Abstract
We study how maternal stress affects offspring outcomes. We find that in-utero exposure to elevated levels of the stress hormone cortisol negatively affects offspring cognition, health and educational attainment. These findings are based on comparisons between siblings which limits variation to short-lived shocks and controls for unobserved differences between mothers that could bias estimates. Our results are consistent with recent experimental results in the neurobiological literature linking exogenous exposure to stress hormones in-utero with declines in offspring cognitive, behavioral and motor development. Moreover, we find that not only are mothers with low levels of human capital characterized by higher and more variable cortisol levels, but that the negative impact of elevated cortisol on their offspring is greater. These results suggest that maternal stress may play a role in the intergenerational persistence of poverty.
I. Introduction
Intergenerational correlations in economic status in the US are high (Corak, 2004; Solon, 1999). Boys born to families with income in the bottom quintile of the income distribution have a 42 percent chance of remaining there as adults and only a five percent chance of reaching the top quintile. Yet little is known about the mechanisms by which parents transmit their economic status to their children. In this work we focus on whether and to what extent exposure to environmental stressors influence offspring outcomes.
We focus on prenatal exposure to stress as a mechanism by which parents affect the human capital and, by extension, the economic outcomes of their children for two reasons. First, poverty is associated with greater levels of stress. The poor, on average, report a greater number of stressful events in their lives and researchers have observed higher levels of the biological markers for stress in low socio-economic status adults. Second, recent evidence in neurobiology based largely on animal experiments suggests that exogenous exposure to stress in-utero negatively affects the cognitive, behavioral and motor development of offspring. Given that cognition is an important determinant of adult human capital and economic status, greater in-utero exposure to stress among the poor has the potential to explain, in part, the intergenerational persistence of poverty in the US.
In this paper we estimate the impact of in-utero exposure to stress on the human capital accumulation (years of schooling) of adult offspring using a unique dataset with detailed information on parental characteristics, including prenatal levels of the hormone cortisol (a marker for stress) and offspring outcomes. Because mothers with greater stress may differ in unobserved ways from those with lower stress, we include maternal fixed effects to address potential omitted variable bias. In this way, we limit identifying variation to temporary shocks during the prenatal period that may also persist short term to the post-natal period, but do not persist long term.1 When we include maternal fixed effects, we find that exposure to elevated cortisol in-utero has a negative and significant impact on offspring educational attainment. To better understand why, we estimate the impact of prenatal stress on two intermediate outcomes: cognition (verbal IQ) and health (a severe chronic condition) at age seven. We find that exposure to prenatal stress negatively and significantly affects offspring IQ and child health at age seven. We follow with a number of robustness checks and an analysis of whether and how prenatal stress relates to prenatal and postnatal investments in children.
Finally, we explore how prenatal stress interacts with maternal human capital. We find that not only are mothers with less schooling more likely to have high measures of the stress hormone cortisol (consistent with existing evidence), and exhibit greater variance, but that conditional on cortisol level, the negative impact of cortisol on offspring outcomes is greater for them. This suggests that mothers with more human capital may have greater resources to protect against the negative effects of stress.
This work has a number of important implications. First, it provides further evidence that the prenatal environment has a lasting impact on offspring outcomes. This is consistent with recent work by economists in this area that has examined the impact of prenatal nutrition, as proxied by birthweight, (Black, Devereaux and Salvanes, 2007), in-utero exposure to the flu (Almond, 2006) and low levels of radiation (Almond, Edlund and Palme, 2009) on short and long term offspring outcomes including cognitive achievement, disability and mortality. Second, it underscores the importance of maternal stress, a difficult to measure environmental factor, in determining offspring human capital, consistent with the growing experimental animal literature. These results also help us to better understand recent findings regarding the negative impact of prenatal exposure to stressful events (eg, family grief, natural disaster, civil conflict) on child outcomes.2 While the authors of these works often suggest that stress may be one of the mechanisms behind the relationship, they lack specific measures of maternal stress. Finally, and perhaps most importantly, the results have implications for our understanding of the various mechanisms by which parental SES and its corresponding greater average levels of stress can negatively affect child outcomes and future economic status by negatively influencing the child’s earliest environmental conditions.3
The rest of the paper is organized as follows. In section II, we provide background information on the relationship between stress, cortisol, prenatal conditions and offspring outcomes. In sections III and IV, we describe our empirical strategy and data, respectively. In section V we present our empirical results, including robustness checks and section VI includes an exploration of interactions between stress and maternal human capital. Section VII concludes.
II. Background on Economic Status, Stress and Prenatal Programming
A. Related Literature on Early Environment Conditions and Child Outcomes
Work on the intergenerational transmission of economic status in the US has produced estimates of intergenerational income elasticities on the order of 0.5 with the highest estimate approaching 0.65 (Solon, 1999; Mazunder, 2005). Most of the existing theoretical and empirical work that has sought to identify the mechanisms of intergenerational transmission of economic status has categorized the mechanisms as either nature (genetic inheritance) or nurture (the environment). With respect to the latter, the importance of the early childhood environment in affecting long term outcomes is now well-established (see Almond and Currie, 2011; Heckman and Masterov, 2007 for excellent summaries). Researchers have also found that even earlier environmental conditions - those found in-utero – exert a strong influence on later long-term outcomes. For example, Almond (2006) and Almond, Edlund and Palme (2009) provide convincing evidence of the importance of the in-utero environment in determining offspring outcomes by estimating negative and significant effects of in-utero exposure to the flu pandemic of 1918, and low levels of radiation, respectively, on cognitive achievement, schooling, rates of disability and welfare receipt. Other related studies include that of Qian and Meng (2009) and Almond, Edlund, Li and Zhang (2007) who find that in-utero exposure to famine conditions in China results in diminished adult offspring outcomes (educational attainment, literacy, health and employment). While this work establishes the importance of the prenatal environment in affecting long term offspring outcomes, these types of shocks are either atypical or immutable from a policy perspective.
The present study differs in important ways from previous work estimating the impact of prenatal conditions on offspring outcomes. First, we focus on offspring exposure to maternal stress as measured by an individual-level biomarker – the hormone cortisol. Second, we focus on stress generated by more common circumstances, not unusual ones such as natural or man-made disasters, generating results that may be more generalizeable. Third, because poverty is associated with higher levels of stress, the results have important implications for our understanding of the mechanisms behind the intergenerational transmission of economic status.
B. SES, Maternal Stress and Offspring Outcomes
Cortisol is a corticosteroid hormone or glucocorticoid produced by the adrenal cortex that is often referred to as the “stress hormone” as it is involved in the physiological response to stress and anxiety and is observed in higher than average levels in those exposed to greater stress (Wust et. al., 2000; Van Eck et al, 1996). While normal circulating levels of the hormone cortisol are necessary for a healthy adaptive response to stress, unusually high (or, in rare cases, unusually low) levels of cortisol have been linked with multiple pathologies.4
There is evidence of a socio-economic gradient in stress. Previous research has found that low income individuals report greater stressful events in their lives (Dohrenwend, 1973; Marmot and Smith, 1991). Consistent with this, medical researchers have established higher than average cortisol levels among those of low economic and social status (Cohen et. al., 2006; Steptoe et al, 2003; Kunkz-Ebrecht, Kirschbaum and Steptoe, 2003). More recently, Chemin, de Laat and Haushofer (2013) find that negative rainfall shocks in Kenya are associated with higher salivary cortisol levels among farmers, but not non-agricultural workers.
Exposure to maternal stress early in life can affect offspring outcomes via two mechanisms that are not mutually exclusive. First, maternal stress can negatively affect maternal behavior towards and investment in her children. Recent work based largely on quasi-experimental and experimental methods shows that stress can lead to “short-sighted and risk averse decision making” (Haushofer and Fehr, 2014).5 Consistent with this, existing work has documented that maternal stress is correlated with less positive parenting, lower levels of cognitive stimulation, and more aggression and conflict in interactions with children (Crnic et al, 1986; Nievar and Luster, 2006; Gutman et al, 2005). Work based on animal experiments shows that experimental manipulation of maternal stress among macaque monkeys negatively affects how they parent their offspring (Rosenblum and Paully, 1984). This behavioral response to stress could operate during either the prenatal or postnatal period.
The second mechanism through which maternal stress can affect offspring outcomes is through prenatal programming, of which cortisol is considered a key agent. Prenatal programming refers to “the action of a factor during a sensitive period or window of fetal development that exerts organizational effects that persist throughout life” (Seckl, 1998). Work based largely on animal studies has established a strong link between exogenous in-utero exposure to stress/cortisol and poor offspring outcomes. These studies generally fall into two categories: those that inject cortisol (glucocorticoids) directly into pregnant animals and those that exogenously expose pregnant animals to an environmental stressor. Examples of the former include Uno et al (1983) who expose fetal rhesus monkeys to high concentrations of cortisol and find that they suffer considerable damage to the hippocampus region of the brain. Welberg, Seckl and Holmes, (2001) likewise administered glucocorticoids to pregnant rats and found that the offspring of exposed rats exhibited behavioral inhibition, and impaired coping and learning in aversive situations.6 An example of the latter type of study is Schneider (1992) who subjected pregnant rhesus monkeys to what they classify as a “mild stressor” and observed impaired motor ability and delays in learning among the offspring.7 In a follow-up to this study, Schneider, Coe and Lubach (1992) successfully mimicked the negative impact of this mild stressor by directly injecting pregnant rhesus monkeys with stress hormones. They observed similar declines in motor and mental development among the prenatally exposed offspring.
Because these latter two studies show that even a mild stressor during the prenatal period results in diminished offspring outcomes similar to exogenous increases in prenatal cortisol, they are key to our ability to extrapolate findings based on animal experiments to humans, for whom only non-experimental evidence is available. In humans, researchers have related exposure to excessive amounts of cortisol in-utero with impaired development of the brain and spinal cord (Yu, Lee, Lee and Son, 2004). Researchers have also linked stress and elevated cortisol in late pregnancy with poor mental and motor development of human offspring at three and eight months (Huizink et al, 2003.) But non-experimental studies based on humans suffer from problems of endogeneity that experimental studies based on animals do not. In the next section we describe the threats to identification in non-experimental settings and our estimation strategy in greater detail.
III. Empirical strategy
In non-experimental settings, estimates of the impact of maternal stress on offspring outcomes likely suffer from endogeneity. This is because women with higher stress levels (as measured by cortisol) may differ in important, unobserved ways from women with lower stress and these unobserved factors may be responsible for the negative effects on offspring observed. For example, genes affect baseline cortisol levels (Wust et al, 2000). If the genetic composition of women with high cortisol is correlated with other heritable traits (such as IQ) this will bias the estimated impact of cortisol on offspring cognition. To address this problem, we identify the impact of cortisol on offspring outcomes using mother fixed effects. In so doing we control for any fixed unobservable differences between mothers (such as genetics) that might bias the results.
In a fixed effect framework, identification derives from differences in stress during the prenatal period between siblings. To better understand why cortisol levels may differ across two pregnancies of the same mother, we look at corresponding changes in underlying characteristics or conditions of the family for the sample of mothers with large changes in prenatal cortisol between births (results presented in Section V). In this sample, “high cortisol” siblings are characterized by lower family income during the prenatal period relative to their “low cortisol” siblings, but this difference largely disappears by age seven.8 Thus identifying variation appears to come from temporary shocks to the mother (particularly to her economic status) during the prenatal period that do not persist through childhood and therefore are unlikely to be independently correlated with offspring outcomes. We conduct a number of robustness checks to rule out alternative explanations, including narrowing the sample to closely spaced births to rule out long term changes in underlying conditions.
IV. Data
Description
The data are a subset of the National Collaborative Perinatal Project (NCPP). The NCPP comprised a prospective survey of 55,908 pregnancies between 1959 and 1965 across 12 cities. Women were enrolled primarily though public clinics in the academic medical centers where they sought prenatal care and their children were followed up through age seven.9
In this study we focus on a subset of 1093 children born to mothers enrolled in NCPP through either the Providence or Boston sites for whom follow-up information as adults is available. Children were selected for participation in the adult follow-up survey through a multi-stage sampling procedure which involved a core assessment interview and three component studies. The sampling design included an emphasis on siblings.
Trained interviewers collected information on adult education, employment and income, disease and other characteristics between 2002 and 2004. In this work we focus on educational attainment as our measure of adult economic status primarily because educational attainment is arguably a better measure of permanent income than a single measure of annual income, but also because the offspring adult income measures in these data are heavily top-coded.10 Of the 1093 pregnancies with adult follow-up information, 386 are siblings.11
Measures of Cortisol
Maternal blood/serum collected during the third trimester of pregnancy (between 31 and 36 weeks of pregnancy) was analyzed for cortisol.12 The decision to focus on third trimester cortisol was based on both greater availability of serum samples for this period and prior literature showing links between third trimester stress and hormone levels and offspring neurobehavioral outcomes (Davis et al, 2007; Gutteling et al 2005; O’Conner et al, 2005; Austin et al, 2005). However, there is in fact considerable debate over when during pregnancy maternal stress should matter most. In early gestation, “the brain is susceptible to alternations in its programming because it undergoes a cascade of timed processes of neural proliferation, migration and early differentiation.” But late in gestation “the brain undergoes rapid growth and is particularly susceptible to reductions in oxygen and nutrients” which are also associated with maternal stress (Weinstock, 2008 p3). The empirical evidence with respect to when during gestation stress and/or cortisol seems to matter most is generally inconsistent with some studies finding late gestation to be critical, and other studies finding early gestation to be especially salient (see Weinstock, 2008; Van der Bergh et al, 2005; and Tarabulsy et al, 2014 for reviews).
Cortisol naturally varies over the period of gestation and over the course of the day. In our data, we do have information on the week of gestation the blood was drawn, but not on the time of day. Despite this we believe that this measure of cortisol is still a reasonable signal of true baseline cortisol for two reasons. The first is that the variation in cortisol across individuals is more than three times as large as the variation in cortisol over the course of the day (Chiu et al, 2003). The second is that the distribution of total cortisol in our sample is very similar to the distribution of total cortisol in other samples with more carefully measured levels (Stroud et al, 2007).13 For example, a 2005 study by Solden et al assayed 50 women in the morning of the 32nd week of pregnancy. The average level of total cortisol for the sample was 287 (compared with 282 for ours) and the standard deviation was 92 (compared with 84 for ours). Moreover, we find that the same patterns observed between cortisol levels and maternal characteristics in studies based on fresh, precise measures of cortisol are present in our sample as well. Together these facts suggest that our measures of cortisol are valid measures of true baseline levels, though they are measured with error.
The nature or source of the measurement error, however, affects the interpretation of our estimates. If the measurement error is random (eg there is no relationship between time of day the blood was drawn and unobserved maternal characteristics), then this would introduce classical measurement error and lead to attenuation bias of approximately 0.59 which the inclusion of family fixed effects would likely exacerbate.14 If the measurement error is non-random and covaries with maternal characteristics (eg, there is a relationship between time of day of blood draw and maternal characteristics), then the inclusion of family fixed effects would reduce any attenuation bias from measurement error. We discuss this when interpreting our results in the next section.
Characteristics of the Sample
Appendix Table 1 presents sample means for the full NCPP Boston/Providence sample (column 2), the cortisol sample (column 3) and the sibling sub-sample (column 4). The cortisol sample appears to be a representative sub-sample of the full Boston/Providence NCPP sample (comparing columns 2 and 3). Mothers are similar in terms of education, income, race, age and marital status. As expected, birth outcomes (gestation and weight at birth) are slightly better for the cortisol subsample due to the fact that they were selected based on availability of third trimester maternal serum collected at at least 4 weeks prior to delivery as well as offspring survival to adulthood. This positive sample selection will likely result in estimates that represent a lower bound if one way that prenatal cortisol negatively affects offspring outcomes is through increased probability of prematurity and/or lower birthweight, as has been hypothesized in the literature (IOM, 2006).
Appendix Table 1.
Sample Characteristics
| 1960 Census | Providence & Boston | Cortisol Sample | Sibling Sample | ||
|---|---|---|---|---|---|
|
|
|
|
|
||
| Unweighted | Unweighted | Unweighted | Unweighted | Weighted | |
| Maternal Education | 11.5 | 11.4 | 11.07 | 11.4 | 11.6 |
| Family Income ($2010) | $37,971 | $26,013 | $24,403 | $26,233 | $35,113 |
| Mother Black | 0.03 | 0.12 | 0.13 | 0.08 | 0.03 |
| Maternal Age | 30.6 | 25.2 | 24.9 | 24.6 | 25.4 |
| Mother Single | 0.03 | 0.11 | 0.09 | 0.056 | 0.024 |
| Birthweight (grams) | 3185 | 3307 | 3290 | 3303 | |
| Gestation (weeks) | 36.8 | 40.1 | 39.9 | 40 | |
| Free Cortisol (ng/ml) | 24 | 22 | 20.5 | ||
| Verbal IQ 7 year | 99.9 | 99.2 | 100.2 | 101.8 | |
| Adult Education | 13.1 | 13.2 | 13.3 | ||
| Severe Chronic Condition 7 year | 0.089 | 0.099 | 0.099 | ||
| Observations | 17921 | 1058 | 320 | 320 | |
If we compare women in the sibling sub-sample with those from the larger cortisol sample we see that the sibling subsample is similar in terms of cortisol levels and offspring outcomes but that the mothers in the sibling sample appear slightly less disadvantaged in terms of income, education, marital status and race compared with the full sample. In fact, the sibling sub-sample is slightly more representative of the population of women with young children as measured in the 1960 decennial census, which is more likely to be white and married, older, and less poor (column 1).15 That the NCPP sample is more disadvantaged than the general population of young mothers is not surprising given that the recruitment for subjects in the NCPP was conducted through hospital-based public clinics. To address the generalizability of findings based on a non-random sample of mothers, in a robustness check, we apply weights to the sibling sub-sample so that the sample better reflects the income, race and educational distribution of the population of women with young children as measured by the 1960 census. The weighted means are presented in column 4 and it is evident that the weighted sample more closely resembles the 1960 census sample in terms of socio-economic status.16
Variation in Cortisol Across and Within Families
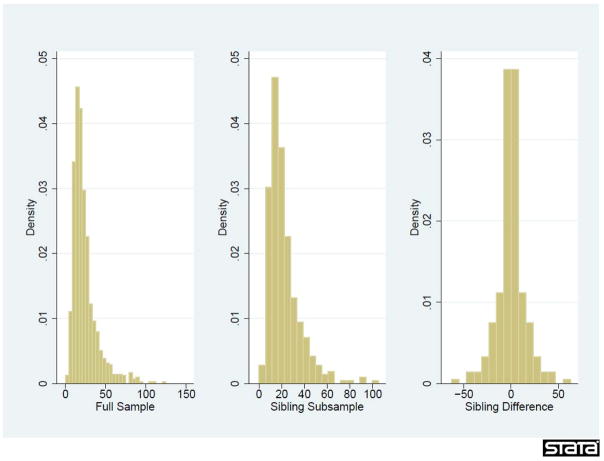
In Figure 1 we present the distribution of free cortisol levels for the whole sample and the sibling subsample.17 They are similar, though there is less variation in the sibling subsample, as expected (μ=22.5 ng/ml and 20.6 ng/ml; σ= 22.6 and 16.3 for the full and sibling sub-samples, respectively).18 There is considerable correlation in the cortisol measures within families (ρ=0.42) which cannot be completely explained by similarities in observable characteristics. When we attempt to match unrelated children based on observable maternal characteristics (maternal age, race, education, marital status, IQ and family income), the correlation in cortisol between matched pairs only reaches 0.16, underscoring a considerable correlation between cortisol levels and unobserved maternal characteristics (such as genetics). Despite the strong correlation in sibling cortisol levels, there is still significant within family variation for identification: the within-family coefficient of variation for the sibling sample is 0.37.
Figure 1.
Distribution of Prenatal Cortisol
V. Results
We proceed in five stages. First, we document a negative relationship between maternal economic status and our marker for stress (cortisol), consistent with existing bio-medical literature. Second, we estimate the impact of prenatal stress on adult educational attainment using OLS and family fixed effects. Third, we explore the mechanisms by which stress affects adult educational attainment by estimating the impact of stress on intermediate outcomes: birth outcomes, cognition and health at age seven. Specifically, we estimate whether prenatal stress negatively affects weight and gestation at birth, verbal IQ and whether child has a severe chronic condition at age seven. Fourth, we conduct robustness checks and explore the relationship between cortisol levels and prenatal and postnatal investments in children. Finally, we explore whether and how maternal human capital interacts with cortisol to affect offspring outcomes.
A. SES and Maternal Cortisol Levels
We document a socioeconomic gradient in maternal cortisol levels in this sample, consistent with existing literature (Cohen et al, 2006; Cohen, Doyle and Baum, 2006; Steptoe et al, 2003; Kunkz-Ebrecht, et al, 2004). We compare maternal/family characteristics of those with “normal prenatal cortisol” (defined as the bottom 80 percent of the distribution of cortisol) to those with “high prenatal cortisol” (defined as the top quintile of the distribution) in Table 1, columns 1–3.19 In the cross-sectional comparisons, we find that mothers with “high prenatal cortisol” levels are more likely to be black and single and have lower income, at both pregnancy and seven years post pregnancy. For example, women with high cortisol are characterized by $5500 less (annual) income at pregnancy, or 21 percent less, than “normal prenatal cortisol” mothers and this difference persists: these same women are characterized by 18 percent less income seven years post-natal. Mothers with elevated cortisol also have fewer years of schooling. All differences are significant except birth order and sibling number.20
Table 1.
Prenatal Cortisol and Maternal Characteristics
|
|
||||||
|---|---|---|---|---|---|---|
| Cross-Sectional Differences in Prenatal Cortisol Levels | Within Family Differences in Prenatal Cortisol Levels | |||||
|
|
|
|||||
| Normal | High | % Difference | Normal | High | % Difference | |
|
|
|
|||||
| Black | 0.08 | 0.17 | 113% | 0.10 | 0.10 | 0% |
| Single during pregnancy | 0.05 | 0.08 | 60% | 0.04 | 0.08 | 85% |
| Single at age 7 | 0.12 | 0.15 | 25% | 0.13 | 0.17 | 28% |
| Income at birth | $ 26,800 | $ 21,147 | −21% | $ 26,570 | $ 22,105 | −17% |
| Income at age 7 | $ 36,800 | $ 30,300 | −18% | $ 35,850 | $ 33,849 | −6% |
| Maternal Education | 11.4 | 10.80 | −5% | 11.28 | 11.28 | 0% |
| Maternal age | 24.7 | 23.60 | −4% | 24.47 | 24.40 | 0% |
| Birth order | 2.9 | 3.00 | 3% | 2.97 | 2.84 | −4% |
| Male | 0.4 | 0.45 | 13% | 0.43 | 0.32 | −26% |
| Observations | 308 | 78 | 27 | 27 | 0 | |
Note: All of the cross family differences with the exception of birth order and male are statistically significant.
None of the differences within family are statistically significant
Not only are low SES mothers characterized by higher average cortisol levels, but low SES mothers (defined as those with less than a high school degree) are also characterized by greater variability in their cortisol levels across siblings. Thirty one percent of high school drop-outs are characterized by at least a standard deviation difference in cortisol levels across siblings (σ=14 ug/ml), compared with 21 percent of mothers who are high school graduates.
In columns 4–6 of Table 1 we explore how family characteristics vary across births in a sample of families with large changes in free cortisol levels between siblings. For these within family comparison, when we compare “high prenatal cortisol” children with their “normal prenatal cortisol” siblings, the difference in real income at pregnancy is 17 percent, similar to the cross sectional difference, but the difference in income at age seven is smaller (6 percent) and insignificant. Note that the difference may have diminished prior to age seven, but the only post-natal income measures that we have in these data were collected at age seven. In contrast, the large difference in the probability of being single while pregnant persists until age seven, but is not statistically significant.21
Importantly, within-family high cortisol is uncorrelated with birth order. Endogenous fertility is potentially a concern here if particularly stressful pregnancies reduce the probability of a future birth. However, there is no evidence of this in our data: high prenatal cortisol levels are not at all predictive of future fertility.22
We conclude that in the cross section, elevated cortisol is associated with race (being black), persistent poverty (at least until age seven), low levels of maternal human capital and being single. Within family, there is some evidence that high prenatal cortisol is associated with a temporary negative shock to income during pregnancy that appears to recover by age seven. These differences underscore the need to include maternal fixed effects to limit comparisons to differences across births to the same mother.
B. Relationship Between Maternal Prenatal Cortisol and Offspring Outcomes: Preliminary Evidence
Before turning to regression analysis, we provide simple non-parametric comparisons of the outcomes of offspring of the same mother. Specifically, we compare years of schooling, gestation and weight at birth, and IQ and health at age seven, of siblings exposed to different levels of prenatal cortisol. Comparing siblings, we find that those exposed to higher prenatal cortisol levels (relative to their siblings) are characterized by slightly lower adult educational attainment and verbal cognition, (but not worse health at age seven) as well as slightly worse birth outcomes (Table 2, top panel).
Table 2.
Within Family Differences in Prenatal Cortisol Levels and Offspring Outcomes
| Lower Cortisol | Higher Cortisol | Difference | Percent Difference | Diff. as Percent Std. Dev. | |
|---|---|---|---|---|---|
| Years of completed schooling | 13.57 | 13.44 | −0.13 | −1% | −7% |
| 7 year IQ | 101.1 | 99.52 | −1.58 | −2% | −11% |
| Any Severe Chronic Health Condition at age 7 | 0.122 | 0.122 | 0.00 | 0% | 0% |
| Gestation | 40.09 | 39.93 | −0.159 | 0% | −8% |
| Birth Weight | 3,338 | 3,309 | −29 | −1% | −6% |
| Low/Normal Cortisol | High Cortisol | Difference | Percent Difference | Diff. as Percent Std. Dev. | |
| Years of completed schooling | 13.61 | 12.54 | −1.07 | −8% | −58% |
| 7 year IQ | 100.3 | 95.2 | −5.12 | −5% | −37% |
| Any Severe Chronic Health Condition at age 7 | 0.081 | 0.121 | 0.04 | 49% | 9% |
| Gestation | 40.18 | 40.00 | −0.185 | 0% | −9% |
| Birth Weight | 3,328 | 3,262 | −66 | −2% | −13% |
However, because existing medical research finds that negative effects of cortisol on mental and motor development occur when cortisol levels either exceed (or, in some rare cases, fall short of) normal circulating levels, we also present differences in offspring outcomes when one sibling is exposed to extremely high levels of prenatal cortisol (defined as in the top quintile). The results are presented in the second panel of Table 2. The differences are large: siblings exposed to very high cortisol levels are characterized by a year less schooling, a verbal IQ that is 5 points lower and a 49 percent increase in the probability of a chronic condition. The birth outcome differences, however, are very small, most likely due to the positive selection on birth outcomes in these data, as noted previously.
We follow these non-parametric, unconditional comparisons with regression analyses that include multiple controls for changing prenatal and post-natal circumstances in the next section.
C. Impact of Maternal Cortisol on Offspring Educational Attainment
In this sub-section we provide empirical evidence based on fixed effect regressions that elevated maternal prenatal cortisol negatively affects offspring human capital accumulation as measured by highest grade completed. Specifically, we create three indicators to represent exposure to free cortisol in-utero: bottom quintile of distribution, the middle 20–80 percent of the distribution (omitted category), and the top quintile of the distribution of cortisol. While this is our preferred specification, we also present results with a linear measure of cortisol.
We include the following controls: maternal race (indicator for black), maternal education, marital status at birth, maternal age at birth, family income during pregnancy, offspring gender, number of siblings at age seven, birth order, whether the husband lives at home with the mother, and the number of times the family moved between birth and age seven (a measure of instability), as well as the week of gestation that the cortisol was measured. We also include an indicator for whether the mother worked during pregnancy (a potential source of stress) and whether there was any pregnancy complication to control for the possibility that the increase in maternal cortisol observed simply reflects maternal anxiety over the health of the fetus. We run three regressions: OLS results based on the full sample (column 1, Table 3), OLS results for the sub-sample of siblings (column 2), and FE results for the subsample of siblings (column 3). The FE regressions include the same controls as the OLS, with the exception of maternal race and education which are fixed across births.
Table 3.
Prenatal Cortisol and Offspring Educational Attainment
| Panel A: Quintiles of Cortisol | (1) | (2) | (3) |
|---|---|---|---|
| Top quintile prenatal cortisol | −0.471 [0.159] | −0.342 [0.183] | −1.148 [0.440] |
| Bottom quintile prenatal cortisol | −0.138 [0.193] | −0.248 [0.191] | −0.241 [0.380] |
| Black | 0.193 [0.192] | 0.177 [0.253] | |
| Maternal education | 0.215 [0.0396] | 0.188 [0.0477] | |
| Mother single at birth | −0.0167 [0.263] | −0.899 [0.370] | −0.211 [0.980] |
| Maternal age at birth | 0.0383 [0.0192] | 0.0313 [0.0220] | −0.138 [0.153] |
| Income at birth in $1000s | 0.0126 [0.00664] | −0.00336 [0.00733] | −0.00537 [0.0122] |
| Mother working while pregnant | 0.106 [0.232] | 0.130 [0.221] | −0.0287 [0.305] |
| Male | −0.507 [0.157] | −0.108 [0.166] | −0.503 [0.231] |
| Birth order | −0.0531 [0.0584] | −0.0729 [0.0779] | 0.240 [0.268] |
| Number of siblings at age 7 | −0.102 [0.0540] | −0.101 [0.0619] | 0.116 [0.176] |
| Husband at home | 0.583 [0.195] | 0.474 [0.249] | −0.327 [0.578] |
| Number moves birth to age 7 | −0.0310 [0.0570] | −0.168 [0.0550] | −0.0437 [0.158] |
| Providence | 0.0124 [0.198] | 0.00634 [0.196] | |
| Pregnancy complication | −0.100 [0.177] | −0.0382 [0.171] | 0.264 [0.276] |
| Gestation at draw date | 0.000178 [0.0499] | −0.0258 [0.0522] | 0.0585 [0.0740] |
| Constant | 10.16 [1.821] | 12.02 [1.763] | 14.76 [4.247] |
| Observations | 980 | 376 | 386 |
| R-squared | 0.206 | 0.234 | 0.111 |
| Panel B: Linear Cortisol | (1) | (2) | (3) |
|---|---|---|---|
| Free cortisol | −0.00500 [0.00417] | −0.00184 [0.00431] | −0.0295 [0.0142] |
| Constant | 10.14 [1.834] | 11.93 [1.772] | 13.66 [4.193] |
| Observations | 977 | 375 | 385 |
| R-squared | 0.199 | 0.228 | 0.097 |
|
| |||
| Sample | Full | Sibling | Sibling |
| Full controls | Y | Y | Y |
| Maternal FE included | N | N | Y |
Note: Robust standard errors in brackets
Being exposed to highly elevated cortisol in-utero has a large negative and significant impact on years of schooling in the OLS regressions. For the full sample, exposure to high cortisol levels results in 0.47 fewer years of schooling, which represents 25 percent of a standard deviation (μ=13.4 and σ=1.9 years of schooling in this sample). For the sibling subsample, the estimate is still large (though smaller, −0.34) but not significantly different from the estimate based on the full sample (Table 3, column 2).
When we include maternal fixed effects to control for all unobserved differences between mothers that may be correlated with cortisol levels while pregnant and offspring outcomes, we find that exposure to elevated levels of cortisol in-utero reduces years of schooling by roughly 1.1 years of schooling, which represents 58 percent of a standard deviation. While these estimated effects may seem large relative to those of most educational interventions (Hanushek, 2006), it is consistent with existing evidence on the impact of prenatal conditions on offspring educational outcomes.23 For example, Almond, Edlund and Palme (2009) find that in-utero exposure to low levels of radiation reduce school test scores by six percentile points – a very large effect relative to most educational interventions. Almond and Mazumder (2011) find that fasting during pregnancy leads to a 20 percent increase in adult disability.
In the second panel of Table 3 we estimate a linear specification. The estimates based on the maternal fixed effect specification with a full set of controls (column 3) suggest that a standard deviation increase in prenatal cortisol leads to a reduction of 0.46 years of schooling, or 25 percent of a standard deviation.
Interpretation of FE Estimates
There are two potential interpretations of our finding that the estimated impact of prenatal cortisol on adult educational attainment increases when we include family fixed effects. The first relates to measurement error in the measure of cortisol used. As noted previously, cortisol naturally varies over the course of the day and it is not known when these measures were drawn. If the time of day that the blood was drawn was not random but correlated with unobservable characteristics of the mother that might affect offspring outcomes, the OLS estimates would be biased. If we assume that these unobservable characteristics remain constant across pregnancies, the inclusion of maternal fixed effects would reduce the bias from such non-random measurement error. This is possible, but we think unlikely given that controlling for maternal work (the most likely characteristic to affect time of day of blood draw) does not alter the results.
A second interpretation or explanation is that the larger FE estimates might reflect a potential misspecification of the OLS regression framework. An alternative model is one in which an individual adjusts to a baseline or personal level of cortisol that may be a function of underlying average levels of stress, genetics or some combination of factors. It is only when mothers are exposed to a new or unexpected stressor that moves them from their usual level that adverse effects are found. There is some support for this model in the psychological and biological literatures. Mineka and Kihlstrom (1978) review a large body of scientific experiments, performed mostly on animals, which finds that unanticipated aversive events have a greater negative impact on observed behavior and health than predictable aversive events which occur with the same frequency. A classic example of this type of study is Rosenblum and Paully (1984) who subject monkeys to three environments: low foraging demand (abundance of food), high foraging demand (scarcity of food) and variable foraging demand (unpredictable periods of abundance and scarcity). They find the greatest negative effects on mother-infant pairs subjected to the variable foraging environments.24
If the true model is one of variable stress, a fixed effect strategy would be the correct specification. By including maternal fixed effects we implicitly identify the impact of deviations from mean (or personal) cortisol levels on outcomes. In contrast, one can interpret the OLS estimates as capturing a mixture of variable stress and chronic stress. Given existing evidence that baseline cortisol varies considerably within a non-stressed pregnant population, this alternative model seems plausible.
With the data we have, there is no way to test empirically whether the true model is one of “variable stress.” We do, however, provide some evidence that would be consistent with a model of this type. Specifically, we limit the sample to one randomly selected child in each family (from the sibling subsample) and define two measures for each child. The first is his or her own absolute level of prenatal cortisol and the second is a relative measure defined as the difference between his level and the level of his sibling (who is excluded from the regression sample in column 2). We then regress the focal child’s educational attainment on both these measures (own absolute level and relative difference) and a full set of controls in an OLS framework.
The results (Appendix Table 2) suggest that the relative measure of cortisol is more highly correlated with offspring educational attainment than the absolute measure. The coefficient on the relative measure of cortisol in column 2 is large and significant (−0.023): a one standard deviation increase in this relative measure (σ=17) is associated with −.39 fewer years of school. In contrast, the coefficient on the absolute measure is positive and much smaller than the coefficient on the relative measure (and significant at the 10 percent level). Because these results are based on an OLS specification, we cannot necessarily interpret them as causal. However, they are consistent with a model in which significant variation in prenatal stress negatively affects offspring outcomes.
Appendix Table 2.
Variable Stress Hypothesis
| (1) | (2) | |
|---|---|---|
| Own Cortisol | 0.00802 [0.00619] | 0.0142 [0.00835] |
| Own Cortisol-Sibling Cortisol | −0.0189 [0.00695] | −0.0229 [0.00875] |
| Constant | 11.43 [1.161] | 11.42 [1.567] |
| Observations | 266 | 134 |
| R-squared | 0.271 | 0.299 |
|
| ||
| Sample | Sibling | Sibling-1 Randomly Selected |
| Full controls | Y | Y |
| Maternal FE included | N | N |
Note: Robust standard errors in brackets
Full controls include all controls listed in Table 3, Top Panel.
In the following subsections we explore the mechanisms by which maternal prenatal cortisol affects offspring educational attainment. Specifically, we focus on birth outcomes, child cognition and health as three potential intermediate outcomes affected by prenatal cortisol. We focus on these three intermediate outcomes because they have been shown in the medical literature to be adversely affected by elevated stress and cortisol in-utero and in the economics literature, to affect educational attainment.
D. Prenatal Cortisol and Birth Outcomes
There is evidence that stress in-utero reduces birth weight and increases the probability of prematurity (IOM, 2006; Eskenazi, et al, 2007; Camacho, 2008). Unfortunately, our sample was constructed in such a way as to preclude most premature births since the sample was selected based on availability of third trimester serum drawn at least 4 weeks before the date of delivery (and typically more than 8 weeks before date of delivery) to prevent the cortisol measure from capturing the stress of delivery. This will likely prevent us from estimating any significant effects on birth outcomes. Indeed, we find that prenatal cortisol has no large or significant impact on birth weight or gestation in either the OLS or FE models with a full set of controls (Table 4). One implication of this is that our estimates of the impact of cortisol on other offspring outcomes likely reflect a lower bound.
Table 4.
Prenatal Cortisol of Birth Outcomes
| Panel A: Quintiles of Cortisol | (1) BW |
(2) Gestation |
(3) BW |
(4) Gestation |
(5) BW |
(6) Gestation |
|---|---|---|---|---|---|---|
| Top quintile prenatal cortisol | −50.60 [39.52] | −0.128 [0.177] | −99.36 [62.73] | −0.238 [0.285] | −45.55 [73.30] | −0.286 [0.331] |
| Bottom quintile prenatal cortisol | 68.79 [45.78] | 0.0392 [0.182] | 39.33 [64.68] | −0.365 [0.279] | 64.83 [67.82] | 0.0618 [0.458] |
| Observations | 981 | 980 | 376 | 376 | 386 | 386 |
| R-squared | 0.067 | 0.057 | 0.099 | 0.052 | 0.078 | 0.108 |
| Panel B: Linear Cortisol | (1) BW |
(2) Gestation |
(3) BW |
(4) Gestation |
(5) BW |
(6) Gestation |
|---|---|---|---|---|---|---|
| Free cortisol | −1.012 [1.119] | 3.59e–05 [0.00565] | −1.493 [1.786] | 0.00274 [0.00996] | 0.643 [2.385] | −0.00328 [0.0133] |
| Constant | 2,981 [344.1] | 37.70 [1.395] | 3,601 [488.2] | 38.08 [2.127] | 4,429 [898.1] | 42.56 [4.666] |
| Observations | 978 | 977 | 375 | 375 | 385 | 385 |
| R-squared | 0.062 | 0.057 | 0.091 | 0.047 | 0.072 | 0.104 |
|
| ||||||
| Sample | Full | Full | Sibling | Sibling | Sibling | Sibling |
| Full controls | Y | Y | Y | Y | Y | Y |
| Maternal FE included | N | N | N | N | Y | Y |
Note: Robust standard errors in brackets
Full controls include all controls listed in Table 3, Top Panel
E. Prenatal Cortisol and Child IQ
Next we estimate the impact of prenatal maternal cortisol on child cognition measured at age seven.25 Specifically we estimate the impact of cortisol on verbal IQ because of evidence that those with Cushings disease (a hormonal disorder caused by prolonged exposure of the body’s tissues to high levels of the hormone cortisol) are characterized by deficits in verbal learning and other verbal functions but not non-verbal functions (Starkman et al 2001).26
The results suggest that exposure to elevated cortisol in-utero (Table 5, top panel) appears to have a large negative and significant impact on verbal IQ. Specifically, exposure to cortisol in the top quntile of the distribution is associated with a 6 point lower verbal IQ (43 percent of a standard deviation) in the fixed effect regression with a full set of controls. This compares with an estimated 3.5 lower IQ points in the OLS with full controls (column 1). It is important to note that verbal IQ comprises only half the total IQ score. As such, the impact of exposure to elevated prenatal cortisol on full IQ at age seven is roughly half the size of the verbal estimates (3.4 points).
Table 5.
Prenatal Cortisol and Offspring 7 Year IQ
| Panel A: Quintiles of Cortisol | (1) | (2) |
|---|---|---|
| Top quintile prenatal cortisol | −3.575 [1.724] | −6.056 [2.428] |
| Bottom quintile prenatal cortisol | −1.858 [1.702] | 1.706 [2.510] |
| Observations | 363 | 372 |
| R-squared | 0.301 | 0.140 |
| Panel B Linear Cortisol | (1) | (2) |
|---|---|---|
| Free cortisol | −0.0521 [0.0514] | −0.123 [0.0916] |
| Observations | 362 | 371 |
| R-squared | 0.294 | 0.113 |
|
| ||
| Sample | Sibling | Sibling |
| Full controls | Y | Y |
| Maternal FE included | N | Y |
Note: Robust standard errors in brackets
Full controls include all controls listed in Table 3, Top Panel
Given the high degree of correlation between child IQ and years of education in these data (ρ=.45 for verbal IQ at age seven), we conclude that the adverse effect of exposure to elevated cortisol in-utero on adult years of education is likely mediated in part by the negative impact on verbal cognitive functioning.
F. Prenatal Cortisol and Child Health at Age Seven
We also estimate the impact of exposure to elevated stress in-utero on child health as measured by whether the child has a severe chronic condition at age seven as determined by medical examination of the child (Table 6).27 In these regressions, we include two additional controls: gestation and birth weight. In our sample, 90 percent of the children have no chronic conditions. The results differ somewhat from those for child IQ. In the non-linear regressions, we find that very high cortisol is associated with a 0.058 increase in the probability of a chronic condition in the fixed effect setting, but the estimate is very imprecise. The results from the linear specification suggest that a linear increase in cortisol positively and significantly raises the probability of a chronic condition. In the fixed effect regressions, a standard deviation increase in cortisol is significantly predictive of an increase in the probability of a chronic condition of .08. However, these results are precise only at the 10 percent level and the precision is very much sensitive to how the condition(s) is measured. As a result, we take this only as suggestive evidence that exposure to cortisol in-utero has a negative effect on offspring health that may not necessarily be evident at birth and that this may represent a second mechanism through which exposure to prenatal cortisol negatively affects completed years of schooling among offspring.
Table 6.
Prenatal Cortisol and Offspring Health (Chronic Conditions) at Age 7
| Dependent variable=number of chronic conditions, age 7 | ||
|---|---|---|
|
| ||
| Panel A: Quintiles of Cortisol | (1) | (2) |
| Top quintile prenatal cortisol | 0.0555 [0.0458] | 0.0583 [0.106] |
| Bottom quintile prenatal cortisol | 0.0635 [0.0446] | −0.0209 [0.103] |
| Observations | 363 | 372 |
| R-squared | 0.066 | 0.117 |
| Panel B: Linear Cortisol | (1) | (2) |
|---|---|---|
| Free cortisol | 0.00118 [0.00141] | 0.00530 [0.00309] |
| Observations | 362 | 371 |
| R-squared | 0.060 | 0.144 |
|
| ||
| Sample | Sibling | Sibling |
| Full controls | Y | Y |
| Maternal FE included | N | Y |
Note: Robust standard errors in brackets
Full controls include all controls listed in Table 3, Top Panel, as well as birth weight and gestation
Interpretation and Robustness
How should we interpret these results? One interpretation is that of a causal impact of prenatal cortisol on offspring outcomes, consistent with neurobiological evidence. A second interpretation is that the underlying stressor is the cause of the decline in offspring human capital and that maternal cortisol levels are merely a proxy for unmeasured stressors, despite the inclusion of controls that are more comprehensive than usual. Both interpretations generate the same policy implication: reducing the sources of everyday stress faced by parents will improve offspring outcomes. The main difference between the two interpretations has to do with the timing. The first interpretation implies that the prenatal period is critical and that post-natal stress doesn’t matter while the latter would be consistent with prenatal and/or postnatal stress mattering.
We conduct a number of robustness checks attempt to shed some light on the issue of timing by trying to isolate short lived changes in stress levels around the prenatal period in two ways. First, we include controls for two potential sources of postnatal stress: income and marital status at age seven (Table 7, Panel A) and there is no change in the estimated effects of prenatal cortisol on educational attainment, child IQ or health.
Table 7.
Robustness Checks
| Panel A: Control for Income and Marital Status at Age 7 | (1) Education |
(2) IQ |
(3) Chronic |
|---|---|---|---|
| Top quintile prenatal cortisol | −1.066 [0.455] | −6.313 [2.326] | |
| Bottom quintile prenatal cortisol | −0.311 [0.387] | 1.426 [2.547] | |
| Free cortisol | 0.00517 [0.00312] | ||
| Constant | 12.47 [4.969] | 47.67 [33.72] | 1.615 [1.057] |
| Observations | 363 | 361 | 360 |
| R-squared | 0.113 | 0.212 | 0.140 |
| Panel B: Birthspacing<2.5 years | (1) Education |
(2) IQ |
(3) Chronic |
|---|---|---|---|
| Top quintile prenatal cortisol | −0.665 [0.546] | −8.392 [2.609] | |
| Bottom quintile prenatal cortisol | −0.537 [0.502] | −2.800 [2.875] | |
| Free cortisol | 0.00196 [0.00366] | ||
| Constant | 18.11 [9.001] | 106.5 [51.41] | −2.026 [1.645] |
| Observations | 198 | 192 | 191 |
| R-squared | 0.051 | 0.229 | 0.111 |
| Panel C: Control for Season of Birth | (1) Education |
(2) IQ |
(3) Chronic |
|---|---|---|---|
| Top quintile prenatal cortisol | −1.169 [0.441] | −5.866 [2.427] | |
| Bottom quintile prenatal cortisol | −0.212 [0.381] | 1.860 [2.538] | |
| Free cortisol | 0.00465 [0.00273] | ||
| Constant | 14.42 [4.254] | 74.97 [33.84] | 0.263 [1.110] |
| Observations | 386 | 372 | 371 |
| R-squared | 0.113 | 0.152 | 0.151 |
| Panel D: Control for Testosterone | (1) Education |
(2) IQ |
(3) Chronic |
|---|---|---|---|
| Top quintile prenatal cortisol | −1.146 [0.439] | −6.077 [2.407] | |
| Bottom quintile prenatal cortisol | −0.239 [0.381] | 1.687 [2.496] | |
| Free cortisol | 0.00521 [0.00309] | ||
| Testosterone (ng/ml) | −0.218 [0.321] | −1.721 [1.941] | −0.0642 [0.0575] |
| Constant | 14.97 [4.259] | 74.70 [34.38] | 0.364 [1.091] |
| Observations | 386 | 372 | 371 |
| R-squared | 0.113 | 0.144 | 0.114 |
| Panel E: Weighted Regressions | (1) Education |
(2) IQ |
(3) Chronic |
|---|---|---|---|
| Top quintile prenatal cortisol | −0.965 [0.526] | −7.025 [3.487] | |
| Bottom quintile prenatal cortisol | −0.261 [0.400] | −0.756 [4.673] | |
| Free cortisol | 0.00359 [0.00361] | ||
| Constant | 12.73 [5.023] | 44.63 [55.88] | −0.602 [1.552] |
| Observations | 377 | 364 | 363 |
| R-squared | 0.845 | 0.881 | 0.606 |
| Panel F: Child Height in cm. at Age 7 (Falsification) | (1) | (2) | |
|---|---|---|---|
| Top quintile prenatal cortisol | −0.943 [0.916] | ||
| Bottom quintile prenatal cortisol | −2.097 [1.220] | ||
| Free cortisol | −0.0461 [0.0273] | ||
| Constant | 117.1 [13.45] | 116.8 [14.01] | |
| Observations | 372 | 371 | |
| R-squared | 0.071 | 0.054 | |
|
| |||
| Sample | Sibling | Sibling | Sibling |
| Full controls | Y | Y | Y |
| Maternal FE included | Y | Y | Y |
Note: Robust standard errors in brackets
Average child height at age 7 is 120 cm
Second, we present estimates of the impact of prenatal cortisol on offspring outcomes limiting the sample to siblings spaced at most two and a half years apart. In this way we further limit variation in the estimation sample to short-lived shocks to stress. The average spacing in this sample is 2.1 years and in limiting the sample this way we reduce the sample size to fewer than 192 siblings (96 pairs). When we do, the point estimates increase in some cases (IQ) and decrease in others (education and health) and in the latter are also imprecise, most likely due to the reduction in sample size. We cannot rule out that the estimates for this reduced sample are statistically equivalent to those based on the full sample.
We conduct a number of additional robustness checks. Since previous work has found season of birth to be correlated with offspring outcomes in humans and scientists have found seasonal patterns in cortisol levels in both animals and adult males (Laurence et al, 2008; Walker et al, 1997)28, we attempt to rule out the possibility that seasonal variation is driving our results. To do so, we control for season of birth in FE regressions of the impact of cortisol on education, IQ and health and present the results in Panel C of Table 7. The inclusion of three indicators for season of birth does not change the point estimates but does reduce precision.29
We also rule out the possibility that our estimates of the impact of cortisol on offspring outcomes are driven by other circulating hormones that are often correlated with cortisol levels. Sex hormones, testosterone in particular, are correlated with cortisol levels and have been hypothesized to affect offspring outcomes (Romano, Leoni and Saino, 2006). To identify the impact of cortisol separate from testosterone, we control for testosterone levels in the regressions presented in Panel D of Table 7. When we control for testosterone levels, the estimated impact of cortisol on education, IQ and health at age seven is unchanged.
As discussed previously, the sample was non-randomly selected. As a result, we constructed weights based on the 1960 census for metropolitan Massachusetts (includes Boston) and Rhode Island (includes Providence). The weights, once applied, should generate results that are more generalizeable to the population of mothers at the time. The weighted regression estimates, presented in panel E of Table 7 are slightly smaller than the non-weighted regressions and in the case of chronic conditions, is no longer significant.
In the last panel of Table 7 we present estimates of the impact of prenatal cortisol levels on height at age seven measured in centimeters as a falsification exercise. The point estimates are very small (−0.943 for elevated cortisol), given a mean height of 120 cm, and imprecise.
Finally, we consider the possibility that prenatal cortisol influences the probability of future fertility. To do so we regress whether the mother had a future birth (we have completed fertility through age seven of the focal child) on measures of prenatal cortisol (linear and quartiles) in estimates not presented here. Estimated effects are very small and imprecise for both the full sample and the sibling subsample.
G. Prenatal Stress and Parental Investments
We examine whether prenatal maternal cortisol is correlated with either pre or post natal investments in the child. The former includes: 1) weight gain during pregnancy, 2) smoking during pregnancy, 3) the number of prenatal visits. The latter include: 1) the mother’s “responsiveness” to her child’s needs, as assessed by a psychologist in the eighth month post-birth, 2) whether as part of the psychological examination, the interviewer concludes that the child faces an “unfavorable emotional environment” at one year of age and 3) whether the child attended preschool at age 4.
We present OLS and FE regression estimates of the impact of prenatal cortisol on the above measures of parental investments (Table 8). For the prenatal investments, the point estimates suggest a negative relationship between elevated cortisol and each of the prenatal investments, but none of the estimates is significant. We also try factor analysis for the prenatal investments, but still find negative but insignificant effects. For the postnatal investments, there is no consistent relationship with elevated cortisol.30
Table 8.
Prenatal Cortisol and Prenatal and Postnatal Child Investments
| Panel A Quintiles of Cortisol | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight Gain | Smoker | Prenatal Visits | Mother Unresponsive | Unfavorable Emotional Env | Nursery School | |||||||
| OLS | FE | OLS | FE | OLS | FE | OLS | FE | OLS | FE | OLS | FE | |
| Top quintile prenatal cortisol | −1.102 [0.975] | −0.562 [2.440] | 0.0648 [0.0642] | 0.0456 [0.0327] | −0.105 [0.419] | −0.630 [0.823] | −0.00300 [0.0265] | −0.0951 [0.0640] | −0.0292 [0.0324] | −0.0272 [0.0725] | 0.0241 [0.0430] | 0.0743 [0.0740] |
| Bottom quintile prenatal cortisol | −0.260 [1.031] | 3.030 [2.664] | 0.0263 [0.0682] | 0.0725 [0.0669] | 0.101 [0.445] | −0.0369 [0.796] | 0.00492 [0.0271] | −0.00853 [0.0700] | −0.0143 [0.0168] | 0.0178 [0.0689] | −0.0235 [0.0343] | 0.0254 [0.0455] |
| Observations | 358 | 368 | 376 | 386 | 375 | 385 | 376 | 386 | 376 | 386 | 346 | 355 |
| R-squared | 0.122 | 0.775 | 0.049 | 0.957 | 0.195 | 0.846 | 0.063 | 0.686 | 0.202 | 0.737 | 0.101 | 0.840 |
| Panel B Linear Cortisol | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight Gain | Smoker | Prenatal Visits | Mother Unresponsive | Unfavorable Emotional Env | Nursery School | |||||||
| OLS | FE | OLS | FE | OLS | FE | OLS | FE | OLS | FE | OLS | FE | |
| Free cortisol | 0.000986 [0.0279] | −0.0426 [0.0548] | 0.00143 [0.00136] | 0.000511 [0.000724] | −0.00271 [0.0119] | −0.00784 [0.0181] | 0.000570 [0.000889] | −0.000163 [0.00238] | −0.000560 [0.000442] | −0.000644 [0.000792] | 0.00109 [0.000919] | 0.00130 [0.00165] |
| Observations | 358 | 368 | 376 | 386 | 375 | 385 | 376 | 386 | 376 | 386 | 346 | 355 |
| R-squared | 0.119 | 0.771 | 0.048 | 0.957 | 0.194 | 0.845 | 0.065 | 0.678 | 0.201 | 0.737 | 0.102 | 0.839 |
|
| ||||||||||||
| Sample | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling |
| Full controls | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Maternal FE included | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y |
Note: Robust standard errors in brackets
Full controls include all controls listed in Table 3, Top Panel.
Maternal Responsiveness is measured at 4 months of age and is an aggregate of maternal responsiveness to the child, maternal focus on the child, child’s appearance (neat to unkempt) and attendtion paid to child
Unfavorable emotional environment is measured at age 1 and is the interviewers positive response to the question of whether there is an unfavorabl emotional environment at home
Nervous Condition is whether the mother reports a nervous condition any time from the birth of the child to age 7
Weight gain is maternal weight gain during pregnancy
VI. Maternal Human Capital, Stress and Offspring Human Capital
Finally, we explore how maternal human capital interacts with stress to affect offspring outcomes. To do so, we present fixed effect estimates of the impact of elevated cortisol on offspring education stratified by maternal education (less than a HS degree, only a HS degree, at least a HS degree). The results very clearly show that the negative impact of elevated cortisol on offspring outcomes is greatest for mothers with the lowest levels of human capital (Table 9). The results indicate that a child born to a mother without a HS degree and exposed to elevated prenatal stress will received nearly 2 fewer years of schooling, compared with his sibling. For mothers with a HS degree, the impact is roughly half the size (the t statistic is only 1.6).
Table 9.
Does Maternal Human Capital Moderate the Impact of Prenatal Cortisol on Offspring Outcomes?
| Dependent Variable= offspring education in years | (1) Mother <HS |
(2) Mother HS |
(3) Mother >=HS |
|---|---|---|---|
| Top quintile prenatal cortisol | −1.871 [0.768] | −0.873 [0.596] | −0.797 [0.560] |
| Bottom quintile prenatal cortisol | −0.113 [0.951] | −0.345 [0.429] | −0.437 [0.433] |
| Constant | 24.68 [8.547] | 9.580 [5.694] | 9.003 [5.702] |
| Observations | 143 | 177 | 233 |
| R-squared | 0.844 | 0.821 | 0.802 |
|
| |||
| Sample | Sibling Mother <HS |
Sibling Mother HS |
Sibling Mother >=HS |
| Full controls | Y | Y | Y |
| Maternal FE included | Y | Y | Y |
Note: Robust standard errors in brackets
Full controls include all controls listed in Table 3, Top Panel
What can explain this difference? It is not because the cortisol is higher among those with less than a HS degree: among those with high cortisol, average cortisol is 48.9 for HS dropouts and 48.4 for HS grads. One likely possibility is that mothers with low levels of human capital when exposed to a stressful event, have fewer resources, mental and otherwise, to draw upon. While only suggestive, we look at how the probability of providing a poor emotional environment at age 1 changes when stressed by maternal education. For HS drop-outs, this probability increases by 12.5 percentage points for siblings exposed to high prenatal cortisol relative to their “non-stressed” siblings. For those with at least a HS degree, the increase is less than half that, 4.8 percentage points.
One implication of these findings is that exposure to stress may play a role in the intergenerational transmission of human capital. In Table 10 we present intergenerational elasticities in human capital (education). We regress ln(offspring years of schooling) on ln(maternal years of schooling) and a full set of controls (but not fixed effects) in column 1, Table 10. The estimated elasticity suggests that a 1 percent increase in maternal education leads to a 0.122 percent increase in offspring education. In column 2, we include an indicator for elevated cortisol. While elevated cortisol is negatively related to offspring human capital, controlling for exposure to stress does little to the main effect of maternal education: the coefficient on ln(maternal education) is virtually unchanged (estimates of 0.122 and 0.119). However, when we include an interaction between maternal education and elevated cortisol in column (3), the interaction term is large and significant. We find that among the non-stressed, there is a moderate amount of transmission of human capital from mother to child, but when stressed, the degree of transmission doubles. More specifically, when non-stressed, a ten percent increase in maternal education translates to a one percent increase in offspring education; when stressed, a ten percent increase in maternal education translates to a 2.2 percent increase in offspring education.
Table 10.
Stress and Intergenerational Correlations in Human Capital
| Dependent Variable= Ln(offspring education) | (1) | (2) | (3) |
|---|---|---|---|
| Ln(maternal education) | 0.122 [0.0243] | 0.119 [0.0240] | 0.0958 [0.0257] |
| Ln(maternal education)*high cortisol | 0.131 [0.0559] | ||
| High (top quintile) prenatal cortisol | −0.0202 [0.0100] | −0.326 [0.134] | |
| Ln(maternal education)*cortisol | |||
| Cortisol | |||
| Constant | 2.279 [0.0654] | 2.272 [0.103] | 2.346 [0.108] |
| Observations | 981 | 981 | 981 |
| R-squared | 0.194 | 0.198 | 0.205 |
|
| |||
| Sample | Full | Full | Full |
| Full controls | Y | Y | Y |
| Maternal FE included | N | N | N |
Note: Robust standard errors in brackets
Full controls include all controls listed in Table 3, Top Panel
1 percent increase in maternal education leads to a 0.122 percent increase in offspring education if highly stressed, 1 percent increase in maternal education leads to a 0.22 percent increase in offspring education
VII. Conclusions
In this paper we explore the role of maternal stress in affecting offspring outcomes. Specifically, we show that in-utero exposure to elevated levels of the stress hormone cortisol negatively affects offspring human capital as measured by years of schooling, cognition and health. While the effects may seem large compared with many educational interventions (Hanushek, 2006), they are comparable to the considerably larger estimates of the impact of prenatal conditions (Almond and Mazumder, 2011 and Almond, Edlund and Palme, 2009) and early childhood interventions (Currie, 2001) on offspring educational outcomes. The results are generally non linear – the negative effects are concentrated among those with the highest cortisol levels. Cortisol levels in the normal range appear to have less of an effect on long term outcomes. Moreover, not only the levels but also the variation in stress appears to matter, suggesting that shocks to stress levels have a particularly negative impact on offspring outcomes. These results are consistent with the emerging neurobiology research showing that exogenous in-utero exposure to stress or cortisol impairs the developing brain of the fetus. But prenatal programming need not be the only mechanism at play, changes in the post natal environment as a result of maternal stress may also play a role.
Our results have important implications. First, they support hypotheses put forth by others that maternal stress may play a part in the documented relationship between exposure to stressful events early in life (including prenatal), such as bereavement, civil conflict or natural disasters, and poor offspring outcomes. Second, they shed light on a potential mechanism behind large intergenerational correlations in economic status. While we find that women with lower levels of human capital face more stressors, this alone does not explain why their children have worse outcomes. Rather, the consequences of elevated stress are greater for the offspring of women with less human capital. This is consistent with women with less human capital having fewer resources to combat the negative effects of stress. These results suggest that reducing stressors faced by women with low levels of human capital may be particularly effective in lowering the observed intergenerational correlations in human capital and better enabling children born into poverty to escape it as adults.
Footnotes
Throughout the paper we refer to our measure of stress as prenatal stress for simplicity and because it was measured during the prenatal period, but it could also reflect some post natal stress and so the measure should be interpreted accordingly.
Examples of family grief include Black, Devereux and Salvanes (2014) and Persson and Rossin-Slater (2014); examples of natural disasters include Currie and Rossin-Slater (2013), Simeonova (2011) and Glynn et al (2001); examples of the civil conflict include Camacho (2008) who looks at land mines, Lauderdale (2006) who examines pregnancies during the attacks of 9/11 and Mansour and Rees (2011) who examine the impact of the Intifada on birth outcomes.
Perhaps the most relevant work in this regard is that of Almond, Hoynes and Schanzenbach (2011) who find that exposure to food stamps in-utero increases birth weight.
Elevated cortisol has been implicated in hypertension, cardiovascular disease, insulin resistance, obesity, diabetes, infection illness and depressive disorder, among others as well as the development of atypical emotional, behavioral and cognitive functioning (Ousova et al, 2004; Walker et al, 1998; Steptoe et al, 2002; McEwen, 1998; Van Goozen et al; 1998).
Haushofer and Fehr (2014) provide an excellent summary of this work.
They concluded that prenatal programming of the HPA axis was responsible for the outcome based on studies of the areas of the brain affected.
A mild stressor consists of removal from one’s home cage to be confined to a smaller cage for ten minutes each day and subjected to three unpredictable noise bursts.
In the data, we only have measures of income and marital status during pregnancy and at age seven.
Women who planned to put their children up for adoption and women who arrived at the hospital for delivery without any prenatal care were excluded from the study. Limiting the sample in this way reduces considerably the number of women with “unwanted pregnancies.” As such, we reduce any potential upward bias that could arise from unwanted pregnancies if they are associated with greater stress and fewer parental investments (Joyce, Kaestner and Korenman, 2000).
While the educational attainment data can be externally validated (eg: the distribution of years of education is similar to that found in the CPS for a similar sample) the income data cannot likely due to confusion regarding the income question in the survey.
Of those with multiple births, we exclude the 15 mothers with 3 or 4 births within the period 1959–1965 as their characteristics are significantly different from the rest of the sample. For example, the mother with 4 births had 17 years of education when the average is 11–12 years in the full sample and the 1960 census.
Blood was assayed for total and free cortisol. The latter refers to the amount of cortisol not bound to the protein CBG (cortical binding globulin) and therefore free to bind to other receptors and affect the body. Free cortisol is typically only 10 percent of total cortisol. In this analysis we focus on free cortisol, consistent with existing medical literature.
We can only compare total cortisol levels across samples as the distribution of free cortisol levels is rarely reported. However, free cortisol is typically between 9 and 10 percent of total cortisol depending on the level of CBG.
We calculate σ2c = 0.036 and σ2c + σ2v =0.0625 (where the former represents the distribution of cortisol without noise and the latter, the variance with noise) based on the following: the mean and standard deviation of 100 random draws of cortisol levels measured at 11 am are .28 and .19, respectively. If we draw 100 random observations from the pool of all measures (taken between waking an 9 pm) the mean and standard deviation are .30 and .25, respectively. Based on this we calculate σc= .19 and σv+ σc =.25 and a reliability ratio of 1.7, which corresponds to an attenuation bias of 0.59.
This comparison is based on women with children less than five years old residing in urban areas of Massachusetts and Rhode Island drawn from the 1960 census. Because of limitations of the census data, we were unable to calculate averages for Providence and Boston only.
Sampling weights equal to one over the probability of being sampled were calculated for 2 racial categories, 3 education categories and 4 income categories for a total of 24 cells.
Free cortisol refers to the amount of circulating cortisol that is not bound to proteins and therefore “free” to interact.
There is one outlier in the sibling cortisol sub-sample which we remove in the regression analyses.
This method of defining “high” and “normal” levels of cortisol is typical of the existing bio-medical literature. It is reassuring, however, to note that the 80 percent cutoff is externally validated as it is very similar to the same cutoff in a dataset based on 50 women in the 32nd week of pregnancy assayed for cortisol in the morning as reported by Solden et al (2005).
Differences in gender are not significant. Ultrasound technology was not available at the time of study so it is not possible that the gender of the fetus was known before birth.
In Table 1, the probability of being single increases considerably between birth and age seven. This is consistent with data from the 1960 and 1970 census which shows that the probability of being single more than doubles over this period, increasing from .032 to 0.085.
When we test this directly we find no evidence of a fertility response to elevated cortisol.
The size of the effect is also comparable to educational interventions geared to the very youngest school children. For example, Gormley and Gayer (2005) find that a year of pre-K increases cognitive/knowledge scores among Kindergarten students by 39 percent of a standard deviation.
The impact of unpredictable events on biomarkers for stress such as cortisol is unclear. There is only one paper that attempts to measure biomarkers among adult monkeys reared as infants in VFD, HFD and LFD environments and the findings are not straightforward, with increases in some baseline markers for stress (CRF) but declines in others (including cortisol) among the VFD reared monkeys (see Coplan et al, 1998).
LeWinn, et al (2008) use a subset of the NCPP sibling sample to produce maternal FE estimates of the impact of an index of free cortisol on child verbal IQ at age7 and the results are very similar.
The verbal IQ score is derived from the Wechsler intelligence tests.
The evidence that physical health affects schooling is limited. In the US, Bleakley (2007) finds that hookworm eradication among children increased school attendance, literacy and long term income. There is more evidence that mental health affects schooling in developed countries (Currie and Stabile, 2006.)
We found only small (3 ng/ml) and insignificant differences in cortisol across seasons.
In results not presented, we also include an indicator for winter birth and interact it with cortisol measures (both linear and quintiles). The effects are small, imprecise and generally offsetting.
It could be that the small sample size simply precludes us from finding an effect. We re-ran the regressions on the full sample of roughly 1000 births without including maternal FE. All but one of the regressions (smoking) showed no change in the estimated coefficient or precision when we moved to the larger sample. For smoking, however, we did find an increase in the relationship between cortisol and the probability of smoking (and the estimates were precise). Given negative selection into smoking, it’s not clear how to interpret these estimates, but the results do not strongly support simply lack of power.
Contributor Information
Anna Aizer, Brown University and NBER.
Laura Stroud, Brown Medical School.
Stephen Buka, Brown University.
References
- Austin Marie Paule, Hadzi-Pavlovic Dusan, Leader Leo, Saint Karen, Parker Gordon. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Human Development. 2005;81(2):183–190. doi: 10.1016/j.earlhumdev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Almond Douglas, Hoynes Hilary, Schanzenbach Diane Whitmore. Inside the War on Poverty: The Impact of Food Stamps on Birth Outcomes. Review of Economics and Statistics. 2011;XCIII(2):387–403. [Google Scholar]
- Almond Douglas. Is the 1918 Influenza Pandemic Over? Long-term Effects of In-utero Influenza Exposure in the Post-1940 U.S. Population. Journal of Political Economy. 2006;114(4):672–712. [Google Scholar]
- Almond Douglas, Edlund Lena, Palme Marten. Chernobyl’s Subclinical Legacy: Prenatal Exposure to Radioactive Fallout and School Outcomes in Sweden. Quarterly Journal of Economics. 2009;124(4):1729–1772. [Google Scholar]
- Almond Douglas, Edlund Lena, Li Hongbin, Zhang Jansen. Long-Term Effects of the 1959–1961 China Famine: Mainland China and Hong Kong. NBER Working Paper 13384 2007 [Google Scholar]
- Almond Douglas, Mazumder Bashkar. Health Capital and the Prenatal Environment: The Effect of Maternal Fasting During Pregnancy. American Economic Journal: Applied Economics. 2011;3(4):56–85. [Google Scholar]
- Almond Douglas, Currie Janet. Human Capital Development before Age Five. Handbook of Labor Economics. 2011;4(B):1315–1486. [Google Scholar]
- Black Sandra, Devereux Paul, Salvanes Kjell. From the Cradle to the Labor Market? The Effect of Birth Weight on Adult Outcomes. Quarterly Journal of Economics. 2007;122(1):409–439. [Google Scholar]
- Black Sandra, Devereux Paul, Salvanes Kjell. Does Grief Transfer Across Generations? In Utero Deaths and Child Outcomes. NBER WP 19979 2014 [Google Scholar]
- Bleakley Hoyt. Disease and development: evidence from hookworm eradication in the American south. Quarterly Journal of Economics. 2007;122(1):73–117. doi: 10.1162/qjec.121.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho Adriana. Stress and Birth Weight: Evidence from Terrorist Attacks. American Economic Review-papers and proceedings. 2008;98(2):511–15. [PubMed] [Google Scholar]
- Chemin Matthieu, de Laat Joost, Haushofer Johannes. Negative rainfall shocks increase levels of the stress hormone cortisol among poor farmers in Kenya. mimeo 2013 [Google Scholar]
- Chiu Simon, Collier Christin, Clark Albert, Wynn-Edwards Katherin. Salivary Cortisol on ROCHE Elecsys immunoassay system: pilot biological variation studies. Clinical Biochemistry. 2003;36(3):211–214. doi: 10.1016/s0009-9120(02)00471-x. [DOI] [PubMed] [Google Scholar]
- Cohen Sheldon, Schwartz Joseph, Epel Elissa, Kirschbaum Clelmens, Sidney Steve, Seeman Teresa. Socioeconomic Status, Race and Diurnal Cortisol Decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Cohen Sheldon, Doyle William, Baum Andrew. Socioeconomic status is associated with stress hormones. Psychosomatic Medicine. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Coplan Jeremy D, Trost Ronald, Owens Michael J, Cooper Thomas B, Gorman Jack, Nemeroff Charles B, Rosenblum Leonard. Cerebrosponal Fluid Concentrations of Somastostatin and Biogenic Amines in Grown Primates Reared by Mothers Exposed to Manipulated Foraging Conditions. Arch Gen Psychiatry. 1998;55(5):473–447. doi: 10.1001/archpsyc.55.5.473. [DOI] [PubMed] [Google Scholar]
- Corak Miles. Do Poor Children Become Poor Adults? Lessons for Public Policy from a Cross-Country Comparison of Earnings Mobility. UNICEF Innocenti Research Center; Florence, Italy: 2004. [Google Scholar]
- Crinik Keith, Greenberg Mark, Slough Nancy. Early stress and social support influences on mothers’ and high-risk infants’ functioning in late infancy. Infant Mental Health Journal. 1986;7(1):19–33. [Google Scholar]
- Currie Janet, Rossin-Slater Maya. Weathering the Storm: Hurricanes and Birth Outcomes. Journal of Health Economics. 2013;32(3):487–503. doi: 10.1016/j.jhealeco.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie Janet, Stabile Mark. Child Mental Health and Human Capital Accumulation: The Case of ADHD. Journal of Health Economics. 2006;25(6):1094–1118. doi: 10.1016/j.jhealeco.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Currie Janet. Early Childhood Intervention Programs: What Do We Know? Journal of Economic Perspectives. 2001;15(2):213–238. [Google Scholar]
- Davis Elysia Poggi, Glynn Laura M, Dunkel-Schetter Christine, Hobel Calvin, Chicz-Demet Aleksandra, Sandman Curt A. Prenatal exposure to maternal cortisol influences infant temperament. Journal of Am Acad of Child and Adolescent Psychiatri. 2007;36(6):737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Dohrenwend Barbara S. Social status and stressful life events. Journal of Personality and Social Psychology. 1973;28(2):225–235. doi: 10.1037/h0035718. [DOI] [PubMed] [Google Scholar]
- Eskenazi Brenda, Marks Amy, Catalano Ralph, Bruckner Tim, Toniolo Paolo. Low Birth Weight in New York City and upstate New York following the events of September 11th. Human Reproduction. 2007;22(11):3013–3020. doi: 10.1093/humrep/dem301. [DOI] [PubMed] [Google Scholar]
- Evans Gary, Schamberg Michelle. Childhood Poverty, Chronic Stress and Adult Working Memory. Proceedings of the National Academy of Sciences. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley William T, Gayer Ted. Promoting School Readiness in Oklahoma: An Evaluation of Tulsa’s Pre-K Program. J Human Resources. 2005;XL(3):533–558. [Google Scholar]
- Glynn Laura M, Wadwha Pathik, Dunkel-Schetter Christine, Chicz-DeMet Aleksandra, Sandman Curt A. When Stress Happens Matters: Effects of Earthquake Timing on Stress Responsivity in Pregnancy. American Journal of Obstetrics and Gynecology. 2001;184(4):637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Gutman Leslie Morrison, McLoyd Vonnie, Tokoyawa Teru. Financial strain, neighborhood stress, parenting behaviors and adolescent adjustment in urban African American families. Journal of Research on Adolescence. 2005;15(4):425–449. [Google Scholar]
- Gutteling Barbara, deWeerth Carolina, Buitelaar Jan. Prenatal Stress and Children’s Cortisol Reaction to the First Day of School. Psychoneuroendocrinology. 2005;30(6):541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hanushek Eric. School Resources. In: Hanushek Eric, Welch Finish., editors. The Handbook of the Economics of Education. Vol. 2. Elseveier; 2006. pp. 865–908. [Google Scholar]
- Haushofer Johanne, Fehr Ernst. On the psychology of poverty. Science. 2014;344(2186):862–867. doi: 10.1126/science.1232491. [DOI] [PubMed] [Google Scholar]
- Heckman James, Masterov Dimirty. The Productivity Argument for Investing in Young Children. NBER Working Paper # 13106 2007 [Google Scholar]
- Huizink Anja, de Medina Pascale Robles, Mulder Eduard, Visser Gerard, Buitelaar Jan. Stress During Pregnancy is Associated with Developmental Outcome in Infancy. Journal of Child Psychology and Psychiatry. 2003;44(6):810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- Behrman RE, Butler AS, editors. Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Press; 2006. [PubMed] [Google Scholar]
- Joyce Theodore, Kaestner Robert, Korenman Sanders. The Effect of Pregnancy Intention on Child Development. Demography. 2000;37(1):83–94. [PubMed] [Google Scholar]
- Kunz-Ebrecht Sabine, Kirschbaum Clemens, Steptoe Andrew. Work Stress, Socioeconomic Status and Neuroendocrine Activation Over the Working Day. Social Science and Medicine. 2004;58(2004):1523–1530. doi: 10.1016/S0277-9536(03)00347-2. [DOI] [PubMed] [Google Scholar]
- Lauderdale Diane. Outcomes for Arabic-Named Women in California Before and After September 11. Demography. 2006;43(1):185–201. doi: 10.1353/dem.2006.0008. [DOI] [PubMed] [Google Scholar]
- LeWinn Kaja, Stround, Laura Molnar, Beth Ware, James Koenen, Buka Stephen. Mimeo. University of California; SF: 2008. Elevated Maternal Cortisol Levels During Pregnancy are Associated with Reduced Childhood IQ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour Hani, Rees Daniel. Prenatal Stress on Birth Weight: Evidence from the al-Aqsa Intifada. Discussion Paper Series, IZA DP No. 5535 2011 [Google Scholar]
- Marmot MG, Smith George Davey, Stansfield Stephen, Patel Chandra, North Fiona, Head Jenny, White Ian, Brunner Eric, Feeney Amanda. Health inequality among British civil servants: The Whitehall II study. The Lancet. 1991;337(8):1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- Mazunder Bhashkar. The apple falls even closer to the tree than we thought: new and revised estimates of the intergenerational inheritance of earnings. In: Bowles Samuel, Gintis Herbert, Groves Melissa Osboure., editors. Unequal Chances: family background and economic success. New York: Russell Sage; 2005. [Google Scholar]
- McEwen Bruce. Protective and Damaging Effects of Stress Mediators. New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mineka Susan, Kihlstrom John. Unpredictable and Uncontrollable Events: A New Perspective on Experimental Neurosis. Journal of Abnormal Psychology. 1978;87(2):256–271. doi: 10.1037//0021-843x.87.2.256. [DOI] [PubMed] [Google Scholar]
- Nievar Angela, Luster Tom. Developmental processes in African American Families: an application of McLoyd’s theoretical model. Journal of Marriage and the Family. 2006;68(2):320–331. [Google Scholar]
- O’Connor Thomas G, Ben-Shlomo Yoav, Hernon Jon, Golding Jean, Adams Diana, Glover Vivette. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Bio Psychiatri. 2005;58(3):211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Ousova O, Guyonnet-Duperat V, Iannuccelli N, Bidanel JP, Milan D, Genet C, Llamas B, Yerle M, Chardon P, Emptoz Bonneto A, Pugeat M, Mormede P, Moisan MP. Corticosteroid binding globulin: A new target for cortisol driven obesity. Mol Endocrinol. 2004;18(7):1687–1696. doi: 10.1210/me.2004-0005. [DOI] [PubMed] [Google Scholar]
- Persson Petra, Rossin-Slater Maya. Family Ruptures and Intergenerational Transmission of Stress. 2014 unpublished manuscript. [Google Scholar]
- Qian Nancy, Meng Xin. The Long Run Impact of Famine on Survivors: Evidence From China’s Great Famine. 2009 unpublished manuscript. [Google Scholar]
- Rablen Matthew, Oswald Andrew. Mortality and Immortality : The Nobel Prize as an Experiment into the Effect of Status on Longevity. Journal of Health Economics. 2008;27(6):1462–1471. doi: 10.1016/j.jhealeco.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Rosenblum Leonard, Paully Gayle. The Effects of Varying Environmental Demands on Maternal and Infant Behavior. Child Development. 1984;55(1):305–314. [PubMed] [Google Scholar]
- Scarr Sandra. Behavior-Genetic and Socialization Theories of Intelligence. In: Sternberg Robert, Grigorenko Elena., editors. Intelligence, Heredity, and Environment. Cambridge University Press; 1997. [Google Scholar]
- Sapolsky Robert, Uno Hideo, Rebert Charles, Finch Caleb. Hippocampal Damage Associated with Prolonged Glucocorticoid Exposure in Primates. The Journal of Neuroscience. 1990;10(9):2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider Mary L, Coe Christopher L, Lubach Gabriele R. Endocrine activation mimics the adverse effects of prenatal stress on the neuromotor development of the infant primate. Developmental Psychobiology. 1992;25(6):427–439. doi: 10.1002/dev.420250604. [DOI] [PubMed] [Google Scholar]
- Schneider Mary L. Prenatal Stress Exposure Alters Postnatal Behavioral Expression Under Conditions of Novelty Change in Rhesus Monkey Infants. Developmental Psychobiology. 1992;25(7):529–540. doi: 10.1002/dev.420250706. [DOI] [PubMed] [Google Scholar]
- Seckl Jonathan. Physiologic Programming of the Fetus. Clinics in Perinatalogy. 1998;25(4):939–964. [PubMed] [Google Scholar]
- Simeonova Emilia. Out of Sight, Out of Mind? The Impact of Natural Disasters on Pregnancy Outcomes. CESifo Economic Studies. 2011;57(3):403–431. [Google Scholar]
- Solon Gary. Intergenerational Mobility in the Labor Market. In: Ashenfelter Eds Orley, Card David., editors. Handbook of Labor Economics. Vol. 3. 1999. [Google Scholar]
- Starkman Monica, Giordani Bruno, Berent Stanley, Schork Anthony, Schteingart David. Elevated Cortisol Levels in Cushing’s Disease Are Associated With Cognitive Decrements. Psychosomatic Medicine. 2001;63(6):985–993. doi: 10.1097/00006842-200111000-00018. [DOI] [PubMed] [Google Scholar]
- Steptoe Andrew, Kunz-Ebrecht Sabine, Owen Natalie, Feldman Pamela, Willemsen Gonneke, Kirschbaum Clemens, Marmot Michael. Socioeconomic Status and Stress-Related Biological Responses Over the Working Day. Psychosomatic Medicine. 2003;65(3):461–470. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- Steptoe Andrew, Marmot Michael. The Role of Psychobiological Pathways in Socio-Economic Inequalities in Cardiovascular Disease. European Heart Journal. 2002;23:13–25. doi: 10.1053/euhj.2001.2611. [DOI] [PubMed] [Google Scholar]
- Stroud Laura, Solomon Catherine, Shenassa Edmund, Papandonatos George, Niaura Raymond, Lipsett Lewis, LeWinn Kaja, Buka Stephen. Long-term stability of maternal prenatal steroid hormones from the National Collaborative Perinatal Project: still valid after all these years. Psychoneuroendocrinology. 2007;32(2):140–50. doi: 10.1016/j.psyneuen.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabulsy GM, Person J, Morel Vaillancourt. Meta analytic findings of the relation between maternal prenatal stress and anxiety and child cognitive outcome. J Dev Behav Pediatr. 2014;35(1):38–43. doi: 10.1097/DBP.0000000000000003. [DOI] [PubMed] [Google Scholar]
- Uno Hideo, Lohmiller Lon, Thieme Carol, Kemnitz Jospeh, Engel Michael, Roecker Ellen B, Ferrell Phillip M. Brain Damage Induced by Prenatal Exposure to Dexamethasone in Fetal Rhesus Macaques. Developmental Brain Research 53(2) 1990:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Van den Bergh Bea RH, Eduard Mulder, Maarten Mennes, Glover Vivette. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanism. A review. Neuroscience and Biobehavioral Reviews. 2005;29(2):237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Van Eck Marleen, Berkhof Hans, Nicolson Nancy, Sulon Jose. The Effect of Perceived Stress, Traits, Mood States and Stressful Daily Events on Salivary Cortisol. Psychosomatic Medicine. 1996;85(5):447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Van Goozen Stephanie, Mathys Walter, Cohen-Kettenis Peggy T, Buitellar Jan K, Van England Herman. Hypothalamic-Pituitary-Adrenal Axis and Autonomic nervous System Activity in Disruptive Children and Matched Controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(11):1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- Walker Brian, Best Ruth, Noon Joseph, Watt Graham CM, Webb David J. Seasonal Variation in Glucocorticoid Activity in Healthy Men. The Journal of Clinical Endocrinology & Metabolism. 1997;82(12):4015–4019. doi: 10.1210/jcem.82.12.4430. [DOI] [PubMed] [Google Scholar]
- Weinstock Marta. The long-term behavioral consequences of prenatal stress. Neuroscience and Biobehavioral Reviews. 2008;32(2008):1073–86. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Seckl Jonathan, Homes Meghan. Prenatal Glucocorticoid Programming of Brian Corticosteroid Receptors and Corticotrophin-Releasing Hormone: Possible Implications for Behaviour. Neuroscience. 2001;104(1):71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Wust Stefan, Federenko Ilona, Hellhammer Dick, Kirschbaum C. Genetic Factors, Perceived Chronic Stress and the Free Cortisol Response to Awakening. Psychoneuroendocrinology. 2000;25(7):707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Yu In Tag, Lee Sang-Hun, Lee Yong-Sung, Son Hyeon. Differential effects of corticosterone and dexamethasone on hippocampal neurogenesis in vitro. Biochemical and biophysical research communications. 2004;317(2):484–90. doi: 10.1016/j.bbrc.2004.03.071. [DOI] [PubMed] [Google Scholar]