Abstract
For more than 6 decades, many patients with advanced chronic kidney disease (CKD) have undergone surgical parathyroidectomy (sPTX) for severe secondary hyperparathyroidism (SHPT) mainly based historical clinical practice patterns, but not on evidence of outcome.We aimed in this meta-analysis to evaluate the benefits and harms of sPTX in patients with SHPT. We searched MEDLINE (inception to October 2016), EMBASE and Cochrane Library (through Issue 10 of 12, October 2016) and website clinicaltrials.gov (October 2016) without language restriction. Eligible studies evaluated patients reduced glomerular filtration rate (GFR), below 60 mL/min/1.73 m2 (CKD 3–5 stages) with hyperparathyroidism who underwent sPTX. Reviewers working independently and in duplicate extracted data and assessed the risk of bias. The final analysis included 15 cohort studies, comprising 24,048 participants. Compared with standard treatment, sPTX significantly decreased all-cause mortality (RR 0.74 [95% CI, 0.66 to 0.83]) in End Stage Kidney Disease (ESKD) patients with biochemical and / or clinical evidence of SHPT. sPTX was also associated with decreased cardiovascular mortality (RR 0.59 [95% CI, 0.46 to 0.76]) in 6 observational studies that included almost 10,000 patients. The available evidence, mostly observational, is at moderate risk of bias, and limited by indirect comparisons and inconsistency in reporting for some outcomes (eg. short term adverse events, including documented voice change or episodes of severe hypocalcaemia needing admission or long-term adverse events, including undetectable PTH levels, risk of fractures etc.). Taken together, the results of this meta-analysis would suggest a clinically significant beneficial effect of sPTX on all-cause and cardiovascular mortality in CKD patients with SHPT. However, given the observational nature of the included studies, the case for a properly conducted, independent randomised controlled trial comparing surgery with medical therapy and featuring many different outcomes from mortality to quality of life (QoL) is now very strong.
Introduction
For many decades surgical parathyroidectomy (sPTX) has been a well-recognised potential clinical intervention for patients with chronic kidney disease (CKD) with unremitting secondary hyperparathyroidism (SHPT), especially for those patients receiving long-term renal replacement therapy [1, 2]. Approximately 5–10 percent of patients with end-stage kidney disease (ESKD) undergo sPTX for severe SHPT [3]; this percentage significantly increases for long-term dialysis survivors. Typically, such patients have biochemical and radiological abnormalities related to SHPT, and important extra skeletal pathology, mostly cardiovascular [4]. Over the last two decades, with the widespread and targeted use of different types of vitamin D therapy, and / or oral calcimimetics, which represent the “medical management” of SHPT, the sPTX rate was expected to fall substantially[5] [6], Two recent reports present contradictory findings in this respect. The first one coming from the DOPPS cohort shows a general trend for increased serum PTH concentrations over the time period covered, while the sPTX rates declined in all regions (1996–2011) [7]. The second study derived from the Healthcare Cost and Utilization Project’s Nationwide Inpatient Sample—a representative national database on hospital stay, coupled with parathyroidectomy data from the US Renal Data System (2002–2011) shows a fairly static rate of sPTX in the US between 2002 and 2011[8].
Currently, it is not yet clear if there is a morbidity-mortality benefit following sPTX for severe SHPT in ESKD. There is a clear potential anaesthetic-surgical risk involved and the DOPPS data referred to above also showed an increased mortality hazard ratio with low serum PTH concentrations (the natural outome of successful parathyroid gland surgery). At the same time, recent guideline recommendations by latest European Renal Best Practice (ERBP) statement downgraded the use of calcimimetics [9]; thus the optimal therapeutic strategy for patients with biochemical changes but without clinical symptoms of SHPT, remains unclear. Whether or not to perform sPTX in CKD and ESKD patients remain a current, controversial and challenging issue. Therefore, clinicians may benefit from a critical systematic summary of the best available evidence regarding the benefits and risks associated with this intervention, to advise their patients accordingly. We conducted a meta-analysis of available evidence to assess the impact of sPTX on the outcomes of CKD /ESKD patients with SHPT compared with matched patients not undergoing sPTX.
Methods
The systematic review and meta-analysis was performed according to a previously published protocol (registration number:CRD42017067736). https://www.crd.york.ac.uk/PROSPERO/display_record.asp? ID = CRD42017067736
Purpose
This review aims to evaluate the benefits and harms of performing PTX in CKD/ESKD patients with secondary hyperparathyroidism.
Data sources/search strategy
We searched MEDLINE (inception to October 2016), the Cochrane Library (Issue 10–12, October 2016) and the website clinicaltrials.gov (October 2016) and EMBASE without language restriction. Hand search for relevant articles was done on reference lists from textbooks, articles, and scientific proceedings. The search terms used and a detailed search strategy is available in the S1 Table.
Study selection
We conducted a systematic review and meta-analysis on observational cohorts studies and randomized controlled trials (RCTs) in adults with CKD 3–5 stages (GFR below 60 mL/min/1.73 m2)) that evaluated the role of parathyroidectomy in determining clinical outcomes in patients with CKD-MBD. Studies enrolling any patient with CKD stages 3–5 (as defined by the Kidney-Disease Outcomes and Quality Initiative [K-DOQI] guidelines: stage 3 = GFR 30–59 ml/min/1.73 m2; stage 4 = GFR 15–29 ml/min/1.73 m2; stage 5 = GFR <15 ml/min/1.73 m2 including those requiring dialysis) with evidence of secondary hyperparathyroidism who had undergone parathyroid surgery were included in this analysis. The surgery itself could be (1) total parathyroidectomy without auto transplantation, (2) total parathyroidectomy with auto transplantation, or, (3) subtotal parathyroidectomy. Patients were compared with control CKD/ESKD patients with non-surgical treatment for SHPT. Patients with CKD/ESKD undergoing surgery for primary hyperparathyroidism and also those undergoing re-operative parathyroidectomy, were excluded.
Data extraction and synthesis
Data extraction was done independently by two authors (IN and MA) using standard data extraction forms. When more than one publication of one study was found, reports were grouped together and only the publication with the most complete data was included. Data extracted included identifying information, aim of the study, details of the study protocol and demographic data. We extracted characteristics of each study including baseline PTH values, baseline clinical characteristics of the study population, known comorbidities, type of study design, types of surgery and use of agents interfering with PTH release and total duration of follow-up. Any unclear or missing information was requested from the authors by written correspondence and any relevant information obtained was included in the review. Disagreements were resolved by consultation between all authors.
Risk of bias
Two reviewers (MA and IN) evaluated the quality of the selected studies independently without blinding to authorship or journal according to recommendations from the Cochrane Collaboration. The quality items assessed were selection bias (random sequence generation, allocation concealment), performance bias (blinding of patients and investigators), detection bias (blinding of outcome assessors), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other forms of bias (significant different group comparisons, funding sources, early termination of a trial). For the observationa studies, the quality was assessed using the Newcastle-Ottawa scale (NOS)[10]. The scale used three categories to evaluate: selection of the study groups, the comparability of the groups and the assesement of outcome. Stars awarded for each quality item serve as a quick visual assessment. Stars are awarded such that the highest quality studies are awarded up to nine stars. Disagreements were resolved by consensus. Publication bias was assessed using the funnel plot technique [11].
Main outcomes and measures
Primary outcomes of this analysis were all-cause mortality: short term and long-term and cardio-vascular mortality from the time of the surgical intervention to the end of follow-up. Secondary outcomes were: (1) QoL, (2) short term adverse events, including documented voice change or episodes of severe hypocalcaemia needing admission, (3) long-term adverse events, including “aparathyroid state” (undetectable PTH levels), fractures, and, (4) postoperative PTH levels. These are all outcomes of definite clinical relevance and importance. Summarized treatment effects were analysed for mortality and cardiovascular (CV) mortality, both postoperative and long-term, using random-effects meta-analysis.
Statistical analysis
We summarized effect estimates using standard and cumulative random effects meta-analysis. We used a random-effects model for meta-analysis and expressed treatment effects as a risk ratio (RR) with 95% confidence intervals (CI).[12]. We used the I2 statistic to assess for inconsistency across individual studies[12]. An I2>50% indicated large inconsistency across studies (heterogeneity) not explained by chance[13]. We considered a p-value below 0.10 to indicate significant heterogeneity. In cumulative meta-analysis, outcome data for all-cause mortality and cardiovascular mortality from all available trials were included sequentially according to the year in which they first became available. All analyses were performed using Review Manager Version 5.2 (The Cochrane Collaboration 2012) and Stata SE software, version 12 (StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP.) [14].
Additional prespecified subgroup analyses were conducted to explore potential causes of heterogeneity for treatment effect on all-cause mortality. Treatment heterogeneity was analysed also in relation with prior medical treatment. The following factors were planned to be investigated in subgroup analyses (1) publication date, (2) use of calcimimetics in the perioperative period, and (3) initial PTH levels (<800 pg/ml vs>800 pg/ml). For all analyses, a two-tailed p-value<0.05 indicated statistical significance.
Results
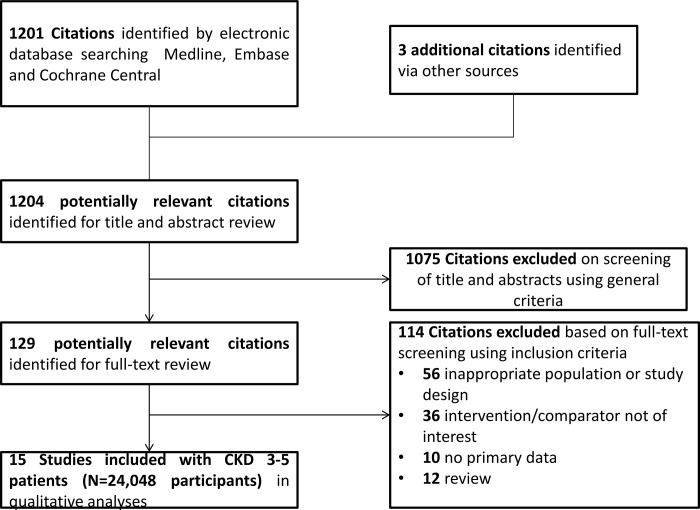
The literature search identified a total of 997 abstracts, of which 15 observational studies comprising 24,048 participants were selected (Fig 1). No randomized cotrolled trial was indentified. The follow-up period varied between 12 and 360 months. Baseline characteristics of the included studies are listed in Tables 1 and 2. These studies included only prevalent dialyzed patients diagnosed with SHPT who underwent subtotal or total parathyroidectomy with or without auto-transplantation. In the majority of included studies, the parathyroidectomy group was compared to matched controls, who had not undergone parathyroidectomy or refused surgery for various reasons and were conservatively managed. In other studies a propensity score matching was used trying to decrease the risk of selection bias, while in others parathyroidectomy group was compared with control patients identified from different registries, matched for age, sex, race, diabetes as cause of kidney failure, years on dialysis, and dialysis modality. The main outcome in the included studies was all-cause mortality, defined as death by any cause. Six studies also reported cardiovascular mortality. In three of them[15–17], the cardiovascular events included sudden death, heart failure, myocardial infarction, peripheral vascular disease and cerebrovascular accident, while in two[18, 19] the definition included only sudden death, cerebrovascular accident and myocardial infarction. Only one study lacked a precise definition for cardiovascular mortality[19]. Six studies [16–18, 20–22] reported data regarding treatments that might interfere with parathyroid function (phosphate binders or vitamin D compounds). No data regarding contemporaneous or parallel-group calcimimetic treatment were reported in any of the included studies. Overall, the 15 included studied had an averaged NOS score of 6.2 stars (of maximum 9 stars) (Table 1) due to lack of or unclear description of follow-up time and lost to follow-up in the included studies.
Fig 1. Selection and description of studies.
Table 1. Demographic and characteristics of studies included in the meta-analysis.
| Reference (first author) | Country | Parients No | Age | Gender (male%) | Newcastle-Ottawa score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PTX | CTRL | PTX | CTRL | PTX | CTRL | Selection | Comparability | Exposure | ||
| Ivarsson etal. 2015 [23] | Sweden | 423 | 1234 | 55.2 | 56 | 48.2 | 50.1 | *** | ** | ** |
| Komaba et al. 2015 [17] | Japan | 4428 | 4428 | 59.1 ± 11.6 | 59.3±12.3 | 55.8 | 55.7 | *** | ** | *** |
| Conzo et al. 2013 [20] | Italy | 30 | 20 | 51.5±10.89 | 55±11.2 | 26.7 | 40 | *** | * | * |
| Sharma et al. 2013 [51] | US | 150 | 1044 | 42.1 | 42.2 | 46.7 | 46.7 | *** | ** | ** |
| Goldstein et al 2013 [21] | Brazil | 123 | 128 | 46 | 50 | 46.3 | 44.5 | *** | * | ** |
| Iwamoto et al 2012 [16] | Japan | 88 | 88 | 60.6±8.4 | 60.5±8.4 | 53.4 | 53.4 | *** | ** | ** |
| Kestenbaun et al. 2004 [24] | US | 4558 | 4558 | 47.6 | 47.6 | 42.5 | 42.5 | *** | ** | * |
| Trombetti et al. 2007 [44] | Switzerland | 40 | 80 | 42.6 | 55 | 45 | 51 | *** | ** | ** |
| Ho LC et al. 2016 [45] | Taiwan | 998 | 998 | 54.7 | 55 | 42.9 | 42.5 | *** | ** | *** |
| Moldovan et al. 2015 [22] | Romania | 26 | 26 | 51.62±9.92 | 49.65±11.49 | 53.84 | 23.07 | *** | * | ** |
| Li-Wedong et al 2016 [46] | China | 53 | 92 | 63.1±13.8 | 53.8±15 | 56.6 | 70.6 | *** | * | * |
| Costa-Hong et al 2007 [18] | Brazil | 50 | 68 | 52 | 59 | 43±10 | 45±12 | ** | ** | * |
| Dussol B et al 2007[47] | France | 19 | 32 | N/A | N/A | N/A | N/A | ** | ** | * |
| Ma T-L et al 2015[48] | Taiwan | 60 | 161 | N/A | N/A | N/A | N/A | ** | ** | * |
| Lin H-C 2014[19] | Taiwan | 30 | 23 | 53.3 ± 13.3 | 53.4 ±13.9 | 43 | 61 | *** | ** | * |
Abbreviations: PTX-parathyroidectomy, CTRL- control
*- Stars awarded for each quality item (Newcastle-Ottawa scale). For each domain, either a "star" or "no star" is assigned, with a "star" indicating that study design element was considered adequate and less likely to introduce bias. For Selection (of the exposed cohort, of the non-exposed cohort, ascertainment of exposure and outcome of interest) a maximum of four stars may be assigned. A maximum of two stars can be given for Comparability and a maximum of 3 stars can be given for Exposure (assessment of outcome, length of follow-up and adequacy offollow-up). A study could receive a maximum of nine stars.
Table 2. Baseline characteristics of the studies included in the meta-analysis.
| Reference (first author) | Design of study | Duration of follow-up (months) | Baseline PTH | Type of surgery | Type of control group | Inclusion criteria | Exclusion criteria | |
|---|---|---|---|---|---|---|---|---|
| PTX | CTRL | |||||||
| Ivarsson et al. 2015 | Cohort-multicenter- prospective | 61.3 | N/A | N/A | Total and subtotal PTX | Between one and five patients randomly matched who had not undergone PTX. The matching criteria were birth year in 10-year categories, sex and cause of ESKD in categories (autosomal dominant polycystic kidney disease, diabetes mellitus, glomerulonephritis, nephrosclerosis, pyelonephritis and other/unknown. | Patients on maintenance dialysis and transplantation with SHPT | Errors in reporting of patient information censoring on the same day as initiation of RRT |
| Komaba et al. 2015 | Cohort-multicenter- prospective | 12 | 96 (28–236) | 669 (570–870) | Total and subtotal PTX | Propensity score-matched patients who had not despite severe SHPT | ≥ 18 years of age with SHPT and were receiving haemodialysis thrice weekly for more than 3 months | No data on demographic characteristics, dialysis prescription, intact PTH levels, or history of PTX |
| Conzo et al. 2013 | One-center retrospective | 60 | 142.08 ± 64.01 | 102.94 ± 32.51 | Total PTX and total PTX with auto-transplantation | Patients with indication for PTX but refusing surgery | SHPT, unresponsive to medical treatment iPTH levels > 53–84, 8 pmol/L, serum P level > 2,09 mmol/l, US enlarged parathyroid glands (> 1 cm or >500 mm3) and persisting clinical symptoms, six months after medical therapy | Renal transplantation, |
| Sharma et al. 2013 | Retrospective and matched-cohort study | 33.6 | N/A | N/A | Near-total parathyroidectomy | For each NTPTX patient, controls were individually matched for age (±2 years), sex, race, diabetes as cause of end-stage renal disease, dialysis duration (vintage), year they started dialysis (±1 year), and dialysis modality | Prevalent haemodialysis or peritoneal dialysis with SHPT | Kidney transplant, no SHPT, no records on dialysis modality |
| Goldstein et al 2013 | Retrospective cohort study | 23 | 1554 | 1360 | Total parathyroidectomy with auto-transplantation | Patients with refractory SHPT not submitted to PTX | PTH greater than 800 pg/ml on calcitriol or in the presence of hyperphosphatemiaand/or hypercalcemia which prevented the use of calcitriol | Kidney transplant and predialysis patients No SHPT |
| Iwamoto et al 2012 | Retrospective cohort study | 53 | 884.5 ± 388.5 | 199.0 ± 120.2 | Total PTX without autotransplantation | Matched patients for sex, age, underlying disease and prior dialysis history | PTH >500 pg/mL and enlarged parathyroid glands confirmed by imaging, enlarged parathyroid gland with imaging and resistant to reduction of iPTH to below 200 pg/mL for hypercalcemia (corrected Ca>11.0 mg/dL) with VDRAs. | N/A |
| Kestenbaum et al. 2004 | prospective cohort study | 53.4 | N/A | N/A | Total+subtotal PTX | Individually matched by age, race, gender, cause of ESKD, dialysis duration, prior transplantation status, and dialysis modality | at least 18 years old and had initiated renal replacement therapy with SHPT | Death, lost to follow-up, or underwent PTX during the first 90 days of renal replacement therapy |
| Trombetti et al. 2007 | retrospective cohort study | 360 | N/A | N/A | Subtotal or total PTX with autotransplantation | two matched controls for each PTX case | ESKD and severe hyperparathyroidism | Kidney transplant, no records, no SHPT |
| Ho LC et al. 2016 | retrospective cohort study | 41.52±30.12 | N/A | N/A | N/A | The parathyroidectomized patients were matched with the controls based on propensity score for parathyroidectomy | Prevalent dialysis with unremitting SHPT | Renal transplantation prior to dialysis or a history of any kind of malignancy before the initiation of long-term dialysis |
| Moldovan et al. 2015 | prospective cohort study | 24 | 2037 | 1282 | Subtotal or total PTX | patients with iPTH over 700 pg/ml, without surgical intervention and treated with specific drugs | severe sHPT, non-responsive to medical treatment with hypercalcemia and hyperphosphatemia | ESKD patients with SHPT and no parathyroid surgery |
| Li-Wedong et al 2016 | prospective cohort study | 12 | 395.3 ± 332.4 | 349.8 ± 334.5 | N/A | Dialysed patient with SHPT | Age>18 years and less than 70 years old. (Duration of HD is more than 3 months. Patients with SHPT (Based on the 2002 KDOQI) | patients with malignant neoplasms, active tuberculosis, AIDS, receiving kidney transplant surgery within one year, pregnancy or lactation, life expectancy being less than 12 months, acute malnutrition, uncontrolled hypertension, severe anemia, serious liver diseases or interrupted follow-up because of all kinds of reasons |
| Costa-Hong et al 2007 | prospective cohort study | N/A | 1278 ±699 | 1243± 753 | Total PTX with autotransplantation in the forearm | Patients who had the diagnosis of medically resistant SHPT and not submitted to PTX | Resistance to medical treatment that was defined as serum levels of parathyroid hormone (PTH) and phosphate greater than 800 pg/mL and 6.5 mg/100 mL, respectively, after a minimum of 6 months of treatment. |
Renal transplantation, previous myocardial revascularization, smokers, individuals using lipid-lowering drugs, patients with diabetes, and those with a history of heart failure, stroke, unstable angina, or myocardial infarction within 12 months preceding the initiation of the study |
| Dussol B et al 2007 | prospective cohort study | 96 | N/A | N/A | Total+subtotal PTX | Patients undergoing chronic hemodialysis treatment | N/A | N/A |
| Ma T-L et al 2015 | Prospective cohort study | 36 | N/A | N/A | N/A | Hemodialysed patients with iPTH values greater than 800 pg/dL | N/A | N/A |
| Lin H-C 2014 | prospective cohort study | 72 | 1011 ±247 | 1007 ± 251 | total PTX with autograft to the brachioradialis muscle in the forearm without arteriovenous shunt. | ESKD patients who were treated with maintenance haemodialysis and who had intact parathyroid hormone (PTH) levels > 800 pg/ml not receiving PTX | Haemodialysis patients with severe secondary hyperparathyroidism. Severe SHPTH was diagnosed when a patient’s PTH level was higher than 800 pg/ml and was associated with the following symptoms: bone and joint pain, muscle weakness, irritability, itching, bone loss, anaemia resistant to erythropoietin, cardiomyopathy or calciphylaxis. | Switched ti peritoneal dialysis Transfer to other hospital Incomplete medical history Received kidney transplant Not eligible for operation Had previous PTX |
Abbreviations: PTH-parathormone, RRT-renal replacement therapy, PTX-parathyroidectomy, SHPT- hyperparathyroidism, ESKD- end–stage kidney disease, VDRAs- vitamin D receptor activators, N/A- not available
Clinical outcomes
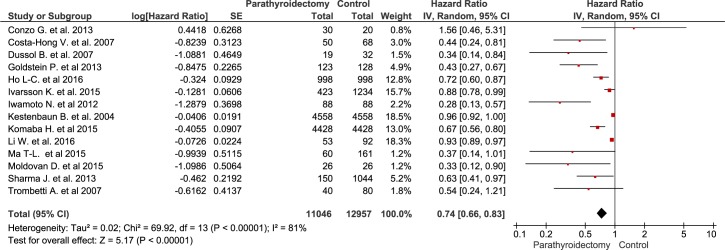
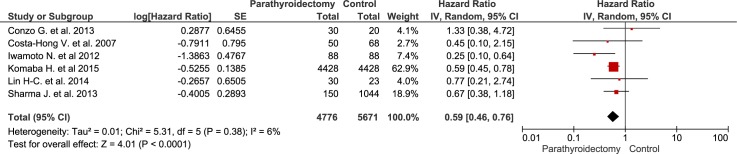
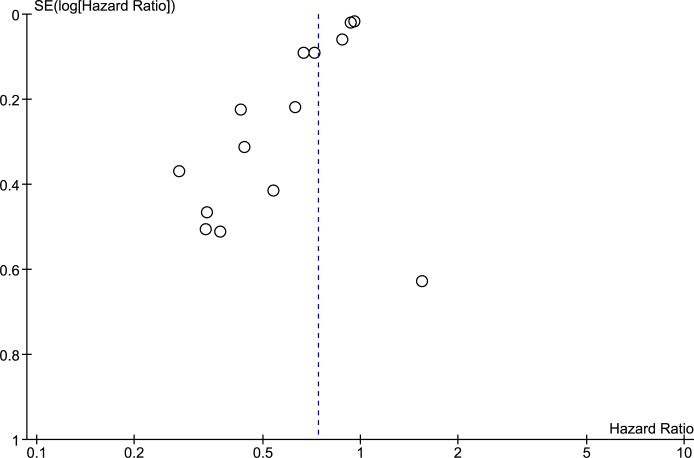
Compared with standard treatment, sPTX was associated with decreased all-cause mortality (RR 0.74 [95% CI, 0.66 to 0.83) in ESKD patients with secondary hyperparathyroidism (Fig 2). Patients undergoing sPTX had decreased cardiovascular mortality (RR 0.59 [95% CI, 0.46 to 0.76]) in 6 observational studies that included almost 10,000 patients (Fig 3). The heterogeneity across included studies is substantial in the analysis of all-cause mortality (I2 = 81%, p < 0.001) and is 6% in the analysis of cardiovascular mortality. The high I2 values for all cause mortality show that most of the variability across studies is due to heterogeneity rather than chance. Because the heterogeneity was found to be higher than expected, the model was switched to a random-effect model by calculating the variance of random-effect components. The funnel plot (Fig 4) shows an asymmetrical plot. This was expected in the presence of the important heterogeneity observed for the all-cause mortality outcome, but there is also a suggestion of missing studies in the middle and right of the plot, broadly in the area of non-significance and this could also imply the presence of reporting bias, with smaller negative studies not having been published.
Fig 2. The effect of parathyroidectomy on all-cause mortality.
Fig 3. The effect of parathyroidectomy on cardiovascular mortality.
Fig 4. Funnel plot for all-cause mortality.
These results were further confirmed by cumulative metanalysis performed in attempt to stratify the studies for trend by time. Cumulative meta-analysis shows that studies conducted since 2012 have collectively signalled benefits for all-cause mortality and since 2015 for cardiovascular mortality (S1 Fig and S2 Fig).
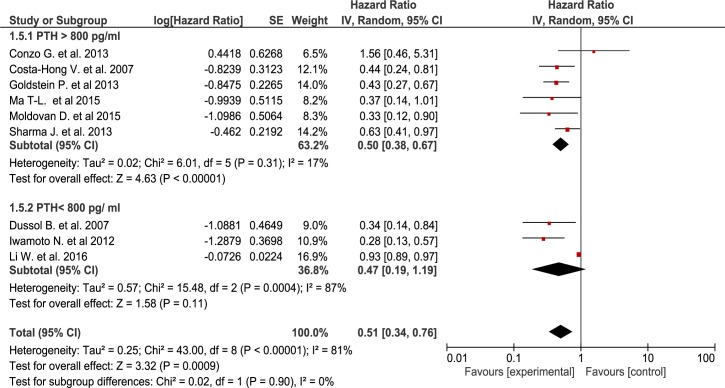
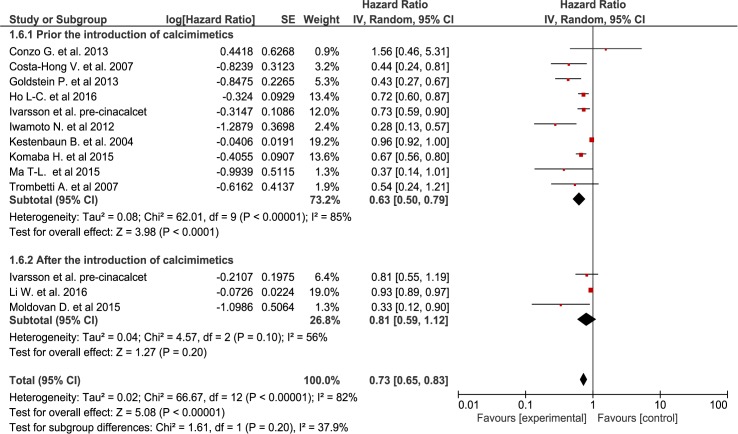
Giving the significant heterogeneity, subgroup analyses were conducted to explore the effect on mortality for different PTH mean values. Thus, after excluding from the analysis studies with lower PTH (n = 3) or non reported baseline PTH (n = 6), we obtained significantly lower heterogeneicity (Chi2 = 6.01, I2 = 17%) while maintaining the same significant association with decreased mortality for the sPTX groups (RR = 0.50[95% CI, 0.38 to 0.67))(Fig 5) although the subgroup differences weren’t statiscally significant. Finally, even though no data regarding the actual use of calcimimetics were reported in the included studies, we assessed the impact of cinacalcet approval and introduction on the market. Thus, knowing that the prescription of calcimimetics was increasingly being part of standard medical care of SHPT from the mid 2000s onwards, we performed a subgroup analysis using as a cutoff the date of calcimimetics introduction in clinical practice for different regions. The results were statistically significant only for the pre-calcimimetic era (RR = 0.63 [95% CI, 0.50 to 0.79]), while in the studies performed in the post-calcimimetics era the advantage of sPTX was smaller and lost statistical significance (RR = 0.81 [95% CI, 0.59 to 1.12])(Fig 6). Once again, the test for subgroup differences did not reached statistical significance.
Fig 5. Subgroup analysis for low and high PTH value at baseline.
Fig 6. Subgroup analysis according to the moment of calcimimetics introduction.
Finally we investigated short-term (30 days–peri-operative) mortality which was described in 2 studies only [23, 24]. Although there were more events reported in the parathyroidectomy group, this difference was not statistically significant (RR 1.43 [95% CI, 0.45 to 4.55]) (Fig 7). In one study, the postoperative deaths among PTX patients were mainly cardiovascular (49.3%) and infectious (18.3%), while in the other study, the main causes of short-term mortality were also dominated by myocardial infarction and infections. Regrettably, no data relating to other specific postoperative complications were reported in the included studies.
Fig 7. The effect of parathyroidectomy on short-term (30-days) mortality.
Discussion
Evidence derived from 15 observational studies including almost 25,000 patients, suggest that sPTX significantly decreased all-cause mortality in ESKD patients with secondary hyperparathyroidism by almost 30 percent (Fig 2). sPTX had also a positive effect on cardiovascular mortality—a 40 percent reduction in 6 observational studies that included almost 10,000 patients (Fig 3). This positive impact of sPTX compared to standard CKD-MBD management was irrespectively of PTH concentration subgroup at the time of surgery (Fig 5) and was not different in studies conducted after the start of the calcimimetic period in clinical practice.
However, no randomized controlled comparing parathyroid surgery with medical therapy for the treatment of SHPT was found, the final analysis comprising only observational studies with their inherent risk of bias. Heterogeneity was considerable for all-cause mortality and this variation between sample estimates may occur for a variety of reasons, including many study design characteristics, different adjustments for confounding, publication date and real-life populations differences across studies.
sPTX was often regarded by pioneer nephrologists as a “last but necessary resort” option for SHPT but one that would very likely be necessary for many patients surviving on dialysis for more than a few years, without a successful renal transplant. Several observational studies indicate that severe hyperparathyroidism may be associated with increased mortality in this population, presumably via a wide range of cardiovascular, metabolic, hematologic, and immunologic abnormalities induced by high concentrations of the uremic toxin PTH [25–28]. With the advent of an orally-active calcimimetic—cinacalcet—there was an additional medical option, besides active vitamin D and surgical interventions to ameliorate progressive SHPT [29]. Both patients and nephrologists in the “calcimimetic dialysis era” would regard the surgical subtotal extirpation of parathyroid tissue only as an acceptable option for severe, progressive, symptomatic and medically non-responsive secondary hyperparathyroidism.
However, there has never been an RCT comparing medical versus surgically-induced reduction in parathyroid activity in dialysis patients. The publication of the EVOLVE study in 2012 was followed in 2015 by a meta-analysis of the medical benefits and risks of using calcimimetics in SHPT[30]–there was no impact on patient survival or outcomes using cinacalcet in largely asymptomatic, biochemically mild-to-moderate, SHPT[31]. There was no similar meta-analysis assessing the impact of sPTX on hard end-points.
Observational studies, with their obvious risk of bias do not unequivocally prove a clinical benefit from SHPT, but nevertheless may suggest a positive outcome. All reports included in our meta-analysed were cohort studies and national databases/ registries. While acknowledging the important limitations of the current analysis, plausibility of a true beneficial effect on mortality of sPTX comes from theoretical and experimental arguments as well, for instance due to reduction in CV disease following better blood pressure control, and decreased hyperphosphataemia [29, 32] [33]. Other potential benefits of parathyroidectomy include: (1) improvements in mineral bone density and reduced risk of pathological fracture–indeed, several single-centre case series have reported increased bone mineral density after parathyroidectomy[34–36]; improvements in erythropoietin-resistant anaemia in patients with marked hyperparathyroidism [37–39]; and (3) improvements in nutritional status and humoral and cellular immunity [14, 40].
Other potential benefits beside “biochemical” improvement might be increased patients QoL, related to the improvement of pruritus, joint and bone pain, or muscle weakness. Although these effect were described in some case series, very regrettably none of the studies included in this meta-analysis specifically and reliably reported important changes in clinical symptoms. A recently published systematic review reported improved QoL in patients treated with sPTX for ESKD-related HPT, whereas cinacalcet did not [41]. However, the difference of impact between sPTx and cinacalcet on QoL has not been compared directly in head-to-head studies.
The immediate and obvious need now is for a well-conceived, well-conducted, independent RCT comparing sPTX with non-surgical therapy for SHPT associated with CKD, ideally over a 5 years time horizon, and featuring many different outcome strata, from mortality, major morbidity, to mental and physical health, QoL, and pharmaco-economics. To the best of our knowledge there is only one published RCT compairing cinacalcet with parathyroidectomy, which included 30 kidney transplanted patients with tertiary hyperparathyroidism and less severe CKD (eGFR>30 ml/min per 1.73 m2÷) [42]. At the end of the follow-up period (12 month), surgery induced greater reduction of iPTH and was associated with a significant increase in femoral neck bone mineral density; vascular calcification remained unchanged in both groups. Another randomized study comparing ultrasonic ablation for the treatment of SHPT with active vitamin D has been completed (ClinicalTrials.gov Identifier: NCT01640184), but these results are not yet published. Currently there is an ongoing RCT comparing cinacalcet with parathyroidectomy in peritoneal dialysis patients and is estimated to be completed by the end of 2017 (ClinicalTrials.gov Identifier: NCT01447368). These studies may help to reduce uncertainty in this area.
Despite an increasing PTH level in CKD patients over the last 15 years, recent data from DOPPS show a decreased rates of parathyroidectomy in all regions[7]. The rise in prescription rates for medications such as cinacalcet and vitamin D analogs, along with higher PTH targets and specifying indications for parathyroid surgery in recent nephrology guidelines [29], have most likely contributed to the decline in sPTX rates. Whether this is an overall benefit to patients remains unclear. Conversely, in Japan there was an increased trend for parathyroidectomy over time [43], probably due to the lower target range for intact PTH in Japanese guidelines than in guidelines used in other countries or to the fact that calcimimetics were available in Japan many years after their approval in Europe or in US.
This meta-analysis has several limitations. The most important of these is the observational design of the included studies with variable duration of follow-up, different indication for sPTX in different areas around the globe, and the variable matching criteria for the control group. The latter received “standard” medical therapy, consisting mostly of vitamin D compounds and/or phosphate binders [16–18, 20–22]; regrettably, some studies did not report any data regarding the treatment of the control group [23, 24, 44–48]. No study mentioned any data about calcimimetic treatment in the included patients; this though is most likely to be related to the fact that at the time of enrolment in these studies, cinacalcet was not yet available in many countries. This meta-analysis was also limited by the methodological quality of studies included; while there was some degree of heterogeneity between studies included in this meta-analysis, most of it could be explained by differences in the methodological quality of the trials. It was not possible to assess thermal, alcohol, or ultrasonographic ablation of parathyroid glands, or, the different surgical options (total vs. subtotal; autoimplantation) in this analysis. Renal transplantation was considered criteria of exclusion in all the included individual studies with one exception where sPTX was not associated with improved survival in patients with renal allograft [23]. This analysis lacked a detailed patient-level analysis of the clinical impacts of the surgery itself. There would most likely in real clinical conditions be some offset in overall benefit of the parathyroidectomy intervention as was showed in a recently analysis of the USRDS database where parathyroidectomy was associated with significant morbidity in the 30 days after hospital discharge and in the year after the procedure. However, due to the study design with the lack of a control group, the authors were not able directly to assess the impact on survival of sPTX [49].
Relatively the same survival benefit was also reported by Chen L. et al. in a recent metanalysis[50]. However, in contrast with this review, our metanalysis comprises 3 more studies with almost 5000 patients more, which may allow the decrease of confidence interval and give more strength to the overall analysis. Furthermore, we also did a analysis of the short-term mortality, and several subgroup analysis in order to explore heterogeneicity.
sPTX remains even in the modern nephrology era a valid and viable therapeutic intervention especially for long-term dialysis patients[7, 8]. Taken together, the results of this new meta-analysis suggests a beneficial effect of sPTX on all-cause and cardiovascular mortality, and maybe more importantly, challenges current practice that positions sPTX as last resort option when medical therapy fails. This meta-analysis (the largest to date) has attempted to gather all available and analyzable data on the impact of parathyroidectomy on hard endpoints–both short and long term all cause and cardiovascular mortality for renal patients with secondary hyperparathyroidism. The case for a properly conducted trial comparing parathyroid surgery with the combination of calcimimetics and vitamin D is now very strong, and the only way to settle this issue.
Supporting information
(DOCX)
(DOCX)
(EPS)
(EPS)
Acknowledgments
A special thank to Angela Webster, Associate Professor Clinical Epidemiology, Nephrologist from the University of Sydney for her valuable commnents and imput in the methodological part of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Stanbury SW, Lumb GA, Nicholson WF. Elective subtotal parathyroidectomy for renal hyperparathyroidism. Lancet (London, England). 1960;1(7128):793–9. Epub 1960/04/09. . [DOI] [PubMed] [Google Scholar]
- 2.Ogg CS. Total parathyroidectomy in treatment of secondary (renal) hyperparathyroidism. British medical journal. 1967;4(5575):331–4. Epub 1967/11/11. ; PubMed Central PMCID: PMCPmc1748802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley RN, Li S, Liu J, Gilbertson DT, Chen SC, Collins AJ. The fall and rise of parathyroidectomy in U.S. hemodialysis patients, 1992 to 2002. Journal of the American Society of Nephrology: JASN. 2005;16(1):210–8. Epub 2004/11/26. doi: 10.1681/ASN.2004020138 . [DOI] [PubMed] [Google Scholar]
- 4.Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C. Left Ventricular Hypertrophy in Chronic Kidney Disease Patients: From Pathophysiology to Treatment. Cardiorenal medicine. 2015;5(4):254–66. Epub 2015/12/10. doi: 10.1159/000435838 ; PubMed Central PMCID: PMCPmc4662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldsmith DJ, Massy ZA, Brandenburg V. The uses and abuses of Vitamin D compounds in chronic kidney disease-mineral bone disease (CKD-MBD). Semin Nephrol. 2014;34(6):660–8. Epub 2014/12/17. doi: 10.1016/j.semnephrol.2014.10.002 . [DOI] [PubMed] [Google Scholar]
- 6.Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney international. 2005;68(4):1793–800. Epub 2005/09/17. doi: 10.1111/j.1523-1755.2005.00596.x . [DOI] [PubMed] [Google Scholar]
- 7.Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(1):98–109. Epub 2014/12/18. doi: 10.2215/CJN.12941213 ; PubMed Central PMCID: PMCPmc4284424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SM, Long J, Montez-Rath ME, Leonard MB, Norton JA, Chertow GM. Rates and Outcomes of Parathyroidectomy for Secondary Hyperparathyroidism in the United States. Clin J Am Soc Nephrol. 2016. Epub 2016/06/09. doi: 10.2215/cjn.10370915 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldsmith D, Covic A, Vervloet M, Cozzolino M, Nistor I. Should patients with CKD stage 5D and biochemical evidence of secondary hyperparathyroidism be prescribed calcimimetic therapy? An ERA-EDTA position statement. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2015;30(5):698–700. Epub 2015/05/01. doi: 10.1093/ndt/gfv050 . [DOI] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 11.Julian PT Higgins SG. Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011.
- 12.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials. 2007;28(2):105–14. Epub 2006/06/30. doi: 10.1016/j.cct.2006.04.004 . [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. Epub 2003/09/06. doi: 10.1136/bmj.327.7414.557 ; PubMed Central PMCID: PMCPmc192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasunaga C, Nakamoto M, Matsuo K, Nishihara G, Yoshida T, Goya T. Effects of a parathyroidectomy on the immune system and nutritional condition in chronic dialysis patients with secondary hyperparathyroidism. American journal of surgery. 1999;178(4):332–6. Epub 1999/12/10. . [DOI] [PubMed] [Google Scholar]
- 15.Conzo G, Perna AF, Savica V, Palazzo A, Della Pietra C, Ingrosso D, et al. Impact of parathyroidectomy on cardiovascular outcomes and survival in chronic hemodialysis patients with secondary hyperparathyroidism. A retrospective study of 50 cases prior to the calcimimetics era. BMC surgery. 2013;13 Suppl 2:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwamoto N, Sato N, Nishida M, Hashimoto T, Kobayashi H, Yamasaki S, et al. Total parathyroidectomy improves survival of hemodialysis patients with secondary hyperparathyroidism. Journal of nephrology. 2012;25(5):755–63. Epub 2011/12/03. doi: 10.5301/jn.5000056 . [DOI] [PubMed] [Google Scholar]
- 17.Komaba H, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Fukagawa M. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney international. 2015;88(2):350–9. Epub 2015/03/19. doi: 10.1038/ki.2015.72 . [DOI] [PubMed] [Google Scholar]
- 18.Costa-Hong V, Jorgetti V, Gowdak LH, Moyses RM, Krieger EM, De Lima JJ. Parathyroidectomy reduces cardiovascular events and mortality in renal hyperparathyroidism. Surgery. 2007;142(5):699–703. doi: 10.1016/j.surg.2007.06.015 . [DOI] [PubMed] [Google Scholar]
- 19.Lin HC, Chen CL, Lin HS, Chou KJ, Fang HC, Liu SI, et al. Parathyroidectomy improves cardiovascular outcome in nondiabetic dialysis patients with secondary hyperparathyroidism. Clinical endocrinology. 2014;80(4):508–15. Epub 2013/10/10. doi: 10.1111/cen.12333 . [DOI] [PubMed] [Google Scholar]
- 20.Conzo G, Perna AF, Savica V, Palazzo A, Della Pietra C, Ingrosso D, et al. Impact of parathyroidectomy on cardiovascular outcomes and survival in chronic hemodialysis patients with secondary hyperparathyroidism. A retrospective study of 50 cases prior to the calcimimetics era. BMC surgery. 2013;13 Suppl 2:S4 Epub 2013/12/07. doi: 10.1186/1471-2482-13-s2-s4 ; PubMed Central PMCID: PMCPmc3851167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldenstein PT, Elias RM, Pires de Freitas do Carmo L, Coelho FO, Magalhaes LP, Antunes GL, et al. Parathyroidectomy improves survival in patients with severe hyperparathyroidism: a comparative study. PloS one. 2013;8(8):e68870 Epub 2013/08/14. doi: 10.1371/journal.pone.0068870 ; PubMed Central PMCID: PMCPmc3734286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moldovan D, Racasan S, Kacso IM, Rusu C, Potra A, Bondor C, et al. Survival after parathyroidectomy in chronic hemodialysis patients with severe secondary hyperparathyroidism. International urology and nephrology. 2015;47(11):1871–7. Epub 2015/09/18. doi: 10.1007/s11255-015-1106-x . [DOI] [PubMed] [Google Scholar]
- 23.Ivarsson KM, Akaberi S, Isaksson E, Reihner E, Rylance R, Prutz KG, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2015;30(12):2027–33. Epub 2015/09/17. doi: 10.1093/ndt/gfv334 ; PubMed Central PMCID: PMCPmc4832998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kestenbaum B, Andress DL, Schwartz SM, Gillen DL, Seliger SL, Jadav PR, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney international. 2004;66(5):2010–6. Epub 2004/10/22. doi: 10.1111/j.1523-1755.2004.00972.x . [DOI] [PubMed] [Google Scholar]
- 25.Massry SG, Smogorzewski M. Mechanisms through which parathyroid hormone mediates its deleterious effects on organ function in uremia. Seminars in nephrology. 1994;14(3):219–31. Epub 1994/05/01. . [PubMed] [Google Scholar]
- 26.Chiu KC, Chuang LM, Lee NP, Ryu JM, McGullam JL, Tsai GP, et al. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism: clinical and experimental. 2000;49(11):1501–5. Epub 2000/11/25. doi: 10.1053/meta.2000.17708 . [DOI] [PubMed] [Google Scholar]
- 27.Massry SG, Alexiewicz JM, Gaciong Z, Klinger M. Secondary hyperparathyroidism and the immune system in chronic renal failure. Seminars in nephrology. 1991;11(2):186–201. Epub 1991/03/01. . [PubMed] [Google Scholar]
- 28.Rao DS, Shih MS, Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. The New England journal of medicine. 1993;328(3):171–5. Epub 1993/01/21. doi: 10.1056/NEJM199301213280304 . [DOI] [PubMed] [Google Scholar]
- 29.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney international Supplement. 2009;(113):S1–130. Epub 2009/08/01. doi: 10.1038/ki.2009.188 . [DOI] [PubMed] [Google Scholar]
- 30.Ballinger AE, Palmer SC, Nistor I, Craig JC, Strippoli GF. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. The Cochrane database of systematic reviews. 2014;12:Cd006254 Epub 2014/12/10. doi: 10.1002/14651858.CD006254.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, Goodman WG, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. The New England journal of medicine. 2012;367(26):2482–94. Epub 2012/11/06. doi: 10.1056/NEJMoa1205624 . [DOI] [PubMed] [Google Scholar]
- 32.Evenepoel P, Rodriguez M, Ketteler M. Laboratory abnormalities in CKD-MBD: markers, predictors, or mediators of disease? Seminars in nephrology. 2014;34(2):151–63. Epub 2014/05/02. doi: 10.1016/j.semnephrol.2014.02.007 . [DOI] [PubMed] [Google Scholar]
- 33.Covic A, Goldsmith DJ, Georgescu G, Venning MC, Ackrill P. Echocardiographic findings in long-term, long-hour hemodialysis patients. Clinical nephrology. 1996;45(2):104–10. Epub 1996/02/01. . [PubMed] [Google Scholar]
- 34.Abdelhadi M, Nordenstrom J. Bone mineral recovery after parathyroidectomy in patients with primary and renal hyperparathyroidism. The Journal of clinical endocrinology and metabolism. 1998;83(11):3845–51. Epub 1998/11/14. doi: 10.1210/jcem.83.11.5249 . [DOI] [PubMed] [Google Scholar]
- 35.Chou FF, Chen JB, Lee CH, Chen SH, Sheen-Chen SM. Parathyroidectomy can improve bone mineral density in patients with symptomatic secondary hyperparathyroidism. Archives of surgery (Chicago, Ill: 1960). 2001;136(9):1064–8. Epub 2001/09/28. . [DOI] [PubMed] [Google Scholar]
- 36.Yano S, Sugimoto T, Tsukamoto T, Yamaguchi T, Hattori T, Sekita KI, et al. Effect of parathyroidectomy on bone mineral density in hemodialysis patients with secondary hyperparathyroidism: possible usefulness of preoperative determination of parathyroid hormone level for prediction of bone regain. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2003;35(4):259–64. Epub 2003/06/05. doi: 10.1055/s-2003-39483 . [DOI] [PubMed] [Google Scholar]
- 37.Rault R, Magnone M. The effect of parathyroidectomy on hematocrit and erythropoietin dose in patients on hemodialysis. ASAIO journal (American Society for Artificial Internal Organs: 1992). 1996;42(5):M901–3. Epub 1996/09/01. . [DOI] [PubMed] [Google Scholar]
- 38.Jemcov TK, Petakov M, Bogdanovic A, Djukanovic L, Lezaic VD. Parathyroidectomy and improving anemia. Archives of surgery (Chicago, Ill: 1960). 2008;143(1):97–8. Epub 2008/01/23. doi: 10.1001/archsurg.2007.26 . [DOI] [PubMed] [Google Scholar]
- 39.Chow TL, Chan TT, Ho YW, Lam SH. Improvement of anemia after parathyroidectomy in Chinese patients with renal failure undergoing long-term dialysis. Archives of surgery (Chicago, Ill: 1960). 2007;142(7):644–8. Epub 2007/07/20. doi: 10.1001/archsurg.142.7.644 . [DOI] [PubMed] [Google Scholar]
- 40.Tzanno-Martins C, Futata E, Jorgetti V, Duarte AJ. Restoration of impaired T-cell proliferation after parathyroidectomy in hemodialysis patients. Nephron. 2000;84(3):224–7. . [DOI] [PubMed] [Google Scholar]
- 41.van der Plas WY, Dulfer RR, Engelsman AF, Vogt L, de Borst MH, van Ginhoven TM, et al. Effect of parathyroidectomy and cinacalcet on quality of life in patients with end-stage renal disease-related hyperparathyroidism: a systematic review. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2017. Epub 2017/04/13. doi: 10.1093/ndt/gfx044 . [DOI] [PubMed] [Google Scholar]
- 42.Cruzado JM, Moreno P, Torregrosa JV, Taco O, Mast R, Gomez-Vaquero C, et al. A Randomized Study Comparing Parathyroidectomy with Cinacalcet for Treating Hypercalcemia in Kidney Allograft Recipients with Hyperparathyroidism. Journal of the American Society of Nephrology: JASN. 2016;27(8):2487–94. Epub 2015/12/10. doi: 10.1681/ASN.2015060622 ; PubMed Central PMCID: PMCPmc4978046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tominaga Y. Current status of parathyroidectomy for secondary hyperparathyroidism in Japan. NDT plus. 2008;1(Suppl 3):iii35–iii8. Epub 2008/08/01. doi: 10.1093/ndtplus/sfn085 ; PubMed Central PMCID: PMCPmc4421124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trombetti A, Stoermann C, Robert JH, Herrmann FR, Pennisi P, Martin PY, et al. Survival after parathyroidectomy in patients with end-stage renal disease and severe hyperparathyroidism. World journal of surgery. 2007;31(5):1014–21. Epub 2007/04/11. doi: 10.1007/s00268-006-0693-1 . [DOI] [PubMed] [Google Scholar]
- 45.Ho LC, Hung SY, Wang HH, Kuo TH, Chang YT, Tseng CC, et al. Parathyroidectomy Associates with Reduced Mortality in Taiwanese Dialysis Patients with Hyperparathyroidism: Evidence for the Controversy of Current Guidelines. Sci Rep. 2016;6:19150 Epub 2016/01/14. doi: 10.1038/srep19150 ; PubMed Central PMCID: PMCPmc4725823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Zhang M, Du S, Yu Y, Liu J, Zhang L, et al. Impact of parathyroidectomy on survival among haemodialysis patients: A prospective cohort study. Nephrology (Carlton, Vic). 2016;21(2):133–8. Epub 2015/07/23. doi: 10.1111/nep.12564 . [DOI] [PubMed] [Google Scholar]
- 47.Dussol B, Morand P, Martinat C, Lombard E, Portugal H, Brunet P, et al. Influence of parathyroidectomy on mortality in hemodialysis patients: a prospective observational study. Renal failure. 2007;29(5):579–86. Epub 2007/07/27. doi: 10.1080/08860220701392447 . [DOI] [PubMed] [Google Scholar]
- 48.Ma TL, Hung PH, Jong IC, Hiao CY, Hsu YH, Chiang PC, et al. Parathyroidectomy Is Associated with Reduced Mortality in Hemodialysis Patients with Secondary Hyperparathyroidism. BioMed research international. 2015;2015:639587 Epub 2015/06/13. doi: 10.1155/2015/639587 ; PubMed Central PMCID: PMCPmc4433652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishani A, Liu J, Wetmore JB, Lowe KA, Do T, Bradbury BD, et al. Clinical outcomes after parathyroidectomy in a nationwide cohort of patients on hemodialysis. Clin J Am Soc Nephrol. 2015;10(1):90–7. Epub 2014/12/18. doi: 10.2215/CJN.03520414 ; PubMed Central PMCID: PMCPmc4284409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farrington K, Covic A, Nistor I, Aucella F, Clyne N, De Vos L, et al. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR<45 mL/min/1.73 m2): a summary document from the European Renal Best Practice Group. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2017;32(1):9–16. Epub 2017/04/10. doi: 10.1093/ndt/gfw411 . [DOI] [PubMed] [Google Scholar]
- 51.Sharma J, Raggi P, Kutner N, Bailey J, Zhang R, Huang Y, et al. Improved long-term survival of dialysis patients after near-total parathyroidectomy. Journal of the American College of Surgeons. 2012;214(4):400–7; discussion 7–8. Epub 2012/04/03. doi: 10.1016/j.jamcollsurg.2011.12.046 ; PubMed Central PMCID: PMCPmc4349349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(EPS)
(EPS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.