Abstract
Pseudomonas aeruginosa is a frequent cause of lung infections, particularly in chronic infections in cystic fibrosis patients. However, treatment is challenging due to P. aeruginosa evasion of the host immune system and the rise of antibiotic resistant strains. Host defense peptides (HDPs) and synthetic derivatives called innate defense regulators (IDRs) have shown promise in several infection models as an alternative to antibiotic treatment. Here we tested peptide IDR-1002 against P. aeruginosa in vitro and in vivo. Treatment of bronchial epithelial cells and macrophages with IDR-1002 or in combination with live P. aeruginosa or its LPS led to the reduction of agonist-induced cytokines and chemokines and limited cell killing by live P. aeruginosa. In an in vivo model using P. aeruginosa combined with alginate to mimic a chronic model, IDR-1002 did not reduce the bacterial burden in the lungs, but IDR-1002 mice showed a significant decrease in IL-6 in the lungs and in gross pathology of infection, while histology revealed that IDR-1002 treated mice had reduced alveolar macrophage infiltration around the site of infection and reduced inflammation. Overall, these results indicate that IDR-1002 has promise for combating P. aeruginosa lung infections and their resulting inflammation.
Introduction
P. aeruginosa is commonly found in the environment but rarely causes infection in healthy individuals. However, in immunocompromised patients or when introduced into wounds, burns, or the urinary tract, P. aeruginosa can cause serious infections and even death [1–3]. Of particular concern are lung infections caused by P. aeruginosa, such as pneumonia or chronic infections in patients with cystic fibrosis (CF) or chronic obstructive pulmonary disease (COPD). P. aeruginosa is also one of the most frequent causes of nosocomial pneumonia in the ICU [2]. In CF and COPD, while initial infection might be similar to acute infections, over time the P. aeruginosa forms biofilms and undergoes a series of adaptations, including the decreased expression of flagella, thus leading to chronic infections. Over 80% of CF patients acquire P. aeruginosa by their mid-twenties, leading to increased hospitalizations and eventually death from loss of lung function [4, 5]. However, the treatment of P. aeruginosa lung infections is hampered by its inherent resistance to antibiotics as well as acquired and adaptive resistance mechanisms [6, 7]. Multi-drug resistant strains of P. aeruginosa are on the rise [8], and it has recently been classified as a serious threat by the U.S. Centers for Disease Control and as a critical threat by the World Health Organization due to the rise of multi-drug resistant strains of P. aeruginosa and a lack of new treatment options [9, 10]. Therefore, there is a need for alternatives to antibiotics for the treatment of P. aeruginosa lung infections.
HDPs, such as the human cathelicidin LL-37, are small, naturally occurring peptides that have shown profound immunomodulatory effects in vivo and under in vivo-like conditions, including a critical role in the host response to microbial infections [11]. These immunomodulatory effects prompt the host immune system to respond to microbial infections, thus limiting the potential development of antimicrobial resistance that occurs from direct targeting of the microbe as well as the negative effects of antibiotics on the host microflora. However, the use of HDPs as exogenous agents has been limited due to toxicity and the relatively expensive process of synthesis for these peptides due to length or the inclusion of more complex chemistry such as disulfide bonds [12, 13]. Therefore, synthetic versions have been developed with similar properties but with reduced size, toxicity, and cost [14–16]. While a handful of synthetic HDPs have been tested against P. aeruginosa lung infections in vivo, they have mostly been ineffective or shown toxic effects [17–19]. Some larger peptides (18-mers or longer) appeared to reduce colony-forming unit (CFU) burden in the lungs, but they were not evaluated for anti-inflammatory or other immunomodulatory effects [20]. Therefore, there is still a need for new peptides for the treatment of P. aeruginosa lung infections.
Synthetic HDPs developed in our laboratory, termed IDRs, are typically only 12 amino acids in length and have been effective in models of cerebral malaria, tuberculosis, and S. aureus-infected wounds and implants, and also have been effective against P. aeruginosa biofilms in vitro [21–25]. However, they have not been tested in vivo against P. aeruginosa infections. One IDR peptide, IDR-1002, has been tested in vivo against S. aureus and E. coli and shown anti-infective therapeutic efficacy [26]. These effects indicated that IDR-1002 might also be beneficial against P. aeruginosa infections.
In the present study, the potential of IDR-1002 as an anti-infective agent against P. aeruginosa lung infections was examined. First, IDR-1002 and the human HDP LL-37 were used in bronchial epithelial cells and macrophages, two key cell populations for the immune response during a lung infection, and their effects on toxicity and cytokine and chemokine release were evaluated alone or in combination with P. aeruginosa or its components. Next, a murine lung model was developed for testing IDR-1002. P. aeruginosa does not normally cause a chronic infection in mice, therefore the bacteria are often embedded in agar or agarose beads and then delivered intratracheally (IT) [27, 28]. Alternatively, a model was developed by Hoffmann et al. using a clinical isolate of P. aeruginosa mixed with alginate isolated from the same strain [29–31]. Alginate is an exopolysaccharide produced by Pseudomonas that is analogous to the alginate derived from seaweed. While both models give the P. aeruginosa some protection from the host immune system, using an actual biofilm matrix component, alginate, instead of agar better reflects the interactions of the host immune system with the bacteria during a chronic infection. To improve the throughput and make the model more representative of the typical route of lung infection, in this study alginate isolated from seaweed was used along with IN administration of the P. aeruginosa and alginate mixture. We also used the chronic epidemic CF patient isolate LESB58, which is now considered to be the first isolated Liverpool Epidemic Strain, a group of highly virulent strains that have increased antibiotic resistance and are associated with increased morbidity and transmission among CF patients [32–34]. LESB58 is a strong biofilm producer, and although it has a flagellum it shows decreased motility [35].
The results showed that IDR-1002 was effective against preventing P. aeruginosa-induced toxicity and could limit the release of pro-inflammatory cytokines. Additionally, while IDR-1002 did not affect the number of lung CFUs in the alginate model, it nevertheless decreased cytokines and damage in the lungs. Overall, the data indicate that IDR peptides could be novel therapeutic or prophylactic agents against P. aeruginosa lung infections.
Materials and methods
Mice and ethics statement
C57Bl/6J mice were ordered from Jackson Laboratory or were bred at the Modified Barrier Facility at the University of British Columbia. Female mice were used between 6–8 weeks of age. All experiments were approved by the UBC Animal Care Committee.
Reagents
LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-NH2) was purchased from CPC Scientific (Sunnyvale, California, USA) and IDR-1002 (VQRWLIVWRIRK-NH2) was purchased from Kinexus (Vancouver, British Columbia, Canada). Both peptides were synthesized by F-moc chemistry and purified by HPLC to greater than 95% purity. Peptides were stored as desiccated powders at -20°C, then resuspended in endotoxin-free water for experiments and stored at -20°C unless otherwise noted. LPS from P. aeruginosa PAO1 (strain H103) was isolated in the laboratory by the Darveau-Hancock method, as previously described [36].
Cell culture
Human bronchial epithelial cells, 16HBE14o- (HBE) cells, were grown in MEM with Earle’s salts (catalogue #11090, Thermo Fisher Scientific, Waltham, Massachusetts, USA) and mouse macrophage cells, RAW264.7 (RAW) cells, were grown in DMEM (catalogue #10313, Thermo Fisher Scientific), each with the addition of 10% (v/v) heat-inactivated fetal bovine serum (FBS) and 2 mM L-glutamine (Thermo Fisher Scientific). Cells were maintained at 37°C with 5% carbon dioxide and passaged by removing media, washing with PBS (Thermo Fisher Scientific), and adding 0.05% trypsin (Thermo Fisher Scientific) to detach cells. Cells were centrifuged, the supernatant was removed, and cells were resuspended and moved into new flasks.
For experiments, HBE cells were passaged into 48-well plates (1 x 105 cells/ml, 0.5 ml per well) and allowed to grow for approximately two days to achieve greater than 80% confluency in a monolayer. At approximately 2 h before experiments, medium was removed, cells were washed with PBS, then fresh medium supplemented with L-glutamine and 1% FBS was added. Cells were rested for 2 h, then used for experiments. RAW cells were passaged into 48-well plates (1.5 x 105 cells/ml, 0.5 ml per well) and allowed to grow overnight before being used for experiments. For treatments, samples received equivalent volumes of the diluents unless otherwise noted. Supernatants were collected and then stored at -20°C.
Lactate dehydrogenase assay
Supernatants collected from cell cultures were combined at a 1:1 (v/v) ratio with complete lactate dehydrogenase (LDH) reagent (Roche Diagnostics, Basel, Switzerland). After incubating for 15–25 minutes in the dark, plates were read on a Power Wave X340 plate-reader (Bio-Tek Instruments, Winooski, Vermont, USA). Results were normalized to the control sample (0% toxicity) and a sample treated with 2% (v/v) Triton X-100 (100% toxicity).
Bacterial culture
Bacterial strains P. aeruginosa PA103, LESB58, and PAO1 (strain H103) were streaked onto LB plates from frozen stocks and grown overnight at 37°C. The following day, individual CFUs were used to make overnight cultures in LB and grown overnight at 37°C with shaking. Overnight cultures were diluted 1:50 (PA103 and PAO1) or 1:10 (LESB58) and grown to an OD600 reading of approximately 0.5. Bacteria were then washed with endotoxin-free 0.9% sodium chloride saline, centrifuged, supernatant discarded, and the pellet resuspended in endotoxin-free saline to an OD600 of 0.5. Bacteria were then diluted to the appropriate concentration based on previous experiments relating OD600 reading and CFU/ml, and serial dilutions were plated on LB to check the final concentration.
Pseudomonas lung infection model
P. aeruginosa PA103 or LESB58 was prepared as described above, except that for the alginate mouse model bacteria were resuspended in 11 mg/ml sodium alginate (Sigma-Aldrich) prepared in endotoxin-free saline. Mice were anesthetized with 2–5% isoflurane and placed on an intubation stand (BrainTree Scientific, Braintree, Massachusetts, USA). IDR-1002, P. aeruginosa, or appropriate controls were instilled dropwise using a micropipette into the left nostril of each mouse. P. aeruginosa was in a 20 μl volume, while peptide was approximately 10–20 μl depending on the weight of the mouse. Isoflurane was periodically applied to keep the mouse at a steady respiratory rate. After instillation, mice were kept on the stand under isoflurane for 2–3 minutes to ensure absorption of the liquid, then they were fully recovered and returned to their cages.
For sample collection, mice were euthanized with 120 mg/kg of IP injected sodium pentobarbital. Blood was collected from the inferior vena cava and allowed to clot, then centrifuged and serum collected for use in ELISAs. For lung homogenates, the lungs were collected and the lobes separated and placed in 300 μl of PBS, then homogenized on a Mini-Beadbeater-96 (BioSpec, Bartlesville, Oklahoma, USA) for 1 minute. The homogenates were filtered (40 μm filter, #431750, Corning) and the filtrate used for plating on LB as described above, then the remaining liquid was stored at -20°C for use in ELISAs.
For histology, a cannulated needle was used to deliver 4% paraformaldehyde (PFA) to the lungs, then the trachea was tied off with string to prevent leakage and the lungs were placed in 4% PFA. After two days, samples were washed with 70% ethanol. Wax-It Histology Services (Vancouver, British Columbia, Canada) embedded the samples in paraffin, sectioned them, and then stained slides with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), or Alcian blue. The slides were then evaluated and quantified by a trained pathologist.
ELISAs
Samples were stored at -20°C until use in ELISAs. Levels of cytokines and chemokines were measured using eBioscience (San Diego, California, USA) antibodies for murine TNF-α and IL-6 and human IL-6. MCP-1 antibodies were from eBioscience or R&D Systems (Minneapolis, Minnesota, USA). KC (CXCL1) antibodies were from Fitzgerald (Acton, Massachusetts, USA) or R&D Systems. Human IL-8 antibodies were from Invitrogen (Waltham, Massachusetts, USA). Standards were purchased from the same sources. The ELISAs were performed by following the manufacturer protocols with optimization of antibody and sample dilutions, washes, and incubation times. They were developed using TMB (eBioscience) and the reaction stopped with 2 N sulfuric acid. The plates were read on a Power Wave X340 plate-reader (Bio-Tek Instruments) and fitted to a 4-parameter standard curve using KC4 software (Bio-Tek Instruments).
Statistical analysis
Data were analyzed using Microsoft Excel 2013 and GraphPad Prism version 7. GraphPad Prism was used to perform an unpaired t-test, one-way ANOVA, or two-way ANOVA as needed. Tukey’s and Dunnett’s multiple comparisons tests were used when comparing the means of all samples or when comparing to only to a control sample, respectively. A repeated measures two-way ANOVA with Sidak’s multiple comparisons test was used to evaluate data taken over multiple time points (i.e., weights and health scores). A value of p ≤ 0.05 was considered statistically significant.
Results
IDR-1002 was not per se pro-inflammatory and it suppressed inflammatory cytokines induced by P. aeruginosa lipopolysaccharide
IDR-1002 (5 x 10−4 μM to 50 μM) or LL-37 (5 x 10−4 μM to 3 μM in HBE cells or 25 μM in RAW cells) was added to HBE cells or RAW cells at the same time as P. aeruginosa lipopolysaccharide (LPS; 10 ng/ml), then supernatants were collected after 24 h and used for ELISAs to measure the production of cytokines and chemokines in response to peptide alone or the combination of peptide and LPS. The maximum concentrations of LL-37 were selected based on the results of LDH cytotoxicity experiments (S1 Fig), with concentrations above 3 μM in HBE cells or 25 μM of LL-37 in RAW cells showing greater than 20% toxicity. In contrast, IDR-1002 treatment produced less than 5% toxicity in both HBE and RAW cells.
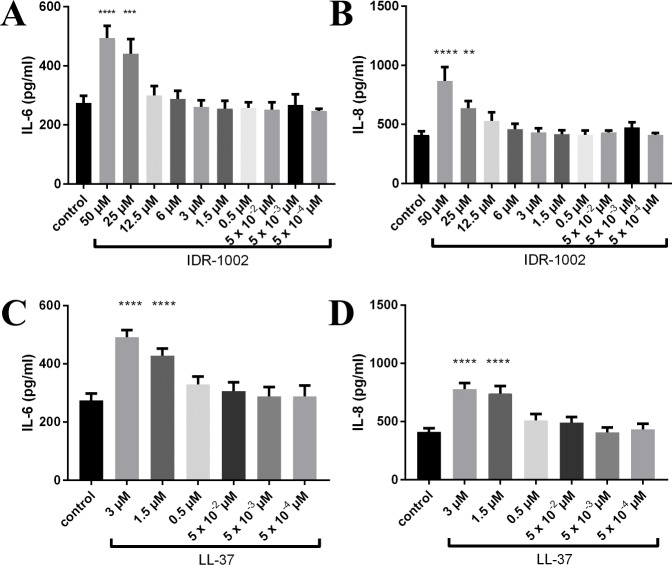
In HBE cells, IL-6 and IL-8 production showed a dose-dependent increase in response to IDR-1002 or LL-37 alone (Fig 1). HBE cells did not demonstrate changes in IL-6 or IL-8 in response to LPS, but the combinations of IDR-1002 or LL-37 with LPS showed virtually identical results to those with peptide alone, with both peptides showing a dose-dependent increase (data not shown).
Fig 1. HBE cells showed increases in IL-6 and IL-8 in response to peptide.
Peptide was added to the cells, then supernatants were collected after 24 h for ELISAs. IDR-1002 significantly increased IL-6 (A) and IL-8 (B), and LL-37 significantly increased IL-6 (C) and IL-8 (D). Data represent mean ± SEM from four independent experiments and were analyzed using two-way ANOVA and Dunnett’s multiple comparisons test. **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 0.0001 compared to control.
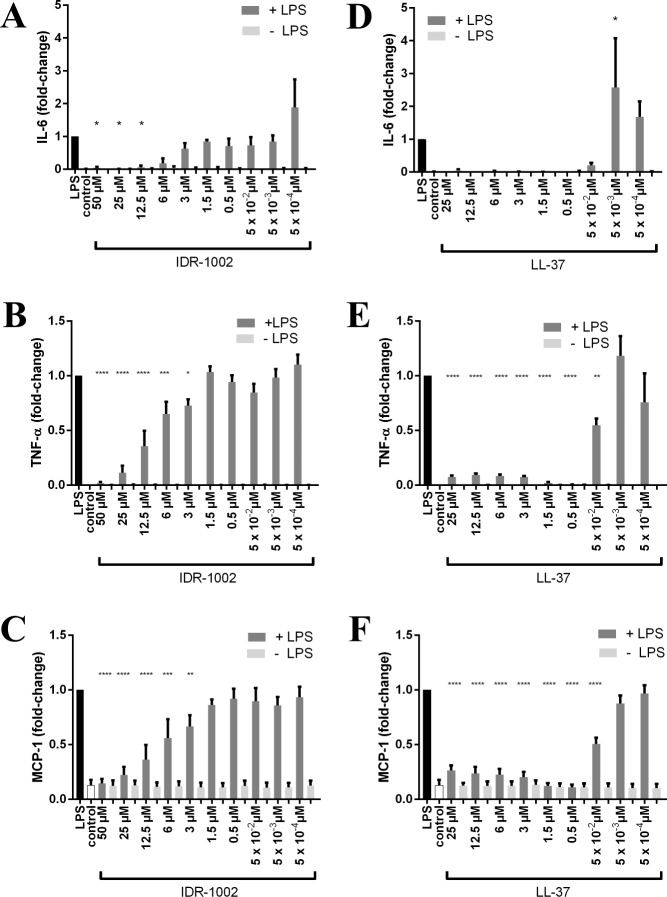
In RAW cells, the levels of pro-inflammatory cytokines TNF-α and IL-6 in the supernatant were below the detectable limit for all doses of IDR-1002 and LL-37 (Fig 2). MCP-1 did not increase in response to either peptide. RAW cells showed significant increases in IL-6, TNF-α, and MCP-1 levels in response to LPS, but when higher doses of IDR-1002 or LL-37 were used in conjunction with LPS, the levels were returned to the baseline seen in the control without LPS. The decreases in cytokine or chemokine concentration were dose-dependent, with higher concentrations of peptide producing greater reductions in the LPS-induced response. However, lower concentrations of LL-37 actually increased the production of cytokines in combination with LPS, with IL-6 showing a significant increase compared to LPS alone.
Fig 2. IDR-1002 and LL-37 reduced LPS-induced cytokines and chemokine MCP-1 in RAW cells.
The listed concentrations of IDR-1002 or LL-37 were added with or without P. aeruginosa LPS (10 ng/ml), then supernatants were collected after 24 h. Without LPS, IDR-1002 and LL-37 did not induce any of the cytokines or chemokine. IDR-1002 decreased LPS-induced IL-6 (A), TNF-α (B), and MCP-1 (C). LL-37 also decreased LPS-induced IL-6 (D), TNF-α (E), and MCP-1 (F). Data represent mean ± SEM from four independent experiments expressed as fold-change relative to LPS and were analyzed using two-way ANOVA and Dunnett’s multiple comparisons test. Only the significance for samples given LPS is displayed. *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 0.0001 compared to LPS.
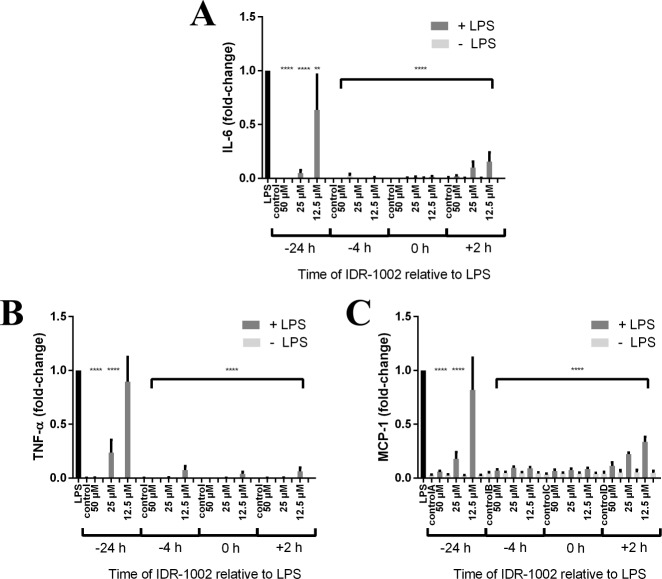
To further examine the effects of IDR-1002 on LPS exposure, RAW cells were treated with 12.5, 25, or 50 μM IDR-1002 at 24 or 4 hours prior to LPS exposure, 2 hours after LPS exposure, or at the same time as LPS as in the previous experiment, with each time point also having an LPS control and a control without LPS. Samples were collected 24 h after LPS addition and used in ELISAs (Fig 3). Intriguingly, IDR-1002 significantly reduced IL-6, TNF-α, and MCP-1 at all times points of application relative to LPS treatment and at most concentrations, with the exceptions being 12.5 μM IDR-1002 given 24 h prior to LPS for MCP-1 and TNF-α. Even when given 2 h after the addition of LPS, IDR-1002 still reduced IL-6, TNF-α, and MCP-1 in a dose-dependent manner with less than half of the cytokines and chemokine produced relative to that due to LPS alone. This demonstrated that IDR-1002 has the potential to be effective as a prophylactic or therapeutic agent.
Fig 3. IDR-1002 reduced LPS-induced inflammatory cytokines and chemokine MCP-1 when added before or after the addition of LPS.
IDR-1002 at 12.5, 25, or 50 μM was added 24 or 4 h prior to P. aeruginosa LPS, at the same time as LPS (0 h), or 2 hours after LPS. Supernatants were collected 24 h after the addition of LPS for use in ELISAs for IL-6 (A), TNF-α (B), and MCP-1 (C). Data represent mean ± SEM from three independent experiments expressed as fold-change relative to LPS at each time point and were analyzed using two-way ANOVA and Dunnett’s multiple comparisons test. Only the significance for samples given LPS is displayed. **: p ≤ 0.01, ****: p ≤ 0.0001 compared to LPS.
IDR-1002 reduced toxicity caused by P. aeruginosa in vitro
While bacterial components such as LPS can cause inflammation, it is also important to examine the effects of living bacteria. After determining the best multiplicity of infection (MOI) and timepoint for RAW and HBE cells (S2 Fig), the effects of peptides on the live P. aeruginosa PA103 were tested. In HBE cells, PA103 (MOI 4) was added at 0 h, and either IDR-1002 (50 μM) or LL-37 (3 μM) was added to the wells at -1 h or 0 h in relation to infection with PA103. Triton X-100 was also added at 0 h. Samples were collected at 6 h post-infection and used for LDH cytotoxicity assays and ELISAs. For RAW cells, the design was similar except that strain PA103 was used at an MOI of 1, LL-37 was used at 12.5 μM, and all samples were collected at 4 h post-infection. In HBE cells, IDR-1002 blocked the cytotoxic effects of PA103, instead showing cytotoxicity levels near those of the control or IDR-1002 by itself (Fig 4A and 4B). The combination of LL-37 and PA103 induced a synergistic effect in increasing cytotoxicity as opposed to reducing the toxicity or simply having an additive effect.
Fig 4. IDR-1002 limited cytotoxicity of P. aeruginosa in HBE and RAW cells, whereas LL-37 increased it.
(A, B) Live P. aeruginosa PA103 (MOI 4) was added to HBE cells at 0 h, IDR-1002 (50 μM) or LL-37 (3 μM) was added at -1 h (A) or 0 h (B), then samples were collected at 6 h. (C, D) Live P. aeruginosa PA103 (MOI 1) was added to RAW cells at 0 h, IDR-1002 (50 μM) or LL-37 (12.5 μM) was added at -1 h (C) or 0 h (D), then samples were collected at 4 h. For both RAW and HBE cells, Triton X-100 was added at 0 h and samples were compared to a control (0% toxicity) and Triton X-100 (100% toxicity) for each time point. Data represent mean ± SEM from four independent experiments and were analyzed using two-way ANOVA and Tukey’s multiple comparisons test. *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 0.0001 when compared to PA103. †††: p ≤ 0.001, ††††: p ≤ 0.0001 when samples compared as indicated in the figure.
In RAW cells, IDR-1002 showed a similar blocking effect for PA103 cytotoxicity, but the combination of PA103 and LL-37 showed an increase in toxicity compared to PA103 alone when the peptide was delivered at -1 h (Fig 4C). However, when LL-37 was delivered at the same time as PA103 in RAW cells, the toxicity of the combination was similar to PA103 alone (Fig 4D).
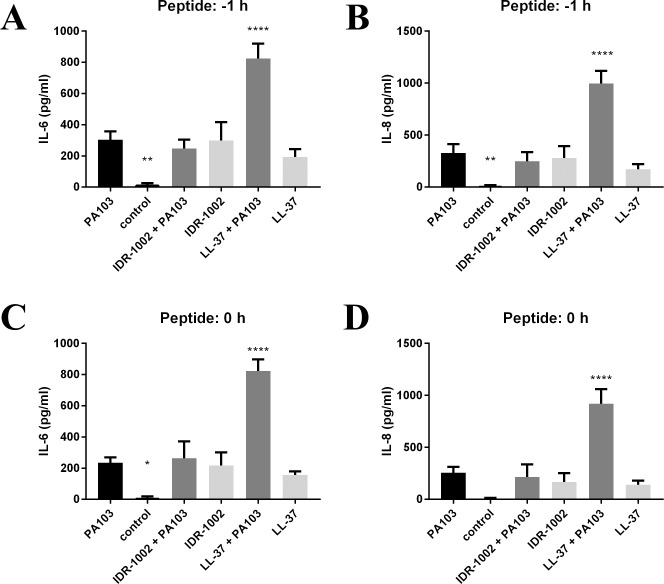
Cytokine and chemokine levels in the supernatants were also examined. In HBE cells, IL-6 and IL-8 showed slight increases when either peptide or PA103 was added, but the combination of LL-37 and PA103 showed significant increases in both markers (Fig 5). IL-6 and TNF-α were below the detectable limit in RAW cell samples, while MCP-1 concentrations were mostly undetectable and all were below 200 pg/ml, whereas the use of LPS for 24 h induced approximately 10 ng/ml of MCP-1.
Fig 5. IL-6 and IL-8 production in HBE cells in response to live P. aeruginosa PA103.
PA103 (MOI 4) was added at 0 h, then IDR-1002 (50 μM) or LL-37 (3 μM) was added at -1 h (A, B) or 0 h (C, D). Samples were collected at 6 h and used for ELISAs for IL-6 (A, C) or IL-8 (B, D). Data represent mean ± SEM from four independent experiments and were analyzed using two-way ANOVA and Dunnett’s multiple comparisons test. *: p ≤ 0.05, **: p ≤ 0.01, ****: p ≤ 0.0001 compared to PA103.
IDR-1002 reduced lesion sizes and lung damage in the alginate model
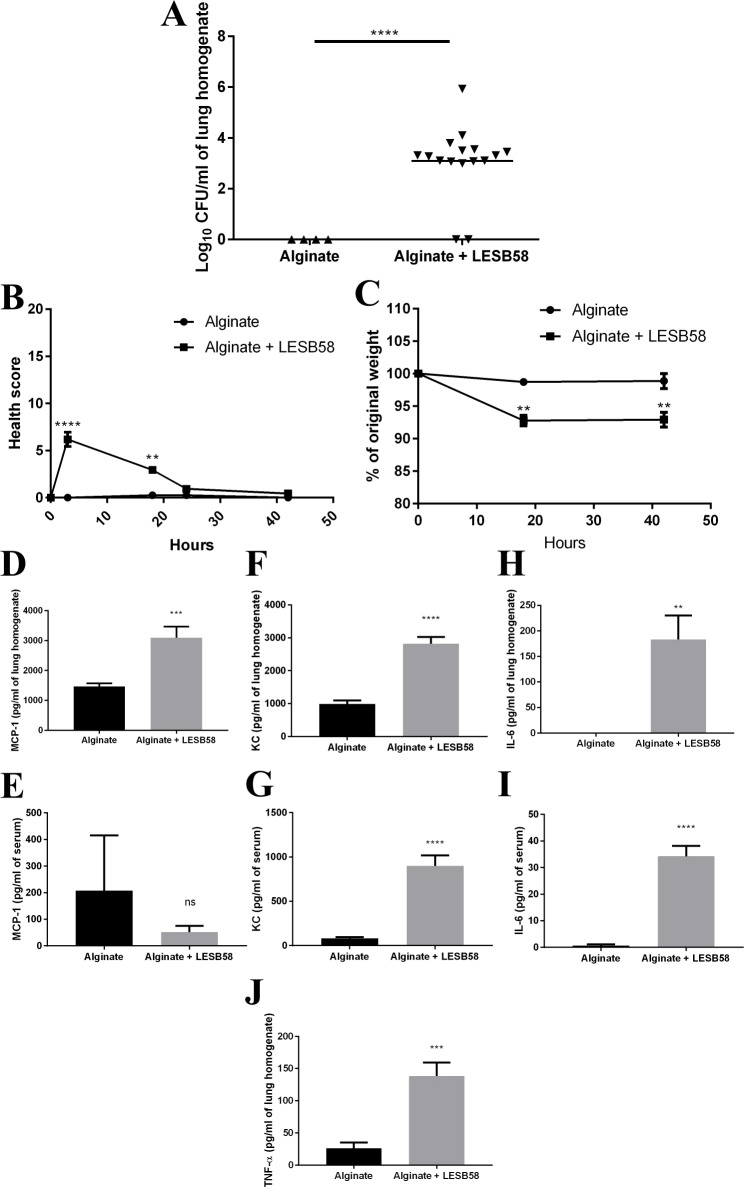
IDR-1002 showed encouraging results prophylactically against P. aeruginosa PA103 in an acute lung model by decreasing CFU burden and inflammatory mediators in the bronchoalveolar lavage fluid (BALF) and serum (S3 Fig). However, the model was not conducive for testing IDR-1002 therapeutically because the mice became sick rapidly (within two hours) and visible signs of infection typically increased over the course of the experiment, making it difficult to humanely anesthetize the mice and deliver IDR-1002 IN even at the early stages of infection. Decreasing the number of bacteria delivered in order to extend the model to multiple days was also ineffective, as the mice simply cleared the bacteria and appeared fully recovered a few hours after infection. Therefore, a lung model was developed based on the work of Hoffmann et al. [29], who used alginate isolated from a clinical strain of P. aeruginosa, then mixed the alginate with the same strain and delivered it IT. In our investigation, we used seaweed alginate to provide consistency and simplicity. It was mixed with the clinical isolate strain P. aeruginosa LESB58, as initial experiments using PA103 did not produce a sustained infection, and the mixture was then delivered IN, which is faster and less invasive than IT. Each mouse received 7 x 106 CFU delivered in a 20 μl volume at 0 h. The mice were monitored at 3, 18, 24, and 42 h post-infection and evaluated for relative temperature, appearance of fur and eyes, activity levels, hunching, and respiratory effort. The mice were euthanized on day 2 at approximately 42 h post-infection. The lungs were homogenized and plated for CFUs. As expected, the mice given only alginate did not have P. aeruginosa in their lungs, while the mice given alginate mixed with P. aeruginosa LESB58 had CFUs recovered from their lungs (Fig 6A). Before homogenization, the lungs of alginate + LESB58 mice also appeared to demonstrate redness around the opening of the trachea into the lungs, which was absent from the mice given only alginate. The alginate + LESB58 mice showed a slight initial worsening of health scores (increased numbers) but recovered by 24 h, although they lost about 6–7% of their initial weight by 18 h after infection and this was maintained throughout the course of the experiment (Fig 6B and 6C). The lung homogenate and serum were used for ELISAs (Fig 6D–6J). Despite the fact that measurements occurred two days after the initial infection, the alginate + LESB58 mice had significant increases in MCP-1, KC, IL-6, and TNF-α in the lungs and in KC and IL-6 in the serum. TNF-α in the serum was below the detectable limit.
Fig 6. P. aeruginosa LESB58 was present two days post-infection when delivered with alginate.
Mice were given alginate or alginate mixed with P. aeruginosa LESB58 (7 x 106 CFU/mouse) IN at 0 h, then euthanized and samples processed at 42 h. (A) CFU counts from the lung homogenate. (B) Health scores over the course of the experiment. (C) Percentage of original weight for mice over the course of the experiment. ELISAs were performed for MCP-1 in lung homogenate (D) and serum (E); KC in lung homogenate (F) and serum (G); IL-6 in lung homogenate (H) and serum (I); and TNF-α in lung homogenate (J). Data represent mean ± SEM for n = 4 or 16 mice per condition from the combination of two experiments and were analyzed using unpaired t-test with Welch’s correction for 6A and 6D-6J, and with a repeated measures two-way ANOVA with Sidak’s multiple comparison for 6B and 6C. **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 0.0001.
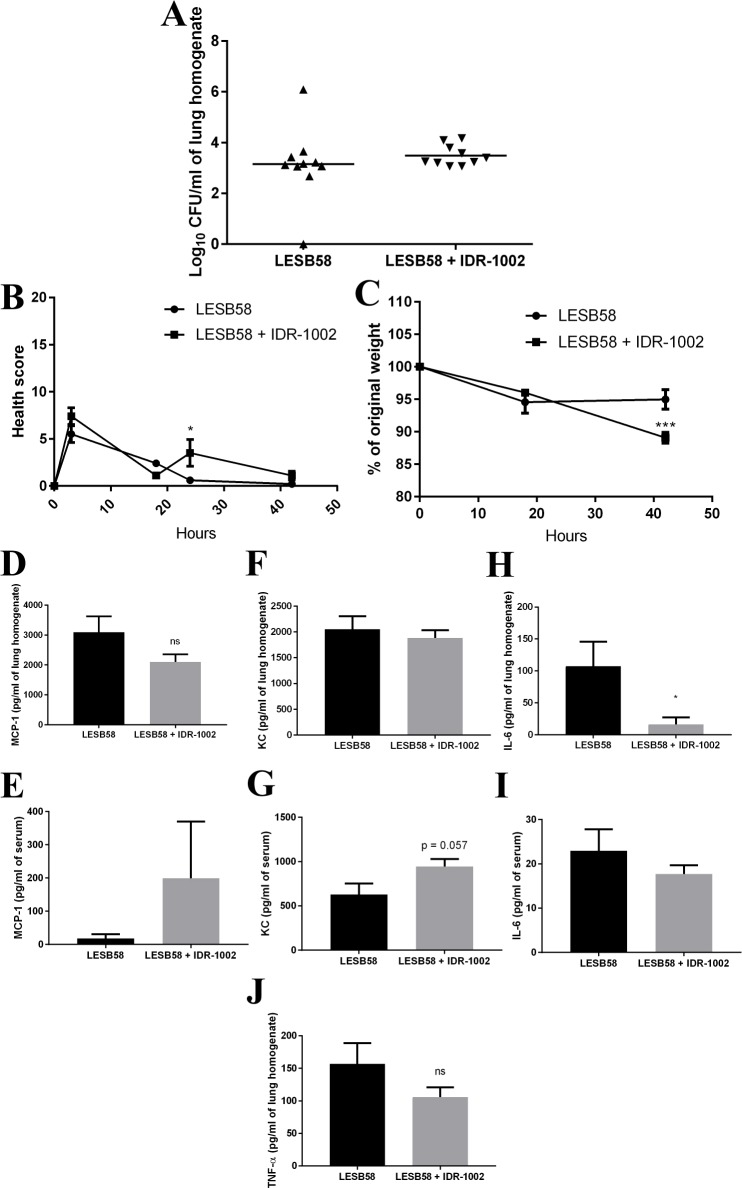
Mice were given 7 x 106 CFUs of P. aeruginosa LESB58 in alginate IN at 0 h, then at 18 h post-infection mice were given IDR-1002 (resuspended in endotoxin-free water) at 12 mg/kg IN while vehicle-control infected mice received an equivalent volume of endotoxin-free water IN. Endotoxin-free water was used for the control mice (rather than a scrambled peptide) because peptides demonstrate a broad range of activities and it is difficult, if not impossible, to conclude that a scrambled peptide is truly a negative control with no effects in vivo. Additionally, the use of scrambled peptides relates to the historical concept of distinct HDP and receptor interactions, but it now appears likely that HDPs bind to anionic surfaces rather than discrete binding sites [37, 38]. Mice were then euthanized at 42 h and the lungs homogenized and plated for CFUs. The number of recovered CFUs was indistinguishable between the two groups (Fig 7A). Mice actually showed a slight, but significant, worsening of health scores 6 h after being treated with IDR-1002 (i.e., at the 24 h time point) when compared to the vehicle-control mice given endotoxin-free water, although all mice including those given IDR-1002 recovered by 42 h (Fig 7B). The IDR-1002 treated mice also demonstrated an 11% decrease in weight by 42 h, while the control mice only had a 5.5% decrease (Fig 7C). The lung homogenate and serum were also used in ELISAs (Fig 7D–7J). In the IDR-1002 treated mice, IL-6 in the lung homogenate was significantly decreased, while the MCP-1 and TNF-α also trended towards showing decreases.
Fig 7. IDR-1002 decreased cytokines and chemokines in the alginate model.
Mice were given alginate mixed with P. aeruginosa LESB58 (7 x 106 CFU/mouse) IN at 0 h, then IDR-1002 (12 mg/kg) or vehicle-control were given IN at 18 h post-infection, then euthanized and samples processed at 42 h. (A) CFU counts from the lung homogenate. (B) Health scores over the course of the experiment. (C) Percentage of original weight for mice over the course of the experiment. ELISAs were performed for MCP-1 in lung homogenate (D) and serum (E); KC in lung homogenate (F) and serum (G); IL-6 in lung homogenate (H) and serum (I); and TNF-α in lung homogenate (J). Data represent mean ± SEM for n = 9 or 10 mice per condition from the combination of two experiments and were analyzed using unpaired t-test for 7A and 7D-7J, and with a repeated measures two-way ANOVA with Sidak’s multiple comparison for 7B and 7C. *: p ≤ 0.05.
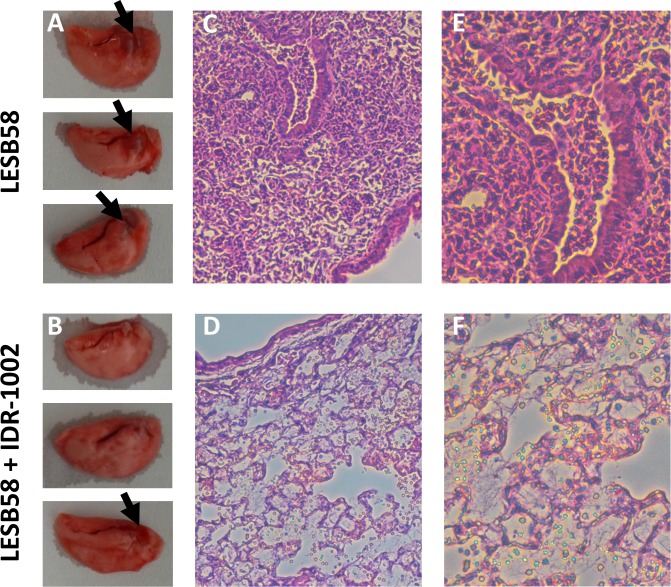
Vehicle-control mice showed areas of redness near the opening to the trachea (Fig 8). However, the IDR-1002 treated mice either did not show these lesions or they were greatly reduced. The histopathological results were quantified in a blinded fashion by a trained pathologist (Table 1). Interestingly, while the neutrophil infiltration was similar for both LESB58 and LESB58 + IDR-1002 mice, the mice treated with IDR-1002 showed a decrease in alveolar macrophages at the infection sites. The IDR-1002 treated mice also had reductions both in the percentage of inflammation by area and in the number of mice with airway inflammation. No major differences were seen between vehicle control mice and IDR-1002 mice in the PAS or Alcian blue stained samples (S4 Fig).
Fig 8. IDR-1002 mice demonstrated improved lung appearance in the alginate model.
Mice were given alginate mixed with P. aeruginosa LESB58 (7 x 106 CFU/mouse) IN at 0 h, then IDR-1002 (12 mg/kg) or control IN at 18 h, then euthanized at 42 h. Vehicle control infected mice (A) demonstrated lesions (black arrows), whereas in the IDR-1002 mice (B) the lesions were reduced or eliminated. H&E staining in the control mice (C) showed damage, while in IDR-1002 mice (D) it was reduced. This was also seen with H&E staining in control mice (E) or IDR-1002 mice (F) at 20x.
Table 1. IDR-1002 mice showed decreased inflammation and alveolar macrophages in assessment of histopathology in the alginate model.
| Condition | Histopathology Assessment | |||
|---|---|---|---|---|
| Neutrophilic inflammation (alveolar/ interstitial) | Alveolar macrophages in the area of acute inflammation | Inflammation in airways | Percentage of the section area with inflammation | |
| LESB58 | 3.0 ± 0.0 | 2.2 ± 0.37 | 0.8 ± 0.20 | 19% ± 2.9 |
| LESB58 + IDR-1002 | 2.6 ± 0.4 | 0.8 ± 0.37 | 0.4 ± 0.24 | 14.4% ± 2.6 |
Mice were given alginate mixed with P. aeruginosa LESB58 (7 x 106 CFU/mouse) IN at 0 h, then IDR-1002 (12 mg/kg) or control IN at 18 h, then euthanized at 42 h. Sections of lung were scored for neutrophilic inflammation and alveolar macrophages on a scale of 0–3 with no inflammation/infiltrate = 0, mild = 1, moderate = 2 and severe = 3. Inflammation in airways was assessed as no = 0 and yes = 1. Data represent mean ± SEM for n = 5 mice per condition from one experiment.
Discussion
In both in vitro and in vivo models of P. aeruginosa lung infection, IDR-1002 demonstrated an ability to reduce inflammatory mediators such as TNF-α or IL-6 that are associated with microbial infections. Many P. aeruginosa lung infections, such as the chronic infections seen in CF, are highly inflammatory, and so the ability to target both P. aeruginosa and the associated inflammatory response would be highly beneficial. Importantly, IDR-1002 did not demonstrate any toxicity, whereas natural human HDP LL-37 caused toxicity at higher concentrations in vitro. In RAW cells, IDR-1002 did not induce TNF-α or IL-6, while in HBE cells increases in IL-6 were slight when compared to using similar concentrations of LL-37. IDR-1002 also did not show any toxicity in vivo in the acute P. aeruginosa model, and while mice used in the alginate model were slow to recover and had similar or slightly worse health scores soon after IDR-1002 delivery, their health scores had returned to baseline within a few hours.
IDR-1002 also did not increase MCP-1 levels in RAW macrophages. This is in contrast to previous results with human peripheral blood mononuclear cells (PBMCs) and mouse peritoneal lavage cells, where IDR-1002 strongly induced MCP-1 [21, 26]. However, in agreement with our results, in mouse bone marrow-derived macrophages MCP-1 mRNA was not significantly upregulated at four hours after IDR-1002 exposure [26]. PBMCs contain T cells, B cells, and monocytes, with small numbers of DCs and natural killer cells, while peritoneal lavage mostly contains B cells, T cells, and naïve macrophages [39]. It is possible that the induction of MCP-1 in PBMCs and peritoneal lavage cells is due to its expression by a small population of cells, such as γδ T cells [40], or that it requires the interactions of particular combinations of cell types. Another factor might be the cellular differentiation state, as RAW cells and BMDMs are differentiated macrophages, whereas PBMCs contain monocytes and peritoneal lavage often contains naïve macrophages. IDR-1002 did however increase a different chemokine, IL-8, in the HBE cells, but since these cells do not express MCP-1, its expression could not be examined. Further research into the induction of MCP-1 by IDR-1002 in specific cell populations would provide insights into the mechanisms of this response.
In RAW cells, P. aeruginosa LPS increased the expression of IL-6, TNF-α, and MCP-1, but in the presence of IDR-1002 these mediators were significantly reduced, even when IDR-1002 was added up to 24 h prior to, or 2 h after, the addition of LPS. LPS uptake is substantially complete within an hour in mouse monocytic cells [41], indicating that IDR-1002 inhibition of LPS responses is likely due to effects on signaling rather than direct interaction with LPS. Consistent with this, it was reported that IDR-1002 added 1 h prior to LPS stimulation in RAW cells limited the translocation of NF-κB to the nucleus by inhibiting the phosphorylation of the NF-κB translocation inhibitor, IκBα, and this mechanism might also be involved in the results seen here [42].
In the presence of live P. aeruginosa in RAW cells, LL-37 did not decrease its toxic effects, and actually produced a slight increase in P. aeruginosa PA103 toxicity when added one hour prior to bacteria. In contrast, IDR-1002 caused a significant reduction in cytotoxicity almost to baseline. In HBE cells, IDR-1002 blocked the cytotoxic effects of PA103, instead showing cytotoxicity levels near those of the control or IDR-1002-treated cells, while LL-37 with PA103 led to a greater than two-fold synergistic increase in cytotoxicity. The combination of LL-37 and PA103 also significantly increased IL-6 and IL-8 expression by nearly three-fold, whereas IDR-1002 showed no synergistic effect. The synergy of LL-37 and P. aeruginosa PAO1 in inducing cell death in both HBE cells and primary airway epithelial cells has been previously reported and found to be caused by the induction of apoptosis [43]. The effects of IDR-1002 in basically eliminating PA103-induced cytotoxicity might have been due in part to direct antimicrobial killing based on published MIC values [44], although tissue culture medium strongly antagonizes direct antimicrobial activity, or could reflect the lack of effect of this peptide on apoptotic cell death.
The alginate model showed a sustained infection over two days, with infected mice displaying a slight initial increase in health scores within 3 h but completely recovering by 24 h, although they maintained slight weight losses for 42 h. Importantly, significant increases were still seen in inflammatory cytokines in both the lungs and the serum of infected mice after two days when compared to the mice given only alginate. Additional testing of the model, such as extending it to longer time points, will help confirm the model as a prototype for testing agents in chronic P. aeruginosa lung infections, such as those seen in CF. When therapeutic IDR-1002 was given at 18 h post-infection, no changes were seen in the CFU burden. However, the peptide-treated mice had a significant reduction in IL-6 concentrations in the lung homogenate, and the macroscopically visible lesions were either eliminated or greatly reduced, consistent with the in vitro properties of this peptide. Histology revealed that IDR-1002 treatment reduced alveolar macrophage infiltration compared to the vehicle control infected mice. This was consistent with the anti-inflammatory effect of IN-delivered IDR-1002 in the acute P. aeruginosa lung infection model (S3 Fig), which also led to significantly reduced bacterial counts at the highest peptide dose. In contrast, IP-delivered IDR-1002 in the acute model was completely ineffective, with no changes in the CFU burden or lung appearance and no reduction in cytokines or chemokines in the BALF or serum, possibly indicating that IDR-1002 needs to be delivered near the site of infection in order to exert its effects (S5 Fig). In the alginate model, decreases in lung cytokine concentrations and the differences in the lung pathology indicated that, despite the lack of change in the CFU counts, IDR-1002 still seemed to be promoting an anti-inflammatory immune response against the infection. It is possible that mixing bacteria with alginate reduced the access of phagocytic cells to these bacteria, and given that phagocytes appear to be highly influential in protection by IDR peptides [26, 45], this might have impeded the immune response induced by IDR-1002.
In conclusion, this research showed the potential of IDR-1002 as a treatment of P. aeruginosa lung infections. IDR-1002 reduced pro-inflammatory cytokines in vitro and in vivo. As excessive inflammation is a life-threatening feature in chronic P. aeruginosa lung infections, the results are encouraging for the adjunctive use of IDR-1002 against P. aeruginosa infections. Critically, IDR-1002 demonstrated both effectiveness and safety, two features that were limited in previous peptide-based drugs against P. aeruginosa in vivo lung infections [17–20]. Overall, IDR-1002 shows promise as a new agent to combat P. aeruginosa lung infections.
Supporting information
The listed concentrations of IDR-1002 (A, B) or LL-37 (C, D) were added to cells, then supernatants were collected after 24 h and used in an LDH cytotoxicity assay. The horizontal line represents 20% cytotoxicity. Data represent mean ± SEM from four (HBE cells) or five (RAW cells) independent experiments.
(TIF)
Live P. aeruginosa PA103 at MOIs of 0.5, 1, 2, or 4 was added to HBE cells (A) or RAW cells (B) at time 0 h at the same time as control and Triton X-100 samples. Samples were collected at 2, 4, or 6 h and compared to a control and Triton X-100 for each time point in an LDH cytotoxicity assay.
(TIF)
Mice were given water or IDR-1002 (4, 6, or 8 mg/kg) IN at -24 h, given 8 x 105 CFUs of P. aeruginosa PA103 IN at 0 h, then euthanized at 18 h. (A) CFU counts from the BALF. ELISAs were performed for MCP-1 in BALF (B) and serum (C); KC in BALF (D) and serum (E); IL-6 in BALF (F) and serum (G); and TNF-α in BALF (H). Data represent n = 3 mice per condition from one experiment and were analyzed using one-way ANOVA and Dunnett’s multiple comparisons test. *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001.
(TIF)
Mice were given alginate mixed with P. aeruginosa LESB58 (7 x 106 CFU/mouse) IN at 0 h, then IDR-1002 (12 mg/kg) or control IN at 18 h, then euthanized at 42 h. PAS staining in the control mice (A) and IDR-1002 mice (B) and Alcian blue staining in the control mice (C) and IDR-1002 mice (D) appeared similar between the two groups.
(TIF)
Mice were injected IP with saline or IDR-1002 (2 or 4 mg/kg) at -4 h, instilled IN with 4 x 105 CFUs of P. aeruginosa PA103 at 0 h, then euthanized and samples processed at 18 h. (A) CFU counts from the BALF. ELISAs were performed for MCP-1 in BALF (B) and serum (C); KC in BALF (D) and serum (E); IL-6 in BALF (F) and serum (G); and TNF-α in BALF (H). Data represent n = 3 or 4 mice per condition from one experiment and were analyzed using one-way ANOVA and Dunnett’s multiple comparisons test. *: p ≤ 0.05.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Canadian Institutes for Health Research (MOP-74493). KCW was supported by a Cystic Fibrosis Canada doctoral studentship while REWH received a Canada Research Chair in Health and Genomics and is a UBC Killam Professor.
References
- 1.Mayhall CG. The epidemiology of burn wound infections: then and now. Clin Infect Dis. 2003;37(4):543–50. doi: 10.1086/376993 [DOI] [PubMed] [Google Scholar]
- 2.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–54. doi: 10.1086/432803 [DOI] [PubMed] [Google Scholar]
- 3.Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009;2(3):101–11. doi: 10.1016/j.jiph.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 4.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122(1):1–9. [DOI] [PubMed] [Google Scholar]
- 5.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127 [DOI] [PubMed] [Google Scholar]
- 6.Gellatly SL, Hancock REW. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67(3):159–73. doi: 10.1111/2049-632X.12033 [DOI] [PubMed] [Google Scholar]
- 7.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50(1):43–8. doi: 10.1128/AAC.50.1.43-48.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25(10):1353–64. doi: 10.1592/phco.2005.25.10.1353 [DOI] [PubMed] [Google Scholar]
- 9.CDC. Antibiotic resistance threats in the United States. 2013.
- 10.WHO. Antibiotic-resistant priority pathogens list. 2017.
- 11.Hilchie AL, Wuerth K, Hancock REW. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9(12):761–8. doi: 10.1038/nchembio.1393 [DOI] [PubMed] [Google Scholar]
- 12.Vandamme D, Landuyt B, Luyten W, Schoofs L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012;280(1):22–35. doi: 10.1016/j.cellimm.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 13.Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnology. 2006;24:1551–7. doi: 10.1038/nbt1267 [DOI] [PubMed] [Google Scholar]
- 14.Hilpert K, Volkmer-Engert R, Walter T, Hancock REW. High-throughput generation of small antibacterial peptides with improved activity. Nat Biotechnol. 2005;23(8):1008–12. doi: 10.1038/nbt1113 [DOI] [PubMed] [Google Scholar]
- 15.Hilpert K, Elliott MR, Volkmer-Engert R, Henklein P, Donini O, Zhou Q, et al. Sequence requirements and an optimization strategy for short antimicrobial peptides. Chem Biol. 2006;13(10):1101–7. doi: 10.1016/j.chembiol.2006.08.014 [DOI] [PubMed] [Google Scholar]
- 16.Wu M, Hancock REW. Improved derivatives of bactenecin, a cyclic dodecameric antimicrobial cationic peptide. Antimicrob Agents Chemother. 1999;43(5):1274–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mardirossian M, Pompilio A, Crocetta V, De Nicola S, Guida F, Degasperi M, et al. In vitro and in vivo evaluation of BMAP-derived peptides for the treatment of cystic fibrosis-related pulmonary infections. Amino Acids. 2016;48(9):2253–60. doi: 10.1007/s00726-016-2266-4 [DOI] [PubMed] [Google Scholar]
- 18.Song Z, Wu H, Mygind P, Raventos D, Sonksen C, Kristensen HH, et al. Effects of intratracheal administration of novispirin G10 on a rat model of mucoid Pseudomonas aeruginosa lung infection. Antimicrob Agents Chemother. 2005;49(9):3868–74. doi: 10.1128/AAC.49.9.3868-3874.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartlett KH, McCray PB Jr., Thorne PS. Novispirin G10-induced lung toxicity in a Klebsiella pneumoniae infection model. Antimicrob Agents Chemother. 2003;47(12):3901–6. doi: 10.1128/AAC.47.12.3901-3906.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Parente J, Harris SM, Woods DE, Hancock RE, Falla TJ. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob Agents Chemother. 2005;49(7):2921–7. doi: 10.1128/AAC.49.7.2921-2927.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achtman AH, Pilat S, Law CW, Lynn DJ, Janot L, Mayer ML, et al. Effective adjunctive therapy by an innate defense regulatory peptide in a preclinical model of severe malaria. Sci Transl Med. 2012;4(135):135ra64 doi: 10.1126/scitranslmed.3003515 [DOI] [PubMed] [Google Scholar]
- 22.Rivas-Santiago B, Castaneda-Delgado JE, Rivas Santiago CE, Waldbrook M, Gonzalez-Curiel I, Leon-Contreras JC, et al. Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis infections in animal models. PLoS One. 2013;8(3):e59119 doi: 10.1371/journal.pone.0059119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choe H, Narayanan AS, Gandhi DA, Weinberg A, Marcus RE, Lee Z, et al. Immunomodulatory peptide IDR-1018 decreases implant infection and preserves osseointegration. Clin Orthop Relat Res. 2015;473(9):2898–907. doi: 10.1007/s11999-015-4301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinstraesser L, Hirsch T, Schulte M, Kueckelhaus M, Jacobsen F, Mersch EA, et al. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS One. 2012;7(8):e39373 doi: 10.1371/journal.pone.0039373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reffuveille F, de la Fuente-Nunez C, Mansour S, Hancock REW. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob Agents Chemother. 2014;58:5363–5371. doi: 10.1128/AAC.03163-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nijnik A, Madera L, Ma S, Waldbrook M, Elliot MR, Easton DM, et al. Synthetic cationic peptide IDR-1002 provides protection against bacterial infections through chemokine induction and enhanced leukocyte recruitment. J Immunol. 2010;184:2539–50. doi: 10.4049/jimmunol.0901813 [DOI] [PubMed] [Google Scholar]
- 27.Kukavica-Ibrulj I, Levesque RC. Animal models of chronic lung infection with Pseudomonas aeruginosa: useful tools for cystic fibrosis studies. Lab Anim. 2008;42(4):389–412. doi: 10.1258/la.2007.06014e [DOI] [PubMed] [Google Scholar]
- 28.van Heeckeren AM, Schluchter MD. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab Anim. 2002;36(3):291–312. doi: 10.1258/002367702320162405 [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann N, Rasmussen TB, Jensen PO, Stub C, Hentzer M, Molin S, et al. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect Immun. 2005;73(4):2504–14. doi: 10.1128/IAI.73.4.2504-2514.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, Johansen HK, et al. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(-/-) mice. Antimicrob Agents Chemother. 2007;51(10):3677–87. doi: 10.1128/AAC.01011-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabet M, Miller CE, Nolan TG, Senekeo-Effenberger K, Dudley MN, Griffith DC. Efficacy of aerosol MP-376, a levofloxacin inhalation solution, in models of mouse lung infection due to Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53(9):3923–8. doi: 10.1128/AAC.00268-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winstanley C, Langille MG, Fothergill JL, Kukavica-Ibrulj I, Paradis-Bleau C, Sanschagrin F, et al. Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool Epidemic Strain of Pseudomonas aeruginosa. Genome Res. 2009;19(1):12–23. doi: 10.1101/gr.086082.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng K, Smyth RL, Govan JR, Doherty C, Winstanley C, Denning N, et al. Spread of beta-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet. 1996;348(9028):639–42. doi: 10.1016/S0140-6736(96)05169-0 [DOI] [PubMed] [Google Scholar]
- 34.Al-Aloul M, Crawley J, Winstanley C, Hart CA, Ledson MJ, Walshaw MJ. Increased morbidity associated with chronic infection by an epidemic Pseudomonas aeruginosa strain in CF patients. Thorax. 2004;59(4):334–6. doi: 10.1136/thx.2003.014258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kukavica-Ibrulj I, Bragonzi A, Paroni M, Winstanley C, Sanschagrin F, O'Toole GA, et al. In vivo growth of Pseudomonas aeruginosa strains PAO1 and PA14 and the hypervirulent strain LESB58 in a rat model of chronic lung infection. J Bacteriol. 2008;190(8):2804–13. doi: 10.1128/JB.01572-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darveau RP, Hancock REW. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983;155(2):831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Vasil AI, Rehaume L, Mant CT, Burns JL, Vasil ML, et al. Comparison of biophysical and biologic properties of alpha-helical enantiomeric antimicrobial peptides. Chem Biol Drug Des. 2006;67(2):162–73. doi: 10.1111/j.1747-0285.2006.00349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamed MF, Brezden A, Mohammad H, Chmielewski J, Seleem MN. A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci Rep. 2017;7:6953 doi: 10.1038/s41598-017-07440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J Vis Exp. 2010;(35). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schilbach K, Frommer K, Meier S, Handgretinger R, Eyrich M. Immune response of human propagated gammadelta-T-cells to neuroblastoma recommend the Vdelta1+ subset for gammadelta-T-cell-based immunotherapy. J Immunother. 2008;31(9):896–905. doi: 10.1097/CJI.0b013e31818955ad [DOI] [PubMed] [Google Scholar]
- 41.Dunzendorfer S, Lee HK, Soldau K, Tobias PS. TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J Immunol. 2004;173(2):1166–70. [DOI] [PubMed] [Google Scholar]
- 42.Huante-Mendoza A, Silva-Garcia O, Oviedo-Boyso J, Hancock REW, Baizabal-Aguirre VM. Peptide IDR-1002 inhibits NF-κB nuclear translocation by inhibition of IκBα degradation and activates p38/ERK1/2—MSK1-dependent CREB phosphorylation in macrophages stimulated with lipopolysaccharide. Front Immunol. 2016;7 doi: 10.3389/fimmu.2016.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barlow PG, Beaumont PE, Cosseau C, Mackellar A, Wilkinson TS, Hancock REW, et al. The human cathelicidin LL-37 preferentially promotes apoptosis of infected airway epithelium. Am J Respir Cell Mol Biol. 2010;43(6):692–702. doi: 10.1165/rcmb.2009-0250OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wieczorek M, Jenssen H, Kindrachuk J, Scott WRP, Elliot MR, Hilpert K, et al. Structural studies of a peptide with immune modulating and direct antimicrobial activity. Chem Biol. 2010;17:970–80. doi: 10.1016/j.chembiol.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 45.Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, et al. An anti-infective peptide that selectively modulates the innate immune response. Nat Biotechnol. 2007;25(4):465–72. doi: 10.1038/nbt1288 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The listed concentrations of IDR-1002 (A, B) or LL-37 (C, D) were added to cells, then supernatants were collected after 24 h and used in an LDH cytotoxicity assay. The horizontal line represents 20% cytotoxicity. Data represent mean ± SEM from four (HBE cells) or five (RAW cells) independent experiments.
(TIF)
Live P. aeruginosa PA103 at MOIs of 0.5, 1, 2, or 4 was added to HBE cells (A) or RAW cells (B) at time 0 h at the same time as control and Triton X-100 samples. Samples were collected at 2, 4, or 6 h and compared to a control and Triton X-100 for each time point in an LDH cytotoxicity assay.
(TIF)
Mice were given water or IDR-1002 (4, 6, or 8 mg/kg) IN at -24 h, given 8 x 105 CFUs of P. aeruginosa PA103 IN at 0 h, then euthanized at 18 h. (A) CFU counts from the BALF. ELISAs were performed for MCP-1 in BALF (B) and serum (C); KC in BALF (D) and serum (E); IL-6 in BALF (F) and serum (G); and TNF-α in BALF (H). Data represent n = 3 mice per condition from one experiment and were analyzed using one-way ANOVA and Dunnett’s multiple comparisons test. *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001.
(TIF)
Mice were given alginate mixed with P. aeruginosa LESB58 (7 x 106 CFU/mouse) IN at 0 h, then IDR-1002 (12 mg/kg) or control IN at 18 h, then euthanized at 42 h. PAS staining in the control mice (A) and IDR-1002 mice (B) and Alcian blue staining in the control mice (C) and IDR-1002 mice (D) appeared similar between the two groups.
(TIF)
Mice were injected IP with saline or IDR-1002 (2 or 4 mg/kg) at -4 h, instilled IN with 4 x 105 CFUs of P. aeruginosa PA103 at 0 h, then euthanized and samples processed at 18 h. (A) CFU counts from the BALF. ELISAs were performed for MCP-1 in BALF (B) and serum (C); KC in BALF (D) and serum (E); IL-6 in BALF (F) and serum (G); and TNF-α in BALF (H). Data represent n = 3 or 4 mice per condition from one experiment and were analyzed using one-way ANOVA and Dunnett’s multiple comparisons test. *: p ≤ 0.05.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.