Abstract
Mucosal immunization of influenza vaccine is potentially an effective approach for the prevention and control of influenza. The objective of the present study was to evaluate the ability of oral immunization with a non-recombinant Lactococcus lactis displaying HA1/L/AcmA recombinant protein, LL-HA1/L/AcmA, to induce mucosal immune responses and to accord protection against influenza virus infection in mice. The LL-HA1/L/AcmA was orally administered into mice and the immune response was evaluated. Mice immunized with LL-HA1/L/AcmA developed detectable specific sIgA in faecal extract, small intestine wash, BAL fluid and nasal fluid. The results obtained demonstrated that oral immunization of mice with LL-HA1/L/AcmA elicited mucosal immunity in both the gastrointestinal tract and the respiratory tract. The protective efficacy of LL-HA1/L/AcmA in immunized mice against a lethal dose challenge with influenza virus was also assessed. Upon challenge, the non-immunized group of mice showed high susceptibility to influenza virus infection. In contrast, 7/8 of mice orally immunized with LL-HA1/L/AcmA survived. In conclusion, oral administration of LL-HA1/L/AcmA in mice induced mucosal immunity and most importantly, provided protection against lethal influenza virus challenge. These results highlight the potential application of L. lactis as a platform for delivery of influenza virus vaccine.
Introduction
Influenza virus infection results in respiratory illnesses and contributes to a high rate of morbidity and mortality in humans, particularly children, the elderly and immunocompromised individuals. Three influenza pandemics had occurred in the 20th century: 1918 Spanish flu, 1957–1958 Asian influenza and 1968 Hong Kong influenza, while one recently occurred in the 21st century: 2009 pandemic H1N1 [1]. Besides pandemic influenza, seasonal epidemics of influenza is also of great concern, as it causes approximately 3–5 million of severe illness and 250,000–500,000 deaths annually worldwide [2]. Controlling the spread of influenza remains a major challenge. It is undeniable that vaccines, which confer protection against influenza, are most effective.
Currently, there are three types of influenza vaccine available in the market: an inactivated influenza virus vaccine (IIV), a live attenuated influenza virus vaccine (LAIV) and a recombinant vaccine. The IIV is a good inducer of systemic immune response but it is ineffective at stimulating mucosal immune response [3]. LAIV on the other hand, induces both systemic and mucosal immune responses [4]. However, there is an associated risk of the attenuated virus reverting back to its highly virulent form and thus causing an infection [3]. A case of LAIV transmission to a recipient was previously reported [4]. In addition, a study had shown that LAIV increased risk of wheezing within 42 days after vaccination in children <12-months-old [5]. Recombinant vaccine is potentially of advantage especially in the event of a pandemic due to its quick production potential [6]. This vaccine is administered intramuscularly and therefore, induces immune response similar to IIV [7]. However, a few serious adverse events associated with this vaccine such as vasovagal syncope, pericardial effusion and Bell’s palsy were previously reported [8]. Taken together, influenza vaccines that are currently available present several drawbacks, including their limited ability at stimulating mucosal immunity without compromising safety issues. As influenza virus initiates infection at the respiratory tract mucosal surface, it would be advantageous to have a vaccine that is able to induce mucosal immune response, mainly the secretory IgA (sIgA).
One of the current strategies in mucosal vaccine research targeting stimulation of mucosal immunity is oral administration of an antigen using Lactococcus lactis as a delivery platform. L. lactis is a generally recognized as safe bacterium. It has been applied in mucosal vaccine research due to its non-colonizing and non-pathogenic properties. More recently, L. lactis is being extensively explored as effective oral-based vaccine vehicles to deliver antigens of several viruses [9–15], bacteria [16–20] and parasites [21]. Studies have shown that L. lactis expressing and displaying antigens were capable of inducing strong systemic as well as mucosal immune responses [9–12, 21–24].
Considering the role of mucosal immunity in protection against influenza, we have previously developed a non-recombinant L. lactis surface displaying influenza A (H1N1) 2009 hemagglutinin (HA1) using N-acetylmuramidase (AcmA) as a binding domain to anchor HA1 on the bacterial cell wall [25]. The amount of HA1 bound on L. lactis was improved using a single-chain variable fragment peptide linker (Gly4Ser)3, to link HA1 to the AcmA binding domain. In the present study, we extended our previous work by optimizing the binding parameters, the amount of HA1 recombinant protein added to L. lactis, the duration of binding and the buffer used for binding. We then assessed the specific immune response elicited upon oral immunization of the non-recombinant L. lactis surface displaying HA1 (LL-HA1/L/AcmA) in mice. In addition, the protective efficacy of LL-HA1/L/AcmA against a lethal dose challenge with influenza virus was also assessed.
Materials and methods
Ethics statement
All procedures involving animals were reviewed and approved by the Faculty of Medicine-Institutional Animal Care and Use Committee (FOM-IACUC) with ethics reference no. 2014-01-07/MMB/R/JPF. Ketamine-xylazine (ketamine: 80–120 mg/kg; xylazine: 10–16 mg/kg) was used to anesthetize the animals in this study. An overdose of ketamine-xylazine (ketamine: 240–360 mg/kg; xylazine: 30–48 mg/kg) was given to euthanize the animals in this study.
Optimization of L. lactis binding conditions
L. lactis displaying HA1/L/AcmA recombinant protein was prepared as previously described [25]. Briefly, an overnight culture of L. lactis MG1363 was subcultured in GM17 broth and incubated at 30°C until the OD600 reading reached 0.5. The L. lactis culture (2 ml) was centrifuged at 2,000 ×g for 10 min and the cell pellet obtained was resuspended in fresh GM17 broth (0.6 ml). Subsequently, refolded HA1/L/AcmA recombinant protein (100 μl) was prepared as previously described [25] and added to the cell suspension and incubated at 30°C for 2 h. The cells were then washed with phosphate buffered saline (PBS) thrice by centrifugation at 2,000 ×g for 10 min. The cell pellet was eventually resuspended in 200 μl of PBS. Optimizations were performed to improve the binding of HA1/L/AcmA to L. lactis by varying the binding parameters, specifically the amount of recombinant protein (25, 20, 15, 10 and 5 μg) added to equal number of L. lactis cells, the duration of binding (1, 2, 3 and 4 h), and the type of binding buffer (GM17 and PBS). Other factors that may influence the binding of recombinant protein onto L. lactis, including pH and temperature were not examined as these factors may affect the integrity of the cell membrane and other biological processes of L. lactis, which may then affect the binding of recombinant proteins on the bacterial cell wall.
After binding, the L. lactis cells pre-mixed with the HA1/L/AcmA were stained and analyzed using flow cytometry as previously described [25]. Briefly, cells were fixed with 200 μl of 4% (w/v) paraformaldehyde for 20 min and blocked with 200 μl of 3% (w/v) bovine serum albumin (BSA) for 30 min. Subsequently, the cells were incubated with 200 μl of mouse monoclonal anti-polyhistidine (Sigma-Aldrich, USA) at 1:1,000 dilution for 1 h, followed by incubation with 200 μl of Alexa Fluor® 488 goat anti-mouse IgG (Invitrogen Life Technologies, USA) at 1:500 dilution in the dark for 1 h. The cells were washed with PBS between steps thrice and recovered by centrifugation at 2,000 ×g for 10 min. The cells were eventually suspended in 1 ml of PBS for examination of stained cells using a fluorescence-activated cell sorting (FACS) CANTO ™ II Flow cytometer (BD Biosciences, USA). A total of 5×104 cells falling into the gate defined on the forward angle light scatter-side angle light scatter plot were acquired to determine the positive cell count and mean fluorescence intensity (MFI).
Immunogen preparation
L. lactis displaying HA1/L/AcmA recombinant protein was prepared using the optimal conditions as determined earlier and scaled-up by proportionally increasing the amount of recombinant protein and L. lactis cells to be used in the binding. The L. lactis displaying HA1/L/AcmA was resuspended in endotoxin-free PBS to a concentration of 1×1011 CFU/ml and 5×1011 CFU/ml for study groups A and B, respectively. The constructed non-recombinant L. lactis displaying HA1/L/AcmA was named LL-HA1/L/AcmA.
Mice immunizations
Six to eight-weeks-old specific-pathogen-free female BALB/c mice were purchased from InVivos Pte Ltd. (Singapore). Animals were housed in specific-pathogen-free conditions with free access to food and water in accordance with Association for the Assessment and Accreditation of Laboratory Animal Care International and FOM-IACUC guidelines of University of Malaya. A total of two study groups were performed to evaluate the immunogenicity of LL-HA1/L/AcmA. In study group A, mice (n = 8) were immunized orally with 0.1 ml of 1×1010 CFU of LL-HA1/L/AcmA (25 µg/dosage) or endotoxin-free PBS by intra-gastric lavage for 3 consecutive days. The immunization regimen was repeated thrice at 2 weeks intervals. In study group B, mice (n = 8) were immunized orally with 0.1 ml of 5×1010 CFU of LL-HA1/L/AcmA (125 μg/dosage), HA1/L/AcmA (125 μg/dosage) or endotoxin-free PBS for 3 consecutive days. The immunization regimen was repeated thrice at 2 weeks intervals. As a positive control, HA1/L/AcmA (50 μg/dosage) emulsified with complete Freund’s adjuvant (CFA; Sigma-Aldrich, USA), HA1/L/AcmA-FA, was administered subcutaneously to a group of mice (n = 8). The immunization was repeated thrice at 2 weeks intervals using incomplete Freund’s adjuvant (IFA; Sigma-Aldrich, USA) instead of CFA.
Sample collection
In study group A, blood and faeces were collected 2 weeks after the last immunization. Thereafter, the mice were euthanized by intraperitoneal injection of an overdose of ketamine-xylazine and samples such as small intestine, bronchoalveolar lavage (BAL) and nasal fluid were collected. In study group B, only blood and faecal pellets were collected. The mice in study group B were not euthanized, but kept for viral challenge.
Briefly, blood sample was collected via tail vein and serum was obtained by centrifugation at 1,000 ×g for 5 min. Faecal pellets (100 mg) were resuspended in 0.5 ml sterile PBS containing 1 mM phenylmethanesulfonyl fluoride (PMSF), vortexed vigorously and faecal extract was obtained by centrifugation at 10,000 ×g for 5 min. Small intestine was harvested and flushed thrice with 1 ml PBS containing 1 mM PMSF. The sample from small intestine was then obtained following centrifugation at 10,000 ×g for 5 min. BAL [26, 27] and nasal fluids [26] were collected as previously described with minor modifications. Briefly, mouse trachea was cannulated with an intravenous catheter (BD Bioscience, USA) and connected to a syringe. BAL fluid was obtained by flushing the lungs with 1 ml cold PBS for 5 times. The washing step was repeated once and lavage fluid recovered from two washings was pooled. Nasal fluid was obtained by flushing the nasopharynx with 1 ml cold PBS for 5 times and nasal wash fluid was recovered from the mouse nostril. The BAL and nasal fluids were centrifuged at 200 ×g for 5 min, and the supernatant samples were collected. All samples were stored in -20°C for subsequent analysis.
Enzyme-linked immunosorbent assay (ELISA) for detection of mucosal and systemic immune response
HA1-specific IgG and IgA antibodies were detected by enzyme-linked immunosorbent assay (ELISA) following the protocol described by Joan et al. [28] with some modifications. Briefly, 96-well microtiter plate (Costar Corporation, USA) was coated with 100 μl of HA1/L/AcmA recombinant protein (1 μg/well) at 4°C for overnight. The wells were blocked with 3% (w/v) BSA for 1 h. Sera or mucosal samples were added and incubated for 1 h. Bound antibody was detected using HRP-conjugated goat anti-mouse IgG (Abcam, UK) at 1:10,000 dilution or HRP-conjugated rat anti-mouse IgA (Abcam, UK) at 1:1,000 dilution. The plates were washed 3 times with PBS between steps. TMB peroxidase substrate system (KPL, USA) was added for detection. After incubation for 20 min, TMB stop solution (KPL, USA) was added and absorbance value at 450 nm was measured using a microplate reader (Tecan, Switzerland). The assay was performed with 3 technical replicates.
H1N1 virus challenge experiment
A stock of mouse adapted A/TN/1-560/2009-MA2(H1N1) influenza virus was kindly provided by Dr. Richard J. Webby (St. Jude Children’s Research Hospital, USA). At 18 days following the last immunization, all mice in study group B were fully anesthetized by intraperitoneal injection of ketamine-xylazine and intranasally inoculated with 50 μl of 10 LD50 of A/TN/1-560/2009-MA2(H1N1) influenza virus. After inoculation, body weight and survival rate of the mice were monitored daily up to 14 days. Mice that showed body weight loss of >20% were considered to have reached the experimental end-point and were euthanized by intraperitoneal injection of an overdose of ketamine-xylazine. No adverse reactions or mortality occurred outside of the humane endpoint was observed throughout the study.
Statistics
Statistical analysis and graphical representations were performed using GraphPad Prism 5 software (GraphPad Software, USA). Data analysis was performed using Student’s t test for the comparison of two groups. The log-rank (Mantel-Cox) test was used for statistical comparison of the survival between groups.
Results
Optimization of the binding of HA1/L/AcmA recombinant protein to L. lactis
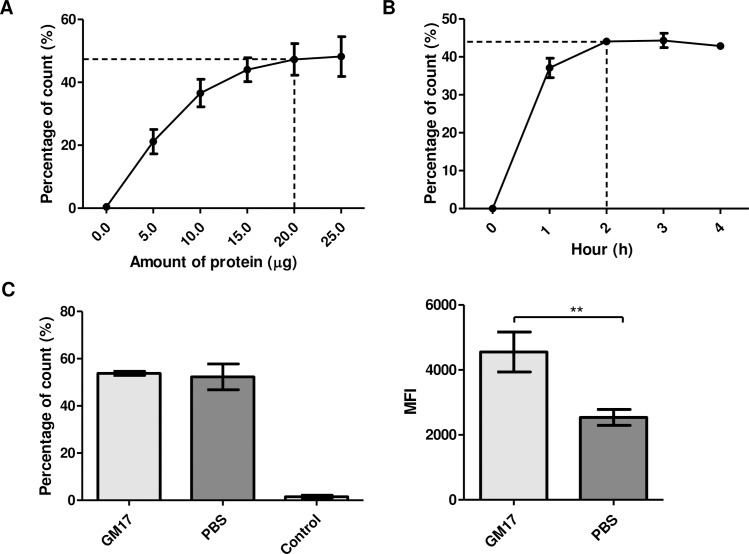
L. lactis surface displaying HA1/L/AcmA recombinant protein was immunofluorescence-labeled with Alexa Fluor 488. The percentage of Alexa Fluor 488-positive gated cells (L. lactis surface displaying HA1/L/AcmA) increased steadily when increasing amount of HA1/L/AcmA recombinant protein was added to 1–3×109 CFU of L. lactis (Fig 1A). The percentage of Alexa Fluor 488-positive gated cells reached plateau when 20 μg of HA1/L/AcmA recombinant protein was used. The results suggested that the optimum amount of HA1/L/AcmA recombinant protein to be incubated with 1–3×109 CFU of L. lactis cells for surface display was 20 μg.
Fig 1. Binding optimization of HA1/L/AcmA recombinant protein to L. lactis.
(A) The percentage of L. lactis surface displaying HA1/L/AcmA recombinant protein after incubation with different amounts of HA1/L/AcmA recombinant protein. (B) The percentage of L. lactis surface displaying HA1/L/AcmA recombinant protein after incubation with HA1/L/AcmA recombinant protein for 1 h, 2 h, 3 h, and 4 h, respectively. (C) The percentage and mean fluorescence intensity (MFI) value of L. lactis surface displaying HA1/L/AcmA recombinant protein after incubation with HA1/L/AcmA recombinant protein in GM17 and PBS, respectively. The data represents mean ± standard deviation. Asterisk indicates statistically significant differences between groups (**P<0.01).
The percentage of L. lactis surface displaying HA1/L/AcmA recombinant protein following incubation of L. lactis with 20 μg of HA1/L/AcmA recombinant protein in GM17 for 1 h, 2 h, 3 h, and 4 h was examined. The percentage of Alexa Fluor 488-positive gated cells reached plateau when 20 μg of HA1/L/AcmA recombinant protein was incubated with L. lactis cells for 2 h, suggesting that incubation for 2 h was optimum for surface display of HA1/L/AcmA recombinant protein (Fig 1B). The binding of HA1/L/AcmA recombinant protein on L. lactis started to dissociate after an incubation period of 3 h.
The suitability of the buffer used for binding of HA1/L/AcmA recombinant protein to L. lactis was also evaluated. The percentage of L. lactis surface displaying HA1/L/AcmA recombinant protein after 2 h incubation with HA1/L/AcmA recombinant protein in GM17 and PBS was 53.8±0.9% and 52.3±5.5%, respectively, suggesting that the number of L. lactis cells detected to have HA1/L/AcmA on its cell wall was similar in both buffers (Fig 1C). Therefore, the MFI value of L. lactis surface displaying HA1/L/AcmA recombinant protein was examined. The MFI value obtained for HA1/L/AcmA recombinant protein binding in GM17 (4552±614.9) was significantly higher in comparison to PBS (2538±243.4). This suggested that more recombinant proteins were bound per L. lactis cells in GM17 and hence, was a better binding buffer.
Immune response induced by oral immunization with LL-HA1/L/AcmA in mice
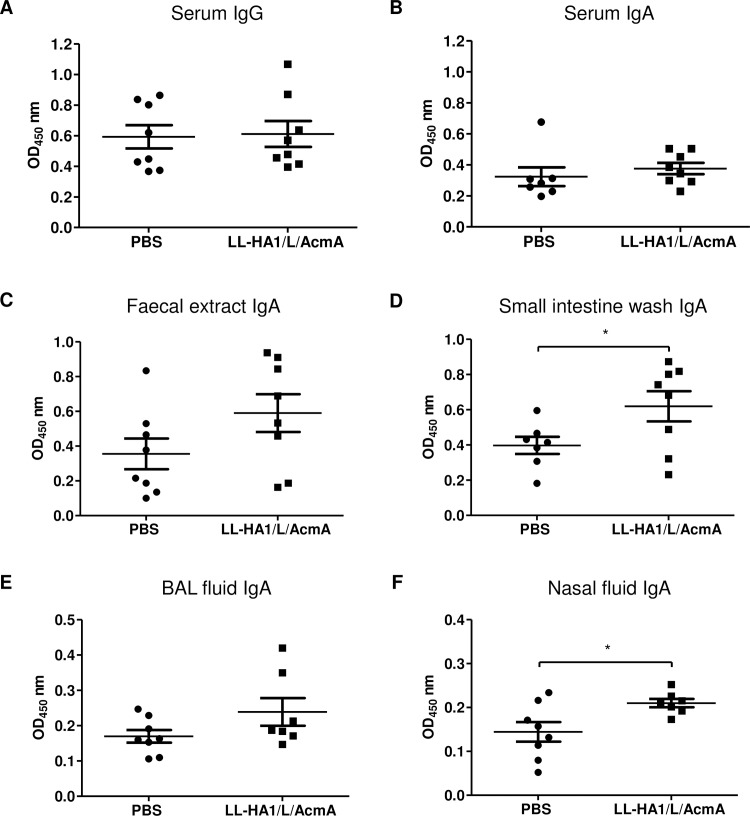
Study group A was a preliminary study to evaluate HA1-specific immune responses towards L. lactis surface displaying HA1/L/AcmA recombinant protein, LL-HA1/L/AcmA. HA1/L/AcmA-specific serum IgG and IgA were measured as the main readout for systemic immunity. There were no differences in the serum IgG (Fig 2A) and IgA (Fig 2B) in mice immunized with LL-HA1/L/AcmA (mean OD: 0.61±0.08 and 0.38±0.04, respectively) compared to the PBS-treated group (mean OD: 0.59±0.07 and 0.32±0.06, respectively). One significant outlier was detected in the data set for serum IgA of PBS-treated group using Grubbs’ test (p<0.05) and was excluded in the statistical analysis. Overall, results suggested that there were no stimulation of serological immune responses upon oral immunization of LL-HA1/L/AcmA in mice, or the immune responses were below the detection limit of the assay.
Fig 2. HA1/L/AcmA-specific IgG and IgA detected by ELISA.
Mice (8 mice/group) were orally immunized with PBS or LL-HA1/L/AcmA, and samples were collected 2 weeks after the last immunization. HA1/L/AcmA-specific (A) IgG and (B) IgA in serum at 1:10 dilution were determined, in addition to HA1/L/AcmA-specific IgA in (C) faecal sample (1:5 dilution), (D) small intestine wash (neat), (E) BAL fluid (neat) and (F) nasal fluid (neat). One significant outlier was removed from the data set for serum IgA and small intestine wash of PBS-treated group, as well as BAL and nasal fluid of LL-HA1/L/AcmA-treated group (Grubbs’ test, p<0.05), respectively. Data are presented as mean ± standard error of the mean. Asterisks indicate statistically significant differences between the corresponding groups (*p<0.05).
HA1/L/AcmA-specific sIgA in faecal extract, small intestine wash, BAL fluid and nasal fluid were measured as main readout of mucosal immunity. The HA1/L/AcmA-specific sIgA in faecal sample (Fig 2C) was noted to be higher, but there was no statistically difference between mice immunized with LL-HA1/L/AcmA (mean OD: 0.59±0.11) and the PBS-treated group (mean OD: 0.36±0.09). In the data set for small intestine wash, one significant outlier was detected in PBS-treated group using Grubbs’ test (p<0.05) and was removed from the statistical analysis. The HA1/L/AcmA-specific sIgA in small intestine wash (Fig 2D) of mice immunized with LL-HA1/L/AcmA (mean OD: 0.62±0.09) was detected to be significantly higher (p<0.05) than PBS-treated group (mean OD: 0.40±0.05). In both data set for BAL and nasal fluid of mice immunized with LL-HA1/L/AcmA, one significant outlier was detected using Grubbs’ test (p<0.05) in each of these groups and were excluded in the statistical analysis. The HA1/L/AcmA-specific sIgA in BAL fluid (Fig 2E) was noted to be higher, but not statistically different in mice immunized with LL-HA1/L/AcmA (mean OD: 0.24±0.03) when compared to the PBS-treated group (mean OD: 0.17±0.01). In contrast, mice immunized with LL-HA1/L/AcmA developed a significantly higher HA1/L/AcmA-specific sIgA in nasal fluid (p<0.05) (mean OD: 0.21±0.01) compared to PBS-treated group (mean OD: 0.14±0.02) (Fig 2F). Collectively, results suggested that oral immunization with LL-HA1/L/AcmA in mice stimulated a significant level of mucosal immunity in the gastrointestinal tract and respiratory tract.
In study group B, mice were immunized orally with a five-fold higher dosage of LL-HA1/L/AcmA to determine if a higher dose would elicit a greater immune response. For comparison, additional control groups of mice were included: mice orally administered with HA1/L/AcmA recombinant protein only (125 μg/dosage) and mice subcutaneously immunized with HA1/L/AcmA (50 μg/dosage) emulsified with Freund’s adjuvant (HA1/L/AcmA-FA).
There were no significant differences in the HA1/L/AcmA-specific serum IgG (Fig 3A) and serum IgA (Fig 3B) for mice orally immunized with LL-HA1/L/AcmA (mean OD: 0.49±0.13 and 0.24±0.07, respectively) when compared to PBS-treated group (mean OD: 0.38±0.02 and 0.20±0.04, respectively). The same was observed for mice orally immunized with HA1/L/AcmA recombinant protein only (mean OD: 0.45±0.06 and 0.12±0.01, respectively) in comparison to PBS-treated group. One significant outlier was detected in the data set for serum IgA of PBS-treated group using Grubbs’ test (p<0.05) and was excluded in the statistical analysis. Another significant outlier was detected in the data set for serum IgG of mice immunized with LL-HA1/L/AcmA using Grubbs’ test (p<0.05), but was not removed as an outlier because this mouse showed detectable HA1/L/AcmA-specific serum IgG and IgA response, which increased with each subsequent immunization. There were also no significant differences in serum IgG and IgA levels between mice orally immunized with LL-HA1/L/AcmA and HA1/L/AcmA. In contrast, significantly highest serum IgG (Fig 3A) and IgA (Fig 3B) (p<0.001) were detected in the positive control group, which were mice immunized subcutaneously with HA1/L/AcmA-FA (mean OD: 1.84±0.04 and 0.69±0.07, respectively). Results suggested that there were no stimulation of serological immune responses upon oral immunization of LL-HA1/L/AcmA or HA1/L/AcmA in mice. Alternatively, the responses were below the detection limit of the assay. Nonetheless, subcutaneous immunization of HA1/L/AcmA-FA in mice stimulated good serological immune responses, suggesting that HA1/L/AcmA is highly antigenic.
Fig 3. HA1/L/AcmA-specific serum IgG, serum IgA and faecal IgA detected by ELISA.
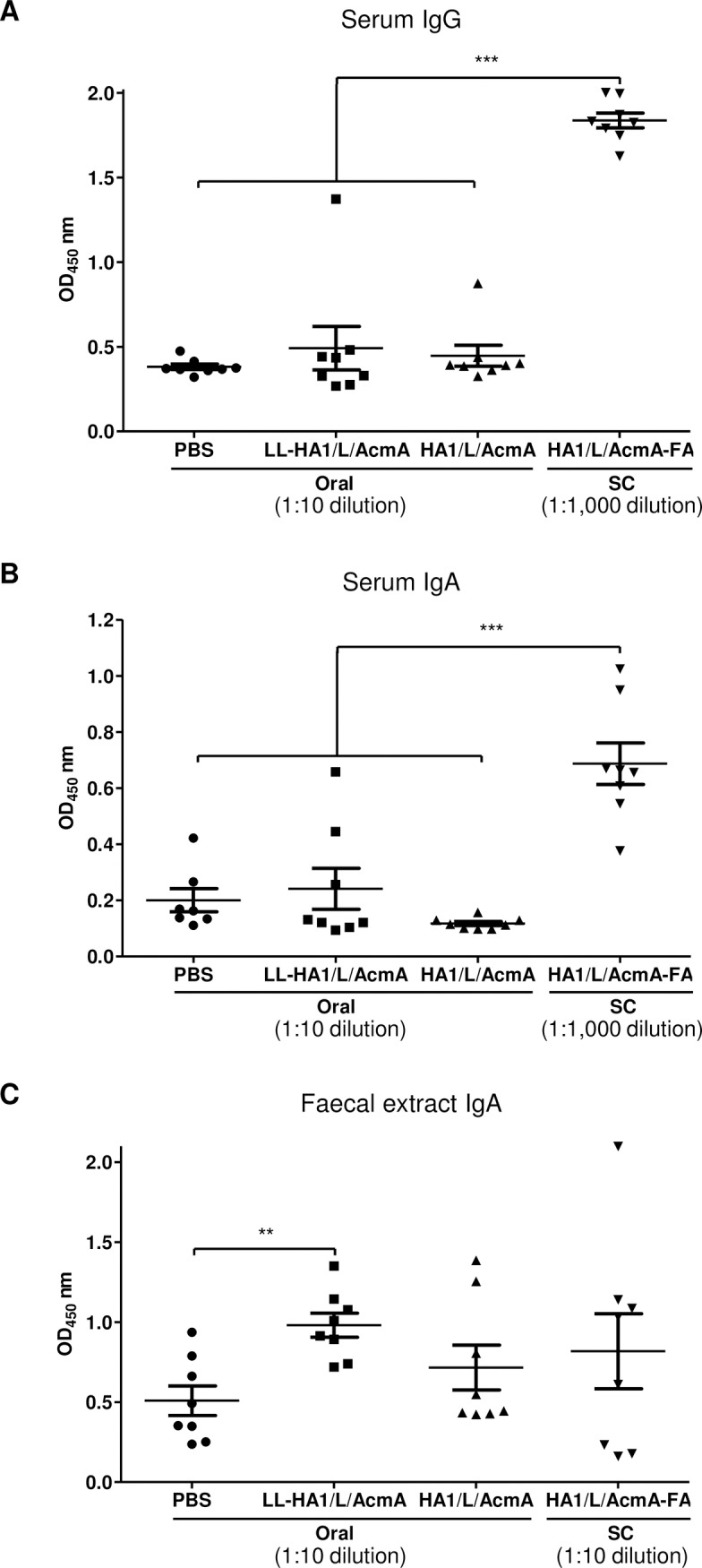
Mice (8 mice/group) were orally immunized with PBS, LL-HA1/L/AcmA or HA1/L/AcmA, or subcutaneously (SC) immunized with HA1/L/AcmA-FA. Serum and faecal samples were collected 2 weeks after the last immunization. (A) HA1/L/AcmA-specific serum IgG, (B) serum IgA and (C) faecal IgA from individual mouse were determined. Data are presented as mean ± standard error of the mean. Asterisks indicate statistically significant differences between the corresponding groups (**p<0.01; ***p<0.001).
Significantly higher HA1/L/AcmA-specific sIgA (Fig 3C) (p<0.01) was detected in faecal sample of mice orally immunized with LL-HA1/L/AcmA (mean OD: 0.98±0.07) than the PBS-treated group (mean OD: 0.51±0.09). A higher dosage of LL-HA1/L/AcmA administered in mice effectively improved mucosal immune response, and this is clearly indicated by the significant stimulation of sIgA detected in the faecal sample (Figs 2C and 3C). The HA1/L/AcmA-specific sIgA in faecal sample of mice orally immunized with LL-HA1/L/AcmA was also higher, but not statistically different compared to mice immunized with HA1/L/AcmA orally (mean OD: 0.72±0.14) or HA1/L/AcmA-FA subcutaneously (mean OD: 0.82±0.23) (Fig 3C). There was no significant difference in the detected HA1/L/AcmA-specific sIgA in faecal sample of mice orally immunized with HA1/L/AcmA from the PBS-treated group, suggesting HA1/L/AcmA without L. lactis as a carrier did not stimulate significant level of HA1/L/AcmA-specific sIgA. The same was noted for mice immunized subcutaneously with HA1/L/AcmA-FA as compared to PBS-treated group. Although mice immunized subcutaneously with HA1/L/AcmA-FA stimulated good serological immune responses, however, it did not stimulate significant level of sIgA in the faecal sample. Taken together, the results suggested that LL-HA1/L/AcmA is a potent inducer of mucosal immunity in the gastrointestinal tract upon oral immunization of LL-HA1/L/AcmA in mice, and L. lactis delivery platform is capable in stimulating mucosal immunity in the gastrointestinal tract.
Protection against lethal challenge
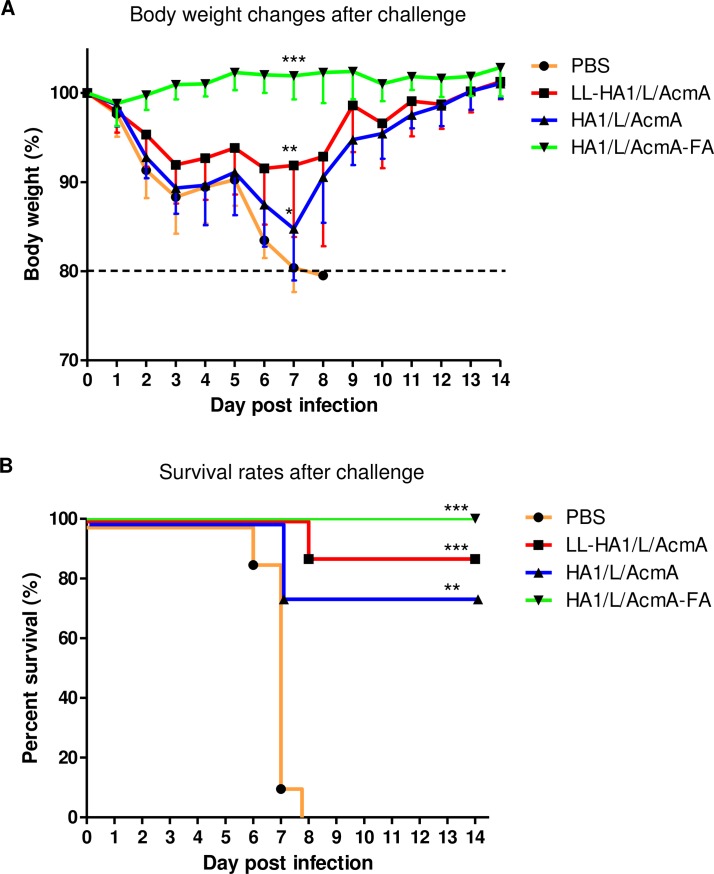
At 18 days following the last immunization, all mice in study group B were challenged intranasally with influenza virus to evaluate the protective efficacy of orally administered LL-HA1/L/AcmA against exposure to influenza virus. Changes in body weight and survival of mice were monitored daily as measure of disease severity. All non-immunized mice were susceptible to influenza infection, showing sickness such as decreased activity and huddling by day 3 post-infection (p.i.). Severe sickness such as hunched posture, ruffled fur and increased body weight loss of up to 20% were observed within day 8 p.i. (Fig 4A). The rate of body weight loss calculated by linear regression analysis was -2.7±0.1 and the maximum percentage change in body weight observed in individual mice over 14 days after lethal challenge with influenza virus was 20.6±0.1% (Table 1). In contrast, body weight of mice in LL-HA1/L/AcmA-treated and HA1/L/AcmA-treated group decreased until day 6 and 7 p.i., respectively, after which mice in both groups recorded an increase in their body weight till day 14 p.i. (Fig 4A). A lower rate of body weight loss was observed in the LL-HA1/L/AcmA-treated (-0.3±0.1) and HA1/L/AcmA-treated group (-0.6±0.1), respectively, as compared to non-immunized mice (-2.7±0.1) (Table 1). Significantly lower percentage change in body weight was noted in LL-HA1/L/AcmA-treated (11.6±2.5%) (p<0.01) and HA1/L/AcmA-treated group (15.7±1.8%) (p<0.05) than the non-immunized mice. In contrast, the positive control group, which were mice immunized subcutaneously with HA1/L/AcmA-FA did not show body weight loss upon exposure to influenza virus (Fig 4A). The mice instead recorded rate of body weight gain of 0.2±0.0 (Table 1). In addition, there was significantly lower percentage change in body weight (p<0.001) in this HA1/L/AcmA-FA-treated group (1.5±0.7%) compared to the non-immunized group (Table 1). These data indicated that mice orally immunized with LL-HA1/L/AcmA or HA1/L/AcmA resulted in significantly less morbidity upon exposure to influenza virus. Mice in the HA1/L/AcmA-treated group suffered more severe sickness as presented by highest rate of body weight loss and percentage change in body weight among the treated group. Subcutaneous immunization of HA1/L/AcmA-FA in mice did not result in any morbidity upon exposure to virus. Collectively, it was demonstrated that oral immunization of LL-HA1/L/AcmA is superior in reducing morbidity upon exposure to influenza virus compared to the HA1/L/AcmA without L. lactis as a carrier.
Fig 4. Body weight and survival rate of mice following lethal challenge with influenza virus.
At 18 days following the last immunization, all mice in study group B were intranasally challenged with 10 LD50 of H1N1/A/TN/1-560/2009-MA2 virus. (A) Body weight and (B) survival of mice orally immunized with PBS, LL-HA1/L/AcmA or HA1/L/AcmA, or subcutaneously immunized with HA1/L/AcmA-FA, was monitored daily for 14 days following virus challenge. The body weight of mice on the day of viral challenge was used as the baseline weight to determine the body weight changes post-viral challenge. Asterisks indicate statistically significant differences in comparison with the PBS-treated group (*p<0.05; **p<0.01; ***p<0.001).
Table 1. Body weight and survival rate of immunized mice upon lethal challenge with influenza virus.
| Immunogen | Rate of body weight lossa) | Rate of body weight recoveryb) | Body weight loss, %c) | No. of survival (%)d) | Mean day of death, p.i.e) |
|---|---|---|---|---|---|
| PBS | -2.7±0.1 | - | 20.6±0.1 | 0/8 (0) | 7.0±0.5 |
| LL-HA1/L/AcmA | -0.3±0.1 | 0.3±0.1 | 11.6±2.5 ** | 7/8 (88) *** | 8.0±0.0 |
| HA1/L/AcmA | -0.6±0.1 | 0.3±0.1 | 15.7±1.8 * | 6/8 (75) ** | 7.0±0.0 |
| HA1/L/AcmA-FA | 0.2±0.0 | 0.2±0.0 | 1.5±0.7 *** | 8/8 (100) *** | >14.0 |
a), b) Rate of body weight loss and body weight recovery was calculated by linear regression analysis. Data are presented as mean ± standard deviation.
c) The maximum percentage change in body weight observed in individual mice over 14 days after lethal challenge with influenza virus. Data are presented as mean ± standard deviation.
d) Total number of mice survived following lethal challenge with influenza virus. Percentage of mice survived following virus challenge is presented in parentheses.
e) Day of death following lethal challenge with influenza virus. Data are presented as mean ± standard deviation.
Asterisks indicate statistically significant differences in comparison to the PBS-treated group (*p<0.05; **p<0.01; ***p<0.001).
The survival rate of immunized mice upon exposure to influenza virus was assessed. Significantly higher survival rate of mice was observed in LL-HA1/L/AcmA-treated (7/8, 88%) (p<0.001) and HA1/L/AcmA-treated group (6/8, 75%) (p<0.01) upon exposure to influenza virus than non-immunized group (Fig 4B; Table 1). Survival rate of mice immunized with HA1/L/AcmA-FA was the highest and significantly different (p<0.001) from non-immunized group, with all mice fully protected from the challenge (8/8, 100%). There were no differences in the survival between all the immunized groups. As for non-immunized group, all mice were not protected (0/8, 0%) and the mean day of death was 7.0±0.5 p.i. The mean day of death LL-HA1/L/AcmA-treated and HA1/L/AcmA-treated group was 8.0±0.0 p.i. and 7.0±0.0 p.i., respectively. Importantly, oral immunization of mice with LL-HA1/L/AcmA or HA1/L/AcmA, or subcutaneous immunization with HA1/L/AcmA-FA provided protection to the animals against a lethal challenge with influenza virus.
Discussion
Influenza virus, a respiratory pathogen, contributes to a high rate of morbidity and mortality in humans globally. As such, while antiviral therapy has been available, vaccination is still the most effective strategy for the prevention and control of influenza. There is growing interest in mucosal vaccines due to their advantages over conventional injectable vaccines. Side effects associated with conventional vaccines, such as local reactions at the injection site are potentially reduced with implementation of mucosal vaccines. The need for needles during vaccine administration is also avoided, eliminating the possibility of blood transmissible infections [29, 30]. Moreover, mucosal vaccination can be easily administered without a trained personnel, thus considered a more favorable approach for mass vaccination [31].
Most pathogens, including influenza viruses, initiate infection by entering the human body through mucosal surfaces. Mucosal vaccination stimulates this natural infection and can provide local immune protection by stimulating sIgA at the mucosal surfaces. SIgA is the most abundant immunoglobulin isotype present in human secretions [32] and is effective in according protection against influenza virus infection [33]. It has been reported to be more important than IgG in the protection of upper respiratory tract, specifically the nose and trachea [34], primarily by reducing virus attachment and preventing internalization of the virus at the mucosal surfaces, thereby preventing infection [34, 35]. In addition, sIgA provides cross-protection against other subtypes of influenza virus [33, 36–38]. Cross-protection is particularly desirable due to the frequent antigenic changes that influenza viruses constantly undergo.
Of the various mucosal immunization approaches, L. lactis is being explored as an effective vaccine vehicle. We previously developed a recombinant L. lactis displaying influenza HA1 and evaluated the immunity elicited by this recombinant construct in mice [28]. This recombinant L. lactis carried the HA1 gene that was genetically introduced into a vector containing an antibiotic resistance gene. The presence of this antibiotic resistance gene could eventually be a matter of concern, particularly the transfer of its antibiotic resistance gene to another organism when L. lactis is released in the field [39]. An approach utilizing a non-recombinant L. lactis will be advantageous as it is likely to overcome this concern, and hence be better accepted by the public. We subsequently developed a non-recombinant L. lactis displaying influenza HA1, LL-HA1/L/AcmA [25], and evaluated its ability in the stimulation of mucosal immunity in this present study.
In the present study, findings of HA1/L/AcmA-specific sIgA in faecal extract, small intestine wash, BAL fluid and nasal fluid demonstrated that oral immunization with LL-HA1/L/AcmA in mice elicited significant level of mucosal immunity in the gastrointestinal tract and the respiratory tract. The result is consistent with previous studies showing that oral immunization with L. lactis displaying antigens induced sIgA at sites other than the gastrointestinal tract [22, 24, 40–42]. The results of sIgA at other mucosal sites could be due to intestinally derived IgA plasma cells expressing CCR10 homing receptor that migrated towards the CCL28 cytokine, which is secreted by mucosal epithelial tissues present at sites such as large intestine, stomach, trachea, bronchi, mammary glands and salivary glands [43]. These migrated intestinal IgA plasma cells then populate the mucosal sites, and secrete HA1/L/AcmA-specific sIgA. Therefore, oral immunization could lead to presence of antigen specific IgA in both intestinal and non-intestinal mucosal tissues [44, 45].
In study group B, higher dosage of LL-HA1/L/AcmA significantly increased the specific sIgA response compared to non-immunized group. The results suggested that sIgA response elicited by LL-HA1/L/AcmA could be improved in a dose-dependent manner. The observed specific sIgA response upon oral immunization with LL-HA1/L/AcmA was consistent with our previously reported findings in which specific sIgA was detected upon oral immunization with recombinant L. lactis expressing HA1 [28].
HA1/L/AcmA-specific serum IgG and IgA were almost absent following immunization with LL-HA1/L/AcmA, with the exception of one mouse (1/8) that elicited detectable serum IgG and IgA upon administration with a higher dosage of LL-HA1/L/AcmA. The result obtained was in contrast with several studies that had demonstrated oral immunization of antigen using L. lactis elicited specific IgG in serum in addition to specific sIgA in faecal extract [9, 11, 12, 46–48]. Although mice immunized orally with LL-HA1/L/AcmA elicited significant mucosal immune response only, and not systemic immune response, more importantly, it was demonstrated that LL-HA1/L/AcmA could provide up to 88% protection in mice against a lethal challenge with influenza virus. An earlier study on mice immunized orally with L. lactis expressing HA of influenza H5N1 showed significant increase in specific serum IgG and intestinal IgA, but IgA was not detected in tracheal mucosal and only 30% mice were protected upon viral challenge [46]. Therefore, in this study, the protection in mice was likely due to the presence of sIgA specific against HA1 in the respiratory tract, which was possibly activated upon oral immunization. It would be beneficial to evaluate the neutralization ability of the sIgA induced by LL-HA1/L/AcmA by measuring its capability to inhibit influenza virus A (H1N1) in vitro, however it could not be examined due to the limitation of samples obtained. Besides sIgA, there are other protective mechanisms, such as T cell-mediated immunity, which may play a protective role by clearing the established infection and reducing disease severity [49, 50]. Therefore, additional studies are needed to elucidate the specific mechanism of protection by LL-HA1/L/AcmA. Nonetheless, the findings obtained highlight the importance of mucosal immunity in the respiratory tract in according protection against influenza virus challenge.
In comparing the importance of L. lactis as an antigen carrier, mice immunized orally with LL-HA1/L/AcmA showed markedly improved immune response than HA1/L/AcmA recombinant protein only, without L. lactis. Mice immunized with LL-HA1/L/AcmA also suffered less sickness and body weight loss upon lethal challenge with influenza virus. These results were in agreement with several earlier studies that oral immunization with L. lactis displaying antigen is more efficient than a simple antigen alone oral immunization [15, 47, 51]. The potential adjuvant effects of using L. lactis in oral immunization could be attributed to the presence of bacterial components that can stimulate innate immunity, leading to adaptive immunity [52]. Besides, L. lactis presenting heterologous antigen as a particle to the immune system, is more superior to soluble antigen presented on its own, particularly if the vaccine is to be delivered orally [53]. As the present study is focused only on the protection of LL-HA1/L/AcmA in mice against homosubtypic influenza virus challenge, heterosubtypic influenza virus challenge can now be explored. Further investigation to surface display antigens from multiple influenza subtypes on a single L. lactis cell as candidate vaccine to render protection against a broad range of influenza subtypes can also be performed.
In conclusion, oral administration of L. lactis surface displaying HA1/L/AcmA recombinant protein induces specific sIgA response in mice that protected against homologous influenza virus challenge. These findings indicate the application of L. lactis as a platform for vaccine delivery and the importance of mucosal immunity in according protection against lethal challenge with influenza virus.
Acknowledgments
We thank Dr. Richard J. Webby and Dr. Wong Sook San from St. Jude Children’s Research Hospital for kindly providing us with the mouse adapted influenza virus. We also thank Dr. Haryanti Azura Mohamad Wali and Dr. Syahar Amir A. Gani from Animal Experimental Unit, Faculty of Medicine, University of Malaya for their assistance in animal handling and care.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was funded in parts by Pharmaceutical & Nutraceutical R&D initiatives fund (09-05-IFN-MEB-005) from Malaysian Institute of Pharmaceuticals and Nutraceuticals, Ministry of Science, Technology and Innovation, the University of Malaya-MOHE High Impact Research Fund (E000013-20001), the University of Malaya Research Grant (FL001-13HTM) to Sazaly AbuBakar, and the University of Malaya Postgraduate Research Fund (PG056-2014B) to Pui-Fong Jee. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhang N, Zheng BJ, Lu L, Zhou Y, Jiang S, Du L. Advancements in the development of subunit influenza vaccines. Microbes Infect. 2015;17(2):123–34. doi: 10.1016/j.micinf.2014.12.006 ; PubMed Central PMCID: PMCPMC4336774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Influenza (Seasonal) 2014 [4 October 2016]. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/.
- 3.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59(1):1–15. . [DOI] [PubMed] [Google Scholar]
- 4.Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist(R); Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71(12):1591–622. doi: 10.2165/11206860-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- 5.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685–96. doi: 10.1056/NEJMoa065368 . [DOI] [PubMed] [Google Scholar]
- 6.Cox MM, Hollister JR. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals: journal of the International Association of Biological Standardization. 2009;37(3):182–9. doi: 10.1016/j.biologicals.2009.02.014 . [DOI] [PubMed] [Google Scholar]
- 7.Haaheim LR, Madhun AS, Cox R. Pandemic influenza vaccines—the challenges. Viruses. 2009;1(3):1089–109. doi: 10.3390/v1031089 ; PubMed Central PMCID: PMC3185517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poles J, Alvarez Y, Hioe CE. Induction of intestinal immunity by mucosal vaccines as a means of controlling HIV infection. AIDS Res Hum Retroviruses. 2014;30(11):1027–40. doi: 10.1089/aid.2014.0233 ; PubMed Central PMCID: PMC4208617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin KQ, Hoshino Y, Toda Y, Igimi S, Kojima Y, Jounai N, et al. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood. 2003;102(1):223–8. doi: 10.1182/blood-2003-01-0110 . [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Zhong J, Huan L. Expression of hepatitis B virus surface antigen determinants in Lactococcus lactis for oral vaccination. Microbiol Res. 2011;166(2):111–20. doi: 10.1016/j.micres.2010.02.002 . [DOI] [PubMed] [Google Scholar]
- 11.Lei H, Sheng Z, Ding Q, Chen J, Wei X, Lam DM, et al. Evaluation of oral immunization with recombinant avian influenza virus HA1 displayed on the Lactococcus lactis surface and combined with the mucosal adjuvant cholera toxin subunit B. Clin Vaccine Immunol. 2011;18(7):1046–51. doi: 10.1128/CVI.00050-11 ; PubMed Central PMCID: PMCPMC3147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marelli B, Perez AR, Banchio C, de Mendoza D, Magni C. Oral immunization with live Lactococcus lactis expressing rotavirus VP8 subunit induces specific immune response in mice. J Virol Methods. 2011;175(1):28–37. doi: 10.1016/j.jviromet.2011.04.011 . [DOI] [PubMed] [Google Scholar]
- 13.Sim AC, Lin W, Tan GK, Sim MS, Chow VT, Alonso S. Induction of neutralizing antibodies against dengue virus type 2 upon mucosal administration of a recombinant Lactococcus lactis strain expressing envelope domain III antigen. Vaccine. 2008;26(9):1145–54. doi: 10.1016/j.vaccine.2007.12.047 . [DOI] [PubMed] [Google Scholar]
- 14.Varma NR, Toosa H, Foo HL, Alitheen NB, Nor Shamsudin M, Arbab AS, et al. Display of the Viral Epitopes on Lactococcus lactis: A Model for Food Grade Vaccine against EV71. Biotechnol Res Int. 2013;2013:431315 doi: 10.1155/2013/431315 ; PubMed Central PMCID: PMCPMC3586462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei H, Liu J, Cheng Y, Sun C, Wang C, Lu Y, et al. Expression of SARS-coronavirus nucleocapsid protein in Escherichia coli and Lactococcus lactis for serodiagnosis and mucosal vaccination. Appl Microbiol Biotechnol. 2005;68(2):220–7. doi: 10.1007/s00253-004-1869-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Audouy SA, van Selm S, van Roosmalen ML, Post E, Kanninga R, Neef J, et al. Development of lactococcal GEM-based pneumococcal vaccines. Vaccine. 2007;25(13):2497–506. doi: 10.1016/j.vaccine.2006.09.026 . [DOI] [PubMed] [Google Scholar]
- 17.Buccato S, Maione D, Rinaudo CD, Volpini G, Taddei AR, Rosini R, et al. Use of Lactococcus lactis expressing pili from group B Streptococcus as a broad-coverage vaccine against streptococcal disease. J Infect Dis. 2006;194(3):331–40. doi: 10.1086/505433 . [DOI] [PubMed] [Google Scholar]
- 18.Lee MH, Roussel Y, Wilks M, Tabaqchali S. Expression of Helicobacter pylori urease subunit B gene in Lactococcus lactis MG1363 and its use as a vaccine delivery system against H. pylori infection in mice. Vaccine. 2001;19(28–29):3927–35. . [DOI] [PubMed] [Google Scholar]
- 19.Robinson K, Chamberlain LM, Schofield KM, Wells JM, Le Page RW. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol. 1997;15(7):653–7. doi: 10.1038/nbt0797-653 . [DOI] [PubMed] [Google Scholar]
- 20.Norton PM, Brown HW, Wells JM, Macpherson AM, Wilson PW, Le Page RW. Factors affecting the immunogenicity of tetanus toxin fragment C expressed in Lactococcus lactis. FEMS Immunol Med Microbiol. 1996;14(2–3):167–77. . [DOI] [PubMed] [Google Scholar]
- 21.Ramasamy R, Yasawardena S, Zomer A, Venema G, Kok J, Leenhouts K. Immunogenicity of a malaria parasite antigen displayed by Lactococcus lactis in oral immunisations. Vaccine. 2006;24(18):3900–8. doi: 10.1016/j.vaccine.2006.02.040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei H, Peng X, Jiao H, Zhao D, Ouyang J. Broadly protective immunity against divergent influenza viruses by oral co-administration of Lactococcus lactis expressing nucleoprotein adjuvanted with cholera toxin B subunit in mice. Microb Cell Fact. 2015;14:111 doi: 10.1186/s12934-015-0287-4 ; PubMed Central PMCID: PMCPMC4524015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei H, Peng X, Zhao D, Jiao H, Ouyang J. Cross-protection of Lactococcus lactis-displayed HA2 subunit against homologous and heterologous influenza A viruses in mice. Arch Virol. 2015;160(12):3011–9. doi: 10.1007/s00705-015-2587-8 . [DOI] [PubMed] [Google Scholar]
- 24.Gao S, Li D, Liu Y, Zha E, Zhou T, Yue X. Oral immunization with recombinant hepatitis E virus antigen displayed on the Lactococcus lactis surface enhances ORF2-specific mucosal and systemic immune responses in mice. Int Immunopharmacol. 2015;24(1):140–5. doi: 10.1016/j.intimp.2014.10.032 . [DOI] [PubMed] [Google Scholar]
- 25.Jee PF, Chen FS, Shu MH, Wong WF, Abdul Rahim R, AbuBakar S, et al. Insertion of single-chain variable fragment (scFv) peptide linker improves surface display of influenza hemagglutinin (HA1) on non-recombinant Lactococcus lactis. Biotechnol Prog. 2017;33(1):154–62. doi: 10.1002/btpr.2400 . [DOI] [PubMed] [Google Scholar]
- 26.Saluja V, Amorij JP, van Roosmalen ML, Leenhouts K, Huckriede A, Hinrichs WL, et al. Intranasal delivery of influenza subunit vaccine formulated with GEM particles as an adjuvant. AAPS J. 2010;12(2):109–16. doi: 10.1208/s12248-009-9168-2 ; PubMed Central PMCID: PMCPMC2844513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arulanandam BP, O'Toole M, Metzger DW. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J Infect Dis. 1999;180(4):940–9. doi: 10.1086/314996 . [DOI] [PubMed] [Google Scholar]
- 28.Joan SS, Pui-Fong J, Song AA, Chang LY, Yusoff K, AbuBakar S, et al. Oral vaccine of Lactococcus lactis harbouring pandemic H1N1 2009 haemagglutinin1 and nisP anchor fusion protein elevates anti-HA1 sIgA levels in mice. Biotechnol Lett. 2016;38(5):793–9. doi: 10.1007/s10529-016-2034-2 . [DOI] [PubMed] [Google Scholar]
- 29.Hauri AM, Armstrong GL, Hutin YJ. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15(1):7–16. doi: 10.1258/095646204322637182 . [DOI] [PubMed] [Google Scholar]
- 30.Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003;9(1):99–103. doi: 10.1038/nm0103-99 . [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Lee KY, Jang YS. Mucosal Immune System and M Cell-targeting Strategies for Oral Mucosal Vaccination. Immune Netw. 2012;12(5):165–75. doi: 10.4110/in.2012.12.5.165 ; PubMed Central PMCID: PMCPMC3509160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–58. doi: 10.1038/nri1777 . [DOI] [PubMed] [Google Scholar]
- 33.Asahi-Ozaki Y, Yoshikawa T, Iwakura Y, Suzuki Y, Tamura S, Kurata T, et al. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J Med Virol. 2004;74(2):328–35. doi: 10.1002/jmv.20173 . [DOI] [PubMed] [Google Scholar]
- 34.Renegar KB, Small PA Jr., Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173(3):1978–86. . [DOI] [PubMed] [Google Scholar]
- 35.Taylor HP, Dimmock NJ. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J Exp Med. 1985;161(1):198–209. ; PubMed Central PMCID: PMCPMC2187541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura S, Funato H, Hirabayashi Y, Kikuta K, Suzuki Y, Nagamine T, et al. Functional role of respiratory tract haemagglutinin-specific IgA antibodies in protection against influenza. Vaccine. 1990;8(5):479–85. . [DOI] [PubMed] [Google Scholar]
- 37.Liew FY, Russell SM, Appleyard G, Brand CM, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984;14(4):350–6. doi: 10.1002/eji.1830140414 . [DOI] [PubMed] [Google Scholar]
- 38.Tamura S, Funato H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, et al. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21(6):1337–44. doi: 10.1002/eji.1830210602 . [DOI] [PubMed] [Google Scholar]
- 39.Sybesma W, Hugenholtz J, Vos WMd, Smid EJ. Safe use of genetically modified lactic acid bacteria in food. Bridging the gap between consumers, green groups, and industry. Electron J Biotechn. 2006;9(4):424–48. doi: 10.2225/vol9-issue4-fulltext-12 [Google Scholar]
- 40.Shi SH, Yang WT, Yang GL, Cong YL, Huang HB, Wang Q, et al. Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarumNC8 expressing hemagglutinin in BALB/c mice. Virology. 2014;464–465:166–76. doi: 10.1016/j.virol.2014.07.011 . [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Yu Q, Gao J, Yang Q. Mucosal and systemic immune responses induced by recombinant Lactobacillus spp. expressing the hemagglutinin of the avian influenza virus H5N1. Clin Vaccine Immunol. 2012;19(2):174–9. doi: 10.1128/CVI.05618-11 ; PubMed Central PMCID: PMCPMC3272930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei H, Peng X, Zhao D, Ouyang J, Jiao H, Shu H, et al. Lactococcus lactis displayed neuraminidase confers cross protective immunity against influenza A viruses in mice. Virology. 2015;476:189–95. doi: 10.1016/j.virol.2014.12.017 . [DOI] [PubMed] [Google Scholar]
- 43.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3(10):822–9. doi: 10.1038/nri1203 . [DOI] [PubMed] [Google Scholar]
- 44.VanCott JL, Brim TA, Lunney JK, Saif LJ. Contribution of antibody-secreting cells induced in mucosal lymphoid tissues of pigs inoculated with respiratory or enteric strains of coronavirus to immunity against enteric coronavirus challenge. J Immunol. 1994;152(8):3980–90. . [PubMed] [Google Scholar]
- 45.Czerkinsky C, Svennerholm AM, Quiding M, Jonsson R, Holmgren J. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect Immun. 1991;59(3):996–1001. ; PubMed Central PMCID: PMCPMC258358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Gao J, Yu Q, Yang Q. Oral immunization with recombinant Lactococcus lactis expressing the hemagglutinin of the avian influenza virus induces mucosal and systemic immune responses. Future Microbiol. 2012;7(8):1003–10. doi: 10.2217/fmb.12.69 . [DOI] [PubMed] [Google Scholar]
- 47.Ahmed B, Loos M, Vanrompay D, Cox E. Oral immunization with Lactococcus lactis-expressing EspB induces protective immune responses against Escherichia coli O157:H7 in a murine model of colonization. Vaccine. 2014;32(31):3909–16. doi: 10.1016/j.vaccine.2014.05.054 . [DOI] [PubMed] [Google Scholar]
- 48.Lei H, Xu Y, Chen J, Wei X, Lam DM. Immunoprotection against influenza H5N1 virus by oral administration of enteric-coated recombinant Lactococcus lactis mini-capsules. Virology. 2010;407(2):319–24. doi: 10.1016/j.virol.2010.08.007 . [DOI] [PubMed] [Google Scholar]
- 49.Chopra A, Saluja M, Tillu G. Diet, Ayurveda and interface with biomedicine. J Ayurveda Integr Med. 2010;1(4):243–4. doi: 10.4103/0975-9476.74423 ; PubMed Central PMCID: PMC3117314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shukla SH, Saluja AK, Pandya SS. Modulating effect of Gmelina arborea Linn. on immunosuppressed albino rats. Pharmacognosy Res. 2010;2(6):359–63. doi: 10.4103/0974-8490.75455 ; PubMed Central PMCID: PMC3111695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Haan A, Haijema BJ, Voorn P, Meijerhof T, van Roosmalen ML, Leenhouts K. Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration. Vaccine. 2012;30(32):4884–91. doi: 10.1016/j.vaccine.2012.04.032 . [DOI] [PubMed] [Google Scholar]
- 52.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–6. doi: 10.1016/j.coi.2010.04.004 . [DOI] [PubMed] [Google Scholar]
- 53.Visweswaran GR, Leenhouts K, van Roosmalen M, Kok J, Buist G. Exploiting the peptidoglycan-binding motif, LysM, for medical and industrial applications. Appl Microbiol Biotechnol. 2014;98(10):4331–45. doi: 10.1007/s00253-014-5633-7 ; PubMed Central PMCID: PMCPMC4004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.