Abstract
BACKGROUND
In extensive-stage small cell lung cancer (SCLC), the combination of pemetrexed plus carboplatin has shown activity and appeared to be well-tolerated. We conducted a trial to confirm the efficacy and to assess the tolerability of this chemotherapy combination.
METHODS
Patients with untreated extensive-stage SCLC were enrolled in this phase 2 open-labeled study. They receive pemetrexed 500 mg/m2 and carboplatin (area under the curve of 5) every 21 days for a maximum 6 cycles. The primary endpoint for this trial was the confirmed response rate and the accrual goal was 70 patients.
RESULTS
Forty-six eligible patients (29 aged <70 years, 17 aged >70 years) were accrued to this study. The efficacy outcomes were similar between the 2 age groups. Overall, the confirmed response rate was 35% (16 of 46; 95% confidence interval [CI], 21%–50%), where all 16 were partial responses. On the basis of these results, we had strong evidence that the study would not meet the preset efficacy criteria and was, therefore, closed before full accrual. The median duration of response was 4.4 months (95% CI, 2.9–5.2). Median overall survival for patients aged <70 years and aged ≥70 years was 9.2 months (95% CI, 5.4–11.6) and 10.8 months (95% CI, 2.2–14.3), respectively. Grade 3 or higher toxicity rates were similar between the younger and older patients. Grade 3/4 and grade 4 hematological toxicities were observed in 46% and 26% of patients, respectively.
CONCLUSIONS
Although well-tolerated, the combination of pemetrexed and carboplatin is not as effective as standard therapy in patients with untreated extensive-stage SCLC.
Keywords: lung cancer, small cell, pemetrexed, carboplatin, extensive stage
Small cell lung cancer (SCLC) accounts for approximately 15% of all types of lung cancers.1 According to the Veterans’ Administration Lung Cancer Group 2-stage classification system, more than two thirds of patients with SCLC are diagnosed with extensive disease also known as extensive stage, defined as a tumor not confined to one hemithorax or that has malignant pleural effusion.2 In patients with extensive-stage SCLC, median survival ranges from 7 months to 12 months, with <5% of patients living beyond 2 years and a 5-year survival rate of 1% to 2%.3,4 Although therapy has significantly improved outcomes of patients with SCLC, long-term survival remains poor.
This tumor is very responsive to initial chemotherapy, with major responses in 70% to 90% of cases. Combination chemotherapy is capable of inducing a rapid tumor regression and improved survival, but relapse and death from chemo-resistant disease occurs in 80% to 90% of patients. In extensive-stage SCLC, treatment mainly comprises platinum-based chemotherapy, which has shown a significant survival advantage compared with patients who did not receive a platinum agent.5 Cisplatin/etoposide remains the first-line therapy for this disease, although carboplatin/etoposide is an acceptable alternative, with equivalent efficacy and less nonhematologic toxicity for patients intolerant of cisplatin.6 Based on the current treatment regimens, overall response rates of 50% to 80% and complete response rates of 0% to 30% have been achieved in patients with extensive-stage SCLC.5,7 A randomized phase 3 study in Japanese patients showed improved survival with cisplatin/irinotecan compared with cisplatin/etoposide. Median survival times were 12.8 months and 9.4 months, and 2-year survival rates were 19.5% and 5.2%, respectively.8 However, 2 US studies could not confirm the results.9,10 Other chemotherapy combinations such as oral topotecan/cisplatin have been tested and found to be inferior to cisplatin/etoposide.11 Another randomized phase 3 study evaluated the efficacy of carboplatin/irinotecan and carboplatin/oral etoposide. Median survival times were 8.5 months and 7.1 months, and 1-year survival rates were 35% and 24%, respectively.12 With the lack of substantial improvement in treatment results, there is a great need for new, active agents against SCLC.
Pemetrexed is a multitargeted antifolate agent that has been approved for treatment of malignant mesothelioma and nonsmall cell lung cancer (NSCLC). Its mechanism of action is the inhibition of thymidylate synthase (TS), dihydrofolate reductase and glycinamide ribonucleotide formyl transferase, enzymes, which are involved in pyrimidine and purine synthesis.13–16 In vitro, pemetrexed and platinum compounds have additive killing effect on human SCLC cell line.17 Socinski et al performed a small and limited institution phase 2 trial with pemetrexed and carboplatin in patients with extensive-stage SCLC and showed a median survival time of 10.4 months with a 1-year survivorship of 39% and a response rate of 39.5% (95% confidence interval [CI], 24.0–56.6).18 The chemotherapy combination was well tolerated with grade 3/4 neutropenia and thrombocytopenia reported at 20% and 22.9%, respectively. The goal of our study was to establish the efficacy of this regimen in a cooperative group setting and to evaluate the tolerability of this combination in all patients, especially the elderly. Often, elderly patients may experience more toxicity with treatment because of pre-existing illnesses, decreased clearance of chemotherapy, and limited marrow reserves.19
MATERIALS AND METHODS
Patients
Adult patients with previously untreated histologically proven extensive-stage SCLC and a life expectancy of ≥12 weeks were eligible. They had to be able to take folic acid, vitamin B12 supplementation or dexamethasone, and permanently discontinue aspirin dose ≥1.3 g/day for ≥10 days before and after pemetrexed disodium treatment. The disease was required to be measurable with at least 1 lesion, whose longest diameter can be accurately measured as ≥2.0 cm with conventional techniques or as ≥1.0 cm with spiral computer tomography (CT). An Eastern Cooperative Oncology Group (ECOG) performance status of0 to 2 and adequate hematologic, renal, and hepatic function were required (hemoglobin, ≥9.0 g/dL; absolute neutrophil count, ≥1500/μL; platelet count, ≥100 000/μL; creatinine clearance, ≥45 mL/min using the Cockcroft-Gault formula20; total bilirubin, ≤1.5 × upper limits of normal [ULN] or direct bilirubin ≤ULN; and transaminases, ≤ 3 × ULN or ≤ 5 × ULN if liver involvement). Patients with cytologically proven malignant pleural effusions were eligible but clinically significant effusions had to be drained before treatment (eg, symptomatic pleural effusion). Palliative radiation therapy except to the chest was allowed with patients enrolled and chemotherapy starting 1 day after completion of radiotherapy.
Patients with central nervous system (CNS) metastases treated with whole brain radiation (WBRT) could be enrolled after completion of WBRT, with chemotherapy beginning as early as the next day after completion of WBRT. However, patients with symptomatic, untreated, or uncontrolled CNS metastases or seizure disorder were excluded. The study also excluded patients with clinically significant infection, active second primary malignancy, concurrent chemotherapy, immunotherapy, hormonal therapy or radiotherapy, uncontrolled hypertension with medications, poorly controlled diabetes mellitus, extensive and symptomatic interstitial fibrosis of the lung, use of St. John’s Wort, and use of medications known to be strong inducers or inhibitors CYP3A4 metabolism. Other contraindications included pregnancy, major surgery, open biopsy, or significant traumatic injury ≤4 weeks before registration, minor surgery ≤2 weeks before registration, or any of the following concurrent and/or uncontrolled medical conditions: hypertension, angina pectoris, history of congestive heart failure ≤3 months, unless ejection fraction >40%, cardiac arrhythmias, myocardial infarction ≤3 months, poorly controlled diabetes, interstitial pneumonia, or extensive and symptomatic interstitial fibrosis of the lung. The institutional review board of each study site approved the study and informed consent was obtained from each trial participant.
Study Design and Treatment
This study was a phase 2, open-labeled trial. Patients had to take folic acid at 350–1000 μg by mouth daily for at least 5 of the 7 days immediately preceding the first dose of pemetrexed disodium until 3 weeks after the last dose of pemetrexed disodium. Vitamin B12 intramuscular injection at 1000 μg was administered on the same day as the start of folic acid or 7 days before the first dose of pemetrexed disodium if the patient was on an adequate vitamin supplement. The injections were continued every 9 weeks until 3 weeks after the last dose of pemetrexed disodium. Oral dexamethasone at 4 mg twice a day was started the day before, day of, and day after all doses of pemetrexed disodium every 21 days. On Day 1 of each cycle, pemetrexed disodium, 500 mg/m2 in 100 mL of normal saline was infused intravenously over 10 minutes via an automatic dispensing pump followed by carboplatin, area under the concentration curve (AUC) 5 (AUC = 5) which was administered in 250 mL of 0.9% saline or 5% dextrose intravenously over 30 minutes. The dose of carboplatin (AUC = 5) was chosen because of its use in prior studies with a similar patient population.18,21 Cycles were repeated every 21 days for a maximum of 6 cycles.
Response Assessment and Toxicity
Tumor assessments were performed every 6 weeks with radiographic imaging using computer tomography (CT) scan, magnetic resonance imaging (MRI), or chest x-ray. Disease response was determined according to the RECIST criteria.22 The cytological confirmation of the neoplastic origin of any effusion that appeared or worsened during treatment when the measurable tumor had met criteria for response or stable disease was mandatory to differentiate between response or stable disease (an effusion may be a side effect of the treatment) and progressive disease. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (CTCAE) v.3.0 grading system and was defined as any adverse event that was possibly, probably, or definitely related to the study treatment.
Statistical Methods
The primary endpoint for this trial was the confirmed response rate, calculated as the number of evaluable patients with a confirmed response (ie, a partial response, [PR] or complete response, [CR] that held for at least 4 weeks), divided by the total number of evaluable patients. For patients younger than the age of 70, a 1-stage design was used to test whether there was sufficient evidence to determine that the confirmed response rate was at least 60% (ie, clinically promising) versus at most 40% (ie, clinically inactive). This study had 94% power to detect a confirmed response rate of 60%, with a 0.11 level of significance. For this age cohort, we planned to accrue a total of 46 evaluable patients. If at least 23 of all 46 evaluable patients had a confirmed response, this would be considered adequate evidence of promising activity and would warrant further testing of this regimen in subsequent studies. A similar 1-stage design was used for patients aged 70 and older. This study had 79% power to detect a confirmed response rate of 60%, with a 0.11 level of significance. For this age cohort, we planned to accrue a total of 24 evaluable patients. If at least 13 of all 24 evaluable patients had a confirmed response, this would be considered adequate evidence of promising activity and would warrant further testing of this regimen in subsequent studies.
Secondary endpoints included toxicities, duration of response, time-to-disease progression, and overall survival. These endpoints were analyzed between the age groups (aged <70 years vs aged ≥70 years), where the Fisher exact test was used to compare categorical data (ie, response, toxicity, gender, etc.) and the Wilcoxon rank sum test was used to compare continuous data (ie, age). The commonly occurring grade 3 + toxicities (ie, adverse events at least possibly related to the study treatment) were reported. Kaplan-Meier methodology was used to describe the distribution of time-to-disease progression, survival, and duration of response.23 The log-rank test was used to compare the time-to-disease progression and overall survival by age cohort. Statistical tests were 2-sided, with P < .05 considered statistically significant. P-values were not adjusted for multiple comparisons. Statistical analyses were performed using SAS software (SAS institute, Cary, NC).
RESULTS
Patient Characteristics
Between April 5, 2006 and September 14, 2007, 50 patients were enrolled in this study. Two patients were deemed ineligible (1 had received prior chemotherapy, and the other had a performance status of 3), and 2 other patients canceled before receiving any treatment, leaving a total of 46 evaluable patients (from 24 treatment locations) who were included in this analysis. The patients were analyzed in 2 age cohorts, <70 years and ≥70 years, with 29 and 17 patients, respectively. Patient characteristics were similar between the 2 groups (see Table 1) and overall median age was 66 years. In the younger cohort, median age was 62 years (range, 48–69 years), 59% were men, 97% had a performance status (PS) of 0 or 1, and 76% had at least 2 metastatic sites at baseline. In the older cohort, median age was 75 years (range, 70–80 years), 53% were men, 76% had a PS of 0 or 1, and 82% had at least 2 metastatic sites at baseline.
Table 1.
Patient Baseline Characteristics
| Characteristics | <70 Group (N = 29) |
≥70 Group (N = 17) |
Pa |
|---|---|---|---|
| Age, y | <.0001b | ||
| Mean [standard deviation] | 60.7 [5.76] | 74.2 [3.07] | |
| Median | 62.0 | 75.0 | |
| Range | (48.0–69.0) | (70.0–80.0) | |
| Sex | .7647 | ||
| Women | 12 (41.4%) | 8 (47.1%) | |
| Men | 17 (58.6%) | 9 (52.9%) | |
| Performance score | .0929 | ||
| 0 | 12 (41.4%) | 7 (41.2%) | |
| 1 | 16 (55.2%) | 6 (35.3%) | |
| 2 | 1 (3.4%) | 4 (23.5%) | |
| Race | .6077 | ||
| White | 28 (96.6%) | 16 (94.1%) | |
| American Indian or Alaska Native | 1 (3.4%) | 0 (0%) | |
| Not reported | 0 (0%) | 1 (5.9%) | |
| Prior brain metastasis | .1415 | ||
| Yes | 5 (17.2%) | 0 (0%) | |
| No | 24 (82.8%) | 17 (100%) | |
| No. of metastatic sites | .7227 | ||
| 0 or 1 Met | 7 (24.1%) | 3 (17.6%) | |
| ≥2 Mets | 22 (75.9%) | 14 (82.4%) | |
| Prior palliative radiation | 1.0000 | ||
| Yes | 3 (10.3%) | 1 (5.9%) | |
| No | 26 (89.7%) | 16 (94.1%) | |
| Prior whole-brain radiation | .1415 | ||
| Yes | 5 (17.2%) | 0 (0%) | |
| No | 24 (82.8%) | 17 (100%) |
Fisher exact test.
Wilcoxon rank sum test.
Outcome Measures
All 46 patients were evaluable for efficacy outcomes, which were similar between the 2 age groups (see Table 2, Figs. 1 and 2). Overall, the confirmed response rate was 35% (16 of 46; 95% CI, 21–50) with all partial responses (PR). The median duration of response was 4.4 months (95% CI, 2.9–5.2). On the basis of these results, we had strong evidence that the study would not meet the efficacy criteria and was closed before full accrual.
Table 2.
Response, Time-to-Progression, and Overall Survival Efficacy Outcomes
| Clinical Outcome | Group Aged <70 Years, N=29 | Group Aged ≥70 Years, N=17 |
|---|---|---|
| Best clinical response | ||
| Stable | 12 (41%) | 5 (29%) |
| Progression | 8 (28%) | 5 (29%) |
| Partial response | 9 (31%) | 7 (41%) |
| Complete response | 0 (0%) | 0 (0%) |
| Confirmed response | ||
| Frequency [95% CI]a | 9 (31%) [15–51] | 7 (41%) [18–67] |
| Duration of responseb | ||
| Progression-freeb | 1 (11%) | 0 (0%) |
| Median, mo [95% CI] | 4.7 [2.9–6.0] | 4.4 [2.8–9.0] |
| Time-to-progression | ||
| 6-month [95% CI] | 23% [11–45] | 28% [12–63] |
| Median, mo [95% CI] | 4.2 [2.5–4.5] | 4.2 [1.4–6.]) |
| Progression-free | 3 (10.3%) | 2 (11.7%) |
| Survival (OS) | ||
| 6-month [95% CI] | 54% [39–76] | 70% [51–96] |
| Median, mo [95% CI] | 9.2 [5.4–11.6] | 10.8 [2.2–14.3] |
| Alive | 6 (20.7%) | 3 (17.6%) |
CI indicates confidence interval.
Fisher exact P comparing the confirmed response rate between the 2 age groups was .53.
Only for those patients that had a confirmed response.
Figure 1.

Time-to-progression by age cohort is shown.
Figure 2.
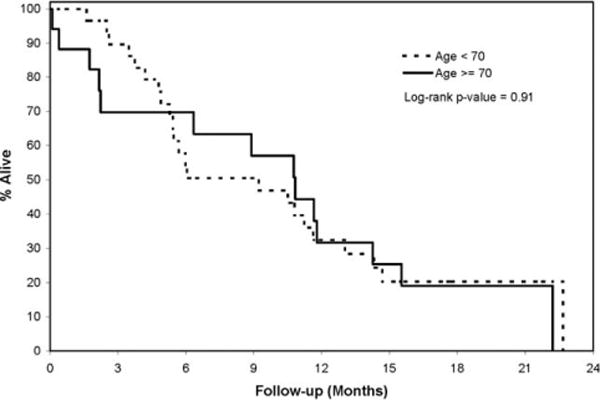
Illustrated is the overall survival by age cohort.
We found 6 (21%) patients who were still alive with a median follow-up of 16.3 months in the younger patient group. Twenty-six patients had progressed, and 23 had died. Median time-to-progression and median overall survival were 4.2 months (95% CI, 2.5–4.5) and 9.2 months (95% CI, 5.4–11.6), respectively. In the older age cohort, 3 (18%) patients were still alive, the median follow-up was 15.7 months, 15 patients had progressed, and 14 had died. Median time-to-progression and median survival was 4.2 months (95% CI, 1.4–6.5) and 10.8 months (95% CI, 2.2–14.3), respectively. Although information on subsequent treatment after the patients had failed front-line therapy were not collected, it is possible that subsequent therapy may have added to the patients’ survival observed in this trial.
Treatment Information
A median of 4 cycles of therapy (range, 1–6 cycles) was administered in the study. Most patients (29 of 46; 63%) discontinued treatment early because of disease progression (19 of 29 [66%] in <70 years age group and 10 of 17 [59%] in the ≥70 years age group). Of the remaining 17 patients, 9 patients completed the study per protocol, 2 patients stopped early because of adverse events, 2 patients refused further treatment, 2 patients died while on study treatment (1 of whom died because of treatment), 1 patient stopped early for financial reasons/insurance issues, and 1 patient stopped early to receive alternative treatment. For patients <70 years, >80% of the treated patients in the first 3 cycles received full-dose pemetrexed (full dose = ≥98% of dose per protocol), and this declined to approximately 60% of the treated patients by Cycles 5 and 6. At least 90% of the treated patients in Cycles 1–3 received full-dose carboplatin, and this decreased to approximately 70% of treated patients for Cycles 4–6. For the older age cohort, at least 90% of the treated patients for each cycle received full-dose pemetrexed, and all of the treated patients for each cycle received full-dose carboplatin.
Toxicities
All 46 eligible patients who received treatment were also evaluable for toxicity analysis. There were no significant differences in grades 3 and 4 toxicities between the 2 age cohorts (see Table 3). Grade 3/4 and grade 4 hematological toxicities were observed in 46% and 26% of patients, respectively. The most commonly occurring grade 3/4 toxicities (see Table 4) included anemia (13%), neutropenia (43%), and thrombocytopenia (24%). Other commonly occurring grade 3/4 nonhematological toxicities included hyperglycemia (7%), fatigue (11%), and anorexia (7%). One patient (aged ≥70 years) died during the study from a grade 5 febrile neutropenia that resulted from bacteremia. No other grade 5 events occurred.
Table 3.
Comparison of Adverse Events (at Least Possibly Related) During Treatment by Age Cohort
| Group Aged <70 Years, N=29 | Group Aged ≥70 Years, N=17 | Pa | |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Grades 3/4/5 overall | .7571 | ||
| No | 11 (37.9) | 8 (47.1) | |
| Yes | 18 (62.1) | 9 (52.9) | |
| Grades 4/5 overall | .4893 | ||
| No | 20 (69) | 14 (82.4) | |
| Yes | 9 (31) | 3 (17.6) | |
| Grades 3/4 hematologic | .7624 | ||
| No | 15 (51.7) | 10 (58.8) | |
| Yes | 14 (48.3) | 7 (41.2) | |
| Grade 4 hematologic | .4893 | ||
| No | 20 (69) | 14 (82.4) | |
| Yes | 9 (31) | 3 (17.6) | |
| Grade 3/4/5 nonhematologic | 1.0000 | ||
| No | 20 (69) | 12 (70.6) | |
| Yes | 9 (31) | 5 (29.4) | |
| Grade 4/5 nonhematologic | .3696 | ||
| No | 29 (100) | 16 (94.1) | |
| Yes | 0 (0) | 1 (5.9) | |
| Grade 5 nonhematologic | .3696 | ||
| No | 29 (100) | 16 (94.1) | |
| Yes | 0 (0) | 1 (5.9) |
Fisher exact P
Table 4.
Grades 3, 4, and 5 Maximal Adverse Events at Least Possibly Related to Treatment
| Age Groups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aged <70 Years Group | Aged ≥70 Years Group | ||||||||||
| Grade | Grade | ||||||||||
| 3 | 4 | 3 | 4 | 5 | |||||||
| Body system | Toxicity | No. | % | No. | % | No. | % | No. | % | No. | % |
| Hematology | Anemia | 4 | 14 | 1 | 3 | 1 | 6 | ||||
| Leukopenia | 3 | 10 | |||||||||
| Lymphopenia | 2 | 7 | |||||||||
| Neutropenia | 7 | 24 | 6 | 21 | 4 | 24 | 3 | 18 | |||
| Thrombocytopenia | 1 | 3 | 7 | 24 | 1 | 6 | 2 | 12 | |||
| Hemorrhage | Colonic hemorrhage | 1 | 3 | ||||||||
| Hepatic | Bilirubin | 1 | 6 | ||||||||
| Infection/Febrile neutropenia | Blood Infection | 1 | 6 | ||||||||
| Metabolic/laboratory | Hyperglycemia | 3 | 10 | ||||||||
| Hyponatremia | 1 | 3 | 1 | 6 | |||||||
| Alk Phosphatase | 1 | 3 | |||||||||
| Neurology | Dizziness | 2 | 7 | ||||||||
| Syncope | 1 | 3 | |||||||||
| Pulmonary | Dyspnea | 1 | 3 | ||||||||
| Constitutional symptoms | Fatigue | 3 | 10 | 2 | 12 | ||||||
| Gastrointestinal | Anorexia | 2 | 7 | 1 | 6 | ||||||
| Diarrhea | 1 | 3 | |||||||||
| Pancreatitis | 1 | 6 | |||||||||
DISCUSSION
This study showed that in patients with untreated extensive-stage SCLC, first-line chemotherapy with pemetrexed and carboplatin is not superior to the current recommended first-line treatment of platinum-based chemotherapy with etoposide. Although the combination of pemetrexed and carboplatin does show activity in extensive-stage SCLC as previously reported by Socinski et al,18 the overall response rate of 35% in our study is the lowest compared with other studies of first-line therapies in untreated extensive-stage SCLC (35%–69%).9,11,21 At 1 year, the overall survival rate across all patients was 32% (95% CI, 21%–49%) which was lower than a previous study, which reported a 1-year overall survival of 39%.18 Median time-to-progression was 4.2 months and at the time of analysis, only 11% of patients remained progression-free.
The poor response rate observed in this study may be due to our study population, which included patients who received prior WBRT, prior palliative radiation, and those who had more than 2 metastatic sites at the time of enrollment. In extensive-stage SCLC, known adverse prognostic factors are metastatic involvement of the central nervous system and the number of metastatic sites.4 The previous study using pemetrexed and carboplatin in untreated extensive-stage SCLC did not identify these patient characteristics.18 The preliminary results of a randomized phase 3 study have also confirmed our study findings, whereby the pemetrexed and carboplatin regimen was shown to be inferior compared with etoposide and carboplatin, with an overall response rate of 24.9% and 40.5%, respectively, and an overall survival of 7.3 months and 9.6 months, respectively (hazard ratio, 1.78; 95% CI, 1.29–2.45).24 As no CRs and only PRs were achieved in our study and the previous study,18 this may be another reason inferior results are seen with pemetrexed and carboplatin when compared with the current platinum-based chemotherapy with etoposide. Limited data have suggested that patients who achieve a CR have a better long-term survival compared with those who have a PR or primary treatment failure.25 In vitro studies of human tumor specimens have shown that low levels of TS gene expression significantly correlated with chemosensitivity to pemetrexed.26 Similarly, in non-SCLC cell lines, high pretreatment TS expression levels conferred resistance to pemetrexed.27 As higher TS expression levels have been found in SCLC compared with carcinoid tumors, this may account for the marginal activity of pemetrexed in combination with carboplatin in extensive-stage SCLC.28,29
The combination of pemetrexed and carboplatin appeared to be well tolerated as demonstrated by our older patient group who received ≥90% of full-dose pemetrexed and 100% of full-dose carboplatin compared with our younger patient group. Similar findings were also observed in a previous study. Hematological toxicities, although present, did not cause significant adverse events, except for 1 patient who died of a grade 5 neutropenic fever. However, grade 3/4 hematological toxicities reported in our study were higher compared with the study by Socinski et al.18
In conclusion, we do not recommend pemetrexed and carboplatin be used as first-line chemotherapy in untreated patients with extensive-stage SCLC. Although this chemotherapy combination shows some activity in SCLC and is well tolerated, it does not appear to improve outcome when compared with the current standard of care. In view of the recently completed trial of single-agent pemetrexed that showed minimal activity in relapsed extensive-stage SCLC,30 this regimen should not be considered in the relapsed setting. There is a pressing need to continue the search for more effective agents in this recalcitrant disease, and, currently, clinical trials with targeted agents are necessary and ongoing.31
Acknowledgments
CONFLICT OF INTEREST DISCLOSURES
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25,224, CA-37,404, CA-35,448, CA-35,269, CA-37,417, CA-35,267, CA-35,195, and CA-60,276. Funding, in part, was also provided by Eli Lilly and Company.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Small Cell Lung Cancer Treatment. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/HealthProfessional/. Accessed April 9, 2009.
- 2.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4:31–42. [PubMed] [Google Scholar]
- 3.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Crowley JJ, Livingston RB. Long-term survival and toxicity in small cell lung cancer. Expanded Southwest Oncology Group experience. Chest. 1991;99:1425–1432. doi: 10.1378/chest.99.6.1425. [DOI] [PubMed] [Google Scholar]
- 5.Pujol JL, Carestia L, Daures JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer. 2000;83:8–15. doi: 10.1054/bjoc.2000.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol. 1994;5:601–607. doi: 10.1093/oxfordjournals.annonc.a058931. [DOI] [PubMed] [Google Scholar]
- 7.Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these 2 regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992;10:282–291. doi: 10.1200/JCO.1992.10.2.282. [DOI] [PubMed] [Google Scholar]
- 8.Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 9.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–2043. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 10.Lara PN, Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–2535. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckardt JR, von Pawel J, Papai Z, et al. Open-label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy-naive patients with extensive-disease small-cell lung cancer. J Clin Oncol. 2006;24:2044–2051. doi: 10.1200/JCO.2005.03.3332. [DOI] [PubMed] [Google Scholar]
- 12.Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol. 2008;26:4261–4267. doi: 10.1200/JCO.2007.15.7545. [DOI] [PubMed] [Google Scholar]
- 13.Adjei AA. Pemetrexed (Alimta): a novel multitargeted antifolate agent. Expert Rev Anticancer Ther. 2003;3:145–156. doi: 10.1586/14737140.3.2.145. [DOI] [PubMed] [Google Scholar]
- 14.Adjei AA. Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent. Clin Cancer Res. 2004;10:4276s–4280s. doi: 10.1158/1078-0432.CCR-040010. [DOI] [PubMed] [Google Scholar]
- 15.Mendelsohn LG, Shih C, Chen VJ, Habeck LL, Gates SB, Shackelford KA. Enzyme inhibition, polyglutamation, and the effect of LY231514 (MTA) on purine biosynthesis. Semin Oncol. 1999;26:42–47. [PubMed] [Google Scholar]
- 16.Schultz RM, Chen VJ, Bewley JR, Roberts EF, Shih C, Dempsey JA. Biological activity of the multitargeted antifolate, MTA (LY231514), in human cell lines with different resistance mechanisms to antifolate drugs. Semin Oncol. 1999;26:68–73. [PubMed] [Google Scholar]
- 17.Kanzawa F, Akiyama Y, Saijo N, Nishio K. In vitro effects of combinations of cis-amminedichloro (2-methylpyridine) platinum (II) (ZD0473) with other novel anticancer drugs on the growth of SBC-3, a human small cell lung cancer cell line. Lung Cancer. 2003;40:325–332. doi: 10.1016/s0169-5002(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 18.Socinski MA, Weissman C, Hart LL, et al. Randomized phase II trial of pemetrexed combined with either cisplatin or carboplatin in untreated extensive-stage small-cell lung cancer. J Clin Oncol. 2006;24:4840–4847. doi: 10.1200/JCO.2006.07.7016. [DOI] [PubMed] [Google Scholar]
- 19.Booton R, Jones M, Thatcher N. Lung cancer 7: management of lung cancer in elderly patients. Thorax. 2003;58:711–720. doi: 10.1136/thorax.58.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 21.Schmittel A, Fischer von Weikersthal L, Sebastian M, et al. A randomized phase II trial of irinotecan plus carboplatin versus etoposide plus carboplatin treatment in patients with extended disease small-cell lung cancer. Ann Oncol. 2006;17:663–667. doi: 10.1093/annonc/mdj137. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National #Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan E, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Socinski MA, Smit EF, Lorigan K, et al. Phase III study of pemetrexed plus carboplatin (PC) versus etoposide plus carboplatin (EC) in chemonaive patients (pts) with extensive-stage disease small cell lung cancer (ED-SCLC): interim results. J Clin Oncol. 2008;26:3543–3551. [Google Scholar]
- 25.Paesmans M, Sculier JP, Lecomte J, et al. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer. 2000;89:523–533. doi: 10.1002/1097-0142(20000801)89:3<523::aid-cncr7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Hanauske AR, Eismann U, Oberschmidt O, et al. In vitro chemosensitivity of freshly explanted tumor cells to pemetrexed is correlated with target gene expression. Invest New Drugs. 2007;25:417–423. doi: 10.1007/s10637-007-9060-9. [DOI] [PubMed] [Google Scholar]
- 27.Giovannetti E, Mey V, Nannizzi S, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol. 2005;68:110–118. doi: 10.1124/mol.104.009373. [DOI] [PubMed] [Google Scholar]
- 28.Ceppi P, Volante M, Ferrero A, et al. Thymidylate synthase expression in gastroenteropancreatic and pulmonary neuroendocrine tumors. Clin Cancer Res. 2008;14:1059–1064. doi: 10.1158/1078-0432.CCR-07-1513. [DOI] [PubMed] [Google Scholar]
- 29.Scagliotti GV, Ceppi P, Capelletto E, Novello S. Updated clinical information on multitargeted antifolates in lung cancer. Clin Lung Cancer. 2009;10:S35–S40. doi: 10.3816/CLC.2009.s.006. [DOI] [PubMed] [Google Scholar]
- 30.Jalal S, Ansari R, Govindan R, et al. Pemetrexed in second line and beyond small cell lung cancer: a Hoosier Oncology Group phase II study. J Thorac Oncol. 2009;4:93–96. doi: 10.1097/JTO.0b013e31818de1e6. [DOI] [PubMed] [Google Scholar]
- 31.PDQ National Cancer Institute Clinical Trials Database. Available at: http://www.cancer.gov/clinical_trials. Accessed April 9, 2009.


