Abstract
Background
5-Aminosalicylic acids (5-ASA) are effective for ulcerative colitis (UC) as a maintenance therapy. It is not clear when and how to reduce a dose of 5-ASA after inducing remission. We aimed to investigate the clinical characteristics and evaluate the risk factors of relapse for UC patients receiving 5-ASA.
Methods
The medical records of prospectively registered UC patients who received oral 5-ASA as maintenance therapy between January and December 31, 2014, were investigated. The patients’ clinical characteristics in a 2-year follow-up were compared between a relapse group and a remission group.
Results
Of 527 UC patients receiving only oral 5-ASA, 390 (74.0%) maintained remission and 137 (26.0%) relapsed during the follow-up period. Multivariable analysis indicated that a shorter duration of disease remission (p < 0.001, OR: 1.24, 95% CI: 1.12–1.38) was statistically significant for each comparison between the remission and relapse groups among all the patients. Risk factors for clinical relapse were a shorter duration of disease remission (p <0.001, OR: 1.17, 95% CI: 1.04–1.33) in the high-dose 5-ASA group and a shorter duration of disease remission (p = 0.003, OR: 1.45, 95% CI: 1.13–1.89) and a history of steroid use (p = 0.048, OR: 4.73, 95% CI: 1.01–22.2) in the low-dose group.
Conclusions
A dose reduction of 5-ASA might be cautiously selected in UC patients with a history of steroid use and a shorter duration of disease remission.
Introduction
Ulcerative colitis (UC) is a chronic idiopathic inflammatory disease of the large intestine that is characterized by periods of remission and relapse [1]. 5-Aminosalicylic acids (5-ASA) are the first-line drug and an effective treatment for induction in patients with mild to moderate UC [2]. Once remission is achieved with any therapeutic agents, up to 70% of patients not receiving maintenance treatment are expected to relapse within a year [3].
The effect of 5-ASA on the maintenance of remission was confirmed in a Cochrane systematic review [4]. The results of a recent meta-analysis indicated that the odds ratio for failure to maintain clinical or endoscopic remission for 5-ASA versus the placebo was 0.47 [5]. A recent study in children newly diagnosed with UC found that 40% of the children started on 5-ASA as the primary maintenance therapy at diagnosis were in corticosteroid-free remission after 1 year of treatment [6].
Although several studies have reported the usefulness for inducing and maintaining remission, there is still little evidence that 5-ASA significantly prevents relapse dose-dependently. Paoluzi et al. observed that 2.4 g daily of oral 5-ASA seems to be better at preventing and delaying relapses of UC than 1.2 g daily [7]. Fockens et al. reported that patients in a group that received 3.0 g showed a lower relapse rate at 1 year than a 1.5 g dose group [8]. Rubin et al. found that long-term higher-dose 5-ASA treatments probably prevented clinical relapse more effectively than a lower dose in UC patients without complete remission [9]. These results suggest that treatments with higher-dose 5-ASA may contribute to a better clinical outcome. However, other studies have shown that the efficacy of preventing relapse was not significantly different in patients even taking more than 2.5 g of 5-ASA daily. Furthermore, the efficacy for maintenance of clinical remission was comparable between patients taking a high dose of 5-ASA and those taking a low dose when treatment adherence was moderate to excellent [10]. These results indicated that it may be difficult to decide when to reduce the dose of 5-ASA after clinical remission has been induced. In addition, some patients want to receive a lower dose of 5-ASA. However, no definitive criteria for dose reduction of 5-ASA in maintenance therapy have been established. In this study, we investigated the clinical characteristics and risk factors of relapse for UC patients who received 5-ASA as maintenance therapy, especially those who received a low dose of 5-ASA.
Materials and methods
Study design and patients
We conducted a review of the medical records of UC patients who visited an outpatient clinic of the Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University Hospital (Tokyo, Japan), between January 1, 2014, and December 31, 2014, from our database of prospectively registered UC patients and who could be evaluated in detail based on their clinical information. We performed detailed analyses of 1325 UC patients who were treated with oral 5-ASA therapy. Three formulations of 5-ASA were identified: Asacol®, Pentasa®, and Salazosulfapyridine®. We excluded patients who received combination therapy for UC (thiopurine, corticosteroid, tacrolimus, cyclosporine, infliximab, adalimumab, and granulocyte apheresis).
Ethical considerations
This study was approved by the Ethics Committee of Keio University School of Medicine (no. 20150210) and was conducted in accordance with the principles of the Declaration of Helsinki. Because no informed consent was required for this retrospective observational study, we obtained the patient’s consent to participate by posting information about this study in Keio University Hospital. Patient records and information were anonymized and de-identified before analysis.
Study procedures
The patients’ demographic data and disease characteristics, including their gender, age, age at onset of symptoms, duration of disease, duration of disease remission, site of the disease, serum albumin (Alb) level, total cholesterol (TC) level, white blood cell (WBC) count, hemoglobin (Hb), platelet (Plt) level, serum C-reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), endoscopic Mayo score (MES), history of steroid use, and history of thioprine use, were collected. We followed the patients’ progress for 2 years from their first visit between January 1, 2014, and December 31, 2014, and classified them into 2 groups as follows: a remission group (partial Mayo (pMayo) score of ≤ 1 for 2 years) and a relapse group (pMayo score of ≥ 3 at least once in 2 years or received new treatment for clinical symptoms of UC). It is difficult to classify pMayo score of 2 into relapse group or remission group. For this reason, patients with pMayo score of 2 at least once during observation period were excluded in this study. PMayo scores were assessed and recorded by physicians at the original time of each visit. Subsequently, patients were subclassified into a high-dose group (HDG) and a low-dose group (LDG) according to the daily dose of 5-ASA. HDG consisted of patients who received at least 3.6 g daily of Asacol®, over 3.0 g daily of Pentasa®, or over 3.0 g daily of Salazosulfapyridine®, and LDG was consisted of others. The patients in HDG and LDG were additionally subclassified into remission groups and relapse groups. In the manner described above, we analysed the patients’ demographic data and disease characteristics between the relapse and remission groups. Information regarding adherence with 5-ASA was also collected. Poor adherence was defined as the presence of one of the following: (i) declined prescriptions two consecutive times in an outpatient clinic, (ii) poor adherence noted in medical records, (iii) self-determined dose reduction or discontinuation of taking medicine, and (iv) hospital visit more than 2 months after the last regular visit.
Statistical analysis
Paired variables were evaluated by Fischer’s exact test or Student’s t-test, and categorical variables were compared using the chi-squared or Kruskal-Wallis tests. Logistic regression was used to perform a multivariate analysis of risk factors for disease relapse, gender, age, age at onset of symptoms, duration of disease, duration of disease remission, MES, dose of 5-ASA (HDG or LDG), history of steroid use, and history of thiopurine use. All of the statistical analyses were performed using IBM SPSS Statistics version 23 (IBM Corp., Armonk, N.Y., USA). Two-sided p values were considered statistically significant at a level of <0.05.
Results
Patient profile
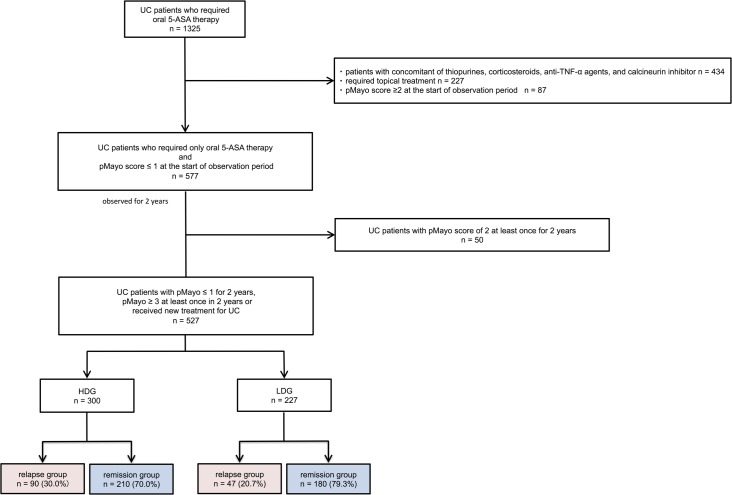
Between January 1, 2014, and December 31, 2014, 1325 patients were treated with oral 5-ASA to maintain clinical remission of UC. Patients with concomitant use of thiopurines (6-mercaptopurine and azathioprine), anti-TNF-α agents (adalimumab and infliximab), and tacrolimus were excluded from this study and 891 UC patients were identified. In our institution, VSL-3, methotrexate and vedolizumab are never used (these agents have not yet been approved in Japan). Of the 891 patients, 227 who received topical treatment, such as a suppository or enema, and 87 patients with a pMayo score of ≥ 2 at the start of observation period were excluded. as a result, a total of 577 patients with a pMayo score of ≤ 1 who were treated with oral 5-ASA alone were identified. Among 577 patients, 50 patients with pMayo score of 2 at least once during observation period were excluded. Consequently, 527 patients were included in this study. Of these, 300 received a high dose of 5-ASA and 227 received a low dose (Fig 1). The baseline characteristics of the 527 patients are shown in Table 1. The mean duration of disease and the duration of disease remission were 14.8 and 4.16 years, respectively. The mean MES was 0.68 (N = 241). Ultimately, 300 and 227 patients were classified into HDG and LDG, respectively (Fig 1). Table 1. showed baseline characteristics of HDG and LDG. Expectedly, there were statistically significant in shorter duration of disease remission, extent of disease, MES, history of steroid use, history of thiopurine use, and clinical relapse between 2 groups.
Fig 1. Flowchart of participants in this study.
A total of 1325 UC patients with oral 5-ASA maintenance therapy were identified. We excluded 661 patients who received thiopurines (6-mercaptopurine and azathioprine), anti-TNF-α agents (adalimumab and Infliximab), tacrolimus, and topical treatment such as a suppository or enema, and 87 patients who had a pMayo score of ≥ 2 at the start of the observation period. Among 577 patients, 50 patients with pMayo score of 2 at least once during observation period were excluded. Consequently, 527 patients were included in this study. Of these, 300 received a high dose of 5-ASA and 227 received a low dose. Ninety patients (30.0%) in HDG and 47 patients (20.7%) in LDG relapsed. The difference in relapse rates between HDG and LDG was statistically significant (p = 0.016).
Table 1. Baseline characteristics of the 527 patients in this study.
| total n = 527 |
HDG n = 300 |
LDG n = 227 |
p value | |
|---|---|---|---|---|
| Male; n (%) | 282 (53.5) | 163(54.3) | 119(52.4) | 0.72 |
| Age, years; mean (SD) | 47.99 (14.6) | 47.3 (14.6) | 48.4(14.6) | 0.40 |
| Age at onset, years; mean (SD) | 33.3 (13.2) | 32.7(13.1) | 34.0(13.4) | 0.30 |
| Duration of disease, years; mean (SD) | 14.8 (9.45) | 14.7 (9.60) | 14.9 (9.29) | 0.79 |
| Duration of disease remission, years; mean (SD) | 4.16 (4.59) | 3.19 (3.84) | 5.44(5.18) | <0.001 |
| Extent of disease | ||||
| Extensive; n (%) | 196 (37.2) | 130(43.3) | 66(29.1) | <0.001 |
| Alb level, g/dl; mean (SD) | 4.33 (0.33) | 4.32 (0.34) | 4.35 (0.32) | 0.32 |
| TC level, mg/dl; mean (SD) | 195.6 (34.2) | 195.2 (33.4) | 196.1 (35.5) | 0.80 |
| WBC counts, 103/μl; mean (SD) | 5.93 (1.72) | 5.86 (1.75) | 6.01 (1.68) | 0.39 |
| Hb, g/dl; mean (SD) | 13.6 (1.75) | 13.5 (1.79) | 13.7 (1.69) | 0.19 |
| Plt level, 104/μl; mean (SD) | 25.9 (6.34) | 26.4 (6.47) | 25.3 (6.13) | 0.080 |
| CRP level, mg/dl; mean (SD) | 0.16 (0.60) | 0.18 (0.76) | 0.13 (0.25) | 0.38 |
| ESR level, mm/hr; mean (SD) | 9.47 (9.50) | 9.64(9.83) | 9.21(9.02) | 0.72 |
| Mayo endoscopic score; mean (SD) | 0.68(0.73) | 0.80(0.70) | 0.49(0.79) | 0.001 |
| Medical history | ||||
| Prednisolone (+); n (%) | 128 (24.8) | 89 (29.7) | 39 (17.2) | 0.001 |
| Thiopurine (+); n (%) | 20(3.8) | 16(5.3) | 4(1.8) | 0.038 |
| Clinical relapse (+); n(%) | 137 (26.0) | 90(30.0) | 47 (20.7) | 0.016 |
n, number; SD, standard deviation; HDG, high dose group; LDG, low dose group; Alb, serum albumin; TC, total cholesterol; WBC, white blood cell count; Hb, haemoglobin; Plt, platelet; CRP serum C-reactive protein; ESR, erythrocyte sedimentation rate.
Risk factors for clinical relapse in all cohorts
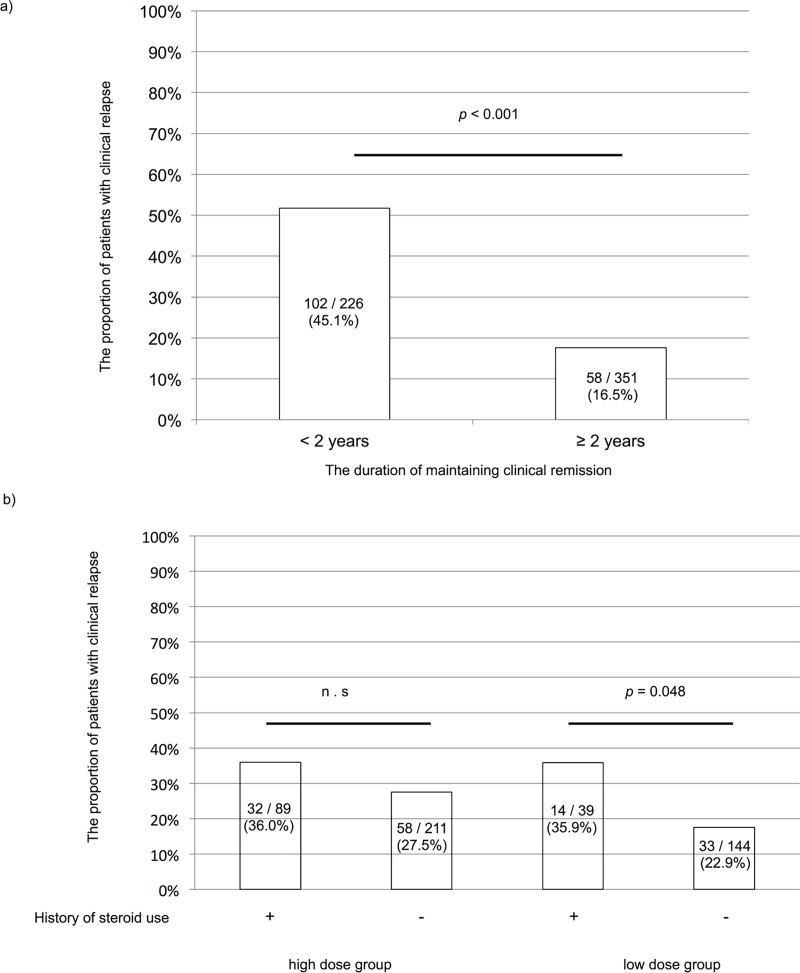
Of the 527 patients, 390 (64.0%) maintained remission and 137 (26.0%) relapsed during the 2-years observational period. Additional treatment for UC in the relapse group was mainly topical treatment (59 patients), corticosteroids (22 patients), and an increased 5-ASA dose (22 patients). Among them, mean dosing period of 5-ASA were 175.6 months (HDG 175.0 months, LDG 176.5 months). As shown in Table 2, age (p = 0.031), duration of disease remission (p < 0.001), serum albumin level (p = 0.043), 5-ASA dose (p = 0.016), gender (p = 0.04) and history of steroid use (p = 0.03) were statistically significant for each comparison between the remission and relapse groups. Multivariate analysis indicated that earlier age at onset (p = 0.023, OR: 1.03, 95% CI: 1.004–1.06), shorter duration of disease remission (p < 0.001, OR: 1.24, 95% CI: 1.12–1.38) and MES (p = 0.021, OR: 1.63, 95% CI: 1.08–2.47) were risk factors for clinical relapse. Dosage of 5-ASA was not statistically significant in multivariable analysis (p = 0.065). As shown in Fig 2A, there were statistically significant results for each comparison between <2 and ≥2 years of disease remission (p < 0.001). The relapse rate was 45.1% among the patients with <2 years of remission and 16.5% among those with ≥2 years of remission.
Table 2. Risk factors for disease relapse in all 527 patients.
| Univariate analyses | Multivariate analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Remission (N = 390) |
Relapse (N = 137) |
OR | 95% CI | p value | OR | 95% CI | p value | |
| Age, years; mean | 48.7 | 45.5 | - | 0.28–5.97 | 0.031 | |||
| Age at onset, years; mean | 33.9 | 31.5 | - | -0.14–5.00 | 0.064 | 1.03 | 1.004–1.05 | 0.023 |
| Duration of disease, years; mean | 15.0 | 14.1 | - | -0.89–2.78 | 0.31 | |||
| Duration of disease remission, years; mean | 4.8 | 2.4 | - | 1.57–3.32 | <0.001 | 1.24 | 1.11–1.38 | <0.001 |
| serum Alb, g/dl; mean | 4.4 | 4.3 | - | 0.003–0.19 | 0.043 | |||
| TC level, mg/dl; mean | 197 | 191 | - | -1.63–14.0 | 0.12 | |||
| WBC counts, 103/μl; mean | 5.9 | 6.1 | - | -0.70–0.13 | 0.18 | |||
| Hb, g/dl; mean | 13.6 | 13.4 | - | -0.15–0.61 | 0.24 | |||
| Plt level, 104/μl; mean | 256 | 266 | - | -23.6–3.99 | 0.16 | |||
| CRP level, mg/dl; mean | 0.17 | 0.15 | - | -0.11–0.15 | 0.73 | |||
| ESR level, mm/h; mean | 9.0 | 10 | - | -4.90–1.48 | 0.29 | |||
| Mayo endoscopic score; mean | 0.60 | 0.89 | - | -0.51–-0.66 | 0.012 | 1.63 | 1.08–2.47 | 0.021 |
| HDG, n | 210 | 90 | 1.64 | 1.10–2.46 | 0.016 | |||
| LDG, n | 180 | 47 | ||||||
| Male, n | 219 | 63 | 1.50 | 1.02–2.22 | 0.04 | |||
| Female, n | 171 | 74 | ||||||
| Previous steroid use (+), n | 82 | 46 | 1.90 | 1.24–2.92 | 0.03 | |||
| Previous steroid use (-), n | 308 | 91 | ||||||
| Previous thiopurine use (+), n | 18 | 7 | 1.11 | 0.45–2.72 | 0.82 | |||
| Previous thiopurine use (-), n | 372 | 130 | ||||||
n, number; SD, standard deviation; HDG, high dose group; LDG, low dose group; Alb, serum albumin; TC, total cholesterol; WBC, white blood cell count; Hb, haemoglobin; Plt, platelet; CRP serum C-reactive protein; ESR, erythrocyte sedimentation rate.
Fig 2. Relationship between the dose of 5-ASA and relapse in participants.
(a) The proportion of patients with clinical relapse was compared between patients with <2 and those with ≥2 years of disease remission. (b) The proportion of patients with clinical relapse was statistically significant higher in low dose group with a history of steroid use than that without a history of steroid use. However, there was no statistically significant difference regardless of the previous steroid use among high dose group.
In the assessment of the relationship between poor adherence and relapse, 73 patients’ adherence was poor, and 49 of those patients relapsed (67.1%). Relapse rates were 100% (35/35) in HDG and 48.7% (13/38) in LDG. Poor adherence was not statistically significant between the remission and relapse groups in 300 HDG patients (p = 0.84). However, poor adherence increased the risk of relapse in LDG patients (p = 0.03).
Risk factors for clinical relapse in HDG
The difference in the relapse rate between HDG and LDG patients was statistically significant. Therefore we next analyzed the risk factors of HDG and LDG patients separately as clinical background was different between HDG and LDG patients.
The numbers of patients in the remission and relapse groups are shown in Fig 1. Of the 300 HDG patients, 210 (70.0%) remained in remission and 90 (30.0%) relapsed. As shown in Table 3, univariate analysis indicated that duration of disease remission (p < 0.001) and MES (p = 0.041) were risk factors for clinical relapse. Multivariate analysis indicated that each comparison between the remission and relapse groups in HDG revealed that a shorter duration of disease remission (p <0.001, OR: 1.17, 95% CI: 1.04–1.33) and MES (p = 0.047, OR: 1.62, 95% CI: 1.01–2.62) correlated with relapse.
Table 3. Risk factors for disease relapse in HDG.
| Univariate analyses | Multivariate analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Remission (N = 210) |
Relapse (N = 90) |
OR | 95% CI | p value | OR | 95% CI | p value | |
| Age, years; mean | 48.2 | 45.5 | - | -0.79–6.10 | 0.13 | |||
| Age at onset, years; mean | 33.1 | 31.8 | - | -1.73–4.48 | 0.38 | |||
| Duration of disease, years; mean | 15.1 | 13.7 | - | -0.85–3.69 | 0.22 | |||
| Duration of disease remission, years; mean | 3.8 | 1.9 | - | 0.96–2.82 | <0.001 | 1.17 | 1.04–1.33 | <0.001 |
| serum Alb, g/dl; mean | 4.3 | 4.3 | - | -0.66–0.119 | 0.57 | |||
| TC level, mg/dl; mean | 198 | 190 | - | -2.10–16.5 | 0.13 | |||
| WBC counts, 103/μl; mean | 5.8 | 6.0 | - | -0.71–0.24 | 0.33 | |||
| Hb, g/dl; mean | 13.5 | 13.4 | - | -0.45–0.52 | 0.87 | |||
| Plt level, 104/μl; mean | 263 | 265 | - | -20.9–16.0 | 0.79 | |||
| CRP level, mg/dl; mean | 0.20 | 0.16 | - | -0.15–0.23 | 067 | |||
| ESR level, mm/h; mean | 9.0 | 11.1 | - | -6.04–1.69 | 0.27 | |||
| Mayo endoscopic score; mean | 0.71 | 1.00 | - | -0.56–0.017 | 0.041 | 1.62 | 1.005–2.62 | 0.047 |
| Male, n | 115 | 48 | 1.05 | 0.65–1.73 | 0.82 | |||
| Female, n | 95 | 42 | ||||||
| Previous steroid use (+), n | 57 | 32 | 1.48 | 0.87–2.51 | 0.14 | |||
| Previous steroid use (-), n | 153 | 58 | ||||||
| Previous thiopurine use (+), n | 11 | 6 | 1.06 | 0.36–3.16 | 0.91 | |||
| Previous thiopurine use (-), n | 169 | 70 | ||||||
n, number; SD, standard deviation; HDG, high dose group; LDG, low dose group; Alb, serum albumin; TC, total cholesterol; WBC, white blood cell count; Hb, haemoglobin; Plt, platelet; CRP serum C-reactive protein; ESR, erythrocyte sedimentation rate.
Risk factors for clinical relapse in LDG
Of the 227 LDG patients, 180 patients (79.3%) maintained remission and 47 (20.7%) relapsed (Fig 1). As shown in Table 4, age at onset (p = 0.025), duration of disease remission (p < 0.001), gender (p = 0.002), history of steroid use (p = 0.001), and poor adherence (p = 0.03) were statistically significant for each comparison between the remission and relapse groups in LDG. Multivariate analysis indicated that shorter duration of disease remission (p = 0.003, OR: 1.45, 95% CI: 1.13–1.89), female (p = 0.019, OR: 4.31, 95% CI 1.26–14.7), and the history of steroid use (p = 0.048, OR: 4.73, 95% CI: 1.01–22.2) were risk factors for clinical relapse. Fig 2B showed relationship between history of steroid use and dosage of 5-ASA. Although there were not statistically significant whether patients had history of steroid use or not in HDG, patients with history of steroid use had higher relapse risk in LDG.
Table 4. Risk factors for disease relapse in LDG.
| Univariate analyses | Multivariate analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Remission (N = 180) |
Relapse (N = 47) |
OR | 95% CI | p value | OR | 95% CI | p value | |
| Age, years; mean | 49.2 | 45.5 | - | -0.14–8.36 | 0.13 | |||
| Age at onset, years; mean | 34.8 | 30.8 | - | 0.17–8.12 | 0.025 | |||
| Duration of disease, years; mean | 15.0 | 14.7 | - | -2.75–3.28 | 0.86 | |||
| Duration of disease remission, years; mean | 3.27 | 6.01 | - | 1.09–4.37 | 0.001 | 1.45 | 1.13–1.89 | 0.003 |
| serum Alb, g/dl; mean | 4.4 | 4.3 | - | -0.012–0.27 | 0.072 | |||
| TC level, mg/dl; mean | 196 | 193 | - | -12.6–20.4 | 0.64 | |||
| WBC counts, 103/μl; mean | 5.9 | 6.4 | - | -1.21–0.21 | 0.16 | |||
| Hb, g/dl; mean | 13.8 | 13.3 | - | -0.027–1.06 | 0.062 | |||
| Plt level, 104/μl; mean | 24.9 | 26.9 | - | -42.3–2.92 | 0.086 | |||
| CRP level, mg/dl; mean | 0.13 | 0.12 | - | -0.079–0.11 | 0.74 | |||
| ESR level, mm/h; mean | 9.1 | 9.6 | - | -6.52–5.64 | 0.88 | |||
| Mayo endoscopic score; mean | 0.46 | 0.63 | - | -0.56–0.22 | 0.373 | |||
| Male, n | 104 | 15 | 2.92 | 1.48–5.77 | 0.002 | 4.31 | 1.26–14.7 | 0.019 |
| Female, n | 76 | 32 | ||||||
| Previous steroid use (+), n | 25 | 14 | 2.63 | 1.24–5.59 | 0.001 | 4.73 | 1.01–22.2 | 0.048 |
| Previous steroid use (-), n | 155 | 33 | ||||||
| Previous thiopurine use (+), n | 4 | 0 | - | - | 0.30 | |||
| Previous thiopurine use (-), n | 176 | 47 | ||||||
n, number; SD, standard deviation; HDG, high dose group; LDG, low dose group; Alb, serum albumin; TC, total cholesterol; WBC, white blood cell count; Hb, haemoglobin; Plt, platelet; CRP serum C-reactive protein; ESR, erythrocyte sedimentation rate
Discussion
5-ASA is effective in both inducing and maintaining remission in patients with mild to moderate UC. Although meta-analysis has shown the usefulness of maintenance in 5-ASA therapy in UC patients [4, 5], the effective dose of 5-ASA has not been well investigated. The Toronto consensus guideline indicated that at least 2 g of oral 5-ASA should be continued to maintain complete remission in patients with oral 5-ASA-induced complete remission of active UC [11]. However, it is not clear whether a higher-dose of 5-ASA is required for a long duration to maintain clinical remission. In addition, risk factors of relapse for UC have not been investigated, particularly in patients who receive a lower dose of 5-ASA. In the present study, we demonstrated that a shorter duration of disease remission, history of steroid use and gender were associated with a higher risk of relapse in patients with LDG. From the results of several studies [12], there are controversies concerning the gender. Therefore, we have thought that gender was not usefully predictive factor of relapse disease. Our study highlights the importance of the duration of disease remission and history of steroid use when considering whether to reduce the dose of 5-ASA.
In our study, the overall relapse rates were 26.0% among all 527 patients over 2 years, whereas Feagan et al. previously reported that 41% of UC patients with oral 5-ASA relapsed [4]. The lower relapse rates in the present study may be explained by our inclusion of more patients with a longer duration of disease remission.
In a recent study, approximately half of the patients who obtained complete remission after daily treatment with 4.8 g of 5-ASA and who received maintenance therapy with 2.4 g had a relapse of the disease within 12 months [9]. These results suggest that patients with a shorter duration of disease remission who receive a lower dose of 5-ASA as maintenance therapy are at high risk of relapse. Thus, it is difficult to decide when the dose of 5-ASA can be reduced even after clinical remission has been achieved. We aimed to determine whether longer clinical remission is related to a better outcome in patients treated with 5-ASA. In our study, Fig 2 indicates that relapse rates were markedly lower after more than 2 years of remission. The results from our study indicated that reducing the dose of 5-ASA within a short duration should not considered after achieving clinical remission.
Several studies have investigated the relapse rate after clinical remission induced by a steroid. Some studies showed that the need for a steroid is related to poor prognosis in UC patients [8, 13, 14]. Khan et al. reported that 65% of patients whose clinical remission was induced by steroids required “re-steroid treatment” within 2 years [15]. Another study reported that the relapse rate after remission was induced by steroids was 72% in a median follow-up of 83 months among UC patients who received 5-ASA as the maintenance treatment and did not receive thiopurine [16]. These results indicate that clinical relapse is frequently observed after clinical remission has been obtained with corticosteroids. In our study, the risk of clinical relapse in the LDG patients was higher in UC patients with a history of steroid use. These data were consistent with those from another study indicating that patients with moderately active UC who had previously been treated with a corticosteroid might benefit from induction therapy with a higher dose of 5-ASA [14]. Because patients with previous use of steroids are at risk for clinical relapse, UC patients who have such a history should not be treated with a reduced dose of 5-ASA.
Thiopurines are also useful as an alternative to 5-ASA for maintenance therapy after remission has been induced by steroids. A meta-analysis found that thiopurines were more effective than placebo for the prevention of UC relapse [17]. Ardizzone et al. reported that thiopurines were significantly more effective than 5-ASA for steroid-dependent UC [18]. However, many adverse effects are associated with thiopurine use, including myelotoxicity, hepatotoxicity, pancreatitis and lymphoma. Almost half of patients who received thiopurine therapy showed adverse effects [19] during the first 12 months after receiving thiopurine. Furthermore, some patients are intolerant to thiopurine, and some do not want to use thiopurine as a maintenance therapy. Therefore, maintenance therapy with an adequate dose of 5-ASA is critical for patients who do not want to receive thiopurine, regardless of the reason.
The present study had some limitations. First, although we analysed a relatively large number of UC patients treated with oral 5-ASA alone, colonoscopy was not done in more than half of the patients. Because our study was performed for patients with clinical remission, the participants in our study didn't necessarily have to undergo annually colonoscopy in the clinical setting. However, we could confirm the endoscopic severity was associated to the prognosis and our results are consistent with many other studies demonstrating that mucosal healing is associated with prolonged disease remission. [12, 20–23]. Second, we neither used a self-administered questionnaire nor confirmed the numbers of prescribed 5-ASA tablets remaining at each visit to assess the adherence. However, we defined poor adherence to medication before the analysis of our findings. Third, our study was performed only in a Japanese population, and we did not confirm our findings in patients of other races.
Conclusion
UC patients with a shorter duration of remission have a high risk of relapse compared to those with a long duration of remission. Therefore, the dose of 5-ASA may be reduced when the duration of disease remission is more than 2 years. In addition, for UC patients with a history of steroid use, the dose of 5-ASA should be reduced with caution because a low dose of 5-ASA increases relapse in those patients.
Supporting information
This is data in our study.
(XLSX)
Acknowledgments
We thank the present and past members of the Keio IBD Group for their continued support.
Abbreviations
- 5-ASA
5-aminosalicylic acids
- UC
ulcerative colitis
- Alb
albumin
- TC
total cholesterol
- WBC
white blood cell count
- Hb
hemoglobin
- Plt
platelet, serum
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- MES
Mayo endoscopic score
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380(9853):1606–19. Epub 2012/08/24. doi: 10.1016/S0140-6736(12)60150-0 . [DOI] [PubMed] [Google Scholar]
- 2.Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. The Cochrane database of systematic reviews. 2012;10:Cd000543 Epub 2012/10/19. doi: 10.1002/14651858.CD000543.pub3 . [DOI] [PubMed] [Google Scholar]
- 3.Hanauer SB. Medical therapy for ulcerative colitis 2004. Gastroenterology. 2004;126(6):1582–92. Epub 2004/05/29. . [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. The Cochrane database of systematic reviews. 2012;10:Cd000544 Epub 2012/10/19. doi: 10.1002/14651858.CD000544.pub3 . [DOI] [PubMed] [Google Scholar]
- 5.Sutherland L, Roth D, Beck P, May G, Makiyama K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. The Cochrane database of systematic reviews. 2002;(4):Cd000544 Epub 2003/01/10. doi: 10.1002/14651858.CD000544 . [DOI] [PubMed] [Google Scholar]
- 6.Zeisler B, Lerer T, Markowitz J, Mack D, Griffiths A, Bousvaros A, et al. Outcome following aminosalicylate therapy in children newly diagnosed as having ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56(1):12–8. Epub 2012/08/01. doi: 10.1097/MPG.0b013e31826ac41a . [DOI] [PubMed] [Google Scholar]
- 7.Paoluzi OA, Iacopini F, Pica R, Crispino P, Marcheggiano A, Consolazio A, et al. Comparison of two different daily dosages (2.4 vs. 1.2 g) of oral mesalazine in maintenance of remission in ulcerative colitis patients: 1-year follow-up study. Aliment Pharmacol Ther. 2005;21(9):1111–9. Epub 2005/04/28. doi: 10.1111/j.1365-2036.2005.02458.x . [DOI] [PubMed] [Google Scholar]
- 8.Fockens P, Mulder CJ, Tytgat GN, Blok P, Ferwerda J, Meuwissen SG, et al. Comparison of the efficacy and safety of 1.5 compared with 3.0 g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Dutch Pentasa Study Group. Eur J Gastroenterol Hepatol. 1995;7(11):1025–30. Epub 1995/11/01. . [DOI] [PubMed] [Google Scholar]
- 9.Rubin DT, Bradette M, Gabalec L, Dobru D, Marquez J, Inglis S, et al. Ulcerative Colitis Remission Status After Induction With Mesalazine Predicts Maintenance Outcomes: the MOMENTUM Trial. Journal of Crohn's & colitis. 2016;10(8):925–33. Epub 2016/02/26. doi: 10.1093/ecco-jcc/jjw049 ; PubMed Central PMCID: PMCPMC4962361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan N, Abbas AM, Koleva YN, Bazzano LA. Long-term mesalamine maintenance in ulcerative colitis: which is more important? Adherence or daily dose. Inflamm Bowel Dis. 2013;19(6):1123–9. Epub 2013/03/22. doi: 10.1097/MIB.0b013e318280b1b8 . [DOI] [PubMed] [Google Scholar]
- 11.Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148(5):1035–58.e3. Epub 2015/03/10. doi: 10.1053/j.gastro.2015.03.001 . [DOI] [PubMed] [Google Scholar]
- 12.Bello C, Belaiche J, Louis E, Reenaers C. Evolution and predictive factors of relapse in ulcerative colitis patients treated with mesalazine after a first course of corticosteroids. Journal of Crohn's & colitis. 2011;5(3):196–202. Epub 2011/05/18. doi: 10.1016/j.crohns.2010.12.011 . [DOI] [PubMed] [Google Scholar]
- 13.Ardizzone S, Petrillo M, Molteni P, Desideri S, Bianchi Porro G. Coated oral 5-aminosalicylic acid (Claversal) is equivalent to sulfasalazine for remission maintenance in ulcerative colitis. A double-blind study. J Clin Gastroenterol. 1995;21(4):287–9. Epub 1995/12/01. . [DOI] [PubMed] [Google Scholar]
- 14.Sandborn WJ, Regula J, Feagan BG, Belousova E, Jojic N, Lukas M, et al. Delayed-release oral mesalamine 4.8 g/day (800-mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology. 2009;137(6):1934–43.e1-3. Epub 2009/09/22. doi: 10.1053/j.gastro.2009.08.069 . [DOI] [PubMed] [Google Scholar]
- 15.Khan N, Abbas A, Williamson A, Balart L. Prevalence of corticosteroids use and disease course after initial steroid exposure in ulcerative colitis. Dig Dis Sci. 2013;58(10):2963–9. Epub 2013/07/03. doi: 10.1007/s10620-013-2748-0 . [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Planella E, Manosa M, Van Domselaar M, Gordillo J, Zabana Y, Cabre E, et al. Long-term outcome of ulcerative colitis in patients who achieve clinical remission with a first course of corticosteroids. Dig Liver Dis. 2012;44(3):206–10. Epub 2011/11/15. doi: 10.1016/j.dld.2011.10.004 . [DOI] [PubMed] [Google Scholar]
- 17.Gisbert JP, Linares PM, McNicholl AG, Mate J, Gomollon F. Meta-analysis: the efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment Pharmacol Ther. 2009;30(2):126–37. Epub 2009/04/28. doi: 10.1111/j.1365-2036.2009.04023.x . [DOI] [PubMed] [Google Scholar]
- 18.Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55(1):47–53. Epub 2005/06/24. doi: 10.1136/gut.2005.068809 ; PubMed Central PMCID: PMCPMC1856376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijer B, Mulder CJ, Peters GJ, van Bodegraven AA, de Boer NK. Efficacy of thioguanine treatment in inflammatory bowel disease: A systematic review. World J Gastroenterol. 2016;22(40):9012–21. Epub 2016/11/12. doi: 10.3748/wjg.v22.i40.9012 ; PubMed Central PMCID: PMCPMC5083806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parente F, Molteni M, Marino B, Colli A, Ardizzone S, Greco S, et al. Are colonoscopy and bowel ultrasound useful for assessing response to short-term therapy and predicting disease outcome of moderate-to-severe forms of ulcerative colitis?: a prospective study. Am J Gastroenterol. 2010;105(5):1150–7. Epub 2009/12/10. doi: 10.1038/ajg.2009.672 . [DOI] [PubMed] [Google Scholar]
- 21.Ardizzone S, Cassinotti A, Duca P, Mazzali C, Penati C, Manes G, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9(6):483–9.e3. Epub 2011/01/05. doi: 10.1016/j.cgh.2010.12.028 . [DOI] [PubMed] [Google Scholar]
- 22.Froslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133(2):412–22. Epub 2007/08/08. doi: 10.1053/j.gastro.2007.05.051 . [DOI] [PubMed] [Google Scholar]
- 23.Arai M, Naganuma M, Sugimoto S, Kiyohara H, Ono K, Mori K, et al. The Ulcerative Colitis Endoscopic Index of Severity is Useful to Predict Medium- to Long-Term Prognosis in Ulcerative Colitis Patients with Clinical Remission. Journal of Crohn's & colitis. 2016;10(11):1303–9. Epub 2016/10/30. doi: 10.1093/ecco-jcc/jjw104 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This is data in our study.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.