Abstract
There are many examples of macroalgae inducing defence in response to small invertebrate herbivores like amphipods, isopods, and gastropods but few cases of induction in response to vertebrate macrograzers like herbivorous fishes. This may be because larger grazers rapidly consume large quantities of seaweed before induction can occur, thus selecting for constitutive rather than induced defences. Alternatively, the pattern could occur because induction due to feeding by macrograzers is less commonly investigated. In Fiji, field assays with the brown macroalga Sargassum polycystum demonstrated that thalli growing in marine protected areas (MPAs) with abundant herbivorous fishes were significantly less palatable than those growing in adjacent fished areas (non-MPAs) with few herbivorous fishes. This significant preference occurred in 11 of 13 trials over 5 time periods and across 3 pairs of MPAs and spatially associated non-MPAs. This preference was not positively associated with algal nitrogen content or with the toughness of algal fronds. When S. polycystum ramets were taken from the non-MPA and half were partially grazed by fishes while the other half were protected from grazing, new growth from the controls was strongly preferred to new growth from the previously grazed ramets although these fronds originated from the same holdfast. This suggests that S. polycystum upregulates defences (probably chemical) in response to grazing by herbivorous fishes. This is one of the few published examples of induction of macroalgal defence in response to feeding by large, mobile grazers. It is unclear whether induced defences against fishes are rare or just under-investigated.
Keywords: Plant-herbivore interaction, Phaeophyte, Ochrophyte, Coral reef
INTRODUCTION
A review from 20 yr ago noted, ‘There are too few studies of chemical induction in marine prey to adequately evaluate the relative importance of large, mobile versus small, sedentary consumers in inducing chemical defences’ (Hay 1996, p. 116). This is still the case. Many publications in the intervening years have documented induced defences in response to grazing by small, sedentary mesograzers (e.g. amphipods, isopods, and snails), but investigations of induction due to mobile, macroherbivores have rarely been published, as noted in meta-analyses of induction studies focused on seaweeds (Toth & Pavia 2007, Jormalainen & Honkanen 2008).
Since Lewis et al. (1987) showed that Padina jamaicensis induced morphological changes in response to fish grazing (growing as creeping filaments in the presence of fishes, but shifting to broad, upright blades in the absence of fishes), there seem to have been only a few published studies documenting a correlation between morphology or metabolite concentration and fish grazing intensity (Paul & Van Alstyne 1988, Coen & Tanner 1989) and, to our knowledge, only one experimental test (Diaz-Pulido et al. 2007). Likewise, reviews (Harvell 1990, Hay 1996) and meta-analyses (Koricheva et al. 2004, Toth & Pavia 2007, Jormalainen & Honkanen 2008) have not covered due to fish grazing, apparently because too few studies exist. It has become the ‘accepted wisdom’ that mesograzers induce defences while large, mobile grazers do not (Hay 1996, Pavia & Toth 2000, Toth et al. 2007, Jormalainen & Honkanen 2008).
In aquatic ecology the thinking supportive of this stems from Hay (1996), who hypothesised that mesograzers would induce defences in their algal prey because they feed on small spatial scales, may remain on one plant for ex tended periods of time and rarely cause prey mortality. Thus, hosts would have time to induce defences before much tissue was consumed. In contrast, large, mobile grazers may consume all, or much, of a palatable alga before the alga could induce defences. Thus, herbivorous fishes that were large and mobile relative to their algal prey were thought to select for constitutive, rather than induced, defences. There are parallels in terrestrial systems where induced chemical defences in response to invertebrate grazers are common and well studied, whereas those induced in response to vertebrate herbivores are rare or unstudied (Karban & Baldwin 1997). It is unclear whether this results from vertebrate herbivores not inducing plant defences, from a lack of investigations involving vertebrate herbivores, or a bias against publishing negative findings.
Despite the limited evidence for induction of algal traits in response to large, mobile grazers, several observations suggest that it may be beneficial for macroalgae to induce defences in response to vertebrate herbivores. Firstly, fish grazing is not spatially or temporally uniform (Hay et al. 1983, Diaz-Pulido et al. 2007). Palatable algae in areas continually accessible to grazers may be rapidly consumed (even to local extinction), but those in areas of spatial or temporal escape experience periodic and non-fatal bouts of feeding, allowing time to induce defences before another feeding bout commences (Hay 1981, Hay et al. 1983). Variability in grazing by herbivorous fishes occurs due to wave action (Vergés et al. 2011), turbulence, depth, topographic structure of the habitat (Hay 1981), and across seasons (Carpenter 1986). This variation provides temporal or spatial escapes, and in such situations, it may be advantageous to induce rather than exhibit constitutive defences because induction would avoid the cost of continuous production and maintenance of defence during times, or locations, of minimal threat (Tollrian & Harvell 1999). Secondly, given that seaweeds, terrestrial plants, and even phytoplankton can chemically sense attacks on neighbouring conspecifics, identify the attacker via chemical cues, and respond by inducing different defences appropriate for that consumer (Karban & Baldwin 1997, Pavia & Toth 2000, Long et al. 2007), it is reasonable to suspect that seaweeds could detect and respond to grazing fishes, as they do for other herbivores. Thirdly, most common reef seaweeds are clonal and many have basal sections that exist within structural refuges in the reef. These protected portions escape consumption by larger herbivores and so might be able to regenerate induced tissue following bouts of even intense grazing (Lewis et al. 1987, Harvell 1990). Finally, most reef fish are resident to an area of reef and feed within a defined home range (Sale 1980, Welsh & Bell wood 2012). Thus, better-defended individual seaweeds that have induced increased defences may be sampled, remembered, and avoided by co-occurring fishes.
In this study we investigated (1) whether herbivorous fishes preferentially fed on Sargassum polycystum fronds collected from areas with few herbivorous fishes versus areas with many, (2) whether the difference in palatability we observed could result from an induced response to fish grazing, and (3) whether differential tissue toughness or nutritional value were associated with feeding preference, as has been demonstrated in some studies of insects, marine invertebrates, or fishes (Littler & Littler 1980, Coley 1983, Burkepile & Hay 2009, Chan et al. 2012).
MATERIALS AND METHODS
Study sites
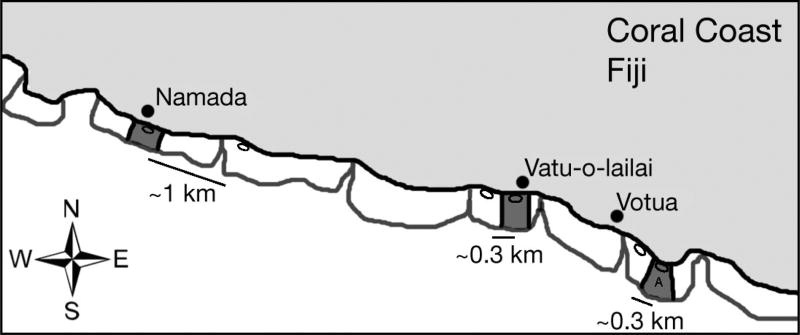
This study was conducted between May 2012 and January 2015. Our study sites were 3 pairs of no-take, marine protected areas (MPAs) and their adjacent fished areas (non-MPAs) in the villages of Votua, Vatu-o-lailai and Namada on the Coral Coast of Fiji’s main island, Viti Levu, between 18° 11’ 23” S, 177° 37’ 15” E (Namada’s MPA) and 18° 12’ 58” S, 177° 43’ 01”E (Votua’s MPA; Fig. 1). Paired MPA and non-MPA sites at each village were separated by ~0.3 to 1 km, and villages were separated from each other by 3.3 to 7.6 km. These sites occur along an 11 km stretch of continuous reef flat habitat, between 1 and 3 m depth at high tide. The MPAs of these 3 villages were established in 2002− 2003 and cover areas of ~0.8, ~0.5 and ~0.5 km2, respectively (Simpson 2010). Surveys conducted near the time of MPA establishment indicated that MPA and non-MPA areas were similarly low in coral cover and high in macroalgal cover (Simpson 2010), suggesting similar physical and biological regimes and selective pressures at that time. However, in the >10 yr since MPA establishment, community composition of the MPAs and associated non-MPAs came to differ dramatically. Biomass and diversity of herbivorous fishes are 7 to 17 times and 2 to 3.3 times greater in the MPAs than the adjacent non-MPAs, respectively (Rasher et al. 2013). Macroalgal cover and diversity are 27 to 61 times and 2.6 to 3.6 times greater in the non-MPAs, respectively, while coral cover is ~3 to 11 times greater in the MPAs than the non-MPAs (Rasher et al. 2013). This difference in macroalgal cover is not the result of differing physical conditions between the MPA and non-MPA areas; caged recruitment and reciprocal transplant experiments between the MPA and non-MPA areas demonstrate that algae recruit and grow as well or better (depending on the village) in the MPA, where seaweeds are scarce, than the non-MPA, where they are abundant, if they are protected from herbivorous fishes (Rasher et al. 2012, Dell et al. 2016). Additionally, isotopic investigations of seaweeds from the sites indicate that MPA and non-MPA areas within a village do not differ in sources of nitrogen (Dell et al. 2015). Despite the similar physical conditions between MPAs and non-MPAs at each village, there is now considerable difference in the selective pressure imposed by the higher densities and diversities of herbivorous fishes in MPA as opposed to non-MPA areas (Rasher et al. 2013).
Fig. 1.
Collection areas inside the marine protected areas (MPAs) (dark grey) and non-MPAs (white) near 3 villages. Modified from Dell et al. (2015). Study sites located between 18° 11’ 23” S, 177° 37’ 15” E (Namada’s MPA) and 18° 12’ 58” S, 177° 43’ 01”E (Votua’s MPA). Distances between the sites of collection (small ovals) in each MPA and non-MPA are shown for each village. A: location where all feeding assays were conducted
Study species
To assess potential differences in palatability and possible induction of defences that might result from differing grazing pressure, we selected the brown macroalga Sargassum polycystum C. Agardh, which is palatable to fishes (Rasher et al. 2013) and occurs in both the MPAs and non-MPAs. S. polycystum is one of the most common macroalgae in the coastal, tropical and sub-tropical Pacific (Chiang et al. 1992). On Fijian reefs, S. polycystum begins to regenerate from perennial holdfasts in December and can grow to 1.5 m long and dominate heavily fished reefs (Rasher et al. 2013) by the end of its growing season (June− July in Fiji). At this point the species may reproduce sexually and uprights senesce (as per De Wreede 1976).
Although 29 species of larger herbivorous fishes occur in the MPAs, 2 unicorn fishes (Naso unicornis and N. lituratus) are the primary consumers of S. poly cystum and brown macroalgae in general (Rasher et al. 2013). Extensive fish surveys in each MPA and non-MPA observed these fishes to be common to abundant in the MPAs and below detection limits in the non-MPAs (see Supplemental Table A1 in Rasher et al. 2013).
Fish feeding assays
To assess the palatability of MPA versus non-MPA S. polycystum to herbivorous fishes in the field, fronds from both the MPA and non-MPA of each village were collected, assembled into pairs of ropes, and these rope pairs were put in Votua’s MPA to be grazed. S. polycystum occurs throughout the non-MPAs but in the MPAs is largely restricted to near-shore, shallow areas. To avoid confounding effects of depth versus MPA status, S. polycystum was first collected from the MPA in each village from as far off shore as possible and then from a comparable depth and distance from shore in the adjacent non-MPA of that village. The upper 15 cm of each frond was collected, rinsed vigorously in seawater to remove epiphytes and particulates and inspected to ensure none remained which could potentially confound results. All algae were visibly free of encrusting organisms and sediment. Fifteen to 20 g of the algae were spun in a salad spinner for 15 revolutions to remove excess water and weighed to the nearest 0.1 g. This quantity was then affixed in 2 segments ~10 cm apart on one end of a 40 cm long 3-strand rope by entwining the algae within the strands. The same was then performed with algae from the other site so that one rope held algae from the MPA and the other from the non-MPA. The weight of algae on each rope was matched to within 1 g and assembled to be visually similar. Twenty rope pairs were prepared, attached to cement weights, and distributed in the MPA to be grazed. Ropes of a pair were separated from each other by ~50 cm, and pairs were positioned ≥2 m apart. Pairs were removed when at least 50% of the macroalgae on either member of a rope pair had been eaten. Pairs were omitted from subsequent analysis if <10% or >90% of the algae on both ropes had been eaten; such events were rare. This eliminated replicates in which relative preference would have been obscured by inadequate or excessive fish grazing. Grazing as says lasted 1 to 4 h, and the rope pairs were monitored frequently during this time so that they could be collected when sufficient grazing had occurred. This frequent monitoring also allowed us to observe that algal fronds were never dislodged by wave action and that all grazing was by fishes and not invertebrate grazers. Following the assay, the algae were then spun again and weighed to measure grazing as grams lost. This assay was repeated with S. polycystum collected from each village multiple times between May 2012 and May 2013 and for one village in January 2015.
Induction experiment
This experiment was conducted to determine whether differential palatability of S. polycystum might be due to induction of defence following initial grazing by herbivorous fishes. In January 2015, we collected 8 ramets of 15 cm in length from each of 25 S. polycystum holdfasts in Votua’s non-MPA, moved them to the MPA where we exposed half (4 from each of the 25 holdfasts) to grazing and protected the rest. Four of the 8 ramets from each holdfast were threaded through a rope and affixed to the sides of cages (40 × 15 × 15 cm with mesh size of 1 cm) so that the upper 5 cm protruded through the mesh while the remainder of the thalli were protected inside the cage. The remaining 4 ramets were threaded through ropes and positioned in separate cages so that they were entirely protected from grazing. All cages (n = 25 pairs) were then distributed in the MPA for a 4 h feeding period on 2 consecutive days, during which time they were frequently monitored to observe grazing. All observed grazing was by fishes and occurred only on the treatment algae. After the grazing period, the entire 5 cm section protruding from the cage had been consumed by fish and the cages were checked for invertebrate grazers; none were found. The algal fronds were measured to give length remaining (in cm) and the ropes were then transferred to separate cages (3 for the treatment ropes and 3 for the controls, each ~1.5 × 1.5 × 1 m in size) where the algae could grow protected from grazing for 2 wk.
After this period, the new growth was measured (in cm) and collected from 3 of the 4 ramets from each rope (~7 g spun wet mass in each replicate) for use in a feeding assay as described above. Because S. polycystum has apical growth, we could identify and use only new growth by taking the uppermost portions of the frond that exceeded that frond’s length at the end of the grazing experiment. Using only new growth avoided confounding the alga’s induced response with the age, or other traits, of basal tissues left after initial grazing on treatment plants. During the 2 wk period of re-growth, some thalli detached from the rope during heavy seas and washed against the side of the cages where they could have been grazed. We excluded these from the subsequent feeding assay leaving n = 16 rather than 25.
Penetrometer measurements of blade toughness
To investigate the potential impact of blade toughness on fish grazing preference, S. polycystum was collected from the MPA and non-MPA of each village in January 2015 for measurement with a penetrometer (methods detailed in Littler & Littler 1980). Such measures of ‘leaf toughness’ have been considered important determinants of grazing in both marine and terrestrial systems (Littler & Littler 1980, Coley 1983).
Briefly, the alga’s blade is clamped between 2 plexiglass sheets that have a predrilled hole where a metal pin supporting a small plastic cup can be positioned so that the pin rests directly on the algal blade. Small lead beads are then slowly added to the cup until the pin pierces the algal blade. The lead beads, needle, and cup are then weighed to determine the mass needed to pierce the blade, thus giving a measurement of algal ‘toughness.’ Eighteen to 20 S. polycystum ramets were collected from the MPA and non-MPA of Votua and Vatu-o-lailai, while 6 and 8 were collected from Namada’s MPA and non-MPA, respectively, as S. polycystum was uncommon there. For consistency, the needle was positioned in the centre of the widest part of a blade growing 1 cm below the apical meristem.
To assess whether fish grazing induced a change in blade toughness in S. polycystum, 1 of the 4 ramets from each replicate in the induction experiment was reserved for penetrometer measurements (the remaining 3 were used in a feeding assay). A total of 23 control and 23 induced ramets from this experiment were measured with the penetrometer.
Nitrogen content and C:N ratio
As nitrogen can be limiting to herbivores (Mattson 1980) and increased N-content of seaweeds commonly increases herbivore preference for, or grazing on, seaweeds (Burkepile & Hay 2009, Chan et al. 2012), we measured nitrogen content and C:N ratios of S. polycystum fronds to determine whether MPA and non-MPA thalli differed in nutritional value. The top 5 cm from 5 S. polycystum ramets were collected from each of the 6 sites in May 2012. These were rinsed vigorously in seawater to remove particulates and epibiota and frozen at −20°C until they were dried at 70°C to a constant weight, ground into a fine powder with a pestle and mortar, and analysed by mass spectrometer for carbon and nitrogen content. Further processing methods are detailed in Dell et al. (2015).
Statistical analyses
Assumptions of normality and homogeneity of variance were assessed using the Shapiro-Wilk and Levene’s test, respectively. Statistical analyses were completed using SPSS version 16.0. Means are reported ±1 SE; α was set to 0.05.
Data from the fish feeding assays satisfied the assumption of normality, or were successfully square-root transformed to do so, and were analysed with independent sample t-tests or t-test for unequal variance when data failed the assumption of homogeneity of variance. All penetrometer data satisfied assumptions of normality (or were successfully log10-transformed to do so) and homogeneity of variance and thus were analysed by independent samples t-test. Percent nitrogen and C:N data were analysed with Mann-Whitney U, 2-tailed tests. Differences between grazing on induced versus control pairs of S. polycystum outplants in the induction experiment satisfied the assumption of normality, so data were analysed by a paired t-test.
RESULTS
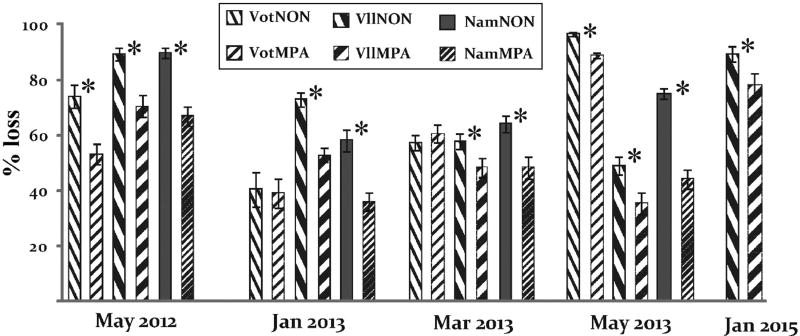
When Sargassum polycystum was collected from MPAs and non-MPAs and placed in the MPA to be grazed, fish consumed significantly more of the non-MPA relative to MPA algae in 11 of the 13 paired assays conducted at 5 time points over 3 yr (p ≤ 0.036; Fig. 2). This difference in consumption was on average ~18% (between ~8 to ~30% over the 11 trials). The preference for non-MPA algae was consistent in the villages of Vatu-o-lailai and Namada, whereas in Votua it occurred in 2 of the 4 trials, both of which were run later in the year (in May; Fig. 2). During the frequent monitoring of these assays, feeding by Naso unicornis and N. lituratus was common, feeding by parrotfishes occurred occasionally, and we never observed feeding by sea urchins, crabs or other noticeable invertebrates.
Fig. 2.
Mean (±1 SE) percentage of Sargassum polycystum from non-marine protected areas (non-MPAs) versus S. polycystum from MPAs consumed by fishes across multiple time points and sets of villages (Vot = Votua; VII = Vatu-o-lailai; Nam = Namada; NON = non-MPA). Fishes preferred S. polycystum from the non-MPAs over those from the MPAs in 11 of 13 trials; n = 12−20 for each assay; asterisks indicate significance at p ≤ 0.05 from independent samples t-tests
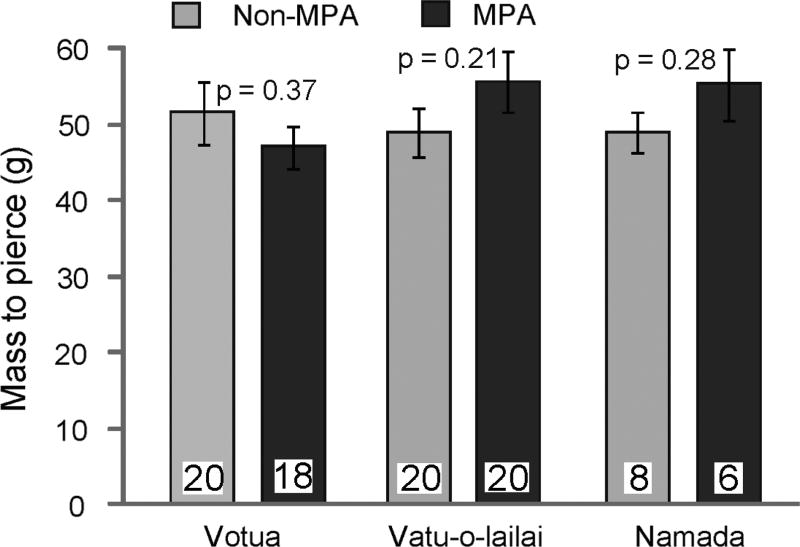
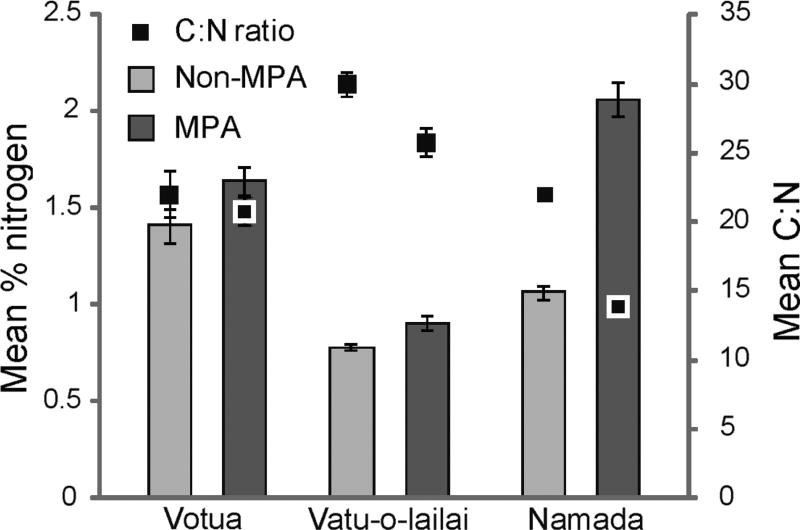
Toughness of S. polycystum blades did not differ significantly between MPA and non-MPA sites (p ≥ 0.214; Fig. 3); thus feeding preference was not related to a change in thallus toughness. Nutritional patterns did differ between MPA and non-MPA sites but in a direction opposite to that of feeding preference; the more nutritious, nitrogen-rich S. polycystum occurred in the MPAs and was consumed significantly less than the lower quality individuals from the non-MPAs. Despite being the less preferred foods, algae from the MPAs were significantly higher in percent nitrogen (dry mass) than those from the associated non-MPAs for the villages of Vatu-o-lailai and Namada (p = 0.016, p = 0.009, respectively; Mann-Whitney U-tests); this contrast was not significant for Votua but trended in the same direction (p = 0.076; Fig. 4). Similarly, contrasts for Vatu-o-lailai and Namada showed the algae from the MPAs had significantly lower C:N ratios than algae from the non-MPAs (p = 0.028, p = 0.009, respectively); again this difference was not significant for Votua (p = 0.602; Fig. 4).
Fig. 3.
Mean blade toughness (mass needed to pierce an algal blade) (±1 SE) as measured with a penetrometer. Light grey bars: blades from non-marine protected areas (non-MPA); dark bars: blades from MPA sites; n is at the base of each column; p-values are from independent sample t-tests
Fig. 4.
Mean nitrogen content and C:N ratio (±1 SE) for Sargassum polycystum. Percent nitrogen (dry mass) was significantly higher in S. polycystum from the marine protected areas (MPA) in 2 of the 3 villages and trended this way in the other (Votua p = 0.076; Vatu-o-lailai p = 0.016; Namada p = 0.009; Mann-Whitney U-tests); significance in C:N ratio on the secondary y-axis followed the same pattern. Light grey bars: non-MPA sites; dark bars: MPA sites; filled squares: C:N ratio for each site. n = 5
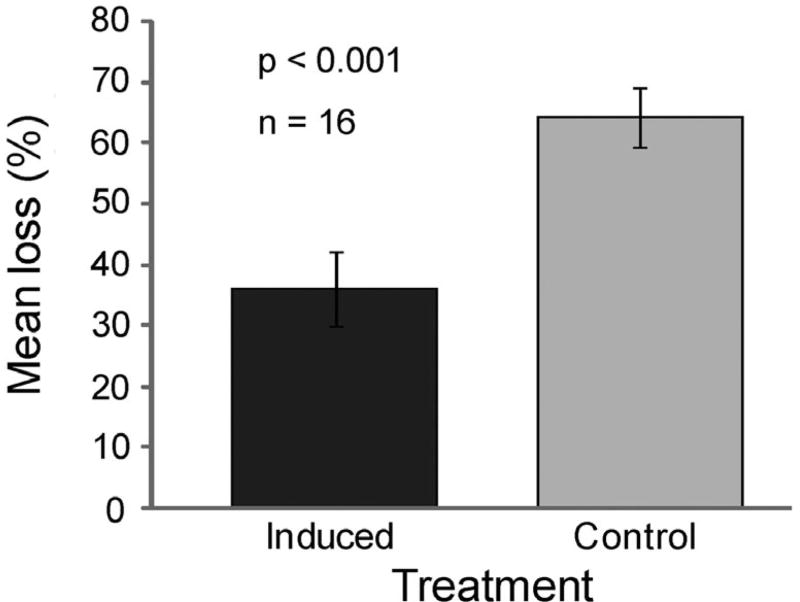
When algal thalli growing in the non-MPA were moved to the herbivore-rich MPA and either protected from or partially exposed to grazing and then allowed to recover for 2 wk, the new growth from thalli previously protected from attack was consumed a significant 78% more than the new growth from fronds that had experienced previous grazing (p < 0.001, paired t-test; Fig. 5). As with the previous feeding assays (MPA versus non-MPA contrasts), this difference in palatability was not correlated with increased toughness; penetrometer measurements did not differ between the 2 groups (induced = 41.4 ± 2.17 SE g; control = 44.7 ± 2.56 g; n = 23; p = 0.337; independent sample t-test).
Fig. 5.
Mean (±1 SE) percent Sargassum polycystum consumption by fish grazing on new growth from control versus induced thalli. Fish preferred new growth from control (light grey bar) versus new growth from previously grazed algae (dark grey bar); n = 16; p < 0.001 from paired samples t-test
DISCUSSION
Over 5 sampling periods and across 3 replicate pairs of MPAs and non-MPAs, herbivorous fishes significantly preferred Sargassum polycystum from the non-MPAs in 11 out of 13 trials (Fig. 2). This occurred despite these S. polycystum fronds growing only a few hundred metres apart on the same reef flat (Fig. 1). Preferential feeding did not occur due to differences in blade toughness, as there were no differences (Fig. 3). Similarly, preferential feeding was not focused on the more nutritionally rich plants (Fig. 4). Nitrogen is commonly a limiting resource for herbivores (Mattson 1980), and in field assays, herbivorous fishes commonly prefer seaweeds that are experimentally enriched with nitrogen (Burkepile & Hay 2009, Chan et al. 2012). In contrast, fishes in our assays were preferentially feeding on non-MPA algae which had lower nitrogen content (significantly so for Vatu-o-lailai and Namada; Fig. 4).
The lower nitrogen content in S. polycystum from the non-MPAs could be caused by greater competition among algae for nutrients in those locations as isotopic studies from these sites indicate that nitrogen sources for paired MPAs and non-MPAs at each village did not differ (Dell et al. 2015). Macroalgal cover in the non-MPAs is 27 to 61 times greater than in the MPAs (Rasher et al. 2013), so increased competition for nutrients could occur and limit nutrient content in non-MPA algae. Furthermore, fish biomass is 7 to 17 times higher in the MPAs than the non-MPAs (Rasher et al. 2013), so there is also greater potential for nitrogenous enrichment from fish excretion in the MPAs (Meyer et al. 1983, Burkepile et al. 2013).
While feeding choices appeared unrelated to changes in blade toughness or nitrogen content, we found they could be explained by induction of defence in S. polycystum following partial grazing (Fig. 5). When S. polycystum ramets were taken from the non-MPA (where there are fewer herbivorous fishes; Rasher et al. 2013) and half were partially grazed by fishes while the other half was protected from fish grazing, the new growth from the protected ramets was preferred to that from the previously grazed algae although they originated from the same holdfast. Penetrometer measurements of blades from treatments versus controls did not differ significantly, and there was no noticeable difference in morphology. This suggests that the induced defences were chemical, but we were unable to conduct chemical investigations at this remote field location. Rigorous bio-assay-guided investigations to determine the role of specific chemical defences in generating these feeding patterns would be valuable.
The magnitude of fish preference for control over induced S. polycystum was greater in the induction experiment (Fig. 5) than the un-manipulated MPA/non-MPA feeding contrasts (Fig. 2)—perhaps because all treatment fronds in the induction experiment had been previously grazed by fishes—while this may not be the case for fronds collected from the MPAs. In the MPAs, S. polycystum survives only in shallow areas where herbivorous fishes have limited access. On these reefs, herbivorous fishes feed primarily at low tide to avoid large predators that occupy the reef flat at high tide, creating spatial escapes from herbivory in shallow habitats (D. B. Rasher et al. unpubl.). Thus, all fronds collected from shallow areas of the MPA may not have been previously grazed. Additionally, in the induction experiment one-third of each frond had been grazed, whereas grazing on the individuals growing in the MPA may have been less severe, perhaps leading to a lower level of induction. It is possible that a threshold of grazing has to occur to cue induction (Rohde & Wahl 2008) and that this threshold is not always reached by S. polycystum growing in the shallow-water escapes of the MPAs. Either of these scenarios could explain the stronger fish preference in the induction experiment relative to the MPA/non-MPA contrasts.
There are numerous previous demonstrations of induced seaweed defences in response to grazing by small, less mobile herbivores (mesograzers such as amphipods, isopods and gastropods) but few demonstrations in response to grazing by larger, more mobile herbivores (Toth & Pavia 2007, Jormalainen & Honkanen 2008). Induction of morphological defence in response to grazing by herbivorous fishes has been reported (Steneck & Adey 1976, Lewis et al. 1987), but to our knowledge, there appear to have been few experimental tests since then (Diaz-Pulido et al. 2007), although some studies have suspected it may be occurring (Paul & Van Alstyne 1988, Coen & Tanner 1989). This highlights a gap in current knowledge and a potential misconception regarding macro algae not inducing defences in response to grazing by larger herbivores. We found that large, mobile grazers could cause macroalga to induce reduced palatability, but more evaluations are needed across more species to determine whether such induction is common or rare. Additionally, rigorous investigation needs to confirm if the induction is chemical and to identify the compounds involved. Those species capable of rapid growth and with extensive portions protected from consumption within the substratum (such as S. polycystum) may be more likely to utilise induced defences because basal portions hidden from grazers may be able to regenerate induced up right portions even if initial uprights are grazed to extinction.
Other studies have documented intraspecific variance in algal palatability to fishes, so induction of defence in response to fish grazing may be more common than currently realised. For example, Keeley et al. (2015) documented that fish consumed S. polycystum transplanted from the intertidal 6 times more rapidly than conspecifics grown <15 m away in the subtidal. This could result from higher grazing in the deeper site causing induction of defence in algae growing there, while less grazing in the intertidal (no fish were observed in that region during the study) left the algae there un-induced.
Although the role of mesograzers in inducing macro algal defences is well established (Toth & Pavia 2007, Jormalainen & Honkanen 2008), differential grazing by invertebrates would not have been responsible for induction in our study. The algae used in the induction experiment were collected from the same holdfast and randomly assigned to treatment or control groups so initial invertebrate densities would not have differed. Similarly, during the experiment, cages were checked during both day and night periods for the presence of herbivorous invertebrates and none were seen. Scars attributable to invertebrate grazing (such as crabs or amphipods) are easily visible on S. polycystum fronds but were not observed, suggesting invertebrate herbivores were uncommon in this experiment. Additionally, if mesograzers were inducing seaweed defences, then one might expect plants from the non-MPA to be more induced and less palatable (we found the opposite) because mesograzer density often scales with macroalgal abundance (Carpenter 1986, Roff et al. 2013) and seaweeds were much more abundant in the non-MPAs (Rasher et al. 2013).
As a final observation, the only previous studies to document induced macroalgal defences in response to vertebrate grazing all detected induction of morphological traits (Steneck & Adey 1976, Lewis et al. 1987, Diaz-Pulido et al. 2007). Likewise in terrestrial systems, morphological or structural defences seem to be induced in response to grazing by large, mobile grazers (thorns or silica; Hanley et al. 2007), while induction of chemical defences (which appears common in response to invertebrate grazing) is rare to absent in response to vertebrate grazing (Bryant et al. 1991). It is unclear whether this is a real pattern in marine and terrestrial systems or a result of bias in investigation, as investigators may focus on invertebrate grazers due to their ease of use in laboratory settings and their importance in agricultural systems.
Here we present one example of induction of macro algal defence caused by vertebrate grazing. Investigations into induction of algal defences have been performed since the 1980s, yet this appears to be one of the few published studies to address induction due to fish grazing. Whether this stems from a bias in interest or the difficulty of publishing negative results is unknown. Regardless of the reason, we are still unable to adequately assess the relative roles of meso- versus macro-grazers in inducing seaweed defences (Hay 1996). More publication of both positive and negative results would assist in this.
Acknowledgments
We thank the Fijian government and the Korolevu-i-wai district elders for granting research permission. Support came from the National Science Foundation (OCE 0929199), the National Institute of Health (U01TW007401 & U19TW007401) and the Teasley Endowment to the Georgia Institute of Technology. This work was completed in accordance with IACUC guidelines; permit A12085.
LITERATURE CITED
- Bryant JP, Kuropat PJ, Reichardt PB, Clausen TP. Controls over the allocation of resources by woody plants to chemical antiherbivore defense. In: Palo RT, Robbins CT, editors. Plant defenses against mammalian herbivory. CRC Press; Boca Raton, FL: 1991. pp. 83–103. [Google Scholar]
- Burkepile DE, Hay ME. Nutrient versus herbivore control of macroalgal community development and coral growth on a Caribbean reef. Mar Ecol Prog Ser. 2009;389:71–84. [Google Scholar]
- Burkepile DE, Allgeier JE, Shantz AA, Pritchard CE, Lemoine NP, Bhatti LH, Layman CA. Nutrient supply from fishes facilitates macroalgae and suppresses corals in a Caribbean coral reef ecosystem. Sci Rep. 2013;3:1493. doi: 10.1038/srep01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RC. Partitioning herbivory and its effects on coral reef algal communities. Ecol Monogr. 1986;56:345–364. [Google Scholar]
- Chan AY, Lubarsky K, Judy KN, Fong P. Nutrient addition increases consumption rates of tropical algae with different initial palatabilities. Mar Ecol Prog Ser. 2012;465:25–31. [Google Scholar]
- Chiang YM, Yoshida T, Ajisaka T, Trono G, Tseng CK, Lu B. Distribution and variation in Sargassum polycystum CA Agardh (Fucales, Phaeophyta) In: Abbott IA, editor. Taxonomy of economic seaweeds with reference to some Pacific and Western Atlantic species. Springer-Verlag; Berlin: 1992. pp. 35–42. [Google Scholar]
- Coen LD, Tanner CE. Morphological variation and differential susceptibility to herbivory in the tropical brown alga Lobophora variegata. Mar Ecol Prog Ser. 1989;54:287–298. [Google Scholar]
- Coley PD. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol Monogr. 1983;53:209–234. [Google Scholar]
- De Wreede RE. The phenology of three species of Sargassum (Sargassaceae, Phaeophyta) in Hawaii. Phycologia. 1976;15:175–183. [Google Scholar]
- Dell C, Montoya JP, Hay ME. Effect of marine protected areas (MPAs) on consumer diet: MPA fish feed higher in the food chain. Mar Ecol Prog Ser. 2015;540:227–234. doi: 10.3354/meps11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell CL, Longo GO, Hay ME. Positive feedbacks enhance macroalgal resilience on degraded coral reefs. PLOS ONE. 2016;11:e0155049. doi: 10.1371/journal.pone.0155049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Pulido G, Villamil L, Almanza V. Herbivory effects on the morphology of the brown alga Padina boergesenii (Phaeophyta) Phycologia. 2007;46:131–136. [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst. 2007;8:157–178. [Google Scholar]
- Harvell CD. The ecology and evolution of inducible defenses. Q Rev Biol. 1990;65:323–340. doi: 10.1086/416841. [DOI] [PubMed] [Google Scholar]
- Hay ME. Spatial patterns of grazing intensity on a Caribbean barrier reef: herbivory and algal distribution. Aquat Bot. 1981;11:97–109. [Google Scholar]
- Hay ME. Marine chemical ecology: What’s known and what’s next? J Exp Mar Biol Ecol. 1996;200:103–134. [Google Scholar]
- Hay ME, Colburn T, Downing D. Spatial and temporal patterns in herbivory on a Caribbean fringing reef: the effects on plant distribution. Oecologia. 1983;58:299–308. doi: 10.1007/BF00385227. [DOI] [PubMed] [Google Scholar]
- Jormalainen V, Honkanen T. Macroalgal chemical defences and their roles in structuring temperate marine communities. In: Amsler CD, editor. Algal chemical ecology. Springer-Verlag; Berlin: 2008. pp. 57–89. [Google Scholar]
- Karban R, Baldwin IT. Induced responses to herbivory. 1. University of Chicago Press; Chicago, IL: 1997. [Google Scholar]
- Keeley KN, Stroh JD, Tran DSC, Fong CR, Fong P. Location, location, location: small shifts in collection site result in large intraspecific differences in macroalgal palatability. Coral Reefs. 2015;34:607–610. [Google Scholar]
- Koricheva J, Nykänen H, Gianoli E. Meta-analysis of trade-offs among plant antiherbivore defenses: Are plants jacks-of-all-trades, masters of all? Am Nat. 2004;163:E64–E75. doi: 10.1086/382601. [DOI] [PubMed] [Google Scholar]
- Lewis SM, Norris JN, Searles RB. The regulation of morphological plasticity in tropical reef algae by herbivory. Ecology. 1987;68:636–641. [Google Scholar]
- Littler MM, Littler DS. The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. Am Nat. 1980;116:25–44. [Google Scholar]
- Long JD, Smalley GW, Barsby T, Anderson JT, Hay ME. Chemical cues induce consumer-specific defenses in a bloom-forming marine phytoplankton. Proc Natl Acad Sci USA. 2007;104:10512–10517. doi: 10.1073/pnas.0611600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson WJ. Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst. 1980;11:119–161. [Google Scholar]
- Meyer JL, Schultz ET, Helfman GS. Fish schools: an asset to corals. Science. 1983;220:1047–1049. doi: 10.1126/science.220.4601.1047. [DOI] [PubMed] [Google Scholar]
- Paul VJ, Van Alstyne KL. Chemical defense and chemical variation in some tropical Pacific species of Halimeda (Halimedaceae; Chlorophyta) Coral Reefs. 1988;6:263–269. [Google Scholar]
- Pavia H, Toth GB. Inducible chemical resistance to herbivory in the brown seaweed Ascophyllum nodosum. Ecology. 2000;81:3212–3225. [Google Scholar]
- Rasher DB, Engel S, Bonito V, Fraser GJ, Montoya JP, Hay ME. Effects of herbivory, nutrients, and reef protection on algal proliferation and coral growth on a tropical reef. Oecologia. 2012;169:187–198. doi: 10.1007/s00442-011-2174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasher DB, Hoey AS, Hay ME. Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology. 2013;94:1347–1358. doi: 10.1890/12-0389.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff G, Wabnitz CC, Harborne AR, Mumby PJ. Macroalgal associations of motile epifaunal invertebrate communities on coral reefs. Mar Ecol (Berl) 2013;34:409–419. [Google Scholar]
- Rohde S, Wahl M. Temporal dynamics of induced resistance in a marine macroalga: time lag of induction and reduction in Fucus vesiculosus. J Exp Mar Biol Ecol. 2008;367:227–229. [Google Scholar]
- Sale PF. Assemblages of fish on patch reefs—predictable or unpredictable? Environ Biol Fishes. 1980;5:243–249. [Google Scholar]
- Simpson R. A case study of the Korolevu-i-wai qoliqoli on the Coral Coast, Fiji. MSc thesis. University of South Pacific; Suva: 2010. Assessing MPA effectiveness through observing the relative abundances of community-selected indicator populations over time. [Google Scholar]
- Steneck RS, Adey WH. Roles of environment in control of morphology in Lithophyllum congestum a Caribbean algal ridge builder. Bot Mar. 1976;19:197–215. [Google Scholar]
- Tollrian R, Harvell CD, editors. The ecology and evolution of inducible defenses. Princeton University Press; Princeton, NJ: 1999. [Google Scholar]
- Toth GB, Pavia H. Induced herbivore resistance in sea weeds: a meta-analysis. J Ecol. 2007;95:425–434. [Google Scholar]
- Toth GB, Karlsson M, Pavia H. Mesoherbivores reduce net growth and induce chemical resistance in natural seaweed populations. Oecologia. 2007;152:245–255. doi: 10.1007/s00442-006-0643-5. [DOI] [PubMed] [Google Scholar]
- Vergés A, Vanderklift MA, Doropoulos C, Hyndes GA. Spatial patterns in herbivory on a coral reef are influenced by structural complexity but not by algal traits. PLOS ONE. 2011;6:e17115. doi: 10.1371/journal.pone.0017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JQ, Bellwood DR. How far do schools of roving herbivores rove? A case study using Scarus rivulatus. Coral Reefs. 2012;31:991–1003. [Google Scholar]