Abstract
Aim
This study used proton magnetic resonance spectroscopy (1H MRS) to measure in vivo brain glutathione (GSH) in adolescents with major depressive disorder (MDD), and explored the relationship between GSH and illness severity and chronicity. Secondarily, associations between GSH and anhedonia, a key symptom of MDD in adolescents, were investigated.
Methods
Occipital cortex GSH levels were obtained in 19 psychotropic medication-free adolescents with MDD (ages 12–21) and compared to those in eight healthy control adolescents. Correlations between GSH levels and anhedonia severity were examined both in the full participant sample and within the MDD group. Within the MDD group, correlations between GSH levels and illness severity and chronicity were assessed.
Results
Occipital GSH levels were lower in adolescents with MDD compared to controls, but did not correlate with anhedonia (either within the MDD group or the full sample), MDD severity, or onset. There were also no group differences in levels of total choline, creatine, and N-acetylaspartate – all neurometabolites that were simultaneously detected with 1H MRS.
Conclusions
Although preliminary, findings add new data to support the role of oxidative stress in MDD and suggest that lower GSH may be a potential marker of MDD early on in the course of illness.
Keywords: anhedonia, mood disorders, occipital lobe, oxidative stress, antioxidants, inflammation
1. Introduction
Major depressive disorder (MDD) is consistently ranked as one of the most common psychiatric conditions among adolescents in the U.S. (Copeland et al., 2011; Kessler et al., 2012; Merikangas et al., 2010) and is accompanied by substantial psychosocial impairment (Asarnow et al., 2005; Jaycox et al., 2009). Additionally, adolescent MDD is associated with high risk for suicide (Asarnow et al., 2008), the second leading cause of death in this age group (Heron, 2016). Despite this clear public health concern, the neurobiological underpinnings and mechanisms of MDD remain poorly understood. Recent data point to the potential role of inflammation and oxidative stress, two closely related events, in the pathophysiology of depression (Gardner and Boles, 2011; Jimenez-Fernandez et al., 2015).
Inflammation is known to increase the production of reactive oxygen and nitrogen species (ROS/RNS). These free radicals can overwhelm cellular antioxidant capacity, leading to oxidative stress, the consequences of which can include mitochondrial dysfunction, further ROS/RNS production, and cell death. Neurons are especially vulnerable to oxidative stress given their high oxygen utilization/metabolism and relatively low levels of antioxidant compounds and enzymes, compared to other tissues (Mytilineou et al., 2002). Indeed, oxidative stress has been implicated in the pathogenesis of most major neurological/neurodegenerative (e.g., Alzheimer’s and Parkinson’s diseases) and neuropsychiatric disorders (Bains and Shaw, 1997; Mahadik and Mukherjee, 1996; Mytilineou et al., 2002; Ng et al., 2008), including adult MDD (Behr et al., 2012; Bilici et al., 2001; Cumurcu et al., 2009; Gibson et al., 2012; Kodydkova et al., 2009). Glutathione (GSH), a tripeptide thiol and the primary living tissue antioxidant, protects cells and their components against oxidative stress to ensure their normal functioning and replication (Bains and Shaw, 1997; Dringen, 2000). GSH is therefore a sensitive and reliably endogenous marker of oxidative stress.
Only a few studies to date have investigated GSH abnormalities in MDD. A postmortem study showed lower GSH levels in the prefrontal cortices of patients with MDD, bipolar disorder, and schizophrenia relative to those of controls (Gawryluk et al., 2011). Other studies examining GSH concentrations in blood serum and plasma have also identified significantly lower GSH in MDD patients compared to healthy controls (Kodydkova et al., 2009; Maes et al., 2011). Further, lower brain GSH levels have been found in a rodent model of depression (de Souza et al., 2006; Zafir and Banu, 2009). Most relevant, using proton magnetic resonance spectroscopy (1H MRS), Shungu and colleagues (2012) measured and then compared occipital cortex (OCC) GSH levels in unmedicated adults with MDD to matched healthy participants and found a 21% lower GSH levels in the MDD patients. Likewise, another recent 1H MRS reported lower in vivo GSH levels in the OCC of unmedicated adults with MDD versus healthy controls (Godlewska et al., 2015). No studies to date have measured GSH levels in adolescents with MDD.
Because MDD typically first develops in adolescence (Kessler et al., 2003), investigations of early-stage MDD may provide important insight into the pathogenesis of the disorder and may help to identify potential biomarkers of risk. The primary objective of the present study was to use 1H MRS to measure in vivo OCC GSH in psychotropic medication-free adolescents with MDD in comparison to healthy adolescents. Based on prior studies, we hypothesized that adolescents with MDD would have lower OCC GSH levels when compared to heathy adolescents. In addition, potential associations between GSH levels and dimensional measures of anhedonia were explored, based on our prior findings of specific links between inflammation and GSH and this core symptom of MDD in adolescents and adults (Gabbay et al., 2012; Gabbay et al., 2010a; Gabbay et al., 2010b; Lapidus et al., 2014). As such, we hypothesized that GSH levels would be inversely related to anhedonia. We chose to examine this relationship in both the MDD group and the full sample, as previous literature suggests that anhedonia may manifest a full range of severity among adolescents, both with and without MDD (Forbes et al., 2010; Keller et al., 2013; Stringaris et al., 2015), and may be a risk factor for future psychopathology (Morgan et al., 2013; Pine et al., 1999; Wilcox and Anthony, 2004). As a final, exploratory aim, we examined the relationship between GSH levels and the duration of MDD since onset in order to assess the effects of disease chronicity on GSH levels.
2. Methods
2.1. Participants
Nineteen adolescents with MDD (mean age = 15.7 years, SD = 2.5; 42% female) and 8 healthy control (HC) participants (mean age = 16.1 years, SD = 3.4; 63% female), ranging in age from 12–21, were recruited in the greater New York City area via clinical referrals from a pediatric psychiatry clinic and local advertisements. The study was approved by Institutional Review Boards at all participating institutions. Participants 18 years of age and older provided written informed consent, and those under age 18 provided assent and a parent or guardian gave signed informed consent.
Adolescents in the MDD group met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria for MDD. Exclusion criteria for all participants consisted of a low IQ (<80) estimated using the Kaufman Brief Intelligence Test (Kaufman and Kaufman, 1990), MRI contraindications, a positive urine toxicology test on the day of the scan, and a positive pregnancy test in females. All participants were free of psychotropic medication for at least thirty days prior to participating in the study. Adolescents with a current or past DSM-IV diagnosis of bipolar disorder, schizophrenia, pervasive developmental disorder, panic disorder, obsessive-compulsive disorder, Tourette’s disorder, or substance use disorder were excluded. HC adolescents did not meet criteria for any current or past DSM-IV diagnosis and were psychotropic medication-naïve.
2.2. Clinical assessments
To assess psychiatric symptoms and exclusionary criteria, a licensed psychiatrist or clinical psychologist administered the Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version for School Aged Children (K-SADS-PL; Kaufman et al., 1997) to the adolescent participants, as well as to a parent or guardian when the participant was under age 18. Detailed information on MDD illness onset and duration was also obtained. To enhance diagnostic reliability, clinical evaluations were discussed between the interviewing clinician and the Primary Investigator (a board-certified child and adolescent psychiatrist).
Depression severity was quantified using the clinician-administered Children’s Depression Rating Scale–Revised (CDRS-R), which assesses depression symptoms across 17 items. CDRS-R scores can range from 17 to 113, with higher scores indicating greater depression severity. The psychometric properties of this scale have been validated both in children and adolescents and are ideal for identifying specific depression symptoms (Mayes et al., 2010).
Participants completed the Snaith-Hamilton Pleasure Scale (SHAPS; Snaith et al., 1995), a self-report measure of anhedonia severity that has been validated for use in clinical and non-clinical populations (Franken et al., 2007), including in adolescents (Leventhal et al., 2015). The SHAPS is a measure of consummatory reward across several domains and is currently considered the “gold standard” for assessing anhedonia in depression (Rizvi et al., 2016).
2.3. Magnetic resonance neuroimaging procedures
All neuroimaging, which included limited structural brain MRI examination and two single-voxel 1H MRS scans, was conducted on a research-dedicated 3.0T GE MR system with an 8-channel phased-array receive head coil and transmit body coil at the Citigroup Biomedical Imaging Center of Weill Cornell Medicine.
2.4. MR imaging and spectroscopy data acquisition and analysis
The structural MRI examination consisted of standard structural T1- and T2-weighted imaging series that were appropriately obliqued for prescribing the MRS voxels, of a T1-weighted spoiled gradient-recalled echo (SPGR) volumetric scan for tissue segmentation, and of an axial fast fluid-attenuated inversion recovery (FLAIR) scan to exclude focal pathology.
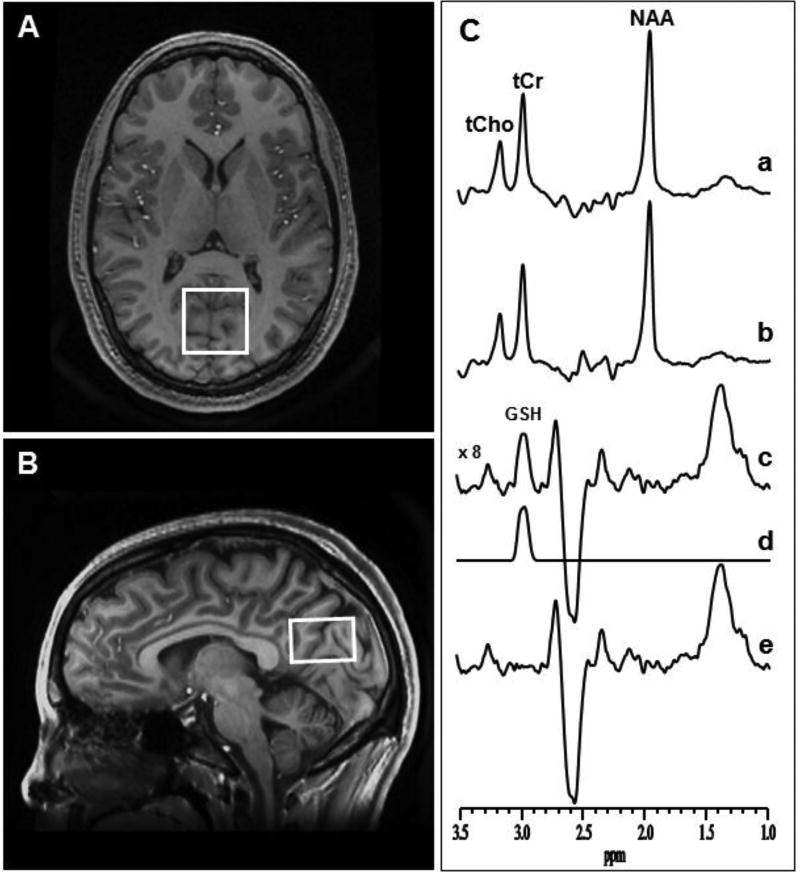
In vivo 1H MRS data were obtained from a 3.0 × 3.0 × 2.0 cm3 voxel prescribed in the OCC. Given that obtaining spectra from several voxels in a single exam requires relatively long scan times, which might not be well tolerated by pediatric participants, we chose to focus only on this one region. The OCC was selected as our region of interest based on our and others’ prior findings of decreased GSH in this region in adults with MDD and associations with anhedonia (Godlewska et al., 2015; Lapidus et al., 2014; Shungu et al., 2012). Additionally, prior studies have documented decreased γ-aminobutyric acid (GABA) in the OCC in adults with MDD (Price et al., 2009; Sanacora et al., 2000). Importantly, our voxel contains the precuneus, which is part of the default mode network (DMN), implicated in MDD (Liu et al., 2017; Peng et al., 2015; Sheline et al., 2010). The standard J-edited spin echo difference method with TE/TR 68/1500 ms was used to measure the levels of reduced GSH, as previously described (Shungu et al., 2012; Terpstra et al., 2006; Weiduschat et al., 2014) and illustrated in Figure 1. Briefly, a pair of frequency-selective inversion pulses were inserted into the standard point-resolved spectroscopy (PRESS) method and applied on alternate scans at the frequency of the GSH α-cysteinyl resonance at 4.56 ppm, while avoiding excitation of oxidized GSH α-cysteinyl at 3.28 ppm (Nepravishta et al., 2012). This resulted in two subspectra in which reduced GSH, but not oxidized GSH, was alternately inverted or not inverted. Subtracting these two subspectra yielded a 1H MR spectrum consisting of only the edited GSH β-cysteinyl resonance at 2.98 ppm. A high test-retest reliability has been reported for detection of GABA with this MRS technique on the same 3.0T GE instrument (Shungu et al., 2016). Spectral data for this study were acquired in 15 minutes using 290 interleaved excitations (580 total) with the editing pulses on or off. The area under the GSH resonance, which is proportional to the concentration of GSH in the voxel-of-interest, was obtained by frequency-domain spectral fitting as previously described (Weiduschat et al., 2014). The derived GSH peak areas were then expressed semi-quantitatively as ratios relative to the unsuppressed intravoxel water (W) signal for normalization across subjects before being used in group analyses.
Figure 1.
[A] Axial and [B] sagittal MR images of a human brain, with depiction of the size, location and angulation of the voxel of interest in the occipital cortex. [C] Demonstration of cortical glutathione (GSH) detection with J-edited 1H MRS: (a) and (b), single-voxel subspectra acquired in 15 min with the editing pulse on and off and 290 (580 total) interleaved averages; spectrum (c), difference between spectra (a) and (b) showing the edited brain GSH resonance at 2.98 ppm; spectrum (d), model fitting of spectrum c to obtain the GSH peak area; spectrum (e), residual of the difference between spectra (c) and (d). NAA, N-acetylaspartate; tCho, total choline; tCr, total creatine.
2.5. Assessment of voxel tissue heterogeneity
To estimate the proportions of gray matter, white matter, and cerebrospinal fluid contained in our voxel of interest, MEDx software (Medical Numerics, Germantown, MD) was used to segment the brain tissue based on the signal-intensity histogram of each participant’s volumetric (SPGR) MRI. In-house software developed in MATLAB (MathWorks, Natick, MA) was then implemented to generate a segmentation mask for each voxel, from which the proportions of gray matter, white matter, and cerebrospinal fluid were determined. These were then compared between the groups and, in case of significant differences, included in the statistical model as covariates.
2.6. Statistical analyses
All statistical analyses were performed in SPSS, version 22. All assumptions of normality were tested prior to statistical analyses using the Shapiro-Wilk test; when assumptions were violated, non-parametric tests were employed. Prior to our analyses, demographic variables (i.e., age, sex, and ethnicity) and 1H MRS voxel tissue heterogeneity (i.e., proportions of gray matter, white matter, and cerebrospinal fluid; mean unsuppressed voxel tissue water signal) were compared between groups to detect any potential confounds.
For our first hypothesis, an independent samples t test was used to compare mean OCC GSH/W levels of adolescents with MDD to HC. To provide a contrast for examination of GSH, group differences in mean OCC levels of total choline (tCho), total creatine (tCr), and N-acetylaspartate (NAA), all neurometabolites that were simultaneously detected with 1H MRS, were also examined. Independent samples t tests were used for normally distributed data (i.e., NAA), and independent samples Mann-Whitney U tests were used for non-normal data (i.e., CHO and CRE). These separate group comparison tests were selected, rather than using multivariate analysis of variance (MANOVA), because these data did not meet normal distributional assumptions. Next, correlation analyses were conducted to assess the associations between OCC GSH/W and SHAPS scores—both in the full participant sample, consisting of both adolescents with MDD and HCs, and within the MDD group only. Lastly, exploratory analyses were conducted to examine correlations among GSH/W and depression severity (CDRS-R scores), time (in months) since first MDD episode onset, and age of illness onset. For all correlation analyses, Pearson correlations (“r”) were used for normally distributed data and Spearman Rank correlations (“rho” or “ρ”) were used for non-normal data.
3. Results
3.1. Participant characteristics
Table 1 provides study participants’ demographic and clinical data. Two-tailed t tests and chi-square tests revealed no significant group differences in age (t = 0.33, p = 0.74), sex (χ2 = 0.94, p = 0.42), or ethnicity (χ2 = 3.50, p = 0.32) between HC and MDD groups. Additionally, OCC GSH/W was not associated with age (ρ = −0.13, p = 0.51) or ethnicity (F = 1.35, p = 0.28). Sex differences in OCC GSH/W approached significance (t = −1.79, p = 0.09). Thus, sex was included as a covariate in correlation analyses. No differences in outcome variables of interest were found between participants who were missing clinical data (e.g., SHAPS, CDRS-R) and those with complete data.
Table 1.
Demographic and clinical data for adolescents with major depressive disorder (MDD) and healthy controls (HC)
| HC (n = 8) | MDD (n = 19) | |
|---|---|---|
| Age in years [Mean ± SD (range)] | 16.1 ± 3.4 (12.6–21.3) | 15.7 ± 2.5 (12.2–19.7) |
| Sex [n (%)] | ||
| Male | 3 (37.5%) | 11 (57.8%) |
| Female | 5 (62.5%) | 8 (42.1%) |
| Ethnicity [n (%)] | ||
| Caucasian | 3 (37.5%) | 6 (31.6%) |
| African American | 4 (50.0%) | 6 (31.6%) |
| Hispanic | 0 (0.0%) | 6 (31.6%) |
| Asian American or Other | 1 (12.5%) | 1 (5.3%) |
| Clinical Measures [Mean ± SD (range)] | ||
| CDRS-R a | 18.7 ± 2.9 (17–25) | 47.6 ± 11.8 (35–79) |
| SHAPS b | 16.8 ± 4.2 (14–24) | 27.6 ± 7.6 (14–45) |
| Comorbid Diagnoses [n (%)] | ||
| Anxiety Disorder c | 0 (0.0%) | 11 (57.8%) |
| Oppositional Defiant Disorder | 0 (0.0%) | 3 (15.8%) |
| Attention Deficit Hyperactivity Disorder | 0 (0.0%) | 4 (21.1%) |
| Eating Disorder, Not Otherwise Specified | 0 (0.0%) | 1 (5.3%) |
| Age of illness onset in years [Mean ± SD (range)] | 13.4 ± 3.3 (5.4–19.1) | |
| Number of months since MDD onset [Mean ± SD (range)] | 26.9 ± 23.2 (1.9–88.1) |
CDRS-R = Children’s Depression Rating Scale—Revised; SHAPS = Snaith-Hamilton Pleasure Scale;
Data missing for 1 HC participant;
Data missing for 1 MDD and 3 HC participants;
Includes Generalized Anxiety Disorder (n = 7), Social Phobia (n = 4), Separation Anxiety Disorder (n = 1), Specific Phobia (n = 1).
3.2. 1H MRS voxel tissue heterogeneity
There were no significant group differences in tissue proportions of gray matter, white matter, cerebrospinal fluid, or mean unsuppressed voxel tissue water signal between participants with MDD and HCs (Table 2).
Table 2.
Neurometabolites, water, gray matter, white matter, and CSF in the occipital cingulate cortex for youth with major depressive disorder (MDD) and healthy controls (HC)
| Mean ± SD | |||
|---|---|---|---|
|
|
|||
| HC | MDD | p-value | |
| Water (ppm) | 1.27×1012 ± 2.24×1011 | 1.38×1012 ± 1.58×1011 | 0.14a |
| Gray matter % | 63.89 ± 4.82 | 60.63 ± 3.20 | 0.11b |
| White matter % | 27.48 ± 3.78 | 29.46 ± 3.17 | 0.21a |
| CSF % | 8.47 ± 3.29 | 9.45 ± 1.52 | 0.44a |
| GSH/W | 2.39×10−3 ± 0.66×10−3 | 1.80×10−3 ± 0.29×10−3 | 0.04a |
| CHO/W | 17.97×10−3 ± 3.82×10−3 | 17.50×10−3 ± 2.27×10−3 | 1.00b |
| CRE/W | 26.31×10−3 ± 5.67×10−3 | 24.47×10−3 ± 1.90×10−3 | 0.62b |
| NAA/W | 46.47×10−3 ± 5.62×10−3 | 46.56×10−3 ± 4.66×10−3 | 0.83a |
ppm = parts per milligram; GSH/W = glutathione level relative to unsuppressed voxel tissue water; CHO/W = choline level relative to unsuppressed voxel tissue water; CRE/W = creatine level relative to unsuppressed voxel tissue water; NAA/W = N-acetylaspartate level relative to unsuppressed voxel tissue water; CSF = cerebrospinal fluid;
t test,
Mann-Whitney U test.
3.3. Group GSH and other neurometabolite level comparisons
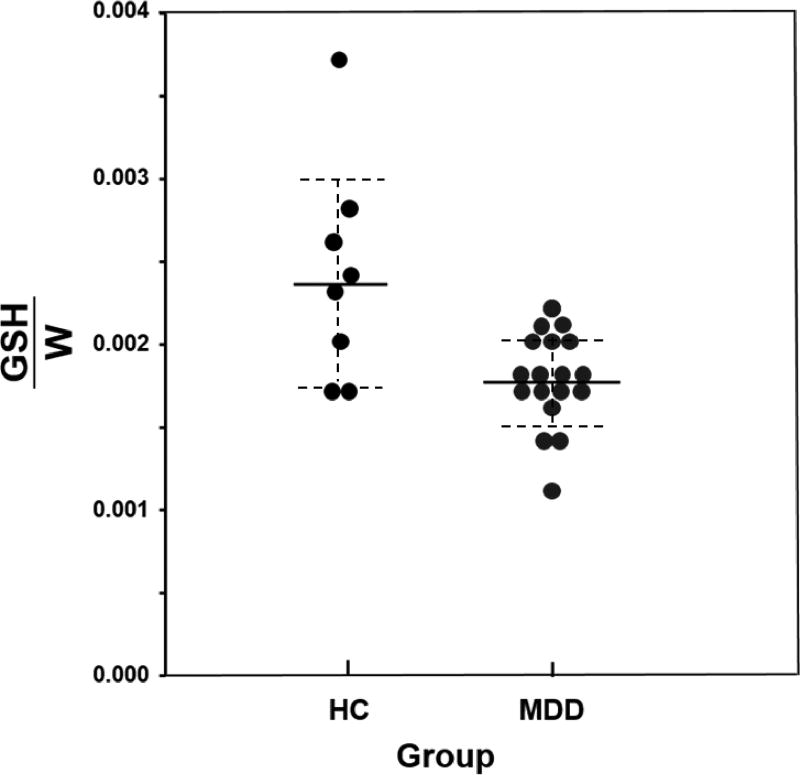
Table 2 also shows the metabolite levels for adolescents with MDD and HCs. Adolescents with MDD had significantly lower OCC GSH/W relative to HCs (Figure 2). There were no group differences in levels of other neurometabolites (CHO/W, CRE/W, and NAA/W) that were simultaneously detected in the OCC.
Figure 2.
Glutathione relative to unsuppressed voxel tissue water (GSH/W) concentrations in the occipital cingulate cortex (OCC) for adolescents with major depressive disorder (MDD) and healthy controls (HC). NOTE: Mean levels are signified by solid lines, and dashed lines indicate error bars (95% confidence interval).
3.4. Associations with anhedonia
OCC GSH/W and SHAPS scores were not significantly correlated in the full sample (ρ = −0.11, p = 0.63), nor were they correlated within the MDD group alone (r = 0.23, p = 0.35). Partial correlations, controlling for sex, were also non-significant in both the full sample (ρ = −0.10, p = 0.68) and the MDD group (r = 0.21, p = 0.42). However, it is important to note that we were missing SHAPS data for three HC participants and one MDD participant; given the small sample size, these results are considered preliminary.
3.5. Relationships with MDD illness severity and duration
In the MDD group, OCC GSH/W was uncorrelated with CDRS-R scores (ρ = −0.08, p = 0.75). When controlling for sex, partial correlations were similar (ρ = −0.02, p = 0.95). OCC GSH/W was also not significantly correlated with illness duration, as indexed by number of months since illness onset (ρ = −0.24, p = 0.33), or with age of illness onset (ρ = −0.17, p = 0.48). When controlling for sex, partial correlations between OCC GSH/W remained uncorrelated with number of months since illness onset (ρ = −0.15, p = 0.57) and age of illness onset (ρ = −0.11, p = 0.66).
4. Discussion
This is the first study that has investigated the potential role of GSH in adolescent MDD. Our sample of psychotropic medication-free adolescents with MDD had lower levels of in vivo GSH/W in the OCC, compared to healthy adolescents. Contrary to hypotheses and results of previous investigations (Gabbay et al., 2012; Gabbay et al., 2010a; Gabbay et al., 2010b; Lapidus et al., 2014), GSH/W levels were not associated with anhedonia severity in the full sample or within the MDD group. GSH/W levels also did not correlate with MDD severity or chronicity.
The main finding of this study points to a cortical GSH deficit in adolescents with MDD. As previously cited, GSH, the primary antioxidant in living tissue, defends cells and their components against oxidant damage by scavenging or detoxifying free radicals. Given that a decrease in GSH is associated with increased consumption of antioxidant reserves, our finding of lower GSH/W in MDD implicates inflammation and increased oxidative stress in the pathogenesis of the disorder. This suggestion is consistent with mounting preclinical data showing links between depression and a number of signs of oxidative damage, including: elevated markers of lipid peroxidation, elevated markers of oxidative DNA damage, abnormal activity of antioxidant enzymes, and decreased levels of peptides and other molecules with antioxidant properties, including GSH (Behr et al., 2012). Clinical evidence of the role of oxidative stress in MDD includes a growing literature that shows increased inflammatory response and oxidative damage in adult MDD (Behr et al., 2012; Bilici et al., 2001; Cumurcu et al., 2009; Gibson et al., 2012; Kodydkova et al., 2009). Additionally, a number of clinical trials found that treatment with antidepressants, especially long-term (12 to 24 weeks) treatment, can reverse the increased oxidative stress observed in individuals with MDD (Behr et al., 2012). Most relevant, two previous 1H MRS studies in adult populations demonstrated lower in vivo cortical GSH levels in MDD patients relative to healthy control participants (Godlewska et al., 2015; Shungu et al., 2012). Despite its smaller sample size, the current study replicates these findings and adds to the literature by implicating oxidative stress in adolescent MDD, early in the course of the illness.
While the evidence implicating oxidative stress in MDD is compelling, it remains unclear whether the observed GSH depletion reflects an underlying vulnerability to MDD or whether it is, instead, a consequence of the disorder. Although our cross-sectional design cannot determine whether GSH levels play an etiological role in MDD, our finding of lower GSH/W in depressed adolescents suggests that depleted GSH is not necessarily the effect of illness chronicity. This finding is further supported by the lack of significant relationships between GSH/W and illness onset and duration among the adolescents with MDD in our study. Although admittedly preliminary, these findings seem to implicate oxidative stress as a potential biomarker or etiological factor among youth with (or potentially at risk for) depression, with clear therapeutic implications. For example, N-acetylcysteine (NAC) may be one such therapeutic strategy for targeting youth MDD, given its role as an antioxidant and glutamatergic modulator. NAC, which restores GSH, has been investigated as a therapeutic agent in adults with several neuropsychiatric disorders (Dean et al., 2011; Deepmala et al., 2015). According to results of a recent meta-analysis, NAC was moderately effective in alleviating depressive symptoms in adults with MDD, bipolar disorder, and other psychiatric conditions (Fernandes et al., 2016). Furthermore, NAC has evidenced antidepressant-like effects in rodent models of depression, directly via its role as an antioxidant (Ferreira et al., 2008; Smaga et al., 2012). While the results have been promising, studies in children and adolescents are limited (Bloch et al., 2013; Hardan et al., 2012). To date, no studies have examined NAC as a treatment for youth depression. Given the present finding of a cortical GSH deficit in adolescents with MDD, future studies evaluating NAC as an antidepressant in this population seem warranted.
The failure to find a significant correlation between GSH/W and anhedonia in the present study is inconsistent with our prior 1H MRS study (Lapidus et al., 2014) in adults with MDD, which documented an inverse relationship between GSH and anhedonia severity. However, the discrepancy may be due to the smaller sample size of the present study. As another present limitation, our single-voxel study of the OCC cannot rule out GSH abnormalities in other brain areas that are implicated in MDD and reward circuitry, such as frontal, limbic, or striatal regions. Third, the present study focused on overall depression severity and the core symptom of anhedonia and did not examine other specific MDD symptoms and/or other phenomenological features associated with depression. Fourth, given the cross-sectional nature of our study, we were unable to assess the temporal trajectory of the relationship between GSH and MDD. Future directions of this research include replicating the investigation in larger samples to confirm the present findings, assessing antioxidant capacity in additional brain regions, and examining and comparing peripheral markers of oxidative stress with temporally concordant 1H MRS measures of brain GSH. Given that recent research has suggested possible MDD subtypes based on divergent patterns of brain function and associated symptomatology (Drysdale et al., 2017), future studies of GSH in depression would also benefit from examining relationships with other depression-related clinical variables (e.g., anxiety). Additionally, future studies would benefit from a longitudinal approach that follows adolescents over time in order to better understand changes in the GSH system and associations with MDD illness trajectories.
Despite these limitations, the present study has contributed evidence in support of a role of oxidative stress in psychiatric disorders and is the first study that has examined GSH in adolescents with MDD, early in the course of this chronic illness. Other strengths include the use of 1H MRS, the only technology that currently allows for the assessment of in vivo GSH levels non-invasively, and inclusion of only psychotropic medication-free participants at the time of the scan to reduce the potential confounding effect of neuro-active medication on GSH. If replicated in a larger sample, the present finding of a cortical GSH deficit early in the course of MDD may provide important information for the development of new assessment, prevention, and treatment paradigms.
Highlights.
Proton magnetic resonance spectroscopy measured occipital cortex glutathione
Adolescents with depression had lower glutathione levels
Glutathione was unrelated to depression chronicity or severity
Glutathione was unrelated to anhedonia severity
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers MH095807, MH101479, R21AT004576]. Funding sources had no involvement in any aspect of study design, data analysis, or manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Freed conducted data analyses and wrote and edited manuscript text. Ms. Hollenhorst conducted literature searches and wrote and edited manuscript sections. Dr. Weiduschat oversaw 1H MRS procedures. Ms. Mao conducted 1H MRS data acquisition, processing, and analysis. Dr. Shungu developed the neuroimaging protocol, supervised 1H MRS procedures, and wrote and edited manuscript text. Dr. Gabbay developed the study protocol, oversaw recruitment, and wrote and edited manuscript text. All authors contributed to and have approved the final manuscript.
References
- Asarnow JR, Baraff LJ, Berk M, Grob C, Devich-Navarro M, Suddath R, Piacentini J, Tang L. Pediatric emergency department suicidal patients: two-site evaluation of suicide ideators, single attempters, and repeat attempters. J Am Acad Child Adolesc Psychiatry. 2008;47:958–966. doi: 10.1097/CHI.0b013e3181799ee8. [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Jaycox LH, Duan N, LaBorde AP, Rea MM, Tang L, Anderson M, Murray P, Landon C, Tang B, Huizar DP, Wells KB. Depression and role impairment among adolescents in primary care clinics. J Adolesc Health. 2005;37:477–483. doi: 10.1016/j.jadohealth.2004.11.123. [DOI] [PubMed] [Google Scholar]
- Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25:335–358. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Behr GA, Moreira JC, Frey BN. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxid Med Cell Longev. 2012:609421. doi: 10.1155/2012/609421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Panza KE, Grant JE, Pittenger C, Leckman JF. N-acetylcysteine in the treatment of pediatric trichotillomania: a randomized, double-blind, placebo-controlled add-on trial. J Am Acad Child Psy. 2013;52:231–240. doi: 10.1016/j.jaac.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W, Shanahan L, Costello EJ, Angold A. Cumulative prevalence of psychiatric disorders by young adulthood: a prospective cohort analysis from the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2011;50:252–261. doi: 10.1016/j.jaac.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci. 2009;63:639–645. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- de Souza FG, Rodrigues MD, Tufik S, Nobrega JN, D'Almeida V. Acute stressor-selective effects on homocysteine metabolism and oxidative stress parameters in female rats. Pharmacol Biochem Behav. 2006;85:400–407. doi: 10.1016/j.pbb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36:78–86. doi: 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepmala, Slattery J, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav R. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, Dubin MJ, Liston C. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes BS, Dean OM, Dodd S, Malhi GS, Berk M. N-acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiat. 2016;77:E457–E466. doi: 10.4088/JCP.15r09984. [DOI] [PubMed] [Google Scholar]
- Ferreira FR, Biojone C, Joca SR, Guimaraes FS. Antidepressant-like effects of N-acetyl-L-cysteine in rats. Behav Pharmacol. 2008;19:747–750. doi: 10.1097/FBP.0b013e3283123c98. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, Dahl RE. Healthy adolescents' neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Psy. 2010;49:162–172. doi: 10.1016/j.jaac.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) J Affect Disord. 2007;99:83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Babb J, Liebes L. The possible role of the kynurenine pathway in anhedonia in adolescents. J Neural Transm. 2012;119:253–260. doi: 10.1007/s00702-011-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM, Babb JS, Hirsch GS, Liebes L. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry. 2010a;51:935–943. doi: 10.1111/j.1469-7610.2010.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Liebes L, Katz Y, Liu S, Mendoza S, Babb JS, Klein RG, Gonen O. The kynurenine pathway in adolescent depression: preliminary findings from a proton MR spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry. 2010b;34:37–44. doi: 10.1016/j.pnpbp.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:730–743. doi: 10.1016/j.pnpbp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Gawryluk JW, Wang JF, Andreazza AC, Shao L, Yatham LN, Young LT. Prefrontal cortex glutathione S-transferase levels in patients with bipolar disorder, major depression and schizophrenia. Int J Neuropsychopharmacol. 2011;14:1069–1074. doi: 10.1017/S1461145711000617. [DOI] [PubMed] [Google Scholar]
- Gibson SA, Korade Z, Shelton RC. Oxidative stress and glutathione response in tissue cultures from persons with major depression. J Psychiatr Res. 2012;46:1326–1332. doi: 10.1016/j.jpsychires.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska BR, Near J, Cowen PJ. Neurochemistry of major depression: a study using magnetic resonance spectroscopy. Psychopharmacology. 2015;232:501–507. doi: 10.1007/s00213-014-3687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Fung LK, Libove RA, Obukhanych TV, Nair S, Herzenberg LA, Frazier TW, Tirouvanziam R. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiatry. 2012;71:956–961. doi: 10.1016/j.biopsych.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. Deaths: Leading Causes for 2014. National Center for Health Statistics. 2016 [PubMed] [Google Scholar]
- Jaycox LH, Stein BD, Paddock S, Miles JN, Chandra A, Meredith LS, Tanielian T, Hickey S, Burnam MA. Impact of teen depression on academic, social, and physical functioning. Pediatrics. 2009;124:596–605. doi: 10.1542/peds.2008-3348. [DOI] [PubMed] [Google Scholar]
- Jimenez-Fernandez S, Gurpegui M, Diaz-Atienza F, Perez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J Clin Psychiatry. 2015;76:1658–1667. doi: 10.4088/JCP.14r09179. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Manual for the Kaufman Brief Intelligence Test. American Guidance Service, MN 1990 [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keller J, Young CB, Kelley E, Prater K, Levitin DJ, Menon V. Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. J Psychiatr Res. 2013;47:1319–1328. doi: 10.1016/j.jpsychires.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Costello EJ, Georgiades K, Green JG, Gruber MJ, He JP, Koretz D, McLaughlin KA, Petukhova M, Sampson NA, Zaslavsky AM, Merikangas KR. Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69:372–380. doi: 10.1001/archgenpsychiatry.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey, R The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kodydkova J, Vavrova L, Zeman M, Jirak R, Macasek J, Stankova B, Tvrzicka E, Zak A. Antioxidative enzymes and increased oxidative stress in depressive women. Clin Biochem. 2009;42:1368–1374. doi: 10.1016/j.clinbiochem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lapidus K, Gabbay V, Mao X, Johnson A, Mourrough JW, Matthew S, Shungu D. In vivo 1H MRS study of potential associations between glutathione, oxidative stress and anhedonia in major depressive disorder. Neurosci Lett. 2014;569:74–79. doi: 10.1016/j.neulet.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Unger JB, Audrain-McGovern J, Sussman S, Volk HE, Strong DR. Measuring anhedonia in adolescents: a psychometric analysis. J Pers Assess. 2015;97:506–514. doi: 10.1080/00223891.2015.1029072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Ma X, Yuan Z, Song LP, Jing B, Lu HY, Tang LR, Fan J, Walter M, Liu CZ, Wang L, Wang CY. Decreased Resting-State Activity in the Precuneus Is Associated With Depressive Episodes in Recurrent Depression. J Clin Psychiatry. 2017;78:e372–e382. doi: 10.4088/JCP.15m10022. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Lower whole blood glutathione peroxidase (GPX) activity in depression, but not in myalgic encephalomyelitis / chronic fatigue syndrome: another pathway that may be associated with coronary artery disease and neuroprogression in depression. Neuro Endocrinol Lett. 2011;32:133–140. [PubMed] [Google Scholar]
- Mahadik SP, Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia: a review. Schizophr Res. 1996;19:1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Mayes TL, Bernstein IH, Haley CL, Kennard BD, Emslie GJ. Psychometric properties of the Children's Depression Rating Scale-Revised in adolescents. J Child Adolesc Psychopharmacol. 2010;20:513–516. doi: 10.1089/cap.2010.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiol Dis. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8:385–387. doi: 10.1016/s1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- Nepravishta R, Sabelli R, Iorio E, Micheli L, Paci M, Melino S. Oxidative species and S-glutathionyl conjugates in the apoptosis induction by allyl thiosulfate. FEBS J. 2012;279:154–167. doi: 10.1111/j.1742-4658.2011.08407.x. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Peng D, Liddle EB, Iwabuchi SJ, Zhang C, Wu Z, Liu J, Jiang K, Xu L, Liddle PF, Palaniyappan L, Fang Y. Dissociated large-scale functional connectivity networks of the precuneus in medication-naive first-episode depression. Psychiatry Res. 2015;232:250–256. doi: 10.1016/j.pscychresns.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am J Psychiat. 1999;156:133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Krystal JH. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Crit Rev Neurobiol. 2000;14:23–45. doi: 10.1615/critrevneurobiol.v14.i1.20. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu DC, Mao X, Gonzales R, Soones TN, Dyke JP, van der Veen JW, Kegeles LS. Brain gamma-aminobutyric acid (GABA) detection in vivo with the J-editing (1) H MRS technique: a comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test-retest reliability. NMR Biomed. 2016;29:932–942. doi: 10.1002/nbm.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, Medow MS, Natelson BH, Stewart JM, Mathew SJ. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012 doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaga I, Pomierny B, Krzyzanowska W, Pomierny-Chamiolo L, Miszkiel J, Niedzielska E, Ogorka A, Filip M. N-acetylcysteine possesses antidepressant-like activity through reduction of oxidative stress: behavioral and biochemical analyses in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:280–287. doi: 10.1016/j.pnpbp.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Belil PVR, Artiges E, Lemaitre H, Gollier-Briant F, Wotke S, Vulser H, Miranda R, Penttila J, Struve M, Fadai T, Kappel V, Grimmer Y, Goodman R, Poustka L, Conrod P, Cattrell A, Banaschewski T, Bokde ALW, Bromberg U, Buchel C, Flor H, Frouin V, Gattinat J, Garavan H, Gowtand P, Heinz A, Ittermann B, Nees F, Papadopoulos D, Paus T, Smolka MN, Walter H, Whelan R, Martinot JL, Schumann G, Paillere-Martinot ML, Consortium, I The brain's response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiat. 2015;172:1215–1223. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra M, Marjanska M, Henry PG, Tkac I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn Reson Med. 2006;56:1192–1199. doi: 10.1002/mrm.21086. [DOI] [PubMed] [Google Scholar]
- Weiduschat N, Mao X, Hupf J, Armstrong N, Kang G, Lange DJ, Mitsumoto H, Shungu DC. Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. Neurosci Lett. 2014;570:102–107. doi: 10.1016/j.neulet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Wilcox HC, Anthony JC. Child and adolescent clinical features as forerunners of adult-onset major depressive disorder: retrospective evidence from an epidemiological sample. J Affect Disorders. 2004;82:9–20. doi: 10.1016/j.jad.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Zafir A, Banu N. Induction of oxidative stress by restraint stress and corticosterone treatments in rats. Indian J Biochem Biophys. 2009;46:53–58. [PubMed] [Google Scholar]