Abstract
Anemia and hemorrhagic shock are leading causes of morbidity and mortality worldwide, and transfusion of human blood products is the ideal treatment for these conditions. As human erythrocytes age during storage in blood banks they undergo many biochemical and structural changes, termed the red blood cell ‘storage lesion’. Specifically, ATP and pH levels decrease as metabolic end products, oxidative stress, cytokines, and cell-free hemoglobin increase. Also, membrane proteins and lipids undergo conformational and organizational changes that result in membrane loss, viscoelastic changes and microparticle formation. As a result, transfusion of aged blood is associated with a host of adverse consequences such as decreased tissue perfusion, increased risk of infection, and increased mortality. This review summarizes current research detailing the known parts of the erythrocyte storage lesion and their physiologic consequences.
Keywords: blood banking, review, storage lesion
Introduction
Hemorrhagic shock is the second-leading overall cause of early death as well as the most common cause of potentially preventable death in traumatically injured patients (Kauvar et al., 2006). In order to ameliorate this shock state, patients must undergo resuscitation in order to restore circulatory volume, reverse metabolic acidosis, and attenuate cellular hypoxia. Although the ideal resuscitation strategy remains controversial, this process commonly involves infusion of crystalloid salt solutions as well as the transfusion of human blood products (Holcomb et al., 2013). The failure to achieve specific endpoints of resuscitation is associated with an increased incidence of multiple organ failure and death (Brakenridge et al., 2011).
Several studies now suggest that transfusion of human blood products, including the use of stored red blood cell units (pRBCs), is the ideal treatment of hemorrhagic shock in the acute setting (Holcomb et al., 2007; Makley et al., 2010a; Holcomb and Pati, 2013; Schorn and Phillippi, 2014). Unfortunately, this use of blood products may result in later harm to the patient. Liberal transfusion strategies for pRBC have been associated with poor clinical outcomes and increased mortality in critically ill patients (Hébert et al., 1999; Vincent et al., 2002; Corwin et al., 2004; Gong et al., 2005; Holst et al., 2014; Robertson et al., 2014). The etiology of this harm is unclear, but it is thought that a combination of transfusion of larger volumes of blood (Sauaia et al., 1994), as well as use of older pRBCs (Weinberg et al., 2008, 2010; Mangalmurti et al., 2009), drive this effect. Due to these concerns, understanding the harm caused by transfusion of pRBC units has become an area of intense study in the care of trauma patients. Unfortunately, most of the literature regarding the adverse effects of transfusing aged pRBCs is retrospective and observational, and thus our understanding of the true effect of the age of pRBCs at the time of transfusion will remain limited until prospective trials are completed.
Standard blood banking inventory management relies on a first in, first out system whereby the oldest viable pRBC units are often used first (Koch et al., 2013). Thus, the average age of transfused pRBCs ranges from 20 to 30 days (Shapiro et al., 2003; Weinberg et al., 2008; Brown et al., 2014). As the life span of erythrocytes is 120 days, each pRBC transfused is a homogenous collection of cells in various phases of the aging process. This means that patients, when transfused, may be exposed to erythrocytes present in pRBCs that range in age from 42 to 162 days in chronological age.
As pRBCs age, they develop changes in biochemical and molecular parameters. Collectively, these changes are known as the red blood cell or erythrocyte ‘storage lesion’ (Koch et al., 2013). Compared to fresh units, transfusion of aged pRBCs has been associated with increased rates of pneumonia, sepsis, multi-organ failure, and mortality (Purdy et al., 1997; Zallen et al., 1999; Offner et al., 2002; Koch et al., 2008; Weinberg et al., 2008). In this review we summarize storage methods of pRBCs, the various molecular components of the red cell storage lesion that result from that storage, as well as the clinical effects that result from transfusion of aged blood.
Blood collection and storage
Human blood is collected from donors as whole blood directly into a solution anticoagulant citrate and nutrient phosphate and dextrose, or by apheresis into a solution of acid citrate dextrose (Hess, 2010a). Blood collected by apheresis is filtered free of most leukocytes and platelets. Whole blood collections are centrifuged to separate the erythrocytes from the anticoagulant solution, plasma, and buffy coat containing leukocytes and platelets. One method, the buffy coat method, involves draining the erythrocytes from the bottom of the collection bag and leaving the buffy coat and plasma behind. The other method requires a gentler centrifugation, which leaves the platelets suspended in the plasma. The platelet-rich plasma is removed and the remaining cells are passed through a leukocyte reducing filter that removes most leukocytes and platelets. The importance of this process, called leukoreduction, is a matter of debate in the transfusion community, and will be discussed in more detail later.
The concentrated erythrocytes are then mixed with a storage solution and stored at 1–6°C for up to 42 days (Wehrli, 2012). The first pRBC additive solution, developed in the late 1970s, contained saline-adenine-glucose (SAG) (Sparrow, 2012). Multiple variations of this formula have been developed in the years since. These additive solutions (AS) all contain membrane protectant sugars, volume for dilution of metabolic waste products, and nutrients, especially adenine (Cancelas et al., 2014). Three of these solutions have been approved for use in the United States (AS-1, AS-3, AS-5), while several others are in use around the world (SAGM, MAP, PAGGSM) (Sparrow, 2012). These storage solutions aid in the preservation of erythrocytes during storage, but aging and degradation still occurs in a predictable fashion, which is the focus of the current review.
Erythrocyte storage lesion
Biochemical parameters
The classic biochemical characteristics of the pRBC storage lesion include decreased ATP, 2,3-diphosphoglycerate, and pH, along with increased potassium, free hemoglobin, and lactate (Bennett-Guerrero et al., 2007; Hess, 2010b). Potassium and other ion imbalances result from the inactivation of membrane pumps during cold storage (Flatt et al., 2014). Erythrocytes lack mitochondria, and thus depend on glycolysis for energy. As erythrocytes age in storage, they metabolize glucose and generate lactate and protons as end products. These initial changes are seen rapidly, often in the first 2 weeks of cold storage (Karon et al., 2009). Excessive 2,3-diphosphoglycerate interacts with membrane proteins, causing structural changes, and also impacts the erythrocyte’s ability to deliver oxygen to the tissues (Tsai et al., 2004; Flatt et al., 2014). While these levels are normalized after transfusion, it takes up to 1 week for a full recovery (Solheim et al., 2004). Stored pRBCs also generate proinflammatory cytokines that have been implicated in subsequent tissue injury after transfusion (Seghatchian, 2006; Belizaire et al., 2012a; Matot et al., 2013).
Complement anaphylatoxins C3a and C5a, as well as the membrane attack complex (MAC), are also found in stored pRBCs (Solheim et al., 2003; Seghatchian, 2006). MAC levels increase as a function of time and have been suggested as the primary complement-mediated contributor to the storage lesion (Hu et al., 2014). The bolus of complement complexes received by a patient during transfusion of aged pRBCs likely leads to the activation of a number of host cell types, including endothelial cells, B cells, and circulating erythrocytes (Stowell et al., 2012), resulting in substantial complement-mediated inflammatory processes (Weinberg et al., 2011).
Morphology
During storage, the erythrocyte structural proteins, lipids, and carbohydrates undergo oxidative injury (Hess, 2010b). There is a time-dependent reduction in glutathione and glutathione-peroxidase activity (Dumaswala et al., 1999), resulting in a sensitivity to oxidation that increases with time of storage (Şekeroğlu et al., 2012). Oxidation of fatty acids results in lipid peroxidation and increased levels of malondialdehyde, which has known cytotoxic effects and can cross-link erythrocyte membrane phospholipids and proteins (Şekeroğlu et al., 2012). Formation of the spectrin-actin-protein 4.1 complex decreases during storage (Wolfe et al., 1986). Band 3, usually freely mobile, aggregates into large oligomers that may result in phospholipid loss (Kriebardis et al., 2007; Karon et al., 2009). This is the result of both protein loss and oxidative cross-linking.
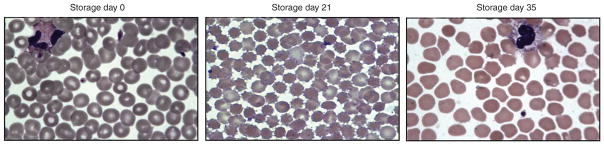
The cumulative result of these changes is that aging erythrocytes lose membrane domain as well as their classic biconcave disc shape, subsequently changing to echinocytes and spherocytes with a loss of their normal deformability (Figure 1) (Izzo et al., 1999; Bennett-Guerrero et al., 2007; Relevy et al., 2008; Karon et al., 2009; Hess, 2010b). Phosphatidylserine, normally on the intracellular side of the plasma membrane, becomes externalized (Hess, 2010b). These membrane changes and increased fragility contribute to increased acidosis and hemolysis observed during the storage of pRBCs, as well as decreased erythrocyte survival following transfusion (Haradin et al., 1969; Berezina et al., 2002; Bennett-Guerrero et al., 2007). A summary of common alterations to erythrocytes during storage can be found in Table 1.
Figure 1.
Light microscopy images demonstrating changes in human erythrocyte morphology during storage. pRBCs were stored in AS-3 at 4°C. Over time, erythrocytes develop loss of biconcave disc shape, subsequently changing to echinocytes and spherocytes. Images adopted from Makley et al. (2010b).
Table 1.
Characteristics of the erythrocyte storage lesion.
| Biochemical | Structural |
|---|---|
| ↓TP | ↓membrane domain |
| ↓2,3 diphosphoglycerate | ↓deformability |
| ↓pH | ↑microparticles |
| ↓nitric oxide | ↑spherocytosis |
| ↑potassium | ↑hemolysis |
| ↑free hemoglobin | ↑band 3 oligomers |
| ↑lactate | ↑ceramide rafts |
| ↑cytokines | ↑external phospatidylserine |
| ↑reactive oxygen species | ↑aggregability |
| ↑soluble lipids | ↑osmotic fragility |
| ↑complement proteins | ↑protein cross-linking |
Microparticles
During storage, pRBCs develop erythrocyte-derived microparticles (MPs) (Xiong et al., 2011; Gao et al., 2013). MPs are sub-cellular particles that range in size from 0.1 to 1.0μm (Lacroix et al., 2010). Although the process of MP formation is incompletely understood, it has been linked to spectrin oxidation (Wagner et al., 1987) as well as ATP depletion (Lutz et al., 1977). Erythrocyte-derived MPs contain hemoglobin, lipids, band 3, glycophorins, actin, lipid raft proteins, caspases, Fas, and extracellular phosphatidylserine, but lack spectrin and ankyrin (Greenwalt et al., 1984; Kriebardis et al., 2008; Koshiar et al., 2014). Stomatin has been implicated in the formation of storage-associated MPs (Salzer et al., 2008; Bosman et al., 2012), suggesting a raft-mediated mechanism of MP formation. The band 3 in MPs shows age-related aggregation and degradation (Bosman et al., 2012). There is also an age-related recruitment of proteins from the plasma, ubiquitin-proteasome system, and small G proteins to MPs (Bosman et al., 2012).
Experimental findings support the hypothesis that MP formation is part of a self-protective mechanism whereby damaged proteins or membrane patches are selected for removal in order to preserve the health of the remaining erythrocyte (Willekens et al., 2008; Bosman et al., 2012). This concept is further supported by the finding that red cell exchange, a procedure for treating patients with complicated sickle cell disease, results in a specific decrease in the number of erythrocyte-derived MPs in the circulation (Mahfoudhi et al., 2012). MP formation from circulating erythrocytes is increased in patients with hemoglobinopathies (Westerman et al., 2008), and by substituting normal erythrocytes for genetically abnormal erythrocytes, MP formation is reduced.
Evaluation of pRBC MPs via flow cytometry, the most common technique utilized in the laboratory setting, is limited because of the small size of these particles (Lacroix et al., 2010). Using techniques such as nanoparticle tracking analysis and dynamic light scattering, the average size of circulating MPs has been determined to range from 100 to 300 nm (Lawrie et al., 2009; Aatonen et al., 2014). However, the lower limit of detection by flow cytometry is 488 nm (Yuana et al., 2011). As such, much of the existing literature characterizing these MPs can only be interpreted as a description of MPs >500 nm. By combining flow cytometry and other available assays it is possible to build a more complete picture regarding these particles, but we are still quite limited by the available technology when it comes to understanding this increasingly important field of study.
Sphingolipids
Sphingolipids are essential eukaryotic cell membrane components and play an important role in cell signaling (Aguilera-Romero et al., 2014). Sphingolipid metabolism appears to play an important role in many of the erythrocyte structural changes that may occur during aging, as shown by Dinkla et al. (2012). By incubating erythrocytes with varying doses of sphingomyelinase, they were able to induce formation of ceramide-enriched platforms, phosphatidylserine exposure, band 3 changes, and CD59 clustering. These changes resulted in decreased cell size, increased cell fragility, and MP formation. Further, because inflammation can increase sphingomyelinase secretion, they suggest this as a contributing mechanism for the anemia of chronic illness. Sphingolipids have also been implicated in membrane changes that occur in sickle cell disease (Awojoodu et al., 2014) and Gaucher disease (Adar et al., 2008). Ceramides have also been suggested to form pores in erythrocyte membranes (Hatakeyama et al., 1999). Finally, mass spectrometry studies have shown phospholipid interactions to be involved in the membrane budding process that drives MP formation (Bicalho et al., 2013). Lipids, specifically sphingolipids, are clearly important components of erythrocyte membrane dynamics involved in the aging process.
Physiologic effects and clinical consequences
Morphology
Under normal flow conditions, erythrocytes, usually 6–9 μm in diameter, are able to flex their discoid shape in order to squeeze through capillary vessels that are only 3–6 μm wide (Stadler and Linderkamp, 1989). The decreased deformability of aged erythrocytes leads to reduced capillary flow and decreased oxygen delivery to tissues (Hess, 2010b). These changes also negatively impact the survival of transfused erythrocytes (Card, 1988). As these damaged cells attempt to pass through capillary beds, they aggregate and break down, resulting in hemolysis, microthrombosis, and occlusion of these tiny vessels. Aged erythrocytes have also been shown to have increased adhesion to endothelial cells (Anniss and Sparrow, 2006; Relevy et al., 2008), likely due to the increased phosphatidylserine on the external erythrocyte membrane (Hess, 2010b). This mechanism has been implicated in the microangiopathy and anemia associated with diabetes, sickle cell disease, and cancer (Klug et al., 1974; Cohen, 1981; Tsukada et al., 2001).
Free hemoglobin
Storage of pRBC units results in increased extracellular hemoglobin. This ‘cell-free hemoglobin’ is actually a combination of MP-bound and free floating hemoglobin and may have significant effects when transfused (Greenwalt et al., 1991). Under normal physiological conditions, nitric oxide (NO) is generated by endothelial nitric oxide synthase and plays a role in controlling blood flow by inducing relaxation of blood vessel smooth muscle (Palmer et al., 1987; Walford and Loscalzo, 2003). When present, cell-free hemoglobin scavenges nitric oxide (NO) at a significantly higher rate than erythrocyte-encapsulated hemoglobin (Vaughn et al., 2000; Azarov et al., 2005). Erythrocyte-derived MPs also contain large concentrations of hemoglobin and play a role in NO consumption (Donadee et al., 2011; Liu et al., 2013). NO scavenging by stored blood supernatant increases with length of storage, and is directly proportional to heme concentration (Donadee et al., 2011). Cell-free hemoglobin plays a role in ROS formation (Donadee et al., 2011), and NO uptake is also affected by the oxygenation status of hemoglobin (Azarov et al., 2005).
The cumulative impact of free hemoglobin in the pRBC unit is that transfusion may result in significant NO scavenging and reduction in the recipient. This reduction in NO is thought to impede endothelial-dependent vasodilation and subsequently affect end-organ perfusion (Bennett-Guerrero et al., 2007; Reynolds et al., 2007; Rigamonti et al., 2008; Neuman et al., 2014). The resultant impaired vasodilation may have a significant clinical impact. Decreased cerebral perfusion may be a reason that cardiac surgery patients receiving older pRBCs are at an increased risk for post-operative delirium (Brown et al., 2014). In a rat model of hemorrhage shock, transfusion of aged pRBCs compared to fresh pRBCs has negative effects on liver perfusion and necrosis (Matot et al., 2013). Baek and coworkers showed that transfusion of aged pRBCs with haptoglobin reduces intravascular hemolysis, acute hypertension, vascular injury, and kidney dysfunction associated with transfusion of cell-free hemoglobin (Baek et al., 2012). Similarly, Baron et al. demonstrated an increase in pulmonary artery pressure following transfusion of old blood, which they were able to mitigate with inhaled NO (Baron et al., 2012). By either scavenging the free hemoglobin or replacing NO it may be possible to ameliorate the negative effects of transfusing aged pRBCs.
One benchmark for pRBC storage is the survival of 75% of erythrocytes up to 24 h after transfusion (Dumont and AuBuchon, 2008). Given that one pRBC contains 220–250 mg of iron, transfusion recipients must rapidly clear greater than 50 mg of iron per pRBC transfused (Ozment and Turi, 2009). Hod et al. have described the effects of non-transferrin-bound iron in both animal models and human trials (Hod et al., 2011, 2010). Following transfusion, iron deposition is visibly evident in the liver, spleen, and kidney. This free iron leads to increased systemic inflammation and has been linked to increased infection susceptibility, specifically Escherichia coli proliferation. This mechanism is likely similar to the increased infection susceptibility seen in hemochromatosis patients (Khan et al., 2007). Clinical studies have correlated age of pRBCs transfused, as well as number of units transfused, with infectious complications (Mynster and Nielsen, 2000; Horvath et al., 2013). Indeed, patients receiving multiple transfusions are also getting a bolus of cell-free hemoglobin, and this appears to have multiple negative consequences.
Microparticles
Transfusion of aged pRBCs has been clinically associated with increased incidence of deep vein thrombosis (Spinella et al., 2009). Elevated levels of erythrocyte-derived MPs in patients have been associated with increased thrombin formation and complement activation (Jy et al., 2013; Koshiar et al., 2014; Zecher et al., 2014). In animal models, transfusion of aged pRBCs causes increased coagulopathy compared to fresh pRBCs (Vlaar et al., 2010). MPs from aged pRBCs have been shown in vitro to induce thrombin generation, potentially due to increased phosphatidylserine expression, possibly in a factor XII-dependent manner (Sweeney et al., 2009; Van Der Meijden et al., 2012; Gao et al., 2013). Coagulopathies associated with microparticles, including those from erythrocytes, are well described and cause significant morbidity.
Transfusion of stored blood in cardiac surgery patients is associated with increased rates of pneumonia (Horvath et al., 2013), and MPs in stored blood may play a role. Aged pRBCs cause increased lung microvascular permeability and neutrophil migration compared to fresh pRBCs (Mangalmurti et al., 2009). This finding is either the result of MP accumulation in aged blood (Belizaire et al., 2012a,b) or other factors in the pRBC supernatant (Silliman et al., 1998). In mouse and rat models, washing aged pRBCs prior to transfusion resulted in diminishing the lung injury (Vlaar et al., 2010; Belizaire et al., 2012a). Initially, the injurious substance was thought to be cytokines or other cell-signaling proteins. However, recent evidence has indicated that MPs are a significant cause of lung inflammation (Belizaire et al., 2012b).
Leukoreduction
The issue of leukoreduction is a hotly debated topic. Blood banks in Canada and most of Europe routinely leukoreduce pRBCs prior to storage, whereas practice patterns in the United States vary. Basic science evidence suggests that leukoreduction improves viscoelastic properties of stored pRBCs as well as reduces immunoglobulins, complement proteins, and cytokines that are generate during storage (Solheim et al., 2003; Seghatchian, 2006; Nagura et al., 2013; Sowemimo-Coker, 2014). From a theoretical standpoint, leukocytes in stored blood are more biologically active than erythrocytes and are responsible for the deleterious production of inflammatory proteins that drive the storage lesion. Thus, by banking blood that has been cleansed of leukocytes, the storage lesion will be greatly improved.
The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists clinical practice guidelines in 2011 recognizes level IIa evidence supporting the use of leukoreduced blood, specifically for patients undergoing cardiac procedures (Ferraris et al., 2011). In clinical studies, leukoreduction has been shown to reduce infectious complications of pRBC transfusion (Fergusson et al., 2004; Blumberg et al., 2007; Friese et al., 2008). Leukoreduction has also been associated with improved mortality (van de Watering et al., 1998; Hébert et al., 2003), decreased length of stay (Fung et al., 2006), fewer febrile transfusion reactions (Hébert et al., 2003; King et al., 2004), and reduced rates of acute respiratory distress syndrome (Plurad et al., 2008) in transfusion recipients. However, there are several clinical studies that show no difference in outcomes with leukoreduction (Sharma et al., 2002; Wallis et al., 2002; Llewelyn et al., 2004; Nathens et al., 2006; Capraro et al., 2007; Phelan et al., 2007; Frietsch et al., 2008; Watkins et al., 2008). As no data suggest that leukoreduction is more harmful than not performing leukoreduction, implementation of practice may revolve cost-effectiveness (Cleemput et al., 2006), as the cost of leukoreducing one unit of pRBCs is between $20 and $40 (van Hulst et al., 2005). While historic opinion has been to purify pRBCs as much as possible to avoid patient harm, further research is necessary to determine when a specific benefit exists for leukoreducing blood for storage. Additional work is needed in this area of controversy as stronger basic and clinical evidence would support universal leukoreduction. If universal leukoreduction is not necessary, the specific population of transfusion recipients who would benefit from leukoreduction needs to be defined.
Conclusion
The ability to acquire, store, and transfuse human blood products is absolutely necessary in modern medicine, especially for the treatment of hemorrhage and anemia. Much progress has been made in understanding the erythrocyte storage lesion and its clinical implications. As erythrocytes age, in storage as well as in circulation, they undergo a variety of biochemical and structural changes. In the body, aging cells are targeted for elimination before becoming harmful. However, upon transfusion, millions of aged and senescent erythrocytes are introduced into a patient who is already under physiologic stress. Subsequently, the burden of acidic, hemolytic, and inefficient erythrocytes can create problems for the new host, such as increased susceptibility to infection and impaired end-organ perfusion. pRBC aging and storage will continue to be a fervent area of research until blood banks around the world are able to adequately meet the needs of their patient populations.
Acknowledgments
TAP was supported in part by grant R01 GM107625 from the National Institute of General Medical Sciences of the US National Institutes of Health. The authors would also like to acknowledge Dr. Erich Gulbins of the University of Duisburg-Essen for his contributions to this project and others.
Biography
![]() From left to right: Richard Hoehn, Peter Jernigan, and Alex Chang are surgical residents at the University of Cincinnati College of Medicine, currently participating in a research fellowship. Their areas of research interest include blood banking, trauma, and sphingolipids. Timothy Pritts is a trauma surgeon at the University of Cincinnati, with research interests including blood banking, shock and resuscitation, lung injury, and traumatic brain injury. Michael Edwards is the chairman of surgery at the University of Cincinnati, with specific research interests including sphingolipids and translational medicine, and is also an avid car racing enthusiast.
From left to right: Richard Hoehn, Peter Jernigan, and Alex Chang are surgical residents at the University of Cincinnati College of Medicine, currently participating in a research fellowship. Their areas of research interest include blood banking, trauma, and sphingolipids. Timothy Pritts is a trauma surgeon at the University of Cincinnati, with research interests including blood banking, shock and resuscitation, lung injury, and traumatic brain injury. Michael Edwards is the chairman of surgery at the University of Cincinnati, with specific research interests including sphingolipids and translational medicine, and is also an avid car racing enthusiast.
Contributor Information
Richard S. Hoehn, Department of Surgery and Institute for Military Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267–0558, USA
Peter L. Jernigan, Department of Surgery and Institute for Military Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267–0558, USA
Alex L. Chang, Department of Surgery and Institute for Military Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267–0558, USA
Michael J. Edwards, Department of Surgery and Institute for Military Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267–0558, USA
Timothy A. Pritts, Department of Surgery and Institute for Military Medicine, University of Cincinnati, 231 Albert Sabin Way, Cincinnati, OH 45267–0558, USA.
References
- Aatonen MT, Ohman T, Nyman TA, Laitinen S, Grönholm M, Siljander PR. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles. 2014;3:10. doi: 10.3402/jev.v3.24692. 3402/jev.v3.24692. Published online 2014 Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar T, Ben-Ami R, Elstein D, Zimran P, Berliner S, Yedgar S, Barshtein G. Increased red blood cell aggregation in patients with Gaucher disease is non-inflammatory. Clin Hemorheol Microcirc. 2008;40:113–118. [PubMed] [Google Scholar]
- Aguilera-Romero A, Gehin C, Riezman H. Sphin-golipid homeostasis in the web of metabolic routes. Biochim Biophys Acta. 2014;1841:647–656. doi: 10.1016/j.bbalip.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Anniss AM, Sparrow RL. Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion. 2006;46:1561–1567. doi: 10.1111/j.1537-2995.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- Awojoodu AO, Keegan PM, Lane AR, Zhang Y, Lynch KR, Platt MO, Botchwey EA. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood. 2014;124:1941–1950. doi: 10.1182/blood-2014-01-543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280:39024–39032. doi: 10.1074/jbc.M509045200. [DOI] [PubMed] [Google Scholar]
- Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesi-ology. 2012;116:637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizaire RM, Makley AT, Campion EM, Sonnier DI, Goodman MD, Dorlac WC, Friend LA, Lentsch AB, Pritts TA. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg. 2012a;73:S128–S133. doi: 10.1097/TA.0b013e3182606301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizaire RM, Prakash PS, Richter JR, Robinson BR, Edwards MJ, Caldwell CC, Lentsch AB, Pritts TA. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J Am Coll Surg. 2012b;214:648–655. doi: 10.1016/j.jamcollsurg.2011.12.032. discussion 656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezina TL, Zaets SB, Morgan C, Spillert CR, Kamiyama M, Spolarics Z, Deitch EA, Machiedo GW. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- Bicalho B, Holovati JL, Acker JP. Phospholipidomics reveals differences in glycerophosphoserine profiles of hypothermically stored red blood cells and microvesicles. Biochim Biophys Acta. 2013;1828:317–326. doi: 10.1016/j.bbamem.2012.10.026. [DOI] [PubMed] [Google Scholar]
- Blumberg N, Zhao H, Wang H, Messing S, Heal JM, Lyman GH. The intention-to-treat principle in clinical trials and meta-analyses of leukoreduced blood transfusions in surgical patients. Transfusion. 2007;47:573–581. doi: 10.1111/j.1537-2995.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Bosman GJ, Lasonder E, Groenen-Döpp YA, Willekens FL, Werre JM. The proteome of erythrocyte-derived microparticles from plasma: new clues for erythrocyte aging and vesiculation. J Proteomics. 2012;76:203–210. doi: 10.1016/j.jprot.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Brakenridge SC, Phelan HA, Henley SS, Golden RM, Kashner TM, Eastman AE, Sperry JL, Harbrecht BG, Moore EE, Cuschieri J, et al. Early blood product and crystalloid volume resuscitation: risk association with multiple organ dysfunction after severe blunt traumatic injury. J Trauma. 2011;71:299–305. doi: 10.1097/TA.0b013e318224d328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Grega M, Selnes OA, McKhann GM, Shah AS, LaFlam A, Savage WJ, Frank SM, Hogue CW, Gottesman RF. Length of red cell unit storage and risk for delirium after cardiac surgery. Anesth Analg. 2014;119:242–250. doi: 10.1213/ANE.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancelas JA, Dumont LJ, Maes LA, Rugg N, Herschel L, Whitley PH, Szczepiokowski ZM, Siegel AH, Hess JR, Zia M. Additive solution-7 reduces the red blood cell cold storage lesion. Transfusion. 2014;55:491–498. doi: 10.1111/trf.12867. [DOI] [PubMed] [Google Scholar]
- Capraro L, Kuitunen A, Vento AE, Suojaranta-Ylinen R, Kolho E, Pettilä V. Universal leukocyte reduction of transfused red cells does not provide benefit to patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2007;21:232–236. doi: 10.1053/j.jvca.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Card RT. Red cell membrane changes during storage. Transfus Med Rev. 1988;2:40–47. doi: 10.1016/s0887-7963(88)70030-9. [DOI] [PubMed] [Google Scholar]
- Cleemput I, Leys M, Ramaekers D, Bonneux L. Balancing evidence and public opinion in health technology assessments: the case of leukoreduction. Int J Technol Assess Health Care. 2006;22:403–407. doi: 10.1017/S0266462306051312. [DOI] [PubMed] [Google Scholar]
- Cohen MH. Influence of tumor burden on red blood cell deformability in small cell lung cancer patients. Ann Clin Res. 1981;13:387–391. [PubMed] [Google Scholar]
- Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, Maclntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- Dinkla S, Wessels K, Verdurmen WP, Tomelleri C, Cluitmans JC, Fransen J, Fuchs B, Schiller J, Joosten I, Brock R, et al. Functional consequences of sphingomyelinase-induced changes in erythrocyte membrane structure. Cell Death Dis. 2012;3:e410. doi: 10.1038/cddis.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaswala UJ, Zhuo L, Jacobsen DW, Jain SK, Sukalski KA. Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Radic Biol Med. 1999;27:1041–1049. doi: 10.1016/s0891-5849(99)00149-5. [DOI] [PubMed] [Google Scholar]
- Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- Fergusson D, Khanna MP, Tinmouth A, Hébert PC. Transfusion of leukoreduced red blood cells may decrease postoperative infections: two meta-analyses of randomized controlled trials. Can J Anaesth. 2004;51:417–424. doi: 10.1007/BF03018302. [DOI] [PubMed] [Google Scholar]
- Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Shore-Lesserson LJ, Goodnough LT, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Flatt JF, Bawazir WM, Bruce LJ. The involvement of cation leaks in the storage lesion of red blood cells. Front Physiol. 2014;5:214. doi: 10.3389/fphys.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese RS, Sperry JL, Phelan HA, Gentilello LM. The use of leukoreduced red blood cell products is associated with fewer infectious complications in trauma patients. Am J Surg. 2008;196:56–61. doi: 10.1016/j.amjsurg.2007.08.063. [DOI] [PubMed] [Google Scholar]
- Frietsch T, Karger R, Schöler M, Huber D, Bruckner T, Kretschmer V, Schmidt S, Leidinger W, Weiler-Lorentz A. Leukodepletion of autologous whole blood has no impact on perioperative infection rate and length of hospital stay. Transfusion. 2008;48:2133–2142. doi: 10.1111/j.1537-2995.2008.01804.x. [DOI] [PubMed] [Google Scholar]
- Fung MK, Moore K, Ridenour M, Mook W, Triulzi DJ. Clinical effects of reverting from leukoreduced to nonleukoreduced blood in cardiac surgery. Transfusion. 2006;46:386–91. doi: 10.1111/j.1537-2995.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Lv L, Liu S, Ma G, Su Y. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sang. 2013;105:11–17. doi: 10.1111/vox.12014. [DOI] [PubMed] [Google Scholar]
- Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- Greenwalt TJ, Bryan DJ, Dumaswala UJ. Erythrocyte membrane vesiculation and changes in membrane composition during storage in citrate-phosphate-dextrose-adenine-1. Vox Sang. 1984;47:261–270. doi: 10.1111/j.1423-0410.1984.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Greenwalt TJ, McGuinness CG, Dumaswala UJ. Studies in red blood cell preservation: 4. Plasma vesicle hemoglobin exceeds free hemoglobin. Vox Sang. 1991;61:14–17. doi: 10.1111/j.1423-0410.1991.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Haradin AR, Weed RI, Reed CF. Changes in physical properties of stored erythrocytes relationship to survival in vivo. Transfusion. 1969;9:229–237. doi: 10.1111/j.1537-2995.1969.tb04929.x. [DOI] [PubMed] [Google Scholar]
- Hatakeyama T, Sato T, Taira E, Kuwahara H, Niidome T, Aoyagi H. Characterization of the interaction of hemolytic lectin CEL-III from the marine invertebrate, Cucumaria echinata, with artificial lipid membranes: involvement of neutral sphingoglycolipids in the pore-forming process. J Biochem. 1999;125:277–284. doi: 10.1093/oxfordjournals.jbchem.a022284. [DOI] [PubMed] [Google Scholar]
- Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Hébert PC, Fergusson D, Blajchman MA, Wells GA, Kmetic A, Coyle D, Heddle N, Germain M, Goldman M, Toye B, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. J Am Med Assoc. 2003;289:1941–1949. doi: 10.1001/jama.289.15.1941. [DOI] [PubMed] [Google Scholar]
- Hess JR. Conventional blood banking and blood component storage regulation: opportunities for improvement. Blood Transfus. 2010a;8(Suppl 3):s9–15. doi: 10.2450/2010.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010b;43:51–59. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon’s perspective. Hematology Am Soc Hematol Educ Program. 2013;2013:656–659. doi: 10.1182/asheducation-2013.1.656. [DOI] [PubMed] [Google Scholar]
- Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. J Am Med Assoc Surg. 2013;148:127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Aneman A, Marianne L, Vang MD, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381–1391. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- Horvath KA, Acker MA, Chang H, Bagiella E, Smith PK, Iribarne A, Kron IL, Lackner P, Argenziano M, Ascheim DD, et al. Blood transfusion and infection after cardiac surgery. Ann Thorac Surg. 2013;95:2194–2201. doi: 10.1016/j.athoracsur.2012.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Patel RP, Weinberg JA, Marques MB, Ramos TN, Barnum SR. Membrane attack complex generation increases as a function of time in stored blood. Transfus Med. 2014;24:114–116. doi: 10.1111/tme.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo P, Manicone A, Spagnuolo A, Lauta VM, Di Pasquale A, Di Monte D. Erythrocytes stored in CPD SAG-mannitol: evaluation of their deformability. Clin Hemorheol Microcirc. 1999;21:335–339. [PubMed] [Google Scholar]
- Jy W, Johansen ME, Bidot C, Horstman LL, Ahn YS. Red cell-derived microparticles (RMP) as haemostatic agent. Thromb Haemost. 2013;110:751–760. doi: 10.1160/TH12-12-0941. [DOI] [PubMed] [Google Scholar]
- Karon BS, Hoyer JD, Stubbs JR, Thomas DD. Changes in Band 3 oligomeric state precede cell membrane phospholipid loss during blood bank storage of red blood cells. Transfusion. 2009;49:1435–1442. doi: 10.1111/j.1537-2995.2009.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- Khan FA, Fisher MA, Khakoo RA. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis. 2007;11:482–487. doi: 10.1016/j.ijid.2007.04.007. [DOI] [PubMed] [Google Scholar]
- King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44:25–29. doi: 10.1046/j.0041-1132.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- Klug PP, Lessin LS, Radice P. Rheological aspects of sickle cell disease. Arch Intern Med. 1974;133:577–590. [PubMed] [Google Scholar]
- Koch CG, Figueroa PI, Li L, Sabik JF, Mihaljevic T, Blackstone EH. Red blood cell storage: how long is too long? Ann Thorac Surg. 2013;96:1894–1899. doi: 10.1016/j.athoracsur.2013.05.116. [DOI] [PubMed] [Google Scholar]
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- Koshiar RL, Somajo S, Norström E, Dahlbäck B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS One. 2014;9:e104200. doi: 10.1371/journal.pone.0104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion. 2007;47:1212–1220. doi: 10.1111/j.1537-2995.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–1953. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- Lacroix R, Robert S, Poncelet P, Dignat-George F. Overcoming limitations of microparticle measurement by flow cytometry. Semin Thromb Hemost. 2010;36:807–818. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- Lawrie AS, Albanyan A, Cardigan RA, Mackie IJ, Harrison P. Microparticle sizing by dynamic light scattering in fresh-frozen plasma. Vox Sang. 2009;96:206–12. doi: 10.1111/j.1423-0410.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhao W, Christ GJ, Gladwin MT, Kim-Shapiro DB. Nitric oxide scavenging by red cell microparticles. Free Radic Biol Med. 2013;65:1164–1173. doi: 10.1016/j.freeradbiomed.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewelyn CA, Taylor RS, Todd AA, Stevens W, Murphy MF, Williamson LM, Group LS. The effect of universal leukoreduction on postoperative infections and length of hospital stay in elective orthopedic and cardiac surgery. Transfusion. 2004;44:489–500. doi: 10.1111/j.1537-2995.2004.03325.x. [DOI] [PubMed] [Google Scholar]
- Lutz HU, Liu SC, Palek J. Release of spectrin-free vesicles from human erythrocytes during ATP depletion. I Characterization of spectrin-free vesicles. J Cell Biol. 1977;73:548–560. doi: 10.1083/jcb.73.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfoudhi E, Lecluse Y, Driss F, Abbes S, Flaujac C, Garçon L. Red cells exchanges in sickle cells disease lead to a selective reduction of erythrocytes-derived blood microparticles. Br J Haematol. 2012;156:545–547. doi: 10.1111/j.1365-2141.2011.08897.x. [DOI] [PubMed] [Google Scholar]
- Makley AT, Goodman MD, Friend LA, Deters JS, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Resuscitation with fresh whole blood ameliorates the inflammatory response after hemorrhagic shock. J Trauma. 2010a;68:305–311. doi: 10.1097/TA.0b013e3181cb4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makley AT, Goodman MD, Friend LA, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Murine blood banking: characterization and comparisons to human blood. Shock. 2010b;34:40–45. doi: 10.1097/SHK.0b013e3181d494fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, et al. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–1166. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matot I, Katz M, Pappo O, Zelig O, Corchia N, Yedgar S, Barshtein G, Bennett-Guerrero E, Abramovitch R. Resuscitation with aged blood exacerbates liver injury in a hemorrhagic rat model. Crit Care Med. 2013;41:842–849. doi: 10.1097/CCM.0b013e3182711b38. [DOI] [PubMed] [Google Scholar]
- Mynster T, Nielsen HJ. The impact of storage time of transfused blood on postoperative infectious complications in rectal cancer surgery. Danish RANX05 Colorectal Cancer Study Group. Scand J Gastroenterol. 2000;35:212–217. doi: 10.1080/003655200750024416. [DOI] [PubMed] [Google Scholar]
- Nagura Y, Tsuno NH, Tanaka M, Matsuhashi M, Takahashi K. The effect of pre-storage whole-blood leukocyte reduction on cytokines/chemokines levels in autologous CPDA-1 whole blood. Transfus Apher Sci. 2013;49:223–230. doi: 10.1016/j.transci.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Nathens AB, Nester TA, Rubenfeld GD, Nirula R, Gernsheimer TB. The effects of leukoreduced blood transfusion on infection risk following injury: a randomized controlled trial. Shock. 2006;26:342–347. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- Neuman R, Hayek S, Rahman A, Poole JC, Menon V, Sher S, Newman JL, Karatela S, Polhemus D, Lefer DJ, et al. Effects of storage-aged red blood cell transfusions on endothelial function in hospitalized patients. Transfusion. 2014 Nov 13; doi: 10.1111/trf.12919. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–6. doi: 10.1001/archsurg.137.6.711. discussion 716–717. [DOI] [PubMed] [Google Scholar]
- Ozment CP, Turi JL. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim Biophys Acta. 2009;1790:694–701. doi: 10.1016/j.bbagen.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Phelan HA, Sperry JL, Friese RS. Leukoreduction before red blood cell transfusion has no impact on mortality in trauma patients. J Surg Res. 2007;138:32–36. doi: 10.1016/j.jss.2006.07.048. [DOI] [PubMed] [Google Scholar]
- Plurad D, Belzberg H, Schulman I, Green D, Salim A, Inaba K, Rhee P, Demetriades D. Leukoreduction is associated with a decreased incidence of late onset acute respiratory distress syndrome after injury. Am Surg. 2008;74:117–123. [PubMed] [Google Scholar]
- Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48:136–146. doi: 10.1111/j.1537-2995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci USA. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigamonti A, McLaren AT, Mazer CD, Nix K, Ragoonanan T, Freedman J, Harrington A, Hare GM. Storage of strain-specific rat blood limits cerebral tissue oxygen delivery during acute fluid resuscitation. Br J Anaesth. 2008;100:357–364. doi: 10.1093/bja/aem401. [DOI] [PubMed] [Google Scholar]
- Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, Baldwin A, Rivera Lara L, Saucedo-Crespo H, Ahmed O, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. J Am Med Assoc. 2014;312:36–47. doi: 10.1001/jama.2014.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer U, Zhu R, Luten M, Isobe H, Pastushenko V, Perkmann T, Hinterdorfer P, Bosman GJ. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion. 2008;48:451–462. doi: 10.1111/j.1537-2995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- Schorn MN, Phillippi JC. Volume replacement following severe postpartum hemorrhage. J Midwifery Womens Health. 2014;59:336–343. doi: 10.1111/jmwh.12186. [DOI] [PubMed] [Google Scholar]
- Seghatchian J. Platelet storage lesion: an update on the impact of various leukoreduction processes on the biological response modifiers. Transfus Apher Sci. 2006;34:125–130. doi: 10.1016/j.transci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Şekeroğlu MR, Huyut Z, Him A. The susceptibility of erythrocytes to oxidation during storage of blood: effects of melatonin and propofol. Clin Biochem. 2012;45:315–9. doi: 10.1016/j.clinbiochem.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Shapiro MJ, Gettinger A, Corwin HL, Napolitano L, Levy M, Abraham E, Fink MP, MacIntyre N, Pearl RG, Shabot MM. Anemia and blood transfusion in trauma patients admitted to the intensive care unit. J Trauma. 2003;55:269–273. doi: 10.1097/01.TA.0000080530.77566.04. discussion 273–274. [DOI] [PubMed] [Google Scholar]
- Sharma AD, Slaughter TF, Clements FM, Sreeram G, Newman MF, Phillips-Bute B, Bredehoeft SJ, Smith PK, Stafford-Smith M. Association of leukocyte-depleted blood transfusions with infectious complications after cardiac surgery. Surg Infect (Larchmt) 2002;3:127–133. doi: 10.1089/109629602760105790. [DOI] [PubMed] [Google Scholar]
- Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim BG, Flesland O, Brosstad F, Mollnes TE, Seghatchian J. Improved preservation of coagulation factors after pre-storage leukocyte depletion of whole blood. Transfus Apher Sci. 2003;29:133–139. doi: 10.1016/S1473-0502(03)00117-4. [DOI] [PubMed] [Google Scholar]
- Solheim BG, Flesland O, Seghatchian J, Brosstad F. Clinical implications of red blood cell and platelet storage lesions: an overview. Transfus Apher Sci. 2004;31:185–189. doi: 10.1016/j.transci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sowemimo-Coker SO. Evaluation of an experimental filter designed for improving the quality of red blood cells (RBCs) during storage by simultaneously removing white blood cells and immunomodulators and improving RBC viscoelasticity and Band 3 proteins. Transfusion. 2014;54:592–601. doi: 10.1111/trf.12330. [DOI] [PubMed] [Google Scholar]
- Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10(Suppl 2):s7–11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler A, Linderkamp O. Flow behavior of neonatal and adult erythrocytes in narrow capillaries. Microvasc Res. 1989;37:267–279. doi: 10.1016/0026-2862(89)90045-9. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Winkler AM, Maier CL, Arthur CM, Smith NH, Girard-Pierce KR, Cummings RD, Zimring JC, Hendrickson JE. Initiation and regulation of complement during hemolytic transfusion reactions. Clin Dev Immunol. 2012;2012:307093. doi: 10.1155/2012/307093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney J, Kouttab N, Kurtis J. Stored red blood cell supernatant facilitates thrombin generation. Transfusion. 2009;49:1569–1579. doi: 10.1111/j.1537-2995.2009.02196.x. [DOI] [PubMed] [Google Scholar]
- Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44:1626–1634. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- Tsukada K, Sekizuka E, Oshio C, Minamitani H. Direct measurement of erythrocyte deformability in diabetes mellitus with a transparent microchannel capillary model and high-speed video camera system. Microvasc Res. 2001;61:231–239. doi: 10.1006/mvre.2001.2307. [DOI] [PubMed] [Google Scholar]
- van de Watering LM, Hermans J, Houbiers JG, van den Broek PJ, Bouter H, Boer F, Harvey MS, Huysmans HA, Brand A. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998;97:562–568. doi: 10.1161/01.cir.97.6.562. [DOI] [PubMed] [Google Scholar]
- Van Der Meijden PE, Van Schilfgaarde M, Van Oerle R, Renné T, ten Cate H, Spronk HM. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012;10:1355–1362. doi: 10.1111/j.1538-7836.2012.04758.x. [DOI] [PubMed] [Google Scholar]
- van Hulst M, Bilgin YM, van de Watering LM, de Vries R, van Oers MH, Brand A, Postma MJ. Cost-effectiveness of leucocyte-depleted erythrocyte transfusion in cardiac valve surgery. Transfus Med. 2005;15:209–217. doi: 10.1111/j.1365-3148.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275:2342–2348. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D. Anemia and blood transfusion in critically ill patients. J Am Med Assoc. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- Vlaar AP, Hofstra JJ, Levi M, Kulik W, Nieuwland R, Tool AT, Schultz MJ, de Korte D, Juffermans NP. Supernatant of aged erythrocytes causes lung inflammation and coagulopathy in a “two-hit” in vivo syngeneic transfusion model. Anesthesiology. 2010;113:92–103. doi: 10.1097/ALN.0b013e3181de6f25. [DOI] [PubMed] [Google Scholar]
- Wagner GM, Chiu DT, Qju JH, Heath RH, Lubin BH. Spectrin oxidation correlates with membrane vesiculation in stored RBCs. Blood. 1987;69:1777–1781. [PubMed] [Google Scholar]
- Walford G, Loscalzo J. Nitric oxide in vascular biology. J Thromb Haemost. 2003;1:2112–2118. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- Wallis JP, Chapman CE, Orr KE, Clark SC, Forty JR. Effect of WBC reduction of transfused RBCs on postoperative infection rates in cardiac surgery. Transfusion. 2002;42:1127–1134. doi: 10.1046/j.1537-2995.2002.00181.x. [DOI] [PubMed] [Google Scholar]
- Watkins TR, Rubenfeld GD, Martin TR, Nester TA, Caldwell E, Billgren J, Ruzinski J, Nathens AB. Effects of leukoreduced blood on acute lung injury after trauma: a randomized controlled trial. Crit Care Med. 2008;36:1493–1499. doi: 10.1097/CCM.0b013e318170a9ce. [DOI] [PubMed] [Google Scholar]
- Wehrli G. Blood banking and transfusion medicine for the nephrologist. Semin Dial. 2012;25:114–118. doi: 10.1111/j.1525-139X.2011.01021.x. [DOI] [PubMed] [Google Scholar]
- Weinberg JA, McGwin G, Marques MB, Cherry SA, Reiff DA, Kerby JD, Rue LW. Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008;65:794–798. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- Weinberg JA, McGwin G, Vandromme MJ, Marques MB, Melton SM, Reiff DA, Kerby JD, Rue LW. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69:1427–31. doi: 10.1097/TA.0b013e3181fa0019. discussion 1431–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JA, Barnum SR, Patel RP. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion. 2011;51:867–873. doi: 10.1111/j.1537-2995.2011.03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman M, Pizzey A, Hirschman J, Cerino M, Weil-Weiner Y, Ramotar P, Eze A, Lawrie A, Purdy G, Mackie I, et al. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br J Haematol. 2008;142:126–135. doi: 10.1111/j.1365-2141.2008.07155.x. [DOI] [PubMed] [Google Scholar]
- Willekens FL, Werre JM, Groenen-Döpp YA, Roerdinkholder-Stoelwinder B, de Pauw B, Bosman GJ. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–556. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- Wolfe LC, Byrne AM, Lux SE. Molecular defect in the membrane skeleton of blood bank-stored red cells. Abnormal spectrin-protein 4.1-actin complex formation. J Clin Invest. 1986;78:1681–1686. doi: 10.1172/JCI112762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z, Cavaretta J, Qu L, Stolz DB, Triulzi D, Lee JS. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 2011;51:610–621. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011;105:396–408. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- Zecher D, Cumpelik A, Schifferli JA. Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arterioscler Thromb Vasc Biol. 2014;34:313–320. doi: 10.1161/ATVBAHA.113.302378. [DOI] [PubMed] [Google Scholar]