Nonetheless, in an effort to find some correlations, we did the following modification: because of the diversity in body build and size among patients, we used the CSA of the L5 vertebral body as an indicator of body size. We then standardized all CT measurements to the L5 vertebral body CSA. Now, we repeated all the linear regression analyses using these standardized measurements. Again, no correlation between cancer progression and any of the standardized CSA measurements or linear measurements was found. Standardization to patient height, as another indicator for body size, also did not yield any correlations between cancer progression and any of the standardized CSA measurements or linear measurements.
Discussion
The findings in this manuscript underscore the fact that pancreatic cancer behavior is complex and cannot be predicted using CT measurements of fat and muscle mass. We used very sophisticated and rigorous measurements, that included 3 cross-sectional and 2 linear measurements that were also normalized to body size, as well as BMI. However, these precise and comprehensive analyses failed to demonstrate any correlation between the progression of pancreatic cancer, including survival, and visceral fat mass, subcutaneous fat mass, muscle mass, or BMI.
More specifically, we found that VFA did not correlate with tumor progression or overall survival, a finding that has been described inconsistently in the literature. Our data should end this debate. The theoretical principle behind this alleged correlation is that visceral fat functions as an endocrine organ, secreting adipokines and inflammatory cytokines such as IL-6 and TNF-α, which promote a milieu that could affect cancer behavior8,12. Epidemiologic studies have shown a link between visceral obesity, obesity (BMI>30), and increased risk of pancreatic cancer6,22,23,25. In 2008, House and colleagues reported an increased rate of perioperative complications and pancreatic fistula in patients with increased visceral adiposity. Visceral fat was approximated by a retrorenal linear measurements. Although they did not study long-term outcomes, the concept was intriguing and inspired further investigation20,26.
Our group and others have investigated the possibility that visceral adiposity could represent a surrogate for pancreatic steatosis (fatty infiltration of the pancreas)24,26. Pancreatic steatosis in pancreatic cancer patients has been demonstrated to correlate with increased angiolymphatic invasion, cancer infiltration of lymph nodes, and worse survival27 Tranchart et al26 found that on preoperative CT, a VFA of >84 cm2 was the only predictor of both pancreatic steatosis and pancreatic fistula after pancraticoduodenectmy, thus adding to the evidence of a potential relationship between VFA, pancreatic steatosis, and outcome.
However, BMI and VFA have been inconsistently linked to oncologic surgical outcomes. Our results suggest there is no correlation between either BMI or VFA and pancreatic cancer progression. In 2012, a group at Memorial Sloan Kettering reported that neither BMI nor VFA (determined by linear measurements of retrorenal fat thickness) correlated with pancreatic tumor progression or with survival in patients undergoing pancreatic resection21. To add to the inconsistency of conclusions, a recent paper evaluated BMI and CT scans in 408 patients with pancreatic cancer and concluded that patients with low BMI had greater 90-day mortality. This paper also suggested that patients with increased subcutaneous fat had better survival and lower risk of complications14. Furthermore, Tsai et al28 found that patients with BMI>30 undergoing pancreaticoduodenectomy have improved long-term survival and lower rates of positive margins.
We did not find any significant association between measured muscle mass and tumor progression or survival. There is some recent literature to suggest that sarcopenia is associated with worse survival in patients with pancreatic adenocarcinoma. Peng et al18 stratified patients into quartiles and separated them by sex, and were able to show sarcopenia was a predictor of inferior survival at 3 years. In another report, Pausch et al14 concluded that patients at risk for lower survival are those with “sarcopenic obesity,” or sacropenia plus obesity. It is not clear whether this “increased risk” resulted from obesity, sarcopenia, or the combination of both. These findings should be further investigated.
Many of the aforementioned studies used different and inconsistent measurements of fat and muscle mass. In most, calculations were based solely on single linear measurements of retrorenal fat thickness. We are the first investigators to use comprehensive and highly precise CT measures of visceral fat and muscle mass, including cross-sectional and volumetric measurements, in an attempt to elucidate relationships with pancreatic cancer. None of the multiple cross-sectional or linear measurements in this study correlated with tumor progression or survival, and neither did BMI. Standardization to patient body size did not change the picture. Despite some existing literature suggesting relationships may exist between pancreatic cancer and fat and muscle mass, we have shown herein that muscle and fat mass do not have meaningful contributions to our ability to predict pancreatic cancer behavior or outcome. It seems this rigorous investigation “closes the door” on this subset.
Apparently, pancreatic cancer biology and behavior are far too complex to predict with a CT scanner. The main focus of pancreatic cancer research should continue to be at the molecular, genetic and immunologic levels, in an attempt to reach a breakthrough knowledge that will improve the universal dismal prognosis associated with this disease.
Acknowledgments
Conflict of interest statement
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 5 December 2016
References
- 1.Sohal DP, Walsh RM, Ramanathan RK, et al. Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. JNCI J Natl Cancer Inst 2014;106:3. [DOI] [PubMed] [Google Scholar]
- 2.Beppu T, Miyamoto Y, Sakamoto Y, et al. Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann Surg Oncol 2014;21(suppl 3):S405–S413. [DOI] [PubMed] [Google Scholar]
- 3.Gish RG, Finn RS, Marrero JA. Extending survival with the use of targeted therapy in the treatment of hepatocellular carcinoma. Gastroenterol Hepatol 2013;9(suppl 2):1–24. [PMC free article] [PubMed] [Google Scholar]
- 4.Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. Chemother Res Pract 2014;2014:357027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leiter U, Meier F, Garbe C. Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete [Targeted therapies for melanoma]. Der Hautarzt 2014;65:600–6. [DOI] [PubMed] [Google Scholar]
- 6.Vongsuvanh R, George J, Qiao L, et al. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett 2013;330:1–10. [DOI] [PubMed] [Google Scholar]
- 7.Ladoire S, Bonnetain F, Gauthier M, et al. Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with antiangiogenic agents. Oncologist 2011;16:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark W, Siegel EM, Chen YA, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg 2013;216:1070–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rickles AS, Iannuzzi JC, Mironov O, et al. Visceral obesity and colorectal cancer: are we missing the boat with BMI? J Gastrointest Surg 2013;17:133–143. Discussion 43. [DOI] [PubMed] [Google Scholar]
- 10.Ballian N, Lubner MG, Munoz A, et al. Visceral obesity is associated with outcomes of total mesorectal excision for rectal adenocarcinoma. J Surg Oncol 2012;105:365–370. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa K, Shimada M, Kurita N, et al. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy-assisted gastrectomy for gastric cancer. Surg Endosc 2011;25:3825–3830. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Lawless MW. Stop feeding cancer: pro-inflammatory role of visceral adiposity in liver cancer. Cytokine 2013;64:626–637. [DOI] [PubMed] [Google Scholar]
- 13.Itoh S, Shirabe K, Matsumoto Y, et al. Effect of body composition on outcomes after hepatic resection for hepatocellular carcinoma. Ann Surg Oncol 2014;21:3063–68. [DOI] [PubMed] [Google Scholar]
- 14.Pausch T, Hartwig W, Hinz U, et al. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery 2012;152(suppl 1):S81–S88. [DOI] [PubMed] [Google Scholar]
- 15.Lieffers JR, Bathe OF, Fassbender K, et al. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harimoto N, Shirabe K, Yamashita YI, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 2013;100:1523–1530. [DOI] [PubMed] [Google Scholar]
- 17.Yip C, Goh V, Davies A, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol 2014;24:998–1005. [DOI] [PubMed] [Google Scholar]
- 18.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan BH, Birdsell LA, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973–6979. [DOI] [PubMed] [Google Scholar]
- 20.House MG, Fong Y, Arnaoutakis DJ, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg 2008;12:270–278. [DOI] [PubMed] [Google Scholar]
- 21.Gaujoux S, Cortes A, Couvelard A, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery 2010;148:15–23. [DOI] [PubMed] [Google Scholar]
- 22.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003;348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 23.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer 2007;120:1993–1998. [DOI] [PubMed] [Google Scholar]
- 24.Mathur A, Hernandez J, Shaheen F, et al. Preoperative computed tomography measurements of pancreatic steatosis and visceral fat: prognostic markers for dissemination and lethality of pancreatic adenocarcinoma. HPB 2011;13:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathur A, Luberice K, Paul H, et al. Increasing body mass index portends abbreviated survival following pancreatoduodenectomy for pancreatic adenocarcinoma. Am J Surg 2015;209:969–973. [DOI] [PubMed] [Google Scholar]
- 26.Tranchart H, Gaujoux S, Rebours V, et al. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg 2012;256:139–145. [DOI] [PubMed] [Google Scholar]
- 27.Mathur A, Zyromski NJ, Pitt HA, et al. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J Am Coll Surg 2009;208:989–994. Discussion 94–6. [DOI] [PubMed] [Google Scholar]
- 28.Tsai S, Choti MA, Assumpcao L, et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg 2010;14:1143–1150. [DOI] [PubMed] [Google Scholar]


