Abstract
Schizophrenia is a disabling, heterogeneous disorder with clinical features that can be parsed into three domains: positive symptoms, negative symptoms and cognitive deficits. Current antipsychotic drugs produce fairly robust clinical benefit against positive symptoms, but typically have minimal therapeutic effects on negative symptoms and cognitive deficits.
Oxytocin (OT) is a nonapeptide that, in addition to its role as a hormone regulating peripheral reproductive-relevant functions, acts as a neurotransmitter in the brain. Several lines of preclinical and clinical research suggest that the OT system may play a role in regulating the expression of schizophrenia-spectrum disorders and that targeting the central OT system may yield novel treatments to address these symptoms.
In this review, we summarize the extant preclinical and clinical evidence relevant to the role of OT in schizophrenia with particular emphasis on its putative therapeutic effects on each of the three above-mentioned clinical domains.
Keywords: oxytocin, schizophrenia, positive symptoms, negative symptoms, cognitive deficits, antipsychotic
Introduction
Schizophrenia (SCZ) is a disabling, heterogeneous disorder whose symptoms can be parsed into three domains: positive symptoms, negative symptoms and cognitive deficits. Positive symptoms include the presence of perceptual aberrations (auditory and visual hallucinations), delusions (fixed, false beliefs), and disorganized behavior or speech. Negative symptoms are composed of deficits in motivation (avolition), experiencing pleasure (anhedonia), seeking social interaction (asociality), verbal communication (alogia), and emotional expression. In addition to these impairments, most people with SCZ also have deficient cognitive processing that further impairs their ability to function. NIMH-MATRICS initiative identified 7 specific domains of cognitive impairment in patients with SCZ, visual and verbal learning and memory, attention/vigilance, working memory, reasoning and problem solving, information processing speed and social cognition (1). Although currently subsumed under a single diagnostic label, in reality there is substantial clinical heterogeneity among patients meeting the criteria for SCZ (2).
Established antipsychotic drugs (APDs) exert their most robust and consistent clinical benefit on the positive symptoms of SCZ, a therapeutic effect associated primarily with their ability to bind mesolimbic dopamine D2 receptors. Whereas inhibition of mesolimbic D2 neurotransmission is the sole mechanism of action for first generation APDs (FGAs), e.g., haloperidol, second generation APDs (SGAs), e.g., clozapine, have additional pharmacological mechanisms most notably binding and blockade of serotonin-2A (5HT2A) receptors (3, 4) while producing minimal extrapyramidal side effects. As the negative symptoms and cognitive deficits are major contributors to poor functioning in patients with SCZ it is highly unfortunate that APDs have, at best, modest therapeutic effects on these domains of the disorder (5, 6). It follows that developing novel, effective treatments for negative symptoms and cognitive deficits is a pressing therapeutic priority.
One promising molecule in addressing this need is oxytocin (OT), a nonapeptide neurohormone with well-known peripheral reproduction-related functions, including induction of uterine contractions and milk letdown. OT also acts as a neurotransmitter in the brain and is now widely recognized to regulate social cognition/affiliation, stress, learning and memory (7).
Evidence from animal studies and several recent small, randomized, double blind, placebo controlled clinical trials in humans suggests that the OT system is a promising therapeutic target for all three of the abovementioned symptom domains of SCZ. In this review, we summarize these data, and address the potential for OT or OT-mimetics1 to provide broad-spectrum benefit in multiple domains of this devastating disorder.
Positive Symptoms
Positive symptoms are a sine qua non of SCZ as, according to the diagnostic criteria of DSM-V, at least one positive symptom must be present to confer this diagnosis. Pathophysiologically, increased (or dysregulated) dopaminergic transmission in the mesolimbic pathway from the ventral tegmental area to the nucleus accumbens is thought to play a crucial role in the generation of positive symptoms (13, 14).
Preclinical Studies of OT’s Effects on Positive-Like Symptoms
Animal models of relevance for positive symptoms of SCZ, although fraught with translational challenges, are valuable tools to screen putative novel APDs and to gain a better understanding of their mechanisms of therapeutic action (2). Since there is currently no reliable way to induce and measure hallmark positive symptoms (e.g. auditory hallucinations, delusional thinking) in animals, investigators seeking to model these symptoms have, mostly, attempted to reproduce the neurochemical perturbations that are thought to underlie their manifestation (2, 15). In this regard, psychostimulants such as amphetamine and cocaine produce mesolimbic hyperdopaminergia, which in turn produce behavioral changes (e.g. hyperactivity). Though hyperactivity is not a characteristic feature of SCZ, in the psychostimulant model it functions as a behavioral surrogate for the underlying abnormality: mesolimbic hyperdopaminergia. Notably, FGAs and SGAs attenuate the hyperactivity associated with the drug-induced hyperdopaminergia (16, 17) suggesting predictive validity of this model for drugs with antipsychotic efficacy.
In a series of studies aimed at investigating OT’s anti-addiction properties, Sarnyai et al. (1990), reported that subcutaneous (SC) OT produced a dose-dependent attenuation of cocaine-induced hyperactivity (18) (see Table 1 for a summary of OT preclinical studies with relevance to the positive symptoms of SCZ). A follow up microdialysis study confirmed these findings were associated with OT’s inhibition of the cocaine-induced increase in nucleus accumbens dopamine levels (19). Importantly, the nucleus accumbens is a brain region highly responsive to the dopamine elevating effects of stimulants, and has been implicated in both the pathophysiology of SCZ (15) as well as the clinical effects of APDs (20). A subsequent, similar study by Qi et al. (21) showed that Intracerebroventricular (ICV) OT dose-dependently blocked methamphetamine-induced hyperactivity as well as the methamphetamine-induced increase in nucleus accumbens and striatal dopamine.
Table 1.
Preclinical studies: therapeutic-like effects of OT on positive-like symptoms, negative-like symptoms and cognitive-like deficits
| Authors | Model/Parameter | Main Findings | Implication |
|---|---|---|---|
| Positive-Like Symptoms | |||
| Sarnyai et al. 1990 (18), Kovacs et al. 1990 (19) | Cocaine-induced hyperactivity Cocaine-induced DA turnover | SC OT blocked cocaine-induced hyperactivity and blocked cocaine-induced increase in NAC but not caudate DA turnover | Suggests OT can inhibit excessive mesolimbic DA consistent with (21, 94–99) |
| Feifel and Reza 1999 (22) | PPI | SC OT blocked PPI deficits induced by indirect DA agonist (Amphetamine) but not direct DA agonist (apomorphine) SC OT blocked PPI deficits induced by NMDA antagonist (dizocilpine) | First demonstrated therapeutic-like effect of OT on a SCZ phenotype Suggest OT inhibits presynaptic not postsynaptic DA function Indicates OT counteracts hypoglutamatergia – profile more similar to SGA than FGA |
| Qi et al. 2008 (21) | MAP-induced hyperactivity | Methamphetamine hyperactivity dose dependently inhibited by SC OT | Similar to (18) |
| Caldwell et al. 2009 (30) | PPI | Mice lacking OT gene exhibited enhanced PPIdeficits after PCP but not amphetamine | Suggests endogenous OT has protective effect against hypoglutamatergia (endogenous APD) |
| Negative-Like Symptoms | |||
| Cushing and Carter 2000(47) | Partner preference | Subchronic peripheral (3 consecutive daily injections) but not acute OT facilitated partner preference in female prairie voles | OT treatments may enhance social bonding. May be therapeutic for asociality. |
| Bowen et al. 2011 (48) | Social Interaction | 10 days of daily OT PND 33–42 (early adolescence) enhanced social interaction on PDN 55 in rat | Subchronic OT during adolescence has an enduring effect on improving social interaction. OT may be developmental treatment for asociality |
| Lukas et al. 2011 (44) | Social preference | OT antagonist reduced social exploration in rats and mice Loss of social preference after single social defeat restored by ICV OT | Supports critical role of endogenous OT in mediating social preference deficits |
| Chang et al. 2012 (45) | Affiliation test | Inhaled OT increased the frequency of prosocial choices associated with reward in rhesus monkeys | Supports OT improving asociality |
| Huang et al. 2012 (46) | Social interaction | Single IN OT treatment facilitated --and chronic IN OT disrupted -- social behaviors in mice and reduced OXTR throughout the brain | Does not support chronic OT improving social interaction; suggests difference between acute and chronic treatment. |
| Bales et al. 2013 (52) | Partner preference test | Single IN OT treatment enhanced social behavior in male voles, whereas chronic IN OT exacerbated social behavior | Similar to (46, 52) |
| Rault et al. 2013 (59) | Social interaction | Chronic OT produced less social contact in pigs (59) | Similar to (46, 52) |
| Teng et al. 2013 (49) | Social interaction | Intermittent OT administration (4 doses separated by 2 days) improved sociability in C58/J and BALB/c mice | Intermittent OT treatment may improve social deficits |
| Bales et al. 2014 (51) | Social interaction | Chronic IN OT in voles produced no effects on reciprocal social interactions or social approach | Similar to (46, 52) |
| Calcagnoli et al. 2014 (54) | Resident intruder test | Chronic OT via osmotic pump enhanced social interaction in male rats | Supports chronic OT facilitates social interaction |
| Meziane et al. 2014 (100) | Social interaction | OT reversed social interaction deficits in MAGEL2 KO adult mice | Suggests OT given in early life can improve social interaction in adulthood |
| Penagarikano et al. 2015 (55) | Social interaction | OT improved social deficits and enhanced endogenous OT production in the brains of CNTNAP2 KO mice with social deficits. OT antagonist blocked rescue of social deficits | Supports OT improving social interaction |
| Keebaugh et al. 2015 (101) | Partner Preference Test | Knockdown of OXTR by RNA interference in the nucleus accumbens produced disrupted partner preference formation in female prairie voles | Supports OXTR expression in this brain region plays a critical role in social attachment |
| Cognitive-Like Deficits | |||
| Popik et al. 1991 (62) | Social recognition | OT infusion into the rat medial preoptic area facilitated social recognition | Supports OT improving social recognition |
| Ferguson et al. 2000, Winslow et al. 2000 (63, 102) | Social recognition | OT KO mice failed to recognize familiar conspecifics; OT rescued deficits | OT necessary for social recognition |
| Ferguson et al. 2001 (66) | Social recognition | Infusion of OT into medial preoptic area restored social recognition in OT KO mice | Similar to (62) |
| Takayanagi et al. 2005 (67) | Social recognition | OXTR KO mice exhibited social recognition deficits | OXTR necessary for social recognition |
| Crawley et al. (65) | Social recognition vs. novel object recognition | Two lines of OT KO mice exhibited deficits in social recognition but not novel object recognition | Supports specificity of OT-dependent social recognition deficits |
| Lee et al. 2008 (68) | Social recognition | Conditional OXTR KO and forebrain specific OXTR KO males exhibited impaired social memory for female mice. | OXTR necessary for social recognition |
| Parr et al. 2013 (69) | Social perception | IN OT significantly reduced monkeys' attention to negative facial expressions, but not neutral social or nonsocial images | First to demonstrate an effect of IN-OT on social perception in monkeys |
| Feifel et al. (Unpublished) | Social recognition | OT restored social recognition in BN rats with natural deficits in this area | Supports OT improving social recognition deficits |
| Feifel et al. 2012 (74) | PPI | OT, but not carbetocin (a long-acting OT analogue), facilitated PPI | Supports OT facilitating natural sensory motor gating deficits |
| Feifel et al. (Unpublished) | PPI | IN OT facilitated PPI in a dose dependent manner in male and female BN rats up to 30 min after treatment | Supports OT facilitating natural sensory motor gating deficits in both male and female rats |
| Tomizawa et al. 2003 (70) | Spatial memory | OT improved long-lasting spatial memory through a MAP kinase cascade | OT also enhances spatial memory |
| Wu et al. 2004 (71) | Spatial learning | OT infused into the rat nucleus basalis of Meynert inhibited spatial learning | OT effects on spatial memory isbrain region dependent |
| Feifel et al. 2014 (72) | Latent inhibition | OT facilitated LI in BN rat | Support for OT improvement in cognitive-like deficits |
| Huang et al. 2012 (46) | PPI | Chronic OT had no effect on PPI in male mice | Does not support chronic OT facilitating PPI (single-dose OT not tested) |
| Meziane et al. 2014 (100) | Social recognition, spatial learning | OT restored social recognition and spatial learning in MAGEL2 KO mice | Supports OT improving social recognition and spatial learning |
| Havranek et al. 2015 (103) | Novel object recognition | Seven days ICV OT in rat increased exploration of a novel versus familiar object | Supports OT facilitation of visual memory |
| Domain Not Specified | |||
| Uvnas-Moberg et al. 1992} (104) | APD effects on pOT | Amperozide and clozapine--but not haloperidol--elevated pOT level | Supports activation of endogenous OT by SGA but not FGAs |
| Kiss et al. 2010 (105) | APD-induced IEG activation | Olanzapine and clozapine--but not haloperidol or risperidone--upregulated IEG expression in the PVN and SON | Supports endogenous OT activation by certain SGAs but not FGAs |
Abbreviations: APD, antipsychotic drug; BN, Brown Norway; CEA, central amygdaloid nucleus; DA, dopamine; FGA, first generation antipsychotic drug; ICV, intracerebroventricular; IEG, immediate early gene; IN, intranasal; KO, knockout; LI, latent inhibition; MAGEL2, melanoma antigen family L2; MAP, mitogen-activated protein kinase; NAc nucleus accumbens; NMDA, N-methyl-D-aspartate OT, oxytocin; OTA, oxytocin antagonist; pOT, plasma oxytocin; PPI, prepulse inhibition; PVN, paraventricular nucleus; SC, subcutaneous; SGA, second generation antipsychotic drug; SON, supraoptic nucleus
Feifel and Reza (22) conducted the first intentional investigation of OT’s antipsychotic potential by examining its ability to reverse deficient prepulse inhibition of the startle reflex (PPI). PPI is a measure of sensorimotor gating, a key process involved in the central nervous system’s processing of information (23). Deficits in PPI have been consistently demonstrated in patients with SCZ and are thought to reflect an underlying abnormality in the brain’s process of gating excessive environmental stimulus, thereby leaving SCZ patients vulnerable to a chaotic internal representation of reality. Deficient PPI is thus considered an ‘endophenotype’ of SCZ (24).
SCZ-like deficits in PPI can be modeled in animals in a number of ways, including the administration of psychotomimetic drugs, such as those that increase mesolimbic dopamine transmission. Importantly, the potency of reversal of PPI deficits by APDs are predictive of their potency against positive symptoms (25). As all established APDs bind D2 receptors, they are able to block disruption of PPI by both direct (e.g. dopamine mimetics) and indirect dopamine agonists (which increase extracellular dopamine levels).
Feifel and Reza demonstrated that subcutaneously (SC) administered OT reversed PPI deficits induced by the indirect-dopamine agonists amphetamine but not by the direct dopamine agonist apomorphine, suggesting OT had the potential to attenuate mesolimbic hyperdopaminergia via presynaptic rather than postsynaptic mechanisms. These findings present the possibility that OT may have a mechanism of action that complements the postsynaptic inhibition of mesolimbic dopamine transmission produced by established APDs. Although it is thought that peptides such as OT do not effectively cross the blood-brain barrier, a recent study demonstrated that intraperitoneal administration of pharmacological doses of OT in rodents produced a rapid, significant increase in brain OT levels (26). This effect may in part be due to stimulation of endogenous OT release in the brain.
In addition to mesolimbic hyperdopaminergia, reduced central glutamatergic function--particularly through the NMDA receptor complex--has also been implicated in positive symptoms (27). In kind, non-competitive NMDA antagonists such as PCP and its analog, dizocilpine (MK801), produce PPI deficits in animals. Whereas both FGAs and SGAs reverse PPI deficits produced by dopamine agonists, only SGAs will reverse PPI deficits produced by non-competitive NMDA antagonists (28). Furthermore, PPI deficits in SCZ patients are consistently restored by treatment with SGAs (29). In their abovementioned study, Feifel and Reza also found that SC OT restored PPI deficits induced by dizocilpine. This finding suggested that OT’s APD-like profile involved more mechanisms than just dopamine inhibition, and that it shared features with the multi-transmitter effects of SGAs (22).
Other supporting data regarding OT and the glutamate system came from Caldwell et al. (30), who found that OT knockout (KO) mice were more sensitive to PCP-induced PPI deficits than wild type mice suggesting that endogenous OT may protect against hypoglutamatergic-induced psychosis. In contrast, KO and wild type mice were equally sensitive to amphetamine-induced PPI disruption, suggesting that the APD-like effects of endogenous OT are specific to the glutamate system.
Although PPI deficits can be induced by a plethora of factors (28), we have limited our discussion in this section to PPI-deficit animal models produced by hyperdopaminergia (e.g., amphetamine and decreased NMDA receptor activation, e.g., with PCP). These psychostimulants often produce positive symptoms in humans that are indistinguishable from the positive symptoms exhibited by patients with SCZ and have, therefore, been extensively used to develop robust, well-accepted animal models of psychosis (15, 31). Other PPI-deficit animal models such as those based on natural deficiencies (e.g., BN rat) are more appropriately included in the “Preclinical Cognitive Deficits” section since it is not clear that the mechanisms underlying these particular PPI deficits are relevant to positive symptoms.
Exogenous OT Effects on Positive Symptoms
In addition to the aforementioned preclinical evidence that OT may counteract central hyperdopaminegia and hypoglutamatergia, the finding that a single administration of intranasal (IN) OT in healthy subjects increased trust of strangers (32, 33) raised the possibility that OT may ameliorate paranoid delusions, which involve clinical levels of mistrust driven by the misattribution of malevolent intentions to others.
The last few years have seen the publication of four small clinical trials designed to primarily investigate the ability of IN OT added-on to stable doses of APDs, to reduce psychosis in SCZ (see Table 2 for a summary of OT clinical trials in SCZ). In the first of these trials, Feifel et al. carried out a clinical proof-of-concept study of OT’s therapeutic potential. In a randomized double blind placebo-controlled crossover trial, IN OT was given twice daily to patients with SCZ for 3 weeks and reported that OT significantly reduced their positive subscale scores on the Positive and Negative Symptoms Scale (PANSS) (34). Improvement in Clinical Global Impression (CGI) scores confirmed the clinical significance of their findings. In a similar trial, Pedersen, et al. (35) reported that twice daily IN OT given to SCZ patients for 2 weeks significantly lowered the PANSS positive subscale scores compared to IN placebo (35). In the largest human trial to date, Modabbernia, et al. (36) gave SCZ patients twice daily IN OT for eight weeks and reported that OT significantly improved PANSS total and positive scores starting 4 weeks after the start of OT treatment. Finally, Lee, at al. (37) gave twice daily IN OT for three weeks to SCZ patients but did not detect a significant change in Brief Psychiatric Rating Scale (BPRS). The negative finding in this study-- in contrast to the earlier three--may be related to their use of the BPRS. Specifically, this group reported the overall score of this 18-item scale, a measure which reflects the summation of various aspects of psychosis. On the other hand, studies with positive findings used the 30-item PANSS, considered the gold standard in SCZ research. Importantly, the PANSS items are readily categorized into separate subscores for positive, negative and general symptoms of SCZ.
Table 2.
Intranasal oxytocin treatment trials in schizophrenia patients stable on antipsychotic drugs: effects on positive symptoms, negative symptoms and cognitive deficits2 (Adapted from Feifel et al Biol Psychiatry 2015)
| Author | N | Dosing/Duration | Results: Positive Symptoms |
Negative Symptoms |
Cognitive Deficits |
|---|---|---|---|---|---|
| Feifel et al. 2010 (34) | 15 outpatients 12 M, 3 F | 40 IU twice daily 3 weeks/ crossover design | OT improved PANSS positive subscale and CGI after 3 weeks | OT improved negative subscale after 3 weeks | See (87) |
| Pederson et al. 2011 (35) | 20 inpatients and outpatients OT 9 M, 2 F PL 8 M, 1 F | 24 IU twice daily 2 weeks | OT improved PANSS positive subscale after 2 weeks | OT improve PANSS negative subscale | Improvement in identification of second false beliefs and trends toward significant improvement in accurate recognition of deception and rating untrustworthy faces as untrustworthy (Brune task) |
| Averbeck et al. 2011 (80) | Exp 1: 30 OT (24 M, 6 F), 29 CL, Exp2: 21 M | 24 IU, single dose | NA | NA | OT treatment improved ability of patients to recognize most emotions (hexagon emotion discrimination test) |
| Goldman et al. 2011 (81) | OT 13, 5 PS, (3 M, 2 F), 8 NPS (4 M, 4 F) 11 CTL | 10 or 20 IU, single dose | NA | NA | 10 IU dose caused decreased emotion recognition due to emotion over identification. 20 IU dose improved emotion recognition PS vs. NPS, specifically around fear recognition |
| Feifel et al. 2012 (87) | See Feifel et al. 2010 | 40 IU twice daily 3 weeks/crossover design | NA | NA | OT improved verbal learning (CVLT) but not working memory (LNS) after 3 weeks |
| Modabbernia et al. 2013 (36) | 40 inpatients 20 OT 17 M, 3 F 20 PL 8 M, 1 F | 40 IU twice daily 8 weeks | OT improved PANSS positive subscale starting at 8 weeks | OT improved PANSS negative subscale after eight weeks | NA |
| Lee et al. 2013 (37) | 12 inpatients 16 outpatients OT 9 M, 4F PL 8 M, 7 F Included schizo-affective | 20 IU twice daily 3 weeks | Positive symptoms (BPRS) not improved vs. PL after 3 weeks | Negative symptoms (BPRS) improved in small group of inpatients patients after 3 weeks | NA |
| Fischer-Shofty et al. 2013 (82) | OT 35 (31 M, 4 F), 48 CTL | 24 IU, single dose | NA | NA | OT improved recognition of kinship (Interpersonal Perception Task) |
| Davis et al. 2013 (83) | OT 11 M, PL 12 M | 40 IU, single dose | NA | NA | OT improved perception of sarcasm, deception and empathy (EPTT, Eckman Facial Recognition Task) |
| Gibson et al. 2014 (38) | 14 outpatients OT 6 M, 2 F PL 5M, 1 F | 24 IU twice daily 6 weeks | Both OT and PL groups exhibited significant improvement in PANSS positive subscale after 6 weeks | OT improved PANSS negative subscale after six weeks | OT but not PL decreased fear after 6 weeks (ER-40) |
| Cacciotti-Saija et al. 2014 (40) | SCT + OT M 18, F 9 SCT + PL 18 M, 7 F | 40 IU twice daily 6 weeks | OT did not improve positive symptoms (SAPS) beyond SCT when given in combination with 6 weeks of SCT | Increased use of IN OT, but not PL was correlated with lower SANS scores | Six weeks of daily OT added to SCT did not enhance the SCT greater than PL |
| Davis et al. 2014 (41) | SCT + OT M 13 SCT + PL M 14 | 40 IU twice weekly 6 weeks | OT did not improve positive symptoms (BPRS) beyond SCT when given in combination with 6 weeks of SCT | Six weeks of OT added to SCT did improve negative symptoms (CAINS) | Six weeks of OT added to SCT enhanced social cognitive benefits (empathic accuracy)greater than PL, lasting at least one month |
| Michalopoulou et al. 2015 (88) | OT 11 M PL 10 M | 20 IU single dose, | NA | NA | OT improved the “executive component” of working memory (Digispan) |
| Shin et al. 2015 (85) | 16 M 16 CTL | 40 IU, single dose cross over design | NA | NA | OT decreased amygdala activity for fearful faces and increased activity (fMRI) for happy faces (Emotion recognition test) |
Abbreviations: BPRS, Brief Psychiatric Research Survey; CAINS, Clinical Assessment Interview for Negative Symptoms; CTL, controls; CVLT, California Verbal Learning Test; EPTT, emotional perspective taking task; ER-40, The Emotion Recognition 40; LNS, Letter Number Sequence; NPS, non poydipsic; PANSS, Positive and Negative Symptom survey; PL, placebo; PS, polydipsic; SANS, Scale for Assessment of Negative symptoms; SAPS, Scale for Assessment of Positive Symptoms; SCT, social cognitive test; SCZ, schizophrenia
In addition to these four clinical trials, three additional clinical trials--designed to investigate the effects of IN OT on social cognition in SCZ--also measured changes in psychosis as a secondary measure. Gibson, et al. (38) gave either six weeks of twice daily IN OT or IN placebo, and found no improvement in PANSS positive scale scores.
Building on OT’s potential as a “cognitive/plasticity enhancer,” (39) two studies have investigated the benefits of OT added to a potent psychosocial treatment. Cacciotti-Salja, et al. tested the addition of twice daily IN OT or IN placebo to six weeks of social cognitive training in patients with early psychosis and found IN OT did not confer any advantage for positive symptoms (measured with the Scale for the Assessment of Positive Symptoms, SAPS) beyond the social cognition training alone (40). Davis et al. gave IN OT to SCZ patients just before twice weekly sessions of a 6-week social cognitive training and did not detect improvement in BPRS (41).
In summary, animal studies consistently support a pharmacological effect of OT predictive of therapeutic effects on positive symptoms: inhibition of excessive mesolimbic dopamine and central hypoglutamatergia. Additionally--at a symptom level--OT’s ability to enhance trust toward strangers (potentially through shifting attributional biases) provides a compelling psychological mechanism for mitigating a clinically disabling positive symptom: paranoid delusions. Three out of four clinical studies carried out primarily to measure the effects of IN OT on psychosis in SCZ patients reported that OT reduced positive symptoms. Three others designed to test OT’s ability to improve social cognition or its ability to enhance social cognitive training, however, did not detect an OT-improvement of positive symptoms.
Negative Symptoms
Negative symptoms refer to collection of deficit features often seen in patients with SCZ (as described above). These symptoms are often seen in the prodromal phase of the illness preceding acute positive symptoms, and are predictive of long-term functioning (42).
Preclinical Studies of OT’s Effects on Negative-Like Symptoms
To date, preclinical studies of OT’s potential therapeutic effects on negative symptoms have been limited to animal models of social withdrawal. These studies suggest that acute OT administration increases social interaction (see Table 1 for a summary of OT preclinical studies with relevance to the negative symptoms of SCZ). For example, Lee et al. (43) reported that a single infusion of OT into the central nucleus of the amygdala reversed PCP-induced social interaction deficits in male rats. Likewise, other studies employing a variety of paradigms in rodents and non-human primates have demonstrated that single dose (44–46) as well as subchronic (47–49) IN OT treatment facilitates social interaction. These findings, together with evidence of pro-social effects following acute IN administration of OT in humans, (50) (vide infra) has fueled enthusiasm that OT or an OT analog could effectively ameliorate asociality, a hallmark negative symptom. Though acute OT effects on social behavior in animals appeared promising, several recent studies investigating chronic OT administration in animals failed to produce the acute OT-induced pro-social effects and in one case even had the opposite effect (46, 51, 52), but see Calcagnoli et al. (53, 54). Although these negative findings using chronic OT have somewhat tempered enthusiasm that OT can be used to treat social motivation deficits in SCZ patients, it is important to note that OT was administered to “normal” animals without social impairments and/or signaling deficits. Therefore, these studies may not adequately model the effects of chronic OT in a clinical population. In that regard, it is notable that, Penagarikano et al 2015, (55) recently reported that chronic OT treatment restored social functioning and enhanced OT production in the brains of Cntnap2 KO mice: a strain that exhibit social deficits and diminished OT signaling.
The effects of OT in animal models of non-social domains of negative symptoms have not been reported. That said, many animal models exist that would enable investigators to test the potential efficacy of OT against non-social negative symptoms. For example, Probabilistic Learning, a task used to measure cross-species reinforcement learning (56), and could be used to test the effects of OT in an animal model with relevance to anhedonia (45). A Progressive Ratio Breakpoint Schedule of Reinforcement--commonly used to assess the reinforcing properties of food or drugs--could be used to test the effects of OT on motivation and hedonic states (57, 58). Exploring the effects of OT in animal models such as these could provide very valuable information relevant to OT’s potential to modulate non-social negative symptoms.
Exogenous OT effects on Negative Symptoms
Within the last five years, six of seven published double-blind randomized clinical trials examining the effects of 2 – 6 weeks of IN OT added to stable doses of atypical APDs in SCZ patients have reported evidence that IN OT improved negative symptoms (see Table 3). Feifel et al. (34), Modabbernia et al. (36) and Gibson et al. (38) all reported that twice daily IN OT for 3 - 6 weeks significantly decreased the PANSS negative subscale and Pederson et al. (35) found that twice daily IN OT for 2 weeks produced a nearly significant reduction in this subscale (P<0.08). Two studies using the SANS, on the other hand, had mixed results. Lee et al. (37) reported an improvement in scores in a subset of patients after twice daily IN OT, whereas Cacciotti-Salja et al. (40) discovered that although daily OT added to social cognition training did not add to the improvement in SANS scores, volume of OT but not placebo, administered IN by patients with SCZ was positively correlated with reduction in negative symptoms as measured by the Scale for Assessment of Negative Symptoms (SANS). In contrast, Davis et al. (41) did not detect any improvement in Clinical Assessment Interview for Negative Symptoms (CAINS) scores when a single dose of IN OT was given twice a week prior to a session of social skills training.
It is notable that these clinical studies reveal promising effects of chronic daily OT on negative symptoms, in contrast with animal studies (46, 51, 52, 59), which revealed no benefit –and potential worsening--of social interaction in rodents after chronic OT administration.
Cognitive Deficits
Exogenous OT effects on cognitive deficits in preclinical and clinical studies are briefly described in Table 1 and Table 3 respectively. For a more detailed description and interpretation of these studies, please see supplemental information.
The Endogenous OT System and Positive Symptoms, Negative Symptoms and Cognitive Deficits
Text and associated table are included in supplemental information.
Conclusions
In a time of flagging innovation and investment in drug discovery, patients and their families desperately need more effective treatments for the broad range of impairments seen in the SCZ-spectrum disorders. The convergent findings described in this review--from preclinical investigations, studies of the OT system in patients with SCZ, and from the recent small randomized double-blind placebo controlled add-on clinical trials—though, not consistently positive, are nevertheless encouraging. They suggest that OT may alleviate positive symptoms and more importantly, that OT may also reduce the debilitating (and to-date treatment resistant) negative symptoms and social cognitive deficits. Importantly, across all clinical studies, IN OT was well tolerated and produced no notable adverse effects. Notwithstanding these encouraging preliminary findings, the potential of OT or OT-mimetics to alleviate symptoms of SCZ-spectrum disorders remains an open question.
Several significant factors have limited the clinical trials conducted thus far from adequately testing the therapeutic potential of OT for SCZ. These clinical trials have each been small and have each only tested one OT dose (range 48 – 80 IU / day) as an add-on therapy to stable regimens of APDs and as a result the optimal therapeutic OT dose, dosing regimen, and time course of OT treatment for each of the three SCZ domains remain to be identified. These limitations are likely driven by a confluence of financial and safety concerns. With regards to the add-on design, few IRB’s will sanction an outpatient placebo-controlled monotherapy trial of an investigational drug in SCZ subjects, especially one with an unproven mechanism of action and it has been the norm for APD monotherapy trials to mandate hospitalization of subjects during the drug washout phase as well as the initial phase of study drug treatment (89). Studies designed in this way are costly and historically have almost exclusively been conducted by pharmaceutical entities seeking regulatory approval for a proprietary agent. In this context, the add-on studies of OT in SCZ that have been conducted represent a pragmatic compromise.
Unfortunately, add-on trials, by their nature, have significant limitations (see (90, 91)). The most serious of these limitations is that they do not test the hypothesis that the added drug is an effective treatment for the clinical disorder in question; rather only whether the add-on drug plus the primary drug is more effective than the primary drug alone. In some studies, OT was administered adjunctive to non-pharmacological interventions in addition to stable regimens of APDs making the inherent effect of OT even more obscure.
A second inherent problem in add-on study designs is that the inclusion criterion for such trials requires recruitment of subjects with--at best--an incomplete response to the primary treatment (in this case an APD) and--at worst--a non-response to it. Incomplete responders have a limited dynamic range to reveal improvement to the add-on therapy compared to untreated patients due to reduced symptoms at baseline. Non-responders belong to a select subset of patients whose symptoms are inherently resistant to APD treatment and thus statistically less likely, than average SCZ patients, to respond to any drug treatment. Given these limitations, it is not surprising that drugs that have been tested as monotherapy as well as add-on treatments, exhibit smaller benefit effect sizes in the add-on role (e.g., (92)).
While OT may ultimately be best suited as an add-on to established APDs, the most direct way to conduct a proof-of-concept study for OT’s putative efficacy against SCZ--and to discern the extent of the ability to ameliorate positive, negative and cognitive symptoms--is by conducting a conventional dose-ranging, monotherapy investigation of OT in APD-free patients with SCZ. Even before conducting such a study, certain gaps in our knowledge about the drug properties of OT should be elucidated and we have discussed these knowledge gaps in a previous paper (93).
In light of the reduced effect size associated with add-on studies, an adequately powered test of OT’s efficacy adjunctive to APDs would require a larger sample size than an OT monotherapy trial. Given the commercial limitations associated with developing OT, a non-proprietary hormone, it is unlikely that funding for such a monotherapy clinical trial will be forthcoming. With these concerns in mind, one could view the extant clinical studies of OT against the core symptoms of SCZ from a hopeful vantage. That is, one could argue that given the abovementioned limitations inherent in their add-on design, and given their small sample sizes and utilization of only one OT dose, it is actually impressive that 3 out of 4 clinical trials designed to specifically test OT’s anti-psychotic effects (versus social cognition effects) have been positive (34–37). That three more recent studies designed to evaluate OT’s effect on social cognition did not find evidence of improvement in positive or negative symptoms is not surprising.
Several OT add-on studies in SCZ are currently underway (clinicaltrials.gov). Though some of them may continue to produce positive findings, given their modest subject recruitment goals and the resulting modest statistical power, in light of the above discussion, they are likely to deliver additional mixed results. Future mixed or predominately negative results from ongoing add-on OT SCZ studies run the risk that potential for stakeholders in this line of research will erroneously conclude that the proposition that OT has novel therapeutic effects for SCZ will have been proven untrue, halting further investigation of what might be an important new treatment.
Supplementary Material
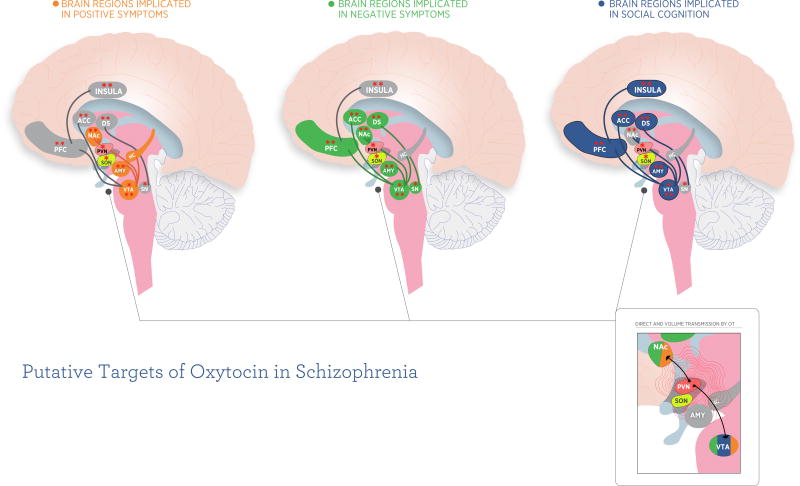
Figure 1.
Putative brain circuits implicated in oxytocin’s effects on 3 domains of schizophrenia. Colored regions in each figure represent brain regions implicated in this symptom domain. Single asterisk represents areas putatively expressing OXTR in humans (based on (106)). Although Loup used older technology to identify OXTR positive brain regions, this is one of only two studies we are aware of that has reported on the localization of OXTR expression in specific human brain nuclei. In the second study, Boccia et al. 2013 (107) did not test their OXTR antibody for specificity in the human brain making their data potentially unreliable. The Loup methodology has been superceded by new molecular biological techniques that have been applied to detecting OXTR expression in rhesus monkey brain (108). Double asterisk signifies brain regions hypothesized to be modulated by single-dose IN OT (in normal controls, and a single imaging study in schizophrenia) in human functional imaging studies. For references, see (85, 86 109–121).
Inset depicts two modes of central oxytocinergic communication: 1) direct transmission via OT neurons; 2) volume transmission via exocytosis and diffusion in the extracellular fluid and cerebrospinal fluid (122–124).
Abbreviations: ACC, anterior cingulate cortex; AMY, amygdala; DS, dorsal striatum; HC, hippocampus; NAc, nucleus accumbens; PFC, prefrontal cortex (including medial prefrontal cortex and orbitofrontal cortex); PVN, paraventricular nucleus; SN, substantia nigra; SON, supraoptic nucleus; VTA, ventral tegmental area
Acknowledgments
DF and PDS are partially funded by NIMH. KSM is supported in part by the Goodenough Neuroscience Research Fund. We thank Maribel Santos for her illustrations.
Footnotes
While OT exerts its primary actions by binding the single oxytocin receptor (OXTR), it also has significant affinity for vasopressin receptors, including the vasopressin-1A (V1A) receptor (the AVPR most abundant in brain) 8. Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, et al. (2012): Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 24:609–628. Experiments disambiguating the relative roles of OXTR versus V1AR in mediating OTs central effects have, in fact, found that AVPR often contribute to OT’s effects, 9. Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, et al. (2011): Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 69:875–882., and sometimes, is solely responsible, 10. Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, et al. (2010): Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 30:8274–8284. 11. Song Z, McCann KE, McNeill JKt, Larkin TE, 2nd, Huhman KL, Albers HE (2014): Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 50C:14–19. That said, the relative role of OXTR versus AVPRs has not been well elucidated in clinical or preclinical studies related to SCZ spectrum disorders. Thus, readers should keep in mind that the experimental effects of OT reviewed herein, may be mediated by OXTR, one of the AVPRs, or both. Readers should also note that the structure, function, and physiology of the OT system are described in detail in other papers in this special Edition and have previously been reviewed by 12. Macdonald K, Feifel D (2012): Oxytocin in schizophrenia: a review of evidence for its therapeutic effects. Acta Neuropsychiatr. 24:130–146.
Several decades ago, investigators in the USSR published two letters {Bujanow, 1972 #23117;Bujanow, 1974 #23116} describing open-label experience using OT to treat patients with “SCZ” and a small randomized study {Bakharev, 1984 #23115} of OT in “SCZ” subjects. These reports suggested that OT had therapeutic effects. However, these reports contain clinical descriptions and terminology that do not correspond to contemporary concepts of SCZ. Furthermore, the rigor of methodology and reporting is well below accepted current standards. These shortcomings significantly limit the value of these early reports to shed light on the effects of OT in SCZ
Disclosures
DF is named inventor of a patent filing by UCSD involving the use of oxytocin. PDS and KM report no biomedical financial interests or potential conflicts of interest.
References
- 1.Young J, Geyer M. Developing treatments for cognitive deficits in schizophrenia: The challenge of translation. J Psychopharmacol. 2015;29:178–196. doi: 10.1177/0269881114555252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feifel D, Shilling PD. Modeling schizophrenia in animals. In: Conn MP, editor. Animal Models for the Study of Human Disease. New York: Elsevier; 2013. pp. 727–748. [Google Scholar]
- 3.Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17:1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger DR, Gallhofer B. Cognitive function in schizophrenia. Int Clin Psychopharmacol. 1997;12(Suppl 4):S29–36. doi: 10.1097/00004850-199709004-00006. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter WT, Koenig JI. The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacology. 2008;33:2061–2079. doi: 10.1038/sj.npp.1301639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarnyai Z, Kovacs GL. Oxytocin in learning and addiction: From early discoveries to the present. Pharmacol Biochem Behav. 2014;119:3–9. doi: 10.1016/j.pbb.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Z, McCann KE, McNeill JKt, Larkin TE, 2nd, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50C:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macdonald K, Feifel D. Oxytocin in schizophrenia: a review of evidence for its therapeutic effects. Acta Neuropsychiatr. 2012;24:130–146. doi: 10.1111/j.1601-5215.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- 14.Winton-Brown TT, Fusar-Poli P, Ungless MA, Howes OD. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014;37:85–94. doi: 10.1016/j.tins.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Segal DS, Geyer MA, Schuckit MA. Stimulant-induced psychosis: an evaluation of animal methods. Essays Neurochem Neuropharmacol. 1981;5:95–129. [PubMed] [Google Scholar]
- 16.Hoffman DC. Typical and atypical neuroleptics antagonize MK-801-induced locomotion and stereotypy in rats. J Neural Transm Gen Sect. 1992;89:1–10. doi: 10.1007/BF01245347. [DOI] [PubMed] [Google Scholar]
- 17.Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacol Biochem Behav. 2006;85:481–491. doi: 10.1016/j.pbb.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarnyai Z, Szabo G, Kovacs GL, Telegdy G. Oxytocin attenuates the cocaine-induced exploratory hyperactivity in mice. Neuroreport. 1990;1:200–202. doi: 10.1097/00001756-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs GL, Sarnyai Z, Barbarczi E, Szabo G, Telegdy G. The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology. 1990;29:365–368. doi: 10.1016/0028-3908(90)90095-9. [DOI] [PubMed] [Google Scholar]
- 20.Schiller D, Zuckerman L, Weiner I. Abnormally persistent latent inhibition induced by lesions to the nucleus accumbens core, basolateral amygdala and orbitofrontal cortex is reversed by clozapine but not by haloperidol. J Psychiatr Res. 2006;40:167–177. doi: 10.1016/j.jpsychires.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol. 2008;376:441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- 22.Feifel D, Reza T. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology. 1999;141:93–98. doi: 10.1007/s002130050811. [DOI] [PubMed] [Google Scholar]
- 23.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies [see comments] Archives of General Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood TA, Swerdlow NR, Gur RE, Cadenhead KS, Calkins ME, Dobie DJ, et al. Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2013;170:521–532. doi: 10.1176/appi.ajp.2012.12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 29.Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int J Neuropsychopharmacol. 2006:1–15. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- 30.Caldwell HK, Stephens SL, Young WS., 3rd Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry. 2009;14:190–196. doi: 10.1038/sj.mp.4002150. [DOI] [PubMed] [Google Scholar]
- 31.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 32.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, et al. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res. 2011;132:50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, et al. Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia : an 8-week, randomized, double-blind, placebo-controlled study. CNS Drugs. 2013;27:57–65. doi: 10.1007/s40263-012-0022-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee MR, Wehring HJ, McMahon RP, Linthicum J, Cascella N, Liu F, et al. Effects of adjunctive intranasal oxytocin on olfactory identification and clinical symptoms in schizophrenia: results from a randomized double blind placebo controlled pilot study. Schizophr Res. 2013;145:110–115. doi: 10.1016/j.schres.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, Pedersen CA. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophr Res. 2014;156:261–265. doi: 10.1016/j.schres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2014 doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cacciotti-Saija C, Langdon R, Ward PB, Hickie IB, Scott EM, Naismith SL, et al. A Double-blind Randomized Controlled Trial of Oxytocin Nasal Spray and Social Cognition Training for Young People With Early Psychosis. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis MC, Green MF, Lee J, Horan WP, Senturk D, Clarke AD, et al. Oxytocin-Augmented Social Cognitive Skills Training in Schizophrenia. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–790. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30:1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- 44.Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci U S A. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia D, et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39:1102–1114. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- 48.Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One. 2011;6:e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, et al. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology. 2013;72:187–196. doi: 10.1016/j.neuropharm.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, et al. Long-term exposure to intranasal oxytocin in a mouse autism model. Translational psychiatry. 2014;4:e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, et al. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry. 2013;74:180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calcagnoli F, Kreutzmann JC, de Boer SF, Althaus M, Koolhaas JM. Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology. 2015;51:112–121. doi: 10.1016/j.psyneuen.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 54.Calcagnoli F, Meyer N, de Boer SF, Althaus M, Koolhaas JM. Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Horm Behav. 2014;65:427–433. doi: 10.1016/j.yhbeh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Science translational medicine. 2015;7:271ra278. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ragland JD, Cools R, Frank M, Pizzagalli DA, Preston A, Ranganath C, et al. CNTRICS final task selection: long-term memory. Schizophr Bull. 2009;35:197–212. doi: 10.1093/schbul/sbn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellenbroek BA, Cools AR. Animal models for the negative symptoms of schizophrenia. Behav Pharmacol. 2000;11:223–233. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Young JW, Geyer MA. Action of modafinil--increased motivation via the dopamine transporter inhibition and D1 receptors? Biol Psychiatry. 2010;67:784–787. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rault JL, Carter CS, Garner JP, Marchant-Forde JN, Richert BT, Lay DC., Jr Repeated intranasal oxytocin administration in early life dysregulates the HPA axis and alters social behavior. Physiol Behav. 2013;112–113:40–48. doi: 10.1016/j.physbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev. 2013;37:2166–2180. doi: 10.1016/j.neubiorev.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 61.Chang SW, Platt ML. Oxytocin and social cognition in rhesus macaques: implications for understanding and treating human psychopathology. Brain Res. 2014;1580:57–68. doi: 10.1016/j.brainres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol. 1991;1:555–560. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- 63.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 64.Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Curr Opin Neurobiol. 2004;14:248–253. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 65.Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parr LA, Modi M, Siebert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys' attention to negative facial expressions. Psychoneuroendocrinology. 2013;38:1748–1756. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, et al. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- 71.Wu W, Yu LC. Roles of oxytocin in spatial learning and memory in the nucleus basalis of Meynert in rats. Regul Pept. 2004;120:119–125. doi: 10.1016/j.regpep.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 72.Feifel D, Shilling PD, Hillman J, Maisel M, Winfield J, Melendez G. Peripherally administered oxytocin modulates latent inhibition in a manner consistent with antipsychotic drugs. Behav Brain Res. 2014;278C:424–428. doi: 10.1016/j.bbr.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9. doi: 10.1016/j.schres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 74.Feifel D, Shilling PD, Belcher AM. The effects of oxytocin and its analog, carbetocin, on genetic deficits in sensorimotor gating. Eur Neuropsychopharmacol. 2012;22:374–378. doi: 10.1016/j.euroneuro.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feifel D, Shilling PD, Melendez G. Clozapine and PD149163 elevate prepulse inhibition in Brown Norway rats. Behav Neurosci. 2011;125:268–272. doi: 10.1037/a0022691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 77.Davis JL, Pico RM, Cherkin A. Dose-dependent and time-dependent action of oxytocin on chick memory. Brain Res. 1983;266:355–358. doi: 10.1016/0006-8993(83)90669-8. [DOI] [PubMed] [Google Scholar]
- 78.Sarnyai Z, Jashar C, Olivier B. Modeling combined schizophrenia-related behavioral and metabolic phenotypes in rodents. Behav Brain Res. 2014 doi: 10.1016/j.bbr.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 79.Evans SL, Dal Monte O, Noble P, Averbeck BB. Intranasal oxytocin effects on social cognition: a critique. Brain Res. 2014;1580:69–77. doi: 10.1016/j.brainres.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2011:1–8. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology (Berl) 2011;216:101–110. doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- 82.Fischer-Shofty M, Brune M, Ebert A, Shefet D, Levkovitz Y, Shamay-Tsoory SG. Improving social perception in schizophrenia: the role of oxytocin. Schizophr Res. 2013;146:357–362. doi: 10.1016/j.schres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF, et al. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res. 2013;147:393–397. doi: 10.1016/j.schres.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 84.Woolley JD, Chuang B, Lam O, Lai W, O'Donovan A, Rankin KP, et al. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology. 2014;47:116–125. doi: 10.1016/j.psyneuen.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin NY, Park HY, Jung WH, Park JW, Yun JY, Jang JH, et al. Effects of Oxytocin on Neural Response to Facial Expressions in Patients with Schizophrenia. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wigton R, Radua J, Allen P, Averbeck B, Meyer-Lindenberg A, McGuire P, et al. Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci. 2015;40:E1–E22. doi: 10.1503/jpn.130289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feifel D, Macdonald K, Cobb P, Minassian A. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr Res. 2012;139:207–210. doi: 10.1016/j.schres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 88.Michalopoulou PG, Averbeck BB, Kalpakidou AK, Evans S, Bobin T, Kapur S, et al. The effects of a single dose of oxytocin on working memory in schizophrenia. Schizophr Res. 2015 doi: 10.1016/j.schres.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feifel D. The Use of Placebo-Controlled Clinical Trials for the Approval of Psychiatric Drugs: Part II-Ethical Considerations Related to the Individual Participant. Psychiatry (Edgmont) 2009;6:19–25. [PMC free article] [PubMed] [Google Scholar]
- 90.Boers M. Add-on or step-up trials for new drug development in rheumatoid arthritis: a new standard? Arthritis Rheum. 2003;48:1481–1483. doi: 10.1002/art.11141. [DOI] [PubMed] [Google Scholar]
- 91.Ottolenghi L, Bertele V, Garattini S. Limits of add-on trials: antirheumatic drugs. Eur J Clin Pharmacol. 2009;65:33–41. doi: 10.1007/s00228-008-0545-z. [DOI] [PubMed] [Google Scholar]
- 92.Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry. 2014;53:153–173. doi: 10.1016/j.jaac.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 93.Macdonald K, Feifel D. Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci. 2013;7:35. doi: 10.3389/fnins.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16:e92–123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Drago F, Caldwell JD, Pedersen CA, Continella G, Scapagnini U, Prange AJ., Jr Dopamine neurotransmission in the nucleus accumbens may be involved in oxytocin-enhanced grooming behavior of the rat. Pharmacol Biochem Behav. 1986;24:1185–1188. doi: 10.1016/0091-3057(86)90168-1. [DOI] [PubMed] [Google Scholar]
- 97.Drago F, Di Leo F, Giardina L. Prenatal stress induces body weight deficit and behavioural alterations in rats: the effect of diazepam. Eur Neuropsychopharmacol. 1999;9:239–245. doi: 10.1016/s0924-977x(98)00032-7. [DOI] [PubMed] [Google Scholar]
- 98.Kovacs G, Telegdy G. Effects of oxytocin, des-glycinamide-oxytocin and anti-oxytocin serum on the alpha-MPT-induced disappearance of catecholamines in the rat brain. Brain Res. 1983;268:307–314. doi: 10.1016/0006-8993(83)90497-3. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 100.Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, et al. An Early Postnatal Oxytocin Treatment Prevents Social and Learning Deficits in Adult Mice Deficient for Magel2, a Gene Involved in Prader-Willi Syndrome and Autism. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 101.Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci. 2015:1–10. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- 103.Havranek T, Zatkova M, Lestanova Z, Bacova Z, Mravec B, Hodosy J, et al. Intracerebroventricular oxytocin administration in rats enhances object recognition and increases expression of neurotrophins, microtubule-associated protein 2, and synapsin I. J Neurosci Res. 2015 doi: 10.1002/jnr.23559. [DOI] [PubMed] [Google Scholar]
- 104.Uvnas-Moberg K, Alster P, Svensson TH. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology (Berl) 1992;109:473–476. doi: 10.1007/BF02247726. [DOI] [PubMed] [Google Scholar]
- 105.Kiss A, Bundzikova J, Pirnik Z, Mikkelsen JD. Different antipsychotics elicit different effects on magnocellular oxytocinergic and vasopressinergic neurons as revealed by Fos immunohistochemistry. J Neurosci Res. 2010;88:677–685. doi: 10.1002/jnr.22226. [DOI] [PubMed] [Google Scholar]
- 106.Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- 107.Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 108.Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mikell CB, McKhann GM, Segal S, McGovern RA, Wallenstein MB, Moore H. The hippocampus and nucleus accumbens as potential therapeutic targets for neurosurgical intervention in schizophrenia. Stereotact Funct Neurosurg. 2009;87:256–265. doi: 10.1159/000225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 111.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 112.Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 113.Viviani D, Terrettaz T, Magara F, Stoop R. Oxytocin enhances the inhibitory effects of diazepam in the rat central medial amygdala. Neuropharmacology. 2010;58:62–68. doi: 10.1016/j.neuropharm.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 114.Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38:962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 115.Zink CF, Meyer-Lindenberg A. Human neuroimaging of oxytocin and vasopressin in social cognition. Horm Behav. 2012;61:400–409. doi: 10.1016/j.yhbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24:645–692. doi: 10.1016/j.euroneuro.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 117.Rosenfeld AJ, Lieberman JA, Jarskog LF. Oxytocin, dopamine, and the amygdala: a neurofunctional model of social cognitive deficits in schizophrenia. Schizophr Bull. 2011;37:1077–1087. doi: 10.1093/schbul/sbq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fujiwara H, Yassin W, Murai T. Neuroimaging studies of social cognition in schizophrenia. Psychiatry and Clnical Neurosciences. 2015 doi: 10.1111/pcn.12258. In Press. [DOI] [PubMed] [Google Scholar]
- 119.Hamilton TJ, Wheatley BM, Sinclair DB, Bachmann M, Larkum ME, Colmers WF. Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proc Natl Acad Sci U S A. 2010;107:18185–18190. doi: 10.1073/pnas.1011558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kanat M, Heinrichs M, Mader I, van Elst LT, Domes G. Oxytocin Modulates Amygdala Reactivity to Masked Fearful Eyes. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kanat M, Heinrichs M, Schwarzwald R, Domes G. Oxytocin attenuates neural reactivity to masked threat cues from the eyes. Neuropsychopharmacology. 2015;40:287–295. doi: 10.1038/npp.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, et al. On the role of volume transmission and receptor-receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain Res. 2012;1476:119–131. doi: 10.1016/j.brainres.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 123.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 124.Veening JG, de Jong T, Barendregt HP. Oxytocin-messages via the cerebrospinal fluid: behavioral effects; a review. Physiol Behav. 2010;101:193–210. doi: 10.1016/j.physbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.