Abstract
Context:
Prolonged physical activity gives rise to variable degrees of body weight and fat loss, and is associated with variability in appetite control. Whether these effects are modulated by postprandial, peptides is unclear. We examined the role of postprandial peptide response in compensatory eating during 12 weeks of aerobic exercise and in response to high-fat, low-carbohydrate (HFLC) and low-fat, high-carbohydrate (LFHC) meals.
Methods:
Of the 32 overweight/obese individuals, 16 completed 12 weeks of aerobic exercise and 16 nonexercising control subjects were matched for age and body mass index. Exercisers were classified as responders or nonresponders depending on net energy balance from observed compared with expected body composition changes from measured energy expenditure. Plasma samples were collected before and after meals to compare profiles of total and acylated ghrelin, insulin, cholecystokinin, glucagon-like peptide 1 (GLP-1), and total peptide YY (PYY) between HFLC and LFHC meals, pre- and postexercise, and between groups.
Results:
No differences between pre- and postintervention peptide release. Responders had greater suppression of acylated ghrelin (P < 0.05) than nonresponders, as well as higher postprandial levels of GLP-1 (P < 0.001) and total PYY (P < 0.001) compared with nonresponders and control subjects.
Conclusion:
No impact on postprandial peptide release was found after 12 weeks of aerobic exercise. Responders to exercise-induced weight loss showed greater suppression of acylated ghrelin and greater release of GLP-1 and total PYY at baseline. Therefore, episodic postprandial peptide profiles appear to form part of the pre-existing physiology of exercise responders and suggest differences in satiety potential may underlie exercise-induced compensatory eating.
We examined peptide responses during 12 weeks of exercise after high- and low-fat meals. Weight losers showed greater suppression of acylated ghrelin and greater release of GLP-1 and PYY at baseline.
The ability of exercise to produce an energy deficit puts it at the forefront of many weight loss and weight maintenance programs (1–3). However, the reality of exercise-producing weight loss is complex, with many exercise-intervention studies showing actual weight loss is somewhat less than the predicted weight loss (4). The individual variability in response to a prescribed, supervised, and measured exercise protocol has been documented by different research groups (5, 6). Furthermore, the fact that some do and some do not lose weight in response to the same stimulus has identified the issue of compensation, which could occur through a number of different mechanisms. Essentially, there are two scenarios: People may compensate for the increased energy expenditure by increasing their energy intake, thereby reducing the possibility of weight loss, or they may compensate for the additional physical activity energy expenditure by decreasing their nonexercise activity thermogenesis for the rest of the day, for example, by increasing their sedentary behavior. Much of the research regarding compensation for exercise has focused on the energy intake side, whereby classification of individuals into noncompensators and responders or compensators and nonresponders has been investigated (6, 7). Our group has previously shown that aerobic exercise affects at least two components of appetite regulation through both increased fasting hunger and an increase in satiety (8). What remains unknown is the possible mechanisms implicated in these changes.
One mechanism could be through changes in gut peptides that are related to appetite control. These peptides are generally categorized into tonic or episodic peptides. Tonic appetite signals are those peptides that reflect the body’s energy stores [e.g., leptin (9), insulin (10) and ghrelin (11)]. These peptides respond to exercise, and the importance of changes in body composition, particularly in fat mass, has been noted (12–14), although there is some evidence for an independent effect of exercise, particularly for insulin (15, 16). In juxtaposition, short-term episodic (i.e., meal-related) signals from the periphery fluctuate throughout the day, particularly in response to consumption of food. Ghrelin, primarily involved in meal initiation and glucagon-like peptide 1 (GLP-1), peptide YY (PYY), and cholecystokinin (CCK) are the primary peptides investigated in response to food consumption. The response of appetite parameters in the period between meals depends on the type, quantity, and quality of food provided, leading to an integrated response via neural and humoral processes (17). The ability of the gastrointestinal tract to recognize the composition of ingested food is paramount for the maintenance of a stable body weight.
A series of studies have investigated the response of these peptides to an acute bout of exercise (18–23). These studies were predominantly in young, healthy, athletic men, however, and few have investigated the effect of longer periods of training, nor have they recognized the importance of investigating the postprandial peptide response to food before and after aerobic exercise. Only one study to date, to our knowledge, has examined the postprandial response before and after a longer-term supervised exercise program (24). The response to a mixed macronutrient, 600 kcal breakfast (17% protein, 35% fat, and 48% carbohydrate) was measured before and after the exercise intervention. Insulin sensitivity was found to be significantly higher after the exercise intervention. There was no effect of exercise on the total or acylated ghrelin postprandial response to feeding. There was no effect of exercise on GLP-1 or total PYY postprandial levels; however, Martins et al. (24) reported a trend for higher GLP-1 level area under the curve approximately 2 hours after food consumption and higher total PYY level between 120 and 180 minutes after food intake.
Studies and consistent evidence in this area are lacking; therefore, the aim of the current study was to investigate the role of postprandial episodic peptides in response to two macronutrient challenges between those who do and do not respond to exercise-induced weight loss. It was hypothesized that responders, nonresponders, and nonexercising control subjects may differ in the postprandial peptide response to high-fat or high-carbohydrate meals because there is evidence that individual peptides respond differently to the macronutrient composition of the food consumed (25). Given the large variability in individual obesity treatment programs, identifying variables or components that may help identify why some people are successful at losing weight compared with those who are not is a clear priority (26).
Methods
Subjects
Thirty-two participants completed the study; 16 (n = 5 men) completed the supervised exercise intervention and 16 (n = 8 men) were recruited as age and body mass index (BMI)-matched control subjects. All participants were initially screened to ensure they met the following inclusion criteria: adult (age 18 to 55 years), BMI (27 to 34.9 kg/m2), nonsmoking, physically inactive (≤2 h/wk of physical activity and not taking part in structured exercise over the previous 6 months), and not taking medication known to affect metabolism or appetite. Answers on screening questionnaires were verified by researchers during the screening process. All study foods were shown to participants to ensure they liked and would eat all of them, and that they had no allergies to any of the foods. None of the participants would be considered restrained eaters, using the Three-Factor Eating Questionnaire (27). Participants were recruited from the University of Leeds, United Kingdom, and surrounding areas using poster advertisements and recruitment e-mails. The study was conducted in accordance with the 1964 Declaration of Helsinki, and all participants provided written informed consent before taking part. Ethical permission was granted by the Leeds National Health Service Research Ethics Committee (no. 09/H1307/7) and the School of Psychology Ethics Committee, University of Leeds. The study was retrospectively registered under international standard trials approval (ISRCTN47291569).
Exercisers
Participants were informed of the general nature of the study—an investigation into exercise and appetite-related peptides—but not the precise aims. The time and physical commitments required from them were made clear. Informed written consent was obtained after the nature and possible consequences of the study were explained.
Nonexercising control subjects
Sixteen participants matched for age and BMI to the exerciser group were recruited as control subjects. Participants were not made aware of the exercise arm of the study but were informed the research was an investigation into the effect of time on appetite-related peptides. Subjects were requested not to change their dietary or exercise patterns for the duration of the study. All procedures and time commitment were made clear before informed written consent was given. Exclusion and inclusion criteria were the same as that of the exercise group.
Design
Participants took part in a 12-week supervised exercise intervention whereby they completed five exercise sessions per week, with each session expending 500 kcal, which was individually calibrated for each participant. The duration and intensity of the exercise sessions were calculated for each participant and recalculated at week 6 to account for changes in body weight and/or cardiovascular fitness. All exercise was recorded using heart rate monitors, and any sessions missed were added on; this ensured all participants had completed the same amount of energy expenditure before the postintervention measures were completed. Indirect calorimetry (Vmax Encore; Carefusion, Basingstoke, UK) was performed every 6 weeks to measure exercise-induced energy expenditure during the exercise sessions. The intensity was designed to be moderate and was set at 70% of the individual’s heart rate maximum (i.e., 220 minus age in years). Participants could choose from a variety of aerobic exercise modes (i.e., treadmill walking or running, cycle ergometer, rowing, or cross-trainer) as long as they kept to their prescribed heart rate. All sessions were supervised within the research unit and recorded using heart rate monitors (RS400; Polar Global, Warwick, UK).
Assessment of maximal oxygen uptake
A maximal fitness test was undertaken every 6 weeks on a treadmill to measure maximum oxygen uptake and calculate the energy expended during exercise. There is a clear linear relationship between oxygen uptake and work rate (i.e., heart rate) (28). The treadmill test was incremental until exhaustion, using both speed and incline according to a validated protocol for a test of maximal fat oxidation rate (29). The treadmill gradient began at 1°, with a speed of 3.5 km/h. Every 3 minutes, the speed was increased by 1.0 km/h until 6.5 km/h was reached. Expired air samples were taken constantly, with heart rate recordings taken during the last minute of the 3-minute intervals. Using the expired air information, if the respiratory quotient was <1, the incline of the treadmill was increased by 2° every 3 minutes. Once a respiratory quotient of 1 was reached, the speed of the treadmill was increased by 1 km/h every minute until exhaustion. Participants were advised to let the researchers know when they thought they were able to continue for only 1 minute more. Strong verbal encouragement was given to the participant to ensure they reached exhaustion.
Assessment of postprandial peptides
To assess the acute and chronic effects of exercise on appetite-related postprandial peptides, two probe-day measurements were used, one with high-fat, low-carbohydrate (HFLC) content (>50% energy from fat) and one with low-fat, high-carbohydrate (LFHC) content (<3% energy from fat). The two probe days were separated by at least 3 days. Participants were provided with a standardized pasta meal on the evening before each test day at weeks 0 and 12, and were then instructed to fast from 10:00 pm the night before the probe day (with the exception of water). The order of the two conditions was randomized to eliminate a condition effect.
Participants arrived at the human-appetite research unit at approximately 8:00 am, when an intravenous cannula was inserted into the antecubital vein for serial measurements of appetite-related peptides. One fasting blood sample was taken before the participant was provided breakfast. The breakfasts were matched for energy (590 kcal) and weight (685 g) but differed in fat and carbohydrate content (HFLC: 50.3% fat, 38.0% carbohydrate, and 11.7% protein; LFHC: 3.2% fat, 83.6% carbohydrate, and 13.2% protein). Both breakfasts consisted of Greek yogurt mixed with cream, banana, honey, raisins, and currants provided to the participant in one bowl to consume together. During pilot testing, the breakfast meals were compared for pleasantness and found to be equipalatable.
Participants were given 10 minutes to consume the breakfast, thereby matching the rate of consumption between individuals. Serial blood samples were taken at 10, 20, 30, 60, 90, 120, and 180 minutes after breakfast. During the 3 hours, participants stayed in the laboratory in separate cubicles to ensure no social influences took place. The cubicles are specifically designed to be devoid of food and time cues so as not to influence the participant.
Samples were analyzed for levels of insulin, total and acylated ghrelin, GLP-1, total PYY, and CCK. Methods of analysis of these peptides can be found in Gibbons et al. (25, 30). Control group data are not available for total and acylated ghrelin, or CCK because funding and time constraints limited the number of peptides that could be measured.
Respective inter- and intra-assay coefficients of variation for total ghrelin were 5.9% and 3.4%; for insulin, GLP-1, and total PYY, 12.5% and 8.3%; and for CCK, 15.6% and 9.4%. Insulin data are presented first as an indicator of the sensitivity of the assays used, because it was expected that differences between two conditions would be most prevalent in this biomarker.
Assessment of subjective appetite
Immediately before each blood sample, appetite sensations were measured using visual analog scales on a handheld computer (31). The scales used included hunger, fullness, and desire to eat.
Food intake
Three hours after consumption of the fixed breakfast, an ad libitum lunch meal was provided. The lunch consisted of two items: a savory component and a sweet component, to reflect a normal lunch meal for the study population. This lunch meal was the same for both the HFLC and LFHC conditions; details of the two components are listed in Table 1. Participants were free to consume as much or as little as they wanted until they were comfortably full.
Table 1.
Nutrient and Energy Composition of the Ad Libitum Lunch
| Risotto | Yogurt | |
|---|---|---|
| Energy, kcal | 811 | 810 |
| Weight, g | 480 | 480 |
| Fat, g | 27.6 | 31.0 |
| Carbohydrate, g | 62.3 | 58.7 |
| Protein, g | 10.1 | 10.3 |
Body composition
After an overnight fast, body weight and composition were measured at baseline and week 12. Body composition was measured using air-displacement plethysmography (Bodpod, Concord, CA).
Responder/nonresponder classification
The responders and nonresponders were retrospectively classified by degree of compensation in response to the negative energy balance induced by the exercise. The degree of compensation was calculated from measured body composition changes relative to predicted energy imbalance if there was no compensation. Predicted energy imbalance was estimated by summing the energy cost of the exercise over 12 weeks for each participant. It was assumed that the energy cost of a 1-kg change of fat mass is 39.9 MJ (9540 kcal) and the energy cost of a 1-kg change in lean mass is 4.72 MJ (1100 kcal) (32). Using this method, participants were divided classified as responders or nonresponders by median split. This implies that these individuals had demonstrated differing degrees of compensation for the exercise-induced negative energy balance.
Statistical analysis
Data are reported as mean ± standard error of the mean throughout. Statistical analyses were performed using SPSS for Windows (version 22; IBM, Armonk, NY). Paired samples t tests were used to compare fasting levels of peptides to ensure the participants started both days in a similar state. Peptide concentrations were then analyzed by repeated measures analysis of variance. There was no significant effect of sex on fasting metabolic or appetite hormone levels; therefore, men and women were analyzed together to improve study power. Owing to the individual variability in blood parameters and peptide levels, the change from fasting at each time point was calculated for each individual, as conducted in our laboratory previously (25, 30). Mean scores on each peptide outcome were calculated for exercising and nonexercising groups (the parameter “group” comprised responders, nonresponders, and control subjects) at baseline and after the 12-week intervention (week: week 0; week 12), before and at seven additional time points after test food intake (time: 0, 10, 20, 30, 60, 90, 120, and 180 minutes) for low-fat and high-fat probe days. Where significant interactions were revealed, these were explored in follow-up analyses using the relevant variable combinations. Statistical significance was accepted at a level of P < 0.05. Where appropriate, Greenhouse-Geisser probability levels were used to adjust for sphericity, and Bonferroni adjustments were applied to control for multiple post hoc comparisons.
Results
Body composition
The participant characteristics for exercise responders, nonresponders, and nonexercise control subjects are listed in Table 2. There were no differences at baseline among groups. There were no differences in total exercise duration or energy expenditure between exercise groups. Responders lost more weight than nonresponders and control subjects, as indicated by differences in weight, BMI, fat mass, and waist circumference. The nonresponders and control subjects did not differ over the 12 weeks except for waist circumference, which was reduced in nonresponders after the intervention.
Table 2.
Defining Body Composition Characteristics Before and After 12-Week Intervention
| Exercisers | Nonexercisers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Responders (n = 8; 2 Men) | Nonresponders (n = 8; 3 Men) | Control Subjects (n = 16; 8 Men) | ||||||||
| Week 0 | Week 12 | Change | Week 0 | Week 12 | Change | Week 0 | Week 12 | Change | Pa | |
| Age, y | 45.8 (2.7) | 45.4 (1.8) | 39.6 (2.5) | |||||||
| Weight, kg | 83.5 (3.1) | 79.9 (3.1) | −3.6 (0.9) | 90.4 (2.7) | 90.4 (2.6) | 0.0 (0.8) | 93.1 (3.6) | 94.0 (3.8) | 0.9 (0.5) | <0.01 |
| BMI, kg/m2 | 29.5 (0.9) | 28.3 (1.1) | −1.2 (0.3) | 30.1 (1.2) | 30.1 (1.3) | 0.0 (0.3) | 30.7 (0.9) | 31.0 (1.0) | 0.3 (0.1) | <0.01 |
| Fat mass, kg | 33.1 (2.5) | 29.0 (3.1) | −4.1 (0.9) | 35.5 (3.3) | 35.0 (3.5) | −0.5 (0.5) | 35.1 (2.2) | 35.8 (2.4) | 0.7 (0.6) | <0.01 |
| Fat-freemass, kg | 50.3 (3.1) | 50.8 (3.2) | 0.5 (0.5) | 54.9 (3.4) | 55.4 (3.1) | 0.5 (0.5) | 58.0 (2.7) | 58.2 (2.8) | 0.2 (0.3) | 0.82 |
| Waist circumference, cm | 99.8 (2.0) | 94.8 (2.3) | −5.0 (0.9) | 103.9 (3.3) | 102.0 (3.6) | −1.9 (0.6) | 104.3 (2.0) | 106.2 (2.3) | 1.9 (0.6) | <0.01 |
| RMR, kcal/d | 1740.5 (116.7) | 1714.1 (92.5) | −26.4 (112.7) | 1655.1 (83.1) | 1761.6 (95.3) | 106.5 (78.3) | 1852.1 (83.1) | 1767.9 (96.7) | −149.9 (71.2) | 0.11 |
| Fitness, mL/kg/min | 29.4 (3.6) | 40.9 (3.1) | 11.5 (1.8) | 36.6 (3.1) | 39.9 (2.1) | 3.3 (3.4) | 0.07 | |||
| Exercise duration, min/12 wk | 2984.6 (132.6) | 2905.9 (167.7) | ||||||||
| ExEE, kcal/12 wk | 24,258.7 (680.9) | 24,708.3 (1097.0) | ||||||||
Data given as no. (standard error of the mean) unless otherwise indicated. There were no baseline differences between groups.
Abbreviations: ExEE, exercise energy expenditure; RMR, resting metabolic rate.
Corresponds to group × time interactions.
Postprandial peptide levels
Fasting peptide levels did not change significantly differently between groups in response to exercise. The peptide response to macronutrient composition has been documented (25, 30), revealing that insulin showed a greater response to the LFHC condition, whereas GLP-1, total PYY, and CCK showed a greater response to an HFLC diet. The analysis in the current study is focused on the pre to postintervention response and the group differences in postprandial peptide response.
Insulin
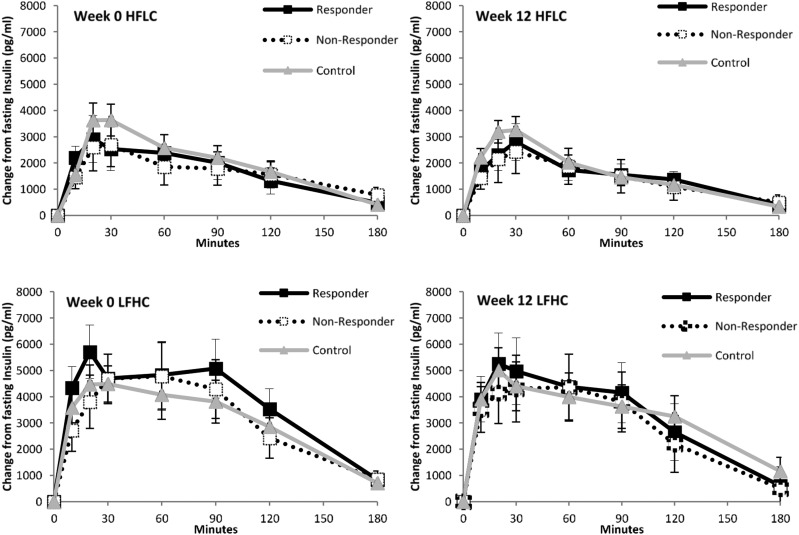
For insulin, there was no main effect of week [F(1,28) = 0.623; P = 0.436] and no main effect of group [F(2,28) = 0.142; P = 0.868] or group interactions (Fig. 1).
Figure 1.
Postprandial profiles of insulin levels in responders and nonresponders to exercise and nonexercising control subjects during HFLC and LFHC conditions before and after the 12-week intervention.
Total and acylated ghrelin
For total ghrelin, there was no main effect of week [F(1,14) = 0.068; P = 0.798] and no main effect of group [F(1,14) = 2.402; P = 0.143] or group interactions.
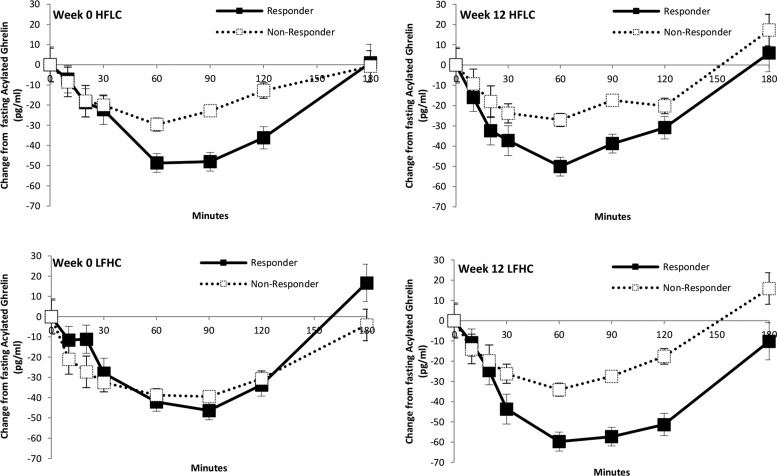
For acylated ghrelin, there was no main effect of week [F(1,13) = 0.072; P = 0.792] and no main effect of group [F(1,13) = 1.004; P = 0.335]. There was a significant time by group interaction [F(6,78) = 4.035; P < 0.05] and the week by condition by time by group was significant [F(6,78) = 2.368; P < 0.05]. The nonresponders showed a blunted suppression of acylated ghrelin to HFLC and LFHC breakfasts except after the LFHC breakfast, before the exercise intervention, when the suppression was similar to that of the responders (Fig. 2).
Figure 2.
Postprandial profiles of acylated ghrelin levels in responders and nonresponders to exercise during HFLC and LFHC conditions before and after the 12-week intervention. *P < 0.05 for between-groups data at individual time points.
GLP-1
For GLP-1, there was no main effect of week [F(1,29) = 0.000; P = 0.994] and there was a main effect of group [F(1,29) = 11.628; P < 0.001], but there were no group interactions. The mean peptide concentrations showed a linear trend for responders to have greater levels of GLP-1 compared with nonresponders (P < 0.01) and control subjects (P < 0.001), whereas nonresponders and control subjects did not differ significantly (P = 0.162). Figure 3 indicates no difference among groups over time, but shows that, overall, responders showed a greater GLP-1 response.
Figure 3.
Postprandial profiles of GLP-1 levels in responders and nonresponders to exercise and nonexercising control subjects during HFLC and LFHC conditions before and after the 12-week intervention. *P < 0.05 for between-groups data at individual time points.
Total PYY
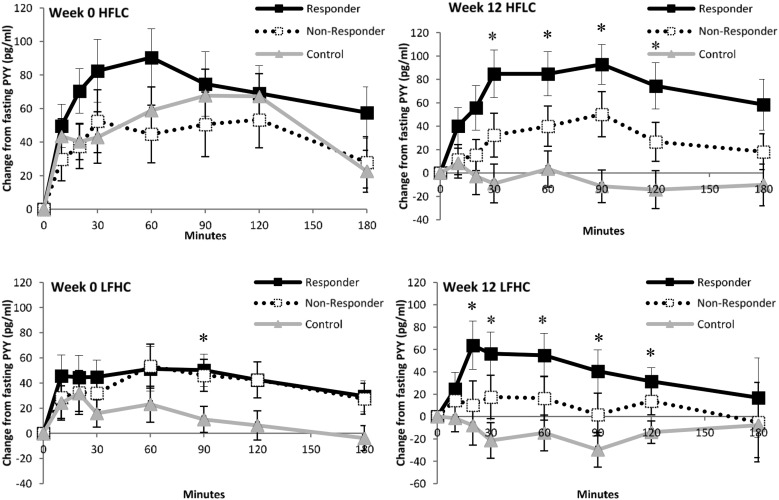
For total PYY, there was a main effect of week [F(1,25) = 6.214; P < 0.05] and a main effect of group [F(1,25) = 16.404; P < 0.001] but no group interactions. The mean peptide concentrations showed a linear trend for responders to have greater levels of total PYY compared with nonresponders (P < 0.05) and control subjects (P < 0.001), and for nonresponders to have higher levels compared with control subjects (P < 0.05). Figure 4 indicates no difference among groups over time, but shows that, overall, responders showed a greater total PYY response.
Figure 4.
Postprandial profiles of total PYY levels in responders and nonresponders to exercise and nonexercising control subjects during HFLC and LFHC conditions before and after the 12-week intervention. *P < 0.05 for between-groups data at individual time points.
CCK
For CCK, there was no main effect of week [F(1,14) = 0.308; P = 0.587] and no main effect of group [F(1,14) = 0.005; P = 0.944] or group interactions.
Discussion
In this study, we demonstrated that appetite-related postprandial peptides may be involved in exercise-induced compensation and differences in peptide profiles between responders and nonresponders precede aerobic exercise. Responders were characterized by a greater suppression of acylated ghrelin and greater release of both GLP-1 and total PYY. These differences were observed irrespective of baseline body composition, test meal composition (HFLC or LFHC), or aerobic exercise. This finding suggests responders and nonresponders have pre-existing differences in physiology that may affect compensatory eating via satiety signaling; and postprandial peptide response, particularly of acylated ghrelin, GLP-1, and total PYY, could be proposed as predictors of weight loss success through aerobic exercise. Appetite-related peptides were responsive to the type of food consumed. In addition, they were associated with the degree of weight loss response to prolonged exercise.
The energy expenditure was fixed and individually calibrated for each participant in this study, and all exercise sessions were supervised and recorded. Therefore, all participants underwent the same challenge to their energy balance system. Interestingly, the response to this challenge resulted in a large variability in body composition changes, something that has been shown by several research groups (5, 6). There is a growing body of research to support the notion that tonic peptides (i.e., peptides related to body weight or body composition) change in response to weight loss. Leptin, insulin, and total ghrelin levels are the forerunners in this evidence and have been shown to respond, in particular, to fat loss (33, 34). There is also evidence of increased insulin sensitivity after exercise regardless of weight loss throughout the intervention (15). There is, however, little consistent evidence of the response of postprandial peptides (i.e., the profile of peptides in response to food before and after exercise interventions). One study using a similar 12-week exercise intervention found that there was a significant reduction in postprandial insulin, no change in postprandial acylated ghrelin, but a possible trend for increased postprandial GLP-1 and total PYY levels in the late satiety period (24), thereby supporting the evidence for an increase in satiety after aerobic exercise. The current study goes a step beyond these findings by demonstrating these differences were present in response to high-fat and high-carbohydrate meals, and by comparing groups of responders and nonresponders and a nonexercising control group.
It is difficult to fully ascertain the role of gut peptides in the control of appetite, particularly when studies using supraphysiological levels are discounted. Current thinking is that some peptides may be linked to the short-term control of appetite (25) and that it is more likely that several peptides have an accumulative effect on appetite and satiety (30). This is a logical progression because many peptides are released into the circulation in the fed state; therefore, corelease of several peptides may more closely represent the physiological fed state. Evidence to support this has been shown in studies in which coinfusing GLP-1 and total PYY peptides simultaneously had a greater effect on ad libitum food intake than either peptide infused alone (35).
The role of gut peptides in weight loss is substantial, particularly in the literature on obesity surgeries. Support for the findings in the current study can be seen in studies showing that those who experience poor weight loss after Roux-en-Y gastric bypass showed attenuated GLP-1 and total PYY postprandial responses (36). However, in the current study, favorable postprandial profiles of acylated ghrelin, GLP-1, and total PYY at baseline predicted success at exercise-induced weight loss.
When the change in variables across exercise interventions are reported in the literature, there is often a difficulty in understanding which change occurs first; for example, does fat mass decrease before leptin levels decrease? Most people would agree with this direction of events; however, they may be concurrent and each is affecting the other throughout the intervention. This study supports the idea that those who respond better to exercise have postprandial peptide responses indicative of improved appetite control before they start the exercise, and this may contribute their ability to lose weight over the course of the intervention. Of the measured variables in this study, we found no other predictors of success in these individuals. Future research should investigate the role of physical activity outside of the exercise intervention to assess whether changes have an effect on compensation and weight change in response to an exercise intervention.
The exercise intervention was not enough on its own to promote changes in body composition in all individuals; this points toward the possible need for additional dietary or behavioral interventions in some people. It is an interesting proposition that by testing the sensitivity of postprandial peptide response, particularly that of acylated ghrelin, GLP1, and total PYY, it may be possible to identify individuals beforehand who may or may not be successful in losing weight through exercise. Nevertheless, it must be pointed out that even though the nonresponders in this study did not show positive changes in body composition, they did benefit from the exercise intervention through increased fitness and improved health markers (i.e., fasting insulin levels) and this message should be communicated rather than changes in weight and body composition.
In conclusion, those who lost weight in response to exercise showed a greater suppression of acylated ghrelin and greater release of GLP-1 and total PYY in response to food. These differences were apparent before and after the intervention; therefore, episodic postprandial peptide profiles may form part of the pre-existing physiology of responders compared with nonresponders and may explain differences in satiety potential underlying exercise-induced compensatory eating.
Acknowledgments
Financial Support: This work was supported by Biotechnology and Biological Sciences Research Council (Diet and Health Research Industry Club) Grant BB/G005524/1 and European Union Seventh Framework Programme (FP7/2007-2013) Grant 266408. E.N. was supported by the NovoNordisk Foundation and Karolinska Institutet Diabetes Theme Center.
Clinical Trial Information: ISRCTN Registry no. 47291569 (registered 14 September 2010).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CCK
- cholecystokinin
- GLP-1
- glucagon-like peptide 1
- HFLC
- high-fat, low-carbohydrate
- LFHC
- low-fat, high-carbohydrate
- PYY
- peptide YY.
References
- 1.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity. Geneva, 3-5 June 1997. Available at: http://apps.who.int/iris/handle/10665/63854. Accessed 20 August 2017. [PubMed] [Google Scholar]
- 2.Jakicic JM, Clark K, Coleman E, Donnelly JE, Foreyt J, Melanson E, Volek J, Volpe SL; American College of Sports Medicine . American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33(12):2145–2156. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine . American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. [DOI] [PubMed] [Google Scholar]
- 4.Ross R, Janssen I.. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc. 2001;33(6 Suppl):S521–527; discussion S528-529. [DOI] [PubMed] [Google Scholar]
- 5.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4(2):e4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes. 2008;32(1):177–184. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly JE, Smith BK. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exerc Sport Sci Rev. 2005;33(4):169–174. [DOI] [PubMed] [Google Scholar]
- 8.King NA, Caudwell PP, Hopkins M, Stubbs JR, Naslund E, Blundell JE. Dual-process action of exercise on appetite control: increase in orexigenic drive but improvement in meal-induced satiety. Am J Clin Nutr. 2009;90(4):921–927. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D Jr. Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 1992;13(3):387–414. [DOI] [PubMed] [Google Scholar]
- 11.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87(1):240–244. [DOI] [PubMed] [Google Scholar]
- 12.Pérusse L, Collier G, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Nadeau A, Zimmet PZ, Bouchard C. Acute and chronic effects of exercise on leptin levels in humans. J Appl Physiol (1985). 1997;83(1):5–10. [DOI] [PubMed] [Google Scholar]
- 13.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133(2):92–103. [DOI] [PubMed] [Google Scholar]
- 14.Leidy HJ, Gardner JK, Frye BR, Snook ML, Schuchert MK, Richard EL, Williams NI. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J Clin Endocrinol Metab. 2004;89(6):2659–2664. [DOI] [PubMed] [Google Scholar]
- 15.Hulver MW, Zheng D, Tanner CJ, Houmard JA, Kraus WE, Slentz CA, Sinha MK, Pories WJ, MacDonald KG, Dohm GL. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283(4):E861–E865. [DOI] [PubMed] [Google Scholar]
- 16.Cox KL, Burke V, Morton AR, Beilin LJ, Puddey IB. Independent and additive effects of energy restriction and exercise on glucose and insulin concentrations in sedentary overweight men. Am J Clin Nutr. 2004;80(2):308–316. [DOI] [PubMed] [Google Scholar]
- 17.Karhunen LJ, Juvonen KR, Huotari A, Purhonen AK, Herzig KH. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept. 2008;149(1-3):70–78. [DOI] [PubMed] [Google Scholar]
- 18.Burns SF, Broom DR, Miyashita M, Mundy C, Stensel DJ. A single session of treadmill running has no effect on plasma total ghrelin concentrations. J Sports Sci. 2007;25(6):635–642. [DOI] [PubMed] [Google Scholar]
- 19.Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. J Appl Physiol (1985). 2007;102(6):2165–2171. [DOI] [PubMed] [Google Scholar]
- 20.Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R29–R35. [DOI] [PubMed] [Google Scholar]
- 21.King JA, Wasse LK, Broom DR, Stensel DJ. The influence of brisk walking on appetite, energy intake and plasma acylated ghrelin. Med Sci Sports Exerc. 2010;42(3):485–492. [DOI] [PubMed] [Google Scholar]
- 22.King JA, Miyashita M, Wasse LK, Stensel DJ. Influence of prolonged treadmill running on appetite, energy intake and circulating concentrations of acylated ghrelin. Appetite. 2010;54(3):492–498. [DOI] [PubMed] [Google Scholar]
- 23.King JA, Wasse LK, Stensel DJ. The acute effects of swimming on appetite, food intake, and plasma acylated ghrelin. J Obes. 2011;2011:351628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J Clin Endocrinol Metab. 2010;95(4):1609–1616. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons C, Caudwell P, Finlayson G, Webb DL, Hellström PM, Näslund E, Blundell JE. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J Clin Endocrinol Metab. 2013;98(5):E847–E855. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Metcalfe LL, Blew RM, Sardinha LB, Lohman TG. Exercise motivation, eating, and body image variables as predictors of weight control. Med Sci Sports Exerc. 2006;38(1):179–188. [DOI] [PubMed] [Google Scholar]
- 27.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. [DOI] [PubMed] [Google Scholar]
- 28.Wyndham CH, Strydom NB, Maritz JS, Morrison JF, Peter J, Potgieter ZU. Maximum oxygen intake and maximum heart rate during strenuous work. J Appl Physiol. 1959;14:927–936. [DOI] [PubMed] [Google Scholar]
- 29.Achten J, Jeukendrup AE. Maximal fat oxidation during exercise in trained men. Int J Sports Med. 2003;24(8):603–608. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons C, Finlayson G, Caudwell P, Webb DL, Hellström PM, Näslund E, Blundell JE.. Postprandial profiles of CCK after high fat and high carbohydrate meals and the relationship to satiety in humans. Peptides. 2016;77:3–8. [DOI] [PubMed] [Google Scholar]
- 31.Gibbons C, Caudwell P, Finlayson G, King N, Blundell J. Validation of a new hand-held electronic data capture method for continuous monitoring of subjective appetite sensations. Int J Behav Nutr Phys Act. 2011;8(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stubbs R, et al. Interactions between energy intake and expenditure in the development and treatment of obesity. Progr Obes Res. 2003;9:418. [Google Scholar]
- 33.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81(12):4406–4413. [DOI] [PubMed] [Google Scholar]
- 34.Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, Jørgensen JO. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf). 2002;56(2):203–206. [DOI] [PubMed] [Google Scholar]
- 35.Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, Dakin CL, Filipsson K, Wang F, Kent AS, Frost GS, Ghatei MA, Bloom SR. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146(12):5120–5127. [DOI] [PubMed] [Google Scholar]
- 36.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–785. [DOI] [PubMed] [Google Scholar]