Abstract
Despite the success of combination antiretroviral therapy (cART), approximately 50% of HIV-1 seropositive individuals develop HIV-1 associated neurocognitive disorders (HAND). Unfortunately, point-of-care screening tools for HAND lack sensitivity and specificity, especially in low-resource countries. Temporal processing deficits have emerged as a critical underlying dimension of neurocognitive impairments observed in HIV-1 and may provide a key target for the development of a novel point-of-care screening tool for HAND. Cross-modal prepulse inhibition (PPI; i.e., auditory, visual, or tactile prepulse stimuli) and gap-prepulse inhibition (gap-PPI; i.e., auditory, visual or tactile prepulse stimuli), two translational experimental paradigms, were used to assess the nature of temporal processing deficits in the HIV-1 transgenic (Tg) rat. Cross-modal PPI revealed a relative insensitivity to the manipulation of interstimulus interval (ISI) in HIV-1 Tg rats in comparison to controls, regardless of prestimulus modality. Gap-PPI revealed an insensitivity to the manipulation of ISI, independent of modality, in HIV-1 Tg rats in comparison to control animals. Manipulation of context (i.e., concurrent visual or tactile stimulus) in auditory PPI revealed a differential sensitivity in HIV-1 Tg animals compared to controls. The potential utility of amodal temporal processing deficits as an innovative point-of-care screening tool was explored using a discriminant function analysis, which diagnosed the presence of the HIV-1 transgene with 97.4% accuracy. Thus, the presence of amodal temporal processing deficits in the HIV-1 Tg rat supports the hypothesis of a central temporal processing deficit in HIV-1 seropositive individuals, heralding an opportunity for the development of a point-of-care screening tool for HAND.
Keywords: HIV-1 associated neurocognitive disorders, Temporal processing, Amodal Gating, Point-of-Care Screening Tool
Introduction
The advent of combination antiretroviral therapy (cART) dramatically decreased the prevalence of HIV-1 associated dementia (HAD), however, HIV-1 associated neurocognitive disorders (HAND) continue to afflict between 40%-70% of HIV-1 seropositive individuals (Ances and Ellis, 2007; Heaton et al. 2010, 2011; Letendre et al. 2010; McArthur et al. 2010). Symptoms of milder forms of HAND include deficits in attention, memory, and executive functions (Chan and Brew, 2014; Cysique and Brew, 2011; Cysique et al. 2004; Heaton et al. 2011; Sacktor and Robertson, 2014). Ideally, a diagnosis of HAND would use a complete neurocognitive battery assessing at least five cognitive abilities (i.e., executive function, speed of information processing, motor skills, etc.; Elbirt et al. 2015; Woods et al. 2009), however, complete assessments are rarely accessible in low-resource countries (Antinori et al. 2007; Joska et al. 2016). Thus, due to the increased prevalence of milder forms of neurocognitive deficits in HIV-1 seropositive individuals, including HAND, the development of point-of-care screening tools may be of great clinical significance (Chan and Brew, 2014; Zipursky et al. 2013).
Point-of-care screening tools currently lack the sensitivity and specificity needed for an accurate diagnosis of HAND (e.g., Overton et al. 2011; Overton et al. 2013; Valcour, 2011a). Specifically, the HIV Dementia Scale (HDS; Power et al. 1995) and International HDS (IHDS; Sacktor et al. 2005) were developed early in the HIV-1 epidemic to screen for HIV-1 associated dementia (HAD), however, neither assessment is able to accurately diagnose HAND (Haddow et al. 2013; Sakamoto et al. 2013; Smith et al. 2003; Zipursky et al. 2013). The Montreal Cognitive Assessment (MoCA; Nasreddine et al. 2005), which is a useful screening tool for other neurodegenerative disorders, is inadequate for the assessment of HAND in HIV-1 seropositive individuals (Joska et al. 2016; Kim et al. 2016; Overton et al. 2013). The Wisconsin Card Sorting Test and Grooved Pegboard Test are able to accurately diagnose HAND (Ku et al. 2014), however, they require specialized equipment, have a long administration time, and there is an absence of normative data, specifically in low-resource countries (Clifford and Ances, 2013; Valcour et al. 2011b). Recent studies suggest the potential utility of computerized and tablet based screening tools for HAND (e.g., CogState (Overton et al., 2011), NeuroScreen (Robbins et al., 2014)), however, they are not without significant limitations (Valcour et al., 2011b). Thus, the development of a brief, reliable point-of-care screening tool for milder neurocognitive impairments observed in HIV-1, including HAND, has the potential for great clinical significance and may have a substantial impact on the lives of HIV-1 seropositive individuals (Chan and Brew, 2014; Zipursky et al. 2013).
Temporal processing deficits, which have been implicated as a fundamental impairment in HAND (Chao et al. 2004; Matas et al. 2010; Moran et al. 2013), may provide the basis for a point-of-care screening tool for HAND. Cross-modal prepulse inhibition (PPI) and gap-prepulse inhibition (gap-PPI), translational experimental paradigms employed in the present study, have commonly been used to assess temporal processing (Hoffman and Searle, 1965; Ison and Hammond, 1971; Ison, 1982). In both PPI and gap-PPI, a salient prestimulus (i.e., tone, air puff, gap in background noise) is presented prior to the startling stimulus (Hoffman and Ison, 1980). Decreases in the auditory startle response (ASR) are dependent upon the interstimulus interval (ISI), or time between the prestimulus and startling stimulus. Regardless of sensory modality, previous studies have revealed the most dramatic decreases in ASR when a prepulse is presented between 30-500 msec prior to the startling stimulus (Campeau and Davis, 1995; Hoffman and Ison 1980; Fitch et al. 2008; Pickney, 1976).
Cross-modal PPI (i.e., auditory or visual prepulse stimuli) has previously been used to assess temporal processing deficits in the HIV-1 transgenic (Tg) rat. Specifically, HIV-1 Tg rats were repeatedly assessed from two to six months of age to characterize auditory and visual PPI in the HIV-1 Tg rat. HIV-1 Tg animals, in comparison to controls, displayed a relative insensitivity to the manipulation of ISI and a lack of perceptual sharpening with age (Moran et al. 2013). Sprague-Dawley rats stereotaxically injected with HIV-1 viral proteins, including Tat and/or gp120, were assessed in auditory PPI, revealing a relative insensitivity to the manipulation of ISI (Fitting et al. 2006a, 2006b, 2008). Temporal processing deficits have also been assessed in the HIV-1 Tg rat using auditory gap-PPI. Specifically, HIV-1 Tg and control animals were assessed using auditory gap-PPI every thirty days from postnatal day (PD) 30 to PD 180 to assess the progression of temporal processing deficits. HIV-1 Tg animals, regardless of sex, exhibited prominent alterations in the development of prepulse inhibition and a differential sensitivity to the manipulation of ISI, in comparison to control animals (McLaurin et al. 2016a). To date, however, the role of sensory modality in temporal processing deficits in the HIV-1 Tg rat has not been systematically evaluated.
The role of sensory modality in the ontogeny of temporal processing has previously been examined (Moran et al. 2015; Parisi and Ison, 1979). The seminal study conducted by Parisi and Ison (1979) reported a linear increase in PPI from PD 12 to PD 18 regardless of modality. However, multiple caveats (i.e., use of a nested design, alterations in prepulse duration) cautioned wide generalization of the results (Parisi and Ison, 1979). Moran et al. (2015) conducted a subsequent study assessing auditory PPI, visual PPI, and tactile PPI beginning at PD 15 in male and female Long-Evans rats. A unique ontogenetic profile was observed dependent upon sensory modality, likely due to sensory maturation.
Thus, the aims of the present study were two-fold. First, to establish the amodal nature and generality of temporal processing deficits in the HIV-1 Tg rat. The HIV-1 Tg rat, which expresses 7 of the 9 HIV-1 genes constitutively throughout development, resembles HIV-1 seropositive individuals on cART. Temporal processing was assessed using cross-modal PPI (i.e., auditory, visual, or tactile prepulse stimuli) and gap-PPI (auditory, visual or tactile prepulse stimuli) between 8 and 10 months of age, prior to any documented signs of clinical wasting (Royal et al. 2012; Peng et al. 2010). Second, to determine the potential utility of temporal processing deficits as a point-of-care screening tool for milder forms of neurocognitive impairment observed in HIV-1, including HAND. A discriminant function analysis was conducted to determine which variables in cross-modal PPI and gap-PPI were best able to correctly identify animals in regards to their genotype (HIV-1 Tg vs. Control). Understanding the amodal nature of temporal processing deficits in the HIV-1 Tg rat is vital to accurately modeling neurocognitive deficits observed in HIV-1 and, most notably, may aid in the development of a point-of-care screening tool for HAND.
Methods
Animals
Temporal processing was assessed in ovariectomized female Fischer (F344/N; Harlan Laboratories Inc., Indianapolis, IN) rats (HIV-1 Tg, n=19; control, n=20) between 8 and 10 months of age using a cross-sectional experimental design. All animals were pair- or group-housed throughout experimentation. Rodent food (2020X Teklad Global Extruded Rodent Diet (Soy Protein-Free)) and water were available ad libitum throughout the experiment.
Animals were kept in AAALAC-accredited facilities according to the guidelines established by the National Institutes of Health (NIH). Environmental conditions for the animal facility were targeted at: 21° ± 2° C, 50% ± 10% relative humidity and a 12-h light:12-h dark cycle with lights on at 0700 h (EST). The project was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina under federal assurance (# A3049-01).
Apparatus
A 10 cm-thick double-walled, 81×81×116-cm isolation cabinet (external dimensions) (Industrial Acoustic Company, INC., Bronx, NY) enclosed the startle platform (SR-Lab Startle Reflex System, San Diego Instruments, Inc., San Diego, CA) instead of the 1.9 cm thick ABS plastic or laminate cabinets offered with this system. Relative to the external environment, 30 dB(A) of sound attenuation was provided in the isolation chamber. An ambient sound level of 22dB(A) was presented in the chamber without any stimuli presented. A high-frequency loudspeaker of the SR-Lab system (model #40-1278B, Radio Shack, Fort Worth, TX), which was affixed inside the chamber 30 cm above the Plexiglas, was used to deliver all auditory prepulse and stimuli (frequency range of 5k-16k Hz). A sound level meter (model #2203, Bruël & Kjaer, Norcross, GA) was used to measure and calibrate all sound levels, with the microphone placed inside the Plexiglas cylinder. Visual prepulse were presented using a white LED light (22 lux; Light meter model #840006, Sper Scientific, Ltd, Scottsdale, AZ). The white LED light was affixed inside the chamber on the wall in front of the test cylinder. Tactile prestimuli (16 p.s.i. air-puff) were presented on the dorsal surface of the rat via a semi-rigid plastic tube (0.64 mm diameter) connected to a compressed air tank via an airline regulator (model #16023, Craftsman, Hoffman Estates, IL). The animal's response to the auditory startle stimulus produced deflection of the test cylinder, which was converted into analog signals by a piezoelectric accelerometer integral to the bottom of the cylinder. Response signals were digitized (12 bit A to D) and saved to a hard disk. The SR-LAB Startle Calibration System was used to calibrate response sensitivities.
Experimental Design
All temporal processing assessments were conducted in a sequential manner as shown in Figure 1.
Figure 1. Sequential experimental design.
Procedure
Habituation
Auditory stimuli were used in a 36-trial startle test session for habituation. Habituation began with a 5-min acclimation period in the dark with 70 dB(A) background white noise. A 100 dB(A) white noise stimulus of 20 msec duration was presented for each trial. The fixed intertrial interval (ITI) was 10 sec.
Cross-Modal Prepulse Inhibition
Prepulse inhibition assessments were conducted using auditory, visual or tactile prepulse stimuli; because of hardware limitations two assessments had to be conducted. First, concurrent visual and auditory prepulse stimuli, arranged using an ABBA counterbalanced order of presentation, were used to test animals for PPI of the ASR. Second, concurrent auditory and tactile prepulse stimuli, also arranged using an ABBA counterbalanced order of presentation, were used to test animals for PPI of the ASR. In both assessments (i.e., concurrent auditory and visual stimulus and concurrent auditory or tactile stimulus) the test session was approximately 30-min. Assessments began with a 5-min acclimation period in the dark with 70 dB (A) background white noise, followed by 6 pulse-only ASR trials with a fixed 10 sec ITI. A total of 72 trials were presented, including an equal number of trials for each modality. A counterbalanced ABBA order of presentation was used to control for the order of sensory modality presentation. Trials had ISIs of 0, 30, 50, 100, 200, and 4000 msec; the 0 and 4000 msec ISI trials served as control trials to provide a reference ASR within cross-modal PPI. Trials were presented in 6-trial blocks interdigitated using a Latin-square experimental design. The ITI was variable from 15 sec to 25 sec. Regardless of modality, prepulse stimuli had a 20 msec duration. Inside the test cylinder, the startle stimulus intensity, which had a 20 msec duration, was 100 dB(A). Mean peak ASR amplitude values were collected for analysis.
Gap-Prepulse Inhibition Test
Multiple sensory modalities (i.e., auditory, visual, tactile) were used to assess gap-prepulse inhibition. Regardless of modality, the test session was approximately 20-min. A 5-min acclimation period in the dark occurred at the beginning of all test sessions. The acclimation period had 70 dB(A) background white noise and was followed by six pulse-only ASR trials with a fixed 10 sec ITI. Thirty-six trials were presented using 6-trial blocks interdigitated using a Latin-square experimental design. A 20-msec gap in background noise/light/air puff was presented dependent upon modality being tested. The 20-msec gap preceded an auditory startle stimulus, which was presented at ISIs of 0, 30, 50, 100, 200, and 4000 msec. Control trials, which included the 0 and 4000 msec ISI trials, provided a reference ASR within gap-PPI. Inside the test cylinder, the auditory startle stimulus had a duration of 20 msec and an intensity of 100dB(A). Mean peak ASR amplitude values were collected for analysis.
Statistical Analyses
Analysis of variance (ANOVA) statistical techniques were used to analyze all data (SPSS Statistics 23, IBM Corp., Somers, NY). For the repeated-measures factors, violations of the compound symmetry assumptions (e.g., trials) were corrected using either orthogonal decompositions or the post-hoc Greenhouse–Geisser df correction factor (Greenhouse and Geisser,1959). The trial-dependent effects of the HIV-1 transgene were assessed using tests of simple main effects and specific linear contrasts (Winer, 1971). Partial eta squared, which indicates the variance accounted for, was used as a measure of effect size, with a maximum of 1. GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA) was used for all regression analyses and graphs. An alpha level of p≤0.05 was considered significant for all statistical tests.
More specifically, a mixed-factor ANOVA was conducted using the mean peak ASR amplitude values. PPI and gap-PPI were analyzed for each prepulse modality (i.e., auditory, visual, tactile). The effect of context (i.e., concurrent visual or tactile stimulus) on auditory PPI was also analyzed using a mixed-factor ANOVA using the mean peak ASR amplitude values. Genotype (HIV-1 Tg vs. control) served as the between-subjects factor. Within subject's factors included context (i.e., concurrent visual or tactile stimulus), ISI and trial, as appropriate.
The diagnostic utility of amodal temporal processing deficits was assessed using a discriminant function analysis to determine which variables in cross-modal PPI and gap-PPI were best able to correctly identify animals in regards to their genotype (HIV-1 Tg vs. Control).
Results
HIV-1 Tg and control animals exhibited significant intrasession habituation
Intrasession habituation, assessed using the mean peak ASR amplitude, for HIV-1 Tg and control animals is illustrated in Figure 2. A linear decrease in mean peak ASR amplitude was observed throughout the habituation session in both control and HIV-1 Tg animals. There was no significant difference between groups in the rate at which mean peak ASR amplitudes decreased [F(1,1400)≤1.0; HIV-1 Tg animals: β=-5.41±5.41 (95% CI), Control animals: β=-5.03±5.03 (95% CI)), indicating no alterations in intrasession habituation between groups.
Figure 2.
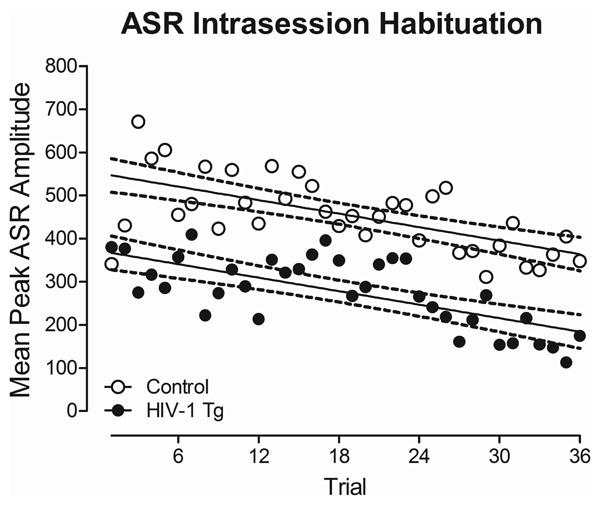
Mean peak ASR amplitude data from the habituation session is presented as a function of genotype (HIV-1 Tg or Control; ± 95% CI). A linear decrease in mean peak ASR amplitude was observed during the habituation session regardless of genotype. The rate of habituation between groups was not significantly different.
HIV-1 Tg animals displayed alterations in prepulse inhibition regardless of modality
In cross-modal PPI, regardless of modality (i.e., auditory, visual, or tactile), HIV-1 Tg animals exhibited a relative insensitivity to the manipulation of ISI in comparison to control animals, illustrated in Figure 3. Both HIV-1 Tg animals and control animals displayed maximal inhibition at the same ISI (i.e., Auditory: 30 msec; Visual: 50 msec; Tactile: 200 msec). However, HIV-1 Tg animals exhibited a relatively flatter ISI function for each modality.
Figure 3.
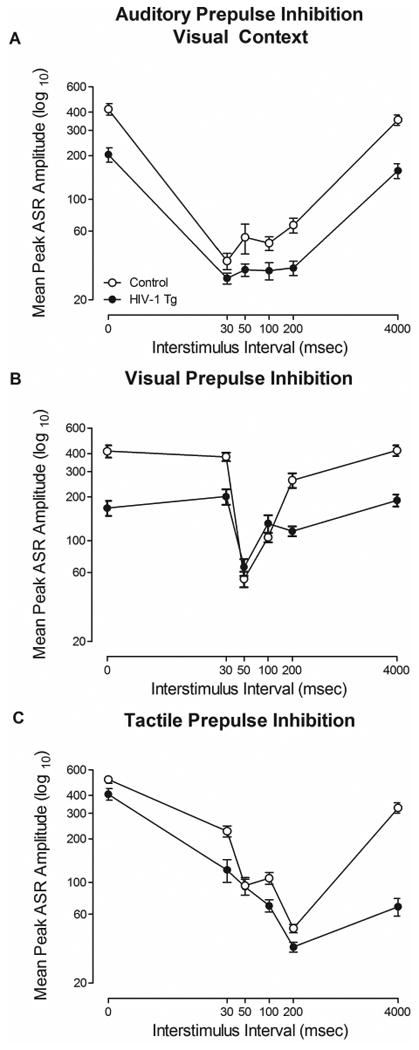
Mean peak ASR startle response for cross-modal prepulse inhibition (PPI; auditory PPI (a), visual PPI (b) and tactile PPI (c)) is illustrated as a function of genotype (HIV-1 Tg or Control; ± SEM). Regardless of sensory modality, HIV-1 Tg animals exhibited a relatively flatter interstimulus interval (ISI) function in comparison to control animals, indicating a relative insensitivity to the manipulation of ISI.
Auditory PPI (Figure 3A) revealed a relative insensitivity to the manipulation of ISI in HIV-1 Tg animals in comparison to control animals. Both HIV-1 Tg and control animals exhibited maximal inhibition at 30 msec, however, HIV-1 Tg animals displayed a relatively flatter ISI function. The overall ANOVA conducted on mean peak ASR amplitude during auditory PPI confirmed these observations, revealing a significant ISI × genotype interaction [F(5,185)=19.8, pGG≤0.001, ηp2=0.348] with a prominent quadratic component [F(1,37)=31.3, p≤0.001, ηp2=0.458]. Main effects of ISI [F(5,185)=134.9, pGG≤0.001, ηp2=0.785] and genotype [F(1,37)=32.0, p≤0.001, ηp2=0.464] were also observed.
In visual PPI, illustrated in Figure 3B, HIV-1 Tg animals exhibited a relative insensitivity to the manipulation of ISI in comparison to control animals. Maximal peak inhibition was observed at 50 msec for both HIV-1 Tg and control animals. HIV-1 Tg animals, however, displayed a relatively flatter ISI function. Observations were confirmed with the overall ANOVA conducted on mean peak ASR amplitude during visual PPI, which revealed a significant ISI × genotype interaction [F(5,185)=21.9, pGG≤0.001, ηp2=0.372] with a prominent quadratic component [F(1,37)=39.1, p≤0.001, ηp2=0.514]. A main effect of ISI [F(5,185)=66.4, pGG≤0.001, ηp2=0.642] and genotype [F(1,37)=29.2, p≤0.001, ηp2=0.441] were also observed.
Tactile PPI revealed a relative insensitivity to the manipulation of ISI in HIV-1 Tg animals (Figure 3C). HIV-1 Tg and control animals exhibited maximal peak inhibition at 200 msec; however, HIV-1 Tg animals exhibited a relatively flatter ISI function. The strong inhibition observed in HIV-1 Tg animals, in comparison to control animals, at 4000 msec provides additional evidence for significant alterations in temporal processing. The overall ANOVA confirmed observations, revealing a significant ISI × genotype interaction [F(5,185)=12.3, pGG≤0.001, ηp2=0.250] with a prominent quadratic component [F(1,37)=18.3, p≤0.001 ηp2=0.332]. Main effects of ISI [F(5,185)=123.1, pGG≤0.001, ηp2=0.769] and genotype [F(1,37)=51.9, p≤0.001, ηp2=0.584] were also observed. Thus, regardless of modality, HIV-1 Tg animals exhibited a relative insensitivity to the manipulation of ISI, supporting the hypothesis of a central temporal processing deficit.
HIV-1 Tg animals exhibited alterations in gap-PPI regardless of modality
Regardless of modality (i.e., auditory, visual, or tactile), HIV-1 Tg animals exhibited alterations in temporal processing in comparison to control animals, assessed using gap-PPI (Figure 4). In auditory and visual gap-PPI, HIV-1 Tg animals exhibited a relative insensitivity to the manipulation of ISI, evidenced by a relatively flatter ISI function. In tactile gap-PPI, HIV-1 Tg animals, in comparison to control animals, exhibited a differential sensitivity to the manipulation of ISI, evidenced by a shift in the point of maximal inhibition.
Figure 4.
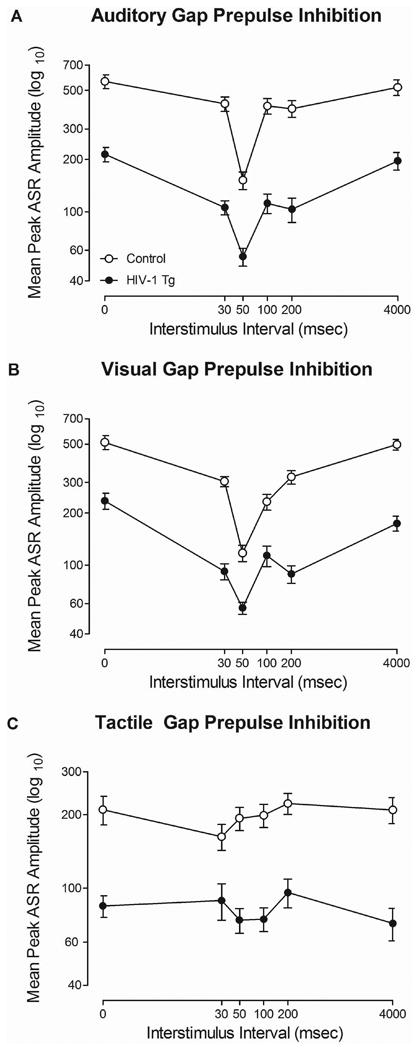
Mean peak ASR startle response for gap-prepulse inhibition (gap-PPI) is presented as a function of genotype (HIV-1 Tg or Control; ± SEM). In auditory gap-PPI (a) and visual gap-PPI (b), HIV-1 Tg animals, in comparison to control animals, exhibited a relative insensitivity to the manipulation of interstimulus interval (ISI), evidenced by a flatter ISI function. In tactile gap-PPI (c), a differential sensitivity to the manipulation of ISI was observed. Control animals exhibited peak inhibition at the 30 msec ISI, while HIV-1 Tg animals exhibited peak inhibition at the 50 msec ISI.
Auditory gap-PPI, illustrated in Figure 4A, revealed a relative insensitivity to the manipulation of ISI in HIV-1 Tg animals, in comparison to control animals. Both HIV-1 Tg and control animals exhibited maximal peak inhibition at 50 msec, however, a relatively flatter ISI function was observed in HIV-1 Tg animals. These observations were confirmed by an overall ANOVA, which revealed a significant ISI × genotype interaction [F(5,185)=11.0, pGG≤0.001, ηp2=0.229] with a prominent quadratic component [F(1,37)=14.4, p≤0.001, ηp2=0.280]. Main effects of ISI [F(5,185)=53.3, pGG≤0.001, ηp2=0.590] and genotype [F(1,37)=49.5, p≤0.001, ηp2=0.572] were also found.
In visual gap-PPI (Figure 4B), HIV-1 Tg animals exhibited a relative insensitivity to the manipulation of ISI in comparison to control animals. Maximal inhibition was observed at the 50 msec ISI for both HIV-1 Tg and control animals. HIV-1 Tg animals, however, displayed a relatively flatter ISI function. The overall ANOVA conducted on mean peak ASR amplitude confirmed these observations, revealing a significant ISI × genotype interaction [F(5,185)=11.8, pGG≤0.001, ηp2=0.242] with a prominent quadratic component [F(1,37)=25.8, p≤0.001, ηp2=0.622]. A main effect of ISI [F(5,185)=56.1, pGG≤0.001, ηp2=0.603] and a main effect of genotype [F(1,37)=82.8, p≤0.001, ηp2=0.691] were also observed.
Tactile gap-PPI revealed a differential sensitivity to the manipulation of ISI in HIV-1 Tg animals, illustrated in Figure 4C. Control animals exhibited maximal inhibition at the 30 msec ISI. HIV-1 Tg animals, in comparison, failed to display any significant inhibition. These observations were confirmed using an ANOVA, which revealed a significant ISI × genotype interaction [F(5,185)=2.7, pGG≤0.035, ηp2=0.067] with a prominent linear component [F(1,37)=4.1, p≤0.05, ηp2=0.100]. Main effects of ISI [F(5,185)=2.8, pGG≤0.029, ηp2=0.070] and genotype [F(1,37)=26.3, p≤0.001, ηp2=0.415] were also observed. Thus, regardless of modality, HIV-1 Tg animals exhibited significant alterations in temporal processing, providing additional evidence for a central temporal processing deficit in HIV-1.
HIV-1 Tg animals exhibited a differential sensitivity to the manipulation of context (i.e., concurrent visual or tactile stimulus)
A differential effect of context (i.e., concurrent visual or tactile stimuli) on auditory PPI was observed in the HIV-1 Tg rat in comparison to control animals, illustrated in Figure 5. Regardless of context, control animals exhibited maximal inhibition at the 30 msec ISI (Figure 5A). HIV-1 Tg animals, in comparison, displayed maximal inhibition at 30 msec in the visual context, but displayed a significant rightward shift to maximal inhibition at 200 msec in the tactile context (Figure 5B), indicative of a differential sensitivity to the manipulation of context. The overall ANOVA confirmed these observations, indicating an ISI × genotype interaction [F(5,185)=42.7, pGG≤0.001, ηp2=0.536] with a prominent quadratic component [F(1,37)=61.7, p≤0.001, ηp2=0.625]. In addition, main effects of modality [F(1,37)=7.1, pGG≤0.012, ηp2=0.160], ISI [F(5,185)=208.4, pGG≤0.001, ηp2=0.849] and genotype [F(1,37)=58.9, p≤0.001, ηp2=0.614] were observed.
Figure 5.
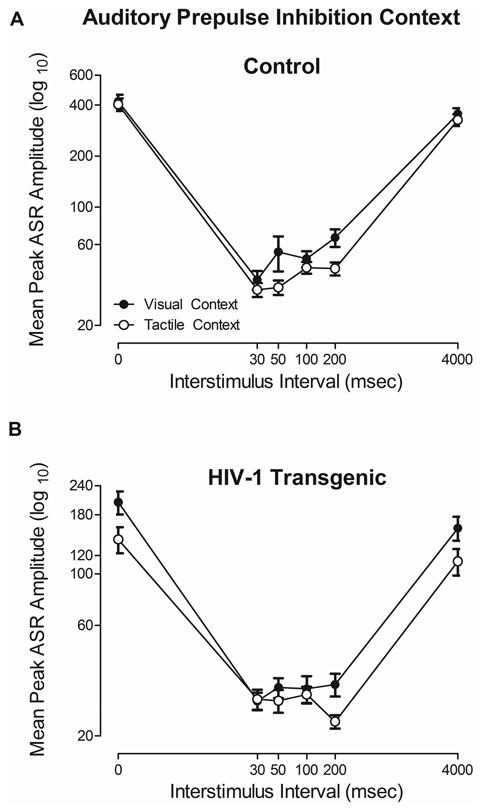
Mean peak ASR startle response for auditory prepulse inhibition (PPI) is presented as a function of context (i.e., concurrent visual or tactile stimulus). HIV-1 Tg animals exhibited a differential sensitivity to the manipulation of context, evidenced by a rightward shift in peak inhibition in the tactile context relative to the visual context. Control animals exhibited peak inhibition at the 30 msec ISI regardless of context.
Amodal temporal processing deficits may accurately predict the presence of the HIV-1 transgene
The potential utility of amodal temporal processing deficits as a point-of-care screening tool for HAND was assessed using a discriminant function analysis to determine which assessments, modalities, and ISI values were best able to identify the presence of the HIV-1 transgene. The use of temporal processing assessments from all three modalities best predicted group membership, as illustrated in Figure 6. A discriminant function analysis based on four variables (Mean Peak ASR Amplitude Values at: Auditory Gap-PPI (30 msec), Auditory PPI (Airpuff context, 200 msec), Tactile PPI (30 msec), and Visual Gap-PPI (30 msec)) maximally separated the HIV-1 Tg and control animals (canonical correlation of 0.90). Each of the predictor variables in the discriminant function analysis contributed to differentiating the treatment groups [Auditory Gap-PPI (30 msec; F(1,37)=55.6, p≤0.001), Auditory PPI (Airpuff context, 200 msec; F(1,37)=22.9, p≤0.001), Tactile PPI (30 msec; F(1,37)=12.8 p≤0.001), and Visual gap-PPI (30 msec; F(1,37)=85.1, p≤0.001)]. Animals were correctly diagnosed for the presence of the HIV-1 transgene (jack-knifed classification) with 97.4% accuracy (Approximation of Wilks' λ of 0.183, χ2(4)=59.5, p≤0.001).
Figure 6.
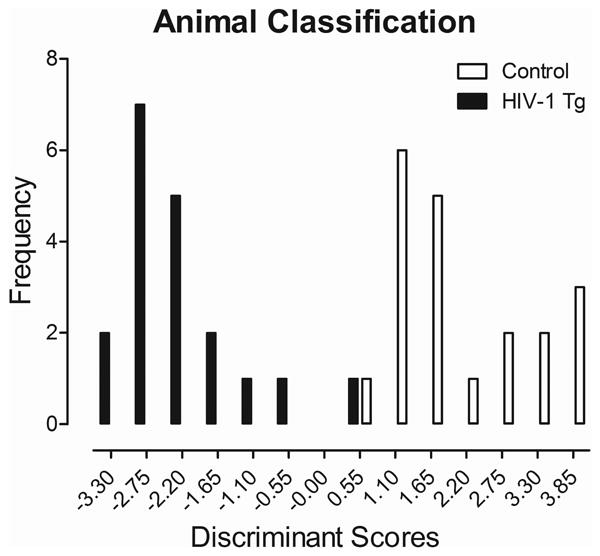
Animal classification is illustrated as a function of the canonical variable representing the simplest linear function that best separated the HIV-1 Tg and control groups (canonical correlation 0.90) and correctly identified (jackknife classification) group membership with 97.4% accuracy (100% of controls, and 94.7% of HIV-1 Tg animals).
Discussion
Amodal temporal processing deficits were detected in the HIV-1 Tg rat using two translational experimental paradigms, including cross-modal PPI and gap-PPI. Cross-modal PPI (i.e., auditory, visual, or tactile prepulse stimuli) revealed a relative insensitivity to the manipulation of ISI, regardless of modality, in HIV-1 Tg rats relative to control animals. Auditory and visual gap-PPI revealed a relative insensitivity to the manipulation of ISI in HIV-1 Tg rats in comparison to control animals. Tactile gap-PPI revealed a differential sensitivity to the manipulation of ISI in HIV-1 Tg animals, relative to controls. A differential sensitivity to the effect of context (i.e., concurrent visual or tactile stimulus) in auditory PPI was also noted in the HIV-1 Tg animals. A discriminant function analysis diagnosed the presence of the HIV-1 transgene with 97.4% accuracy, indicating the potential utility of amodal temporal processing deficits as a point-of-care screening tool for HAND. Evidence for amodal temporal processing deficits in the HIV-1 Tg rat supports the hypothesis of a central temporal processing deficit and heralds an opportunity to develop a point-of-care screening tool for HAND, which has the potential for considerable clinical interest, especially in low-resource countries (Chan and Brew, 2014; Zipursky et al. 2013).
Cross-modal PPI revealed a relative insensitivity to the manipulation of ISI in HIV-1 Tg rats in comparison to control animals, independent of modality. In all sensory modalities, HIV-1 Tg and control animals exhibited maximal inhibition at the same ISI (i.e., 30 msec in auditory PPI; 50 msec in visual PPI; 200 msec in tactile PPI). However, HIV-1 Tg animals exhibited a significantly flatter ISI function relative to controls. Alterations in cross-modal PPI extend previously reported temporal processing deficits observed in adolescent and adult HIV-1 Tg rats (Moran et al. 2013; McLaurin et al. 2016b) to additional sensory modalities, providing additional evidence for the generality of temporal processing deficits.
Auditory and visual gap-PPI revealed a relative insensitivity to the manipulation of ISI in HIV-1 Tg animals in comparison to controls. In both auditory and visual gap-PPI, HIV-1 Tg and control animals exhibited maximal peak inhibition at the same ISI (i.e., 50 msec). However, a significantly flatter ISI function was observed in HIV-1 Tg animals in comparison to controls. Tactile gap-PPI revealed a differential sensitivity to the manipulation of ISI in HIV-1 Tg animals, evidenced by a rightward shift in maximal peak inhibition, relative to controls. Specifically, maximal peak inhibition was observed at the 30 msec ISI in control animals, but a rightward shift to the 50 msec ISI was observed in HIV-1 Tg animals. The present results are an extension of a longitudinal analysis of auditory gap-PPI from PD 30 to PD 180 (McLaurin et al. 2016a) to additional sensory modalities and a more advanced age.
The manipulation of testing context (i.e., concurrent visual or tactile stimulus) in auditory PPI had a differential effect on HIV-1 Tg animals in comparison to control animals. Regardless of context, control animals exhibited maximal inhibition at the 30 msec ISI in auditory PPI. HIV-1 Tg animals displayed a differential sensitivity to context in auditory PPI, evidenced by a rightward shift in maximal inhibition (e.g., 30 msec in the concurrent visual context, 200 msec in the concurrent tactile context). Both HIV-1 Tg and control animals displayed a slight downward shift in the mean peak ASR amplitude curve in the concurrent tactile context in comparison to the concurrent visual context, extending the effect of context, previously reported in adult Long-Evans rats at PD 90 (Moran et al. 2015) to Fischer HIV-1 Tg and control animals.
Translational assessments of temporal processing deficits, including cross-modal PPI and gap-PPI, provide an opportunity for the development of a clinical diagnostic screening tool for HAND. In the present study, a discriminant function analysis was conducted to assess the potential utility of amodal temporal processing deficits as a point-of-care screening tool for the diagnosis of HAND. Presence of the HIV-1 transgene was diagnosed with 97.4% accuracy using assessments from all three modalities (i.e., auditory, visual, or tactile prepulse stimuli).
The eyeblink startle experimental paradigm, which uses electromyography (EMG) to measure the eyeblink component of the ASR, provides a clinically relevant method for the assessment of prepulse inhibition in humans. Previous research reports the use of the eyeblink startle experimental paradigm to assess both PPI (e.g., Minassian et al., 2013) and gap-PPI (e.g., Fournier and Hébert, 2013). Specifically, the eyeblink startle paradigm has been used to assess sensorimotor gating deficits in HIV-1 seropositive individuals. HIV-1 seropositive individuals with HAND displayed significant deficits in sensorimotor gating, which were correlated with deficits in working memory (Minassian et al., 2013). Assessments of prepulse inhibition (i.e., the eyeblink startle experimental paradigm) exhibit characteristics, including brevity (i.e., approximately 15-25 minutes; Fournier and Hébert, 2013; Minassian et al., 2013), repeatability (Braff et al. 1978; Schwarzkopf et al. 1993), and ease of administration, which are critical for the development of a clinically relevant diagnostic screening tool (Myers and Brown, 2006).
Both cross-modal PPI and gap-PPI, implicated as an innovative point-of-care screening tool in the present study, display prominent non-monotonic relationships (i.e., Figures 3, 4). In contrast, gap threshold detection, which relies on the manipulation of gap duration, is another translational assessment of temporal processing that displays a prominent monotonic relationship; as gap duration increases, significant decreases in ASR are observed (Ison et al. 2005; Ison & Bowen, 2000). In a comparable group of HIV-1 Tg and control animals (i.e., similar ages, ovariectomized female animals), auditory gap threshold detection measures diagnosed the presence of the HIV-1 transgene with 91.1% (McLaurin et al. 2016b). The monotonic relationship present in gap threshold detection provides a distinct advantage for the development of a clinical diagnostic screening tool. However, it must be noted that the presence of the HIV-1 transgene can be diagnosed with high accuracy (i.e., ≥ 90%) using assessments of temporal processing (i.e., cross-modal PPI, gap-PPI, and gap threshold detection) regardless of the monotonicity of the function (McLaurin et al. 2016a, 2016b, 2016c). Thus, the present study continues to provide strong evidence for the utility of temporal processing deficits as a clinically relevant diagnostic screening tool for HAND.
The underlying neural mechanisms involved in HAND may be elucidated through the use of translational behavioral assessments, including cross-modal PPI and gap-PPI (Hoffman and Ison, 1980). The neural circuitry involved in the mediation of PPI of the ASR has been established using lesioning (Leitner and Cohen, 1985) and electrical stimulation studies (Li et al. 1998; Li and Yeomans, 2000). Specifically, lesions of the inferior (Leitner and Cohen, 1985; Li et al., 1998) or superior colliculus (Fendt et al., 1994) have been critical in defining the serial circuit involved in PPI. The serial circuitry begins with sensory system input, including auditory prepulses, which are relayed to the inferior colliculus, and visual or tactile prepulses, which are relayed to the superior colliculus. Information is subsequently transmitted from the superior colliculus to the pedunculopine tegmental nucleus, which triggers a cholinergic projection to the caudal pontine reticular nucleus mediating PPI of the ASR (Fendt et al. 1994; Fendt et al. 2001; Koch et al. 1993; Koch and Schnitzler 1997).
Understanding the amodal nature of temporal processing deficits is vital for accurately modeling neurocognitive deficits observed in HIV-1 seropositive individuals and the development of a point-of-care screening tool for HAND. The HIV-1 Tg rat is non-infectious, expresses 7 of the 9 HIV-1 genes constitutively throughout development, and has been promoted as a model for investigating aspects of HAND (Vigorito et al. 2015). The contemporary phenotype of the HIV-1 Tg rat, used in the present study, is a healthier derivation of those originally described (Reid et al. 2001). In the present study, HIV-1 Tg animals, in comparison to F344/N controls, displayed no significant health disparities (i.e., similar growth rates, similar inhibition in visual PPI; Roscoe et al. 2014, Moran et al. 2012, 2013). Despite the differences in temporal processing, HIV-1 Tg rats appear to have intact visual, auditory, and tactile sensory systems, evidenced by the robust inhibition of the ASR to all sensory modalities. Thus, the HIV-1 Tg rat used in the present study in some respects resembles HIV-1 seropositive individuals on cART, making it useful for establishing the nature of neurocognitive impairment in HAND.
Observations of amodal temporal processing deficits in the HIV-1 Tg rat supports the hypothesis of a central temporal processing deficit in HIV-1 seropositive individuals. Cross-modal PPI and gap-PPI were conducted in multiple sensory modalities, including auditory, visual, and tactile, sensory systems which permit punctate prestimuli, enhancing our understanding of the nature of temporal processing deficits in HAND. Temporal processing deficits provide an innovative opportunity for the development of a brief and accurate diagnostic screening tool for HAND, which may be of great clinical significance in the post-cART era (Chan and Brew, 2014; Zipursky et al. 2013).
Acknowledgments
This work was supported in part by grants from NIH (National Institute on Drug Abuse, DA013137; National Institute of Child Health and Human Development, HD043680; National Institute of Mental Health, MH106392) and the interdisciplinary research training program supported by the University of South Carolina Behavioral-Biomedical Interface Program.
References
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurol. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normal and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Prepulse inhibition of the acoustic startle reflex using visual and auditory prepulses: disruption by apomorphine. Psychopharmacology. 1995;17:267–274. doi: 10.1007/BF02246101. [DOI] [PubMed] [Google Scholar]
- Chan P, Brew BJ. HIV associated neurocognitive disorders in the modern antiviral treatment era: Prevalence, characteristics, biomarkers, and effects of treatment. Current HIV/AIDS Reports. 2014;11(3):317–324. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- Chao LL, Lindgren JA, Flenniken DL, Weiner MW. ERP evidence of impaired central nervous system function in virally suppressed HIV patients on antiretroviral therapy. Clin Neurophysiol. 2004;115:1583–1591. doi: 10.1016/j.clinph.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Ances BM. HIV-associated neurocognitive disorder (HAND) Lancet Infect Dis. 2013;13:976–986. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol. 2011;17(2):176–183. doi: 10.1007/s13365-011-0021-x. [DOI] [PubMed] [Google Scholar]
- Elbirt D, Mahlab-Guri K, Bezalel-Rosenberg S, Gills H, Attali M, Asher I. HIV-associated neurocognitive disorders (HAND) Israel Medical Associated Journal. 2015;17:54–59. [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler HU. Sensorimotor gating deficit after lesions of the superior colliculus. NeuroReport. 1994;5:1725–1738. doi: 10.1097/00001756-199409080-00009. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology. 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Threlkeld SW, McClure MM, Peiffer AM. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res Bull. 2008;76:1–7. doi: 10.1016/j.brainresbull.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal injection of the HIV-1 proteins gp12 and Tat: Differential effects on behavior and the relationship to stereological hippocampal measures. Brain Res. 2008;1232:139–154. doi: 10.1016/j.brainres.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006a;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alteration in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006b;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fournier P, Hébert S. Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: Does tinnitus fill in the gap? Hear Res. 2013;295:16–23. doi: 10.1016/j.heares.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Haddow LJ, Floyd S, Copas A, Gilson RJC. A systematic review of the screening accuracy of the HIV dementia scale and international HIV dementia scale. Plos one. 2013;16:e61826. doi: 10.1371/journal.pone.0061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I CHARTER Group, HNRC Group. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in modification of startle reaction in rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychology Review. 1980;87:175–189. [PubMed] [Google Scholar]
- Ison JR, Hammond GR. Modification of startle reflex in rat by changes in auditory and visual environments. J Comp Physiol Psychol. 1971;75:435–452. doi: 10.1037/h0030934. [DOI] [PubMed] [Google Scholar]
- Ison JR. Temporal acuity in auditory function in the rat: Reflex inhibition by brief gaps in noise. J Comp Physiol Psychol. 1982;96:945–954. [PubMed] [Google Scholar]
- Ison JR, Bowen PG. Scopolamine reduces sensitivity to auditory gaps in the rat, suggesting a cholinergic contribution to temporal acuity. Hear Res. 2000;145:169–176. doi: 10.1016/s0378-5955(00)00088-5. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, Rivoli PJ, Moore JT. The behavioral response of mice to gaps in noise depends on its spectral components and its bandwidth. J Acoust Soc Am. 2005;117:3944–3951. doi: 10.1121/1.1904387. [DOI] [PubMed] [Google Scholar]
- Joska JA, Witten J, Thomas KG, Robertson C, Casson-Crook M, Roosa H, Creighton J, Lyons J, McArthur J, Sacktor NC. A comparison of five brief screening tools for HIV-associated neurocognitive disorders in the USA and South Africa. AIDS and Behavior. 2016:1–11. doi: 10.1007/s10461-016-1316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Ku NS, Lee YJ, Ahn JY, Kim SB, Ahn HW, Hong KW, Song JY, Cheong HJ, Kim WJ, Kim JM, Namkoong K, Choi JY, Kim E. Utility of the Montreal Cognitive Assessment (MoCA) and its subset in HIV-associated neurocognitive disorder (HAND) screening. J Psychosomatic Res. 2016;80:53–57. doi: 10.1016/j.jpsychores.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Koch M, Kungel M, Herbert H. Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp Brain Res. 1993;97:71–82. doi: 10.1007/BF00228818. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats: Circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Ku NS, Lee Y, Ahn JY, Song JE, Kim MH, Kim SB, Jeong SJ, Hong KW, Kim E, Han SH, Song JY, Cheong HJ, Song YG, Kim WJ, Kim JM, Smith DM, Choi JY. HIV-associated neurocognitive disorder in HIV-infected Koreans: the Korean NeuroAIDS project. HIV Med. 2014;15:470–477. doi: 10.1111/hiv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner DS, Cohen ME. Role of the inferior colliculus in the inhibition of acoustic startle in the rat. Physiol Behav. 1985;34:65–70. doi: 10.1016/0031-9384(85)90079-4. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- Li L, Priebe PM, Yeomans JS. Prepulse inhibition of acoustic or trigeminal startle of rats by unilateral electrical stimulation of the inferior colliculus. Behav Neurosci. 1998;112:1187–1198. doi: 10.1037//0735-7044.112.5.1187. [DOI] [PubMed] [Google Scholar]
- Li L, Yeomans JS. Using intracranial electrical stimulation to study the timing of prepulse inhibition of the startle reflex. Brain Res Protoc. 2000;5:67–74. doi: 10.1016/s1385-299x(99)00056-2. [DOI] [PubMed] [Google Scholar]
- Matas CG, Silva SM, Marcon Bde A, Goncalves IC. Electrophysiological manifestations in adults with HIV/AIDS submitted and not submitted to antiretroviral therapy. Pro Fono. 2010;22:107–113. doi: 10.1590/s0104-56872010000200007. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders mind the gap. Ann Neurol. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF. Progression of temporal processing deficits in the HIV-1 transgenic rat. Sci Rep. 2016a;6:32831. doi: 10.1038/srep32831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Moran LM, Li H, Booze RM, Mactutus CF. A gap in time: Extending our knowledge of temporal processing deficits in the HIV-1 transgenic rat. J Neuroimmune Pharm. 2016b doi: 10.1007/s11481-016-9711-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF. Selective developmental alterations in the HIV-1 transgenic rat: Opportunities for diagnosis of pediatric HIV-1. J Neuroviol. 2016c doi: 10.1007/s13365-016-0476-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W. Prepulse inhibition in HIV-Associated Neurocognitive Disorders. J Int Neuropsychol Soc. 2013;19:709–717. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012;10:415–424. doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF. Time and time again: Temporal processing demands implicate perceptual and gating deficits in the HIV-1 Transgenic rat. J Neuroimmune Pharm. 2013;8:988–997. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Hord LL, Booze RM, Harrod SB, Mactutus CF. The role of sensory modality in prepulse inhibition: An ontogenetic study. Dev Psychobiol. 2015;58(2):211–222. doi: 10.1002/dev.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Overton ET, Kauwe JS, Paul R, Tashima K, Tate DF, Patel P, Carpenter CC, Patty D, Brooks JT, Clifford DB. Performances on the CogState and standard neuropsychological batteries among HIV patients without dementia. AIDS Behav. 2011;15(8):1902–9. doi: 10.1007/s10461-011-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton ET, Azad TD, Parker N, Demarco Shaw D, Frain J, Spitz T, Westerhaus E, Paul R, Clifford DB, Ances BM. The Alzheimer's disease-8 and Montreal Cognitive Assessment as screening tools for neurocognitive impairment in HIV-infected persons. J Neurovirol. 2013;19(1):109–16. doi: 10.1007/s13365-012-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi T, Ison JR. Development of the acoustic startle response in the rat: Ontogenetic changes in the magnitude of inhibition by prepulse stimulation. Dev Psychobiol. 1979;12(3):219–230. doi: 10.1002/dev.420120305. [DOI] [PubMed] [Google Scholar]
- Peng JS, Vigorito M, Liu XQ, Zhous DJ, Wu XW, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected indiviuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Pickney LA. Inhibition of the startle reflex in the rat by prior tactile stimulation. Anim Learn Behav. 1976;4:476–472. [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: A rapid screening test. J Acq Immune Def Synd. 1995;8:273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins RN, Brown H, Ehlers A, Joska JA, Thomas KGF, Burgess R, Byrd D, Morgello S. A smartphone app to screen for HIV-related neurocognitive impairment. J Mob Technol Med. 2014;3(1):23–26. doi: 10.7309/jmtm.3.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe RF, Jr, Mactutus CF, Booze RM. HIV-1 transgenic female rat: Synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol. 2014;9:642–653. doi: 10.1007/s11481-014-9555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19:1367–1374. [PubMed] [Google Scholar]
- Sacktor N, Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Curr Opin HIV AIDS. 2014;9(6):517–520. doi: 10.1097/COH.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Marcotte TD, Umlauf A, Franklin D, Heaton RK, Ellis RJ, Letendre S, Alexander T, McCutchan JA, Morgan EE, Woods SP, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson D, Grant I CHARTER group. Concurrent classification accuracy of the HIV dementia scale for HIV-associated neurocognitive disorders in the CHARTER cohort. J Acquir Immune Defic Syndr. 2013;62(1):36–42. doi: 10.1097/QAI.0b013e318278ffa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf SB, McCoy L, Smith DA, Boutros NN. Test-retest reliability of prepulse inhibition of the acoustic startle response. Biol Psychiat. 1993;34:896–900. doi: 10.1016/0006-3223(93)90059-m. [DOI] [PubMed] [Google Scholar]
- Smith CA, van Gorp WG, Ryan ER, Ferrando SJ, Rabkin J. Screening subtle HIV-related cognitive dysfunction: The clinical utility of the HIV dementia scale. J Acquir Immune Defic Syndr. 2003;33:116–118. doi: 10.1097/00126334-200305010-00018. [DOI] [PubMed] [Google Scholar]
- Valcour VG. Evaluating cognitive impairment in the clinical setting: Practical screening and assessment tools. Top Antivir Med. 2011a;19:175–180. [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Paul R, Chiao S, Wendelken LA, Miller B. Screening for cognitive impairment in human immunodeficiency virus. Clin Infect Dis. 2011b;53(8):836–842. doi: 10.1093/cid/cir524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, Connaghan KP, Chang SL. The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun. 2015;48:336–349. doi: 10.1016/j.bbi.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. 2nd ed. New York: 1971. [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky AR, Gogolishvili D, Rueda S, Brunetta J, Carvalhal A, McCombe JA, Gill MJ, Rachlis A, Rosenes R, Arbess G, Marcotte T, Rourke SB. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: a systematic review of the literature. AIDS. 2013;27:2385–2401. doi: 10.1097/QAD.0b013e328363bf56. [DOI] [PMC free article] [PubMed] [Google Scholar]



