Abstract
Context:
Hypertension in young women is uncommon compared with young men and older women. Estrogen appears to protect most women against hypertension, with incidence increasing after menopause. Because some premenopausal women develop hypertension, estrogen may play a different role in these women. Genetic variations in the estrogen receptor (ER) are associated with cardiovascular disease. ER-β, encoded by ESR2, is the ER predominantly expressed in vascular smooth muscle.
Objective:
To determine an association of single nucleotide polymorphisms in ESR2 with salt sensitivity of blood pressure (SSBP) and estrogen status in women.
Methods:
Candidate gene association study with ESR2 and SSBP conducted in normotensive and hypertensive women and men in two cohorts: International Hypertensive Pathotype (HyperPATH) (n = 584) (discovery) and Mexican American Hypertension–Insulin Resistance Study (n = 662) (validation). Single nucleotide polymorphisms in ESR1 (ER-α) were also analyzed. Analysis conducted in younger (<51 years, premenopausal, “estrogen-replete”) and older women (≥51 years, postmenopausal, “estrogen-deplete”). Men were analyzed to control for aging.
Results:
Multivariate analyses of HyperPATH data between variants of ESR2 and SSBP documented that ESR2 rs10144225 minor (risk) allele carriers had a significantly positive association with SSBP driven by estrogen-replete women (β = +4.4 mm Hg per risk allele, P = 0.004). Findings were confirmed in Hypertension Insulin–Resistance Study premenopausal women. HyperPATH cohort analyses revealed risk allele carriers vs noncarriers had increased aldosterone/renin ratios. No associations were detected with ESR1.
Conclusions:
The variation at rs10144225 in ESR2 was associated with SSBP in premenopausal women (estrogen-replete) and not in men or postmenopausal women (estrogen-deplete). Inappropriate aldosterone levels on a liberal salt diet may mediate the SSBP.
In two cohorts, the ER-β gene risk allele associated with SSBP but only in the presence of estrogen, potentially secondary to inappropriate aldosterone levels on liberal salt intake.
Before the age of 45, women as compared with men have a lower incidence and prevalence of hypertension, a significant risk factor for cardiovascular disease (CVD); however, as women age, this risk increases (1). One explanation for this age-related development of hypertension seen in women is postulated to involve the decline in estrogen levels after menopause, suggesting that the loss of estrogen unmasks hypertension in these women (2). However, a subset of women develops hypertension prior to menopause, suggesting that estrogen may not be protective in these women. According to the National Health and Nutrition Examination Survey (1998 to 2008), 8% of women with hypertension are between the ages of 22 and 44 years (3). Women who develop hypertension early are at an increased risk of adverse cardiovascular outcomes over time as compared with premenopausal women without hypertension (4). The mechanisms that account for hypertension in premenopausal women who are estrogen-replete are not well understood.
Estrogen’s effects on the cardiovascular system are mediated by two estrogen receptor (ER) subtypes, ER-α (encoded for by the ESR1 gene) and ER-β (encoded for by the ESR2 gene). ESR2 has a high homology with ESR1 DNA/ligand binding domains, but a distinct transcriptional activation domain. Polymorphisms in the ER have been associated with CVD risk in men and women, but most studies have focused on ESR1 genetic variants (5–7). ESR2 is highly expressed in the vasculature, and estrogen binding to this receptor generally leads to vasodilation, yet its role in CVD is not well studied (2, 8). Activation of ESR2 can protect against the adverse effect of CVD caused by aldosterone in animals (9, 10). Moreover, women who are heterozygous for certain genotypic polymorphisms of ESR2 have been reported to be at increased risk of hypertension, especially those who use oral contraceptives (11), suggesting that certain single nucleotide polymorphisms (SNPs) in ESR2 may transform the interaction of estrogen with ESR2 from a protective effect on blood pressure to a detrimental one.
Salt sensitivity of blood pressure (SSBP) is a heritable subset of hypertension and is present in approximately half of those with essential hypertension (12). SSBP may enhance the risk of cardiovascular and kidney-related morbidity in these patients (13). Additionally, normotensive family members of those with SSBP have been found to have SSBP themselves, which predates the development of hypertension, suggesting heritability (12). Accordingly, we hypothesized that genetic variability in the ER subtypes may be associated with SSBP in the presence of estrogen.
We postulate that polymorphic variants within ESR2 may repress estrogen’s ability to bind to its receptor, thereby inhibiting estrogen-induced vasodilation and leading to a loss of its protective effect against hypertension. To determine whether ESR2 plays a particular role in the blood pressure response to changes in salt intake, we examined the relationship between haplotypes and SNPs of ESR2 and SSBP in premenopausal women (estrogen-replete state) and postmenopausal women and/or men (estrogen-deplete state). Because our results demonstrated an association, we then explored whether the mechanism(s) underlying this effect were related to alterations in the renin–angiotensin–aldosterone system (RAAS) response to salt intake. We also examined SNPs of ESR1 to determine whether the findings were specific to ESR2.
Subjects and Methods
Participants
The analysis consisted of 584 female and male and hypertensive and normotensive subjects from the International Hypertensive Pathotype (HyperPATH) cohort and a validation cohort of 662 normotensive and hypertensive Mexican Americans [Hypertension Insulin–Resistance Study (HTN-IR)]. Details of the HyperPATH and HTN-IR cohorts have been described previously (14, 15). In brief, the HyperPATH cohort consists of ∼2000 subjects with and without hypertension. Five international centers contributed to this data set: Brigham and Women’s Hospital (Boston, MA), University of Utah Medical Center (Salt Lake City, UT), Hospital Broussais (Paris, France), Vanderbilt University (Nashville, TN), and University of La Sapiena (Rome, Italy). The HTN-IR cohort consists of normotensive and hypertensive Mexican Americans recruited within the Los Angeles area who had a family history of hypertension.
HyperPATH salt sensitivity protocol
Inclusion and exclusion criteria along with a detailed phenotyping protocol for the HyperPATH cohort are defined in detail elsewhere (12, 14, 16–18). In brief, all subjects received a screening history and physical and laboratory examinations. Hypertension was defined as a seated diastolic blood pressure (DBP) of at least 100 mm Hg on no medications, or 90 mm Hg or more on one antihypertensive medication at the time of screening, or treatment with two or three antihypertensive medications. Subjects on four or more antihypertensive medications were excluded. All antihypertensive medications were washed out for 1 to 3 months prior to dietary salt study, resulting in 100% of subjects being studied off antihypertensive medication. Normotension was defined as blood pressure <135/85 mm Hg with report of no first-degree relatives diagnosed with hypertension before the age of 60. All subjects were between 18 and 65 years old. Race was self-reported. Each participant was placed on both isocaloric liberal salt (200 mmol/d sodium) and restricted salt (10 mmol/d sodium) diets, with each diet containing 100 mmol/d potassium and 1000 mmol/d calcium for 5 to 7 days as an outpatient. The order of the diets was randomized. On the final day of each diet, participants were admitted overnight to the Clinical Research Centers and remained fasting and supine. The next morning, three consecutive systolic blood pressure (SBP) and DBP readings separated by 5 minutes each were obtained using an automated device (Dinamap; Critikon, Tampa, FL), and blood samples were obtained for a variety of analytes (e.g., hormones, metabolic factors, ions, DNA analysis). Subjects underwent 24-hour urine collections to confirm sodium balance (urine sodium ≥ 150 mmol per 24 hours on the liberal salt diet and ≤30 mmol per 24 hours on the restricted salt diet).
For the current study, the subjects selected from the HyperPATH cohort met the following criteria: ESR2 rs10144225 genotype, supine DBP obtained between 7:00 and 8:00 am after an overnight fast and in balance on both the liberal and the restricted salt diets (as defined below), plasma renin activity (PRA) and serum aldosterone obtained at 7:00 to 8:00 am, supine, after an overnight fast on the liberal salt diet. Women on oral contraceptive or hormonal therapy were excluded. Because HyperPATH did not document the age of onset of menopause, subjects were classified according to presumed menopausal status using the average menopausal age of 50 years (average age of menopause in North America and Europe is 50.9 and 50.4 years, respectively) as a surrogate for menopausal status (19). Women <51 years old were classified as premenopausal, whereas women 51 years of age or older were classified as postmenopausal. In some, but not all, of the subjects, follicle-stimulating hormone (FSH) and estradiol values were available in the HyperPATH data set (premenopausal, 158 subjects; postmenopausal, 152 subjects). Data for both hormones were not available on each of these subjects. In aggregate, these data were used to determine the validity of our surrogate for menopausal state: age criterion. Finally, 4% of the hypertensive subjects had mild diabetes mellitus or impaired glucose intolerance.
SSBP was determined by using a continuous variable, for example, the change in either SBP or DBP (ΔSBP or ΔDBP) between the restricted and liberal salt diets (liberal salt-restricted salt). For logistic regression analyses we used our previously reported cut points (ΔSBP = 14 mm Hg and ΔDBP = 9 mm Hg) (17).
Assays were performed in the Brigham Research Assay Core Laboratory by standard immunoassay methods for aldosterone, cortisol, PRA, FSH, and estradiol.
HTN-IR cohort salt sensitivity protocol
All subjects received a screening history and physical and laboratory examinations. Hypertension was defined as SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg or on pharmacological therapy for hypertension with onset before age 65 years with no secondary causes. If clinically permissible, hypertensive individuals were studied at least 2 weeks after washing out antihypertensive medications. Thus, 96% of the subjects in this study were not on antihypertensives. Blood pressures were measured using an automated device (Dinamap; Critikon) in a sitting position. Three blood pressure readings were taken at 5-minute intervals and averages were used (15, 20). SSBP was determined using the Weinberger protocol, a validated procedure that involves an infusion of 1 L of 0.9% saline over 4 hours on day 1, 10 mEq sodium diet, and three doses of furosemide over 10 hours on day 2 in an inpatient setting (21). SSBP was defined as the change in SBP between baseline on day 2 (salt loaded) and the end of salt depletion on day 2 (18). In HTN-IR hormonal treatment was not documented for women and blood hormonal data were not available.
The corresponding Institutional Review Boards approved all studies, and written informed consent was obtained from each participant.
Genotyping
In HyperPATH, DNA was extracted as previously described (18). Genotyping was conducted using a 7600-SNP IlluminaSelect platform (Illumina® platform, San Diego, CA). For ESR2, in the HyperPATH cohort, 24 SNPs were originally selected for tagging to capture common variation using HapMap (November 2011) chromosome 14q23.2 of the ER-β gene. For ESR1, eight SNPs were originally selected for tagging using HapMap (November 2012) chromosome 6q25.1 of the ER-α gene.
Selected SNPs had minor allele frequencies >0.1 at R2 ≥ 0.8 (22). All SNPs had a completion rate of ≥95% and were in Hardy–Weinberg equilibrium. Repeat genotyping in 10% of the subjects demonstrated concordance with the original genotype call. None of these SNPs has been reported in the literature to be associated with SSBP. Therefore, we assessed the relationship between each SNP and SSBP independently.
In HTN-IR, the SNP of interest was extracted from existing genome-wide genotyping data (23).
Statistical analysis
For the haplotype-based analyses, haplotypes were constructed using the Haploview 4.1 program (24). An association between each block with SSBP was assessed using PLINK 1.07 (25). PLINK provides an estimate of the haplotype frequencies via the expectation-maximization algorithm, computing global and haplotype-specific score statistics for tests of association between a trait and weighted haplotype (weighted by their posterior possibility). PLINK is not able to account for relatedness; therefore, the haplotype analysis was conducted in unrelated individuals only. All statistical tests were two-sided. We used STATA version 14.1 for the statistical analysis of individual SNPs. Hardy–Weinberg equilibrium testing was performed for each SNP using a χ2 test. Pairwise linkage (r2) was estimated using Haploview 4.1. Significance at the Bonferroni-corrected level for multiple comparisons to account for three haplotypes was 0.017.
Phenotype/genotype analyses were also used. For the ESR2 SNP, rs10144225, in the haplotype that drove the significant association with the SSBP phenotype, logistic regression analysis accounting for body mass index (BMI), age, sex, race, and underlying diseases (hypertension) was used to analyze SSBP (cut-off value for ΔSBP = 14 mm Hg and for ΔDBP = 9 mm Hg) in risk allele–carrying women with age <51 years old vs all others (17). For blood pressure, the three measurements were averaged. For both ESR2 and ESR1 SNPs, linear regression analysis was performed with the two degree of freedom genotype model (minor allele homozygotes, heterozygote allele carriers, and major allele homozygotes) without adjustment for covariates, and then adjusted for BMI, age, sex, race, and underlying diseases (hypertension). For the aldosterone/renin ratio (ARR), there were three additional adjustments made: serum cortisol, serum potassium, and urine potassium. Group comparisons were performed by t test or two-way analysis of variance, where appropriate. Also, where the sample size of one group was small, nonparametric tests (e.g., Fisher’s exact test) were used. Trend analysis by STATA nptrend command was performed to test for trend across ordered groups.
For the HTN-IR cohort, the R package genome-wide association analyses with family was used to test the association between SSBP and the SNP rs10144225 under an additive genetic model (26). To account for familial correlation within pedigrees, a linear mixed effects model from the lmekin function in the kinship package was used.
Significance at the Bonferroni-corrected level for multiple comparisons was adjusted to P = 0.01. Error was represented by 95% CIs.
Results
Haplotype selection
For our initial analysis in the HyperPATH cohort, SNPs in ESR2 were selected from CEU and YRI populations of HapMap (phase II, November 2012) to maximize available variation using chromosome 14q23.2. Twenty-four SNPs were originally genotyped with 14 SNPs being removed before the start of the analysis. Specifically, three were monomorphic in the population, two had a minor allele frequency ≤ 0.05, one failed Hardy–Weinberg equilibrium, one failed genotype completion rate > 95%, and seven SNPs were in linkage disequilibrium (LD; R2 > 0.80) with other SNPs, resulting in 10 tagging SNPs (Supplemental Table 1 (220.3KB, docx) ). The 10 SNPs captured 100% of the common HapMap white variation in this region. The SNP LD plot from our HyperPATH cohort is shown in Supplemental Fig. 1 (220.3KB, docx) .
We then conducted a haplotype analysis with the SNP LD plot from our HyperPATH population indicating three haplotype blocks. Given the potential sex difference of ESR2 seen in prior studies, we conducted a sex-specific haplotype analysis. We evaluated each global haploblock stratifying the cohort into men or women. In women, global block 1 showed a trend toward significance in association with SSBP (Pglobal = 0.06), which was entirely driven by haplotype number 3 rs1256062G|rs10144225G|rs1256059G|rs17766755G (P = 0.02) with particular influence by SNP rs10144225, as shown in Supplemental Fig. 2 (220.3KB, docx) . This haplotype was rare in the population with a frequency of 9% and a large effect size (β = 4.33). Blocks 2 and 3 did not have significant associations with SSBP in this population (Pglobal = 0.41 and 0.23, respectively). The individual SNP analysis of rs867443, not encompassed within a global block, also did not show an association with SSBP (P = 0.97). Additionally, we did not find a significant association with SSBP in men when conducting the haplotype analysis. We then performed an individual analysis of the SNP driving the significance within global block 1, haplotype number 3, rs10144225G, detailed in the Statistical analysis section above. All participants who carried the rs10144225 minor allele G were referred to as having the risk allele, and nonrisk allele carriers were the participants with major allele A homozygote of ESR2 rs10144225.
Demographic data
Among 584 participants from the HyperPATH cohort, 418 individuals (71.5%) had hypertension. Regarding ethnicity, each subject self-defined whether they were principally of European/Middle Eastern descent (whites) or of African descent (blacks). Most (86.9%) of the participants were whites. Two hundred sixty-six of the participants (45.5%) were women, with 68% of them younger than age 51 years. SBP/DBP on a high-salt diet were 148 ± 1/88 ± 1 mm Hg for the hypertensive group and 111 ± 1/67 ± 1 mm Hg for the normotensive group (P < 0.005 for both SBP and DBP between groups). For the entire cohort, the mean ± standard error of the mean (SEM) of the supine, liberal salt diet hormone levels were: serum aldosterone 4.9 ± 0.12 ng/dL, cortisol 11.1 ± 0.16 μg/dL, and PRA 0.51 ± 0.02 ng/mL/h. Mean age (±SEM) of women was 45.8 ± 0.5 years, and of men was 45.6 ± 0.5 years. The hormonal status for the women <51 years of age included estradiol at 59.4 ± 5.3 pg/mL (n = 99) and FSH at 12.5 ± 1.9 mIU/mL (n = 101), and for those ≥51 years of age included 27.4 ± 6.5 pg/mL (n= 53) and 54.0 ± 4.2 mIU/mL (n = 57), respectively, and differed significantly (P = 0.01 and 0.005, respectively) between age groups. The sex, age, prevalence of hypertension, mean BMI, and baseline blood pressure level did not show any significant difference among genotypes. However, race showed a statistically significant difference among genotype groups. In the Africans, the prevalence of risk allele carriers was significantly higher than that of the nonrisk allele carriers, whereas in the whites the greater number of nonrisk allele carriers was observed (P < 0.0005). Other participant characteristics are summarized in Table 1.
Table 1.
HyperPATH Cohort Baseline Characteristics Defined by rs10144225 Genotype
| Major Allele Homozygotes (AA) (n = 441) | Carrier Allele Heterozygotes (AG) (n = 117) | Minor Allele Homozygotes (GG) (n = 26) | P Value | |
|---|---|---|---|---|
| Sex | ||||
| Females (%) | 201 (45.6) | 50 (42.7) | 15 (57.7) | 0.38a |
| Males (%) | 240 (54.4) | 67 (57.3) | 11 (42.3) | |
| Age, y (range) | 46.08 ± 0.44 (19–65) | 44.88 ± 0.93 (18–65) | 43.9 ± 2.31 (18–65) | 0.21 |
| Female (%) | ||||
| Age < 51 y (“premenopausal”) | 129 (64.1) | 35 (70.0) | 13 (86.7) | 0.17a |
| Age ≥ 51 y (“postmenopausal”) | 72 (35.9) | 15 (30.0) | 2 (13.3) | |
| Race (%) | ||||
| White | 416 (94.3) | 84 (71.8) | 7 (26.9) | <0.001a |
| Africans | 25 (5.7) | 33 (28.2) | 19 (73.1) | |
| Disease status (%) | ||||
| Hypertensive with diabetes mellitus | 13 (2.9) | 2 (1.7) | 1 (3.8) | 0.86 |
| Hypertensive | 303 (68.7) | 83 (70.9) | 16 (61.5) | |
| Normotensive | 125 (28.3) | 32 (27.4) | 9 (34.6) | |
| BMI, kg/m2 (range) | 27.53 ± 0.15 (16.1–40.7) | 27.09 ± 0.37 (18.1–42.4) | 28.50 ± 0.91 (19.2–42.2) | 0.27 |
| SBP on liberal salt, mm Hg | 136.89 ± 1.11 | 139.16 ± 2.11 | 139.01 ± 4.91 | 0.27 |
| DBP on liberal salt, mm Hg | 81.41 ± 0.74 | 83.92 ± 1.31 | 82.60 ± 2.50 | 0.62 |
| Mean aldosterone, ng/dL | 4.94 ± 0.16 | 5.37 ± 0.44 | 4.75 ± 0.85 | 0.52 |
| Mean PRA, ng/mL/h | 0.54 ± 0.02 | 0.44 ± 0.03 | 0.34 ± 0.05 | 0.03 |
| Mean ARR, ng/dL per ng/mL/h | 20.15 ± 1.49 | 26.37 ± 3.61 | 32.83 ± 9.87 | 0.07 |
Data are presented as mean ± SEM and P value reflects analysis of variance test unless otherwise indicated.
χ2 or Fisher’s exact test.
In the HTN-IR cohort, there were 692 Mexican Americans with 578 hypertensive and 114 normotensive individuals, with 59% women. SBP/DBP on the high-salt diet were 153 ± 1/87 ± 1 for the hypertensives and 115 ± 1/71 ± 1 mm Hg for the normotensive group (P < 0.005 for both SBP and DBP between groups). Mean age and BMI of women were 38 ± 1 years and 29.3 ± 0.3 kg/m2, respectively, and of men were 38 ± 1 years and 28.7 ± 0.3 kg/m2, respectively. Data are depicted in Table 2
Table 2.
HTN-IR Cohort Baseline Characteristics Defined by rs10144225 Genotype
| Major Allele Homozygotes (AA) (n = 553) | Carrier Allele Heterozygotes (AG) (n = 135) | Minor Allele Homozygotes (GG) (n = 4) | P Value | |
|---|---|---|---|---|
| Sex | ||||
| Females (%) | 324 (58.6) | 79 (58.5) | 3 (75) | 0.89 |
| Males (%) | 229 (41.4) | 56 (41.5) | 1 (25) | |
| Age, y | 37.8 ± 0.6 | 40.5 ± 1.2 | 31.5 ± 6.2 | 0.08 |
| Female (%) | ||||
| Age < 51 y (“premenopausal”) | 254 (78.4) | 55 (69.6) | 3 (100.0) | 0.08a |
| Age ≥ 51 y (“postmenopausal”) | 70 (21.6) | 24 (30.4) | 0 (0) | |
| Disease status (%) | ||||
| Hypertensive | 78 (14.1) | 36 (26.7) | 0 (0) | 0.002a |
| Normotensive | 475 (85.9) | 99 (73.3) | 4 (100.0) | |
| BMI, kg/m2 | 29.0 ± 0.2 | 29.1 ± 0.4 | 28.0 ± 2.8 | 0.90 |
| SBP, mm Hg | 120.6 ± 0.7 | 124.5 ± 1.8 | 112.9 ± 8.4 | 0.06 |
| DBP, mm Hg | 73.0 ± 0.4 | 75.2 ± 0.9 | 66 ± 1.5 | 0.03 |
Data are presented as mean ± SEM and P value reflects analysis of variance test unless otherwise indicated.
χ2 or Fisher’s exact test.
The relationship between ESR2 rs10144225G and SSBP
There was a statistically significant difference between the minor (risk) allele carriers and major allele homozygotes for diastolic SSBP in univariate analysis that remained significant even after adjusting for age, BMI, race, sex, and disease state (hypertension) [P = 0.001; β = +2.10 mm Hg per risk allele, 95% CI of 0.83 to 3.37)] (Table 3). This relationship was driven by the estrogen-replete subjects (women < 51 years of age) (P = 0.004; β = +4.4 mm Hg per risk allele, 95% CI of 1.46 to 7.33), fully adjusted regression) (Table 3). Then, the subjects were divided into two groups: those who carried no risk allele for ESR2 rs10144225, and those who carried one or two risk alleles. Each group was divided into those who were estrogen replete (women < 51 years old) and those who were estrogen deplete (women ≥ 51 years old and all men). Estrogen-replete subjects had significantly higher odds of diastolic SSBP compared with estrogen-deplete subjects [odds ratio (OR) = 2.41, 95% CI of 1.16 to 5.00, P = 0.01). Importantly, there were no differences between men and women ≥ 51 years old. For systolic SSBP, the estrogen-replete group, but not the estrogen-deplete group, showed significantly association with ESR2 risk allele after being adjusted for age, BMI, race, sex, and disease state (hypertension) (P = 0.02, β = 3.93, 95% CI of 0.44 to 7.42).
Table 3.
Association Between ESR2 rs10144225 and Outcomes in the HyperPATH Cohort
| Regression Model |
Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| Coefficient Value (95% CI) | P Value | Coefficient value (95% CI) | P Value | |
| ΔDBP on Lib–Resa | 1.34 (0.14 to 2.55) | 0.03 | 2.10 (0.83 to 3.37) | 0.001 |
| Estrogen replete | 3.62 (0.48 to 6.77) | 0.02 | 4.4 (1.46 to 7.33) | 0.004 |
| Estrogen deplete | 1.53 (−0.33 to 3.40) | 0.10 | 1.16 (−0.61 to 2.91) | 0.20 |
| PRA on Liba | −0.10 (−0.18 to −0.02) | 0.01 | −0.09 (−0.18 to 0) | 0.04b |
| Estrogen replete | −0.09 (−0.22 to 0.023) | 0.15 | −0.03 (−0.21 to 0.12) | 0.64 |
| Estrogen deplete | −0.11 (−0.21 to −0.011) | 0.03 | −0.10 (−0.21 to −0.008) | 0.05 |
| ARR on Libc | 6.28 (0.91 to 11.64) | 0.02 | 6.74 (0.61 to 12.87) | 0.031 |
| Estrogen replete | 12.96 (5.27 to 20.64) | 0.001 | 15.03 (5.35 to 24.70) | 0.003 |
| Estrogen deplete | 2.15 (−5.01 to 9.32) | 0.55 | 1.62 (−6.23 to 9.48) | 0.68 |
P values were obtained from linear regression analysis.
Abbreviations: Lib, liberal salt diet; Res, restricted salt diet.
Adjustment made for age, sex, BMI, race, and underlying disease.
The statistical significance was lost after race was added in the model.
Adjustment made for age, sex, BMI, race, underlying disease, serum cortisol, serum potassium, and urine potassium level.
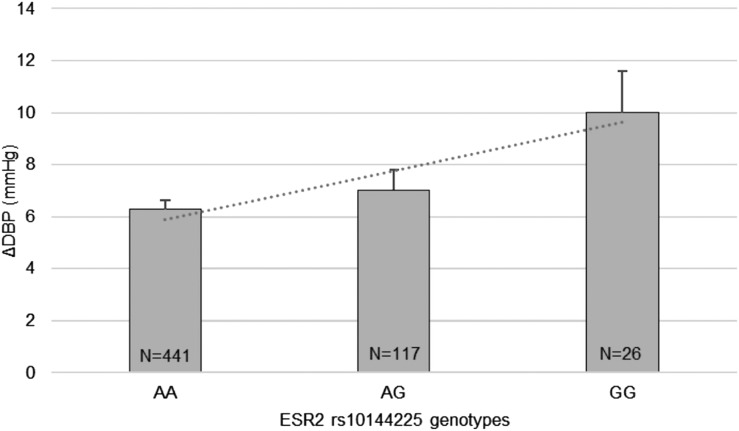
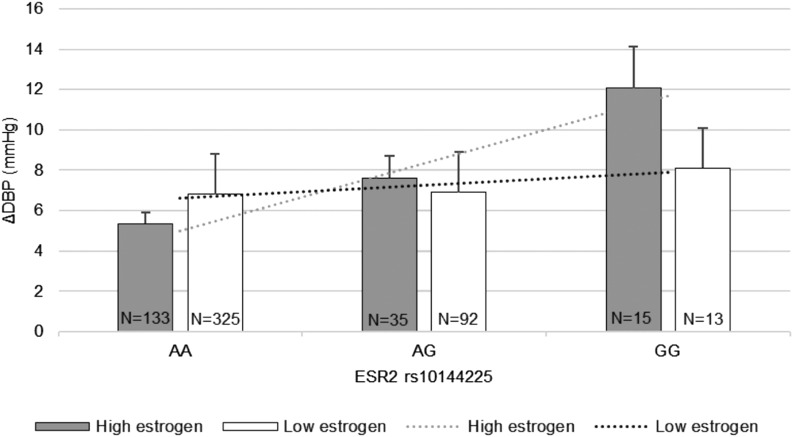
Based on trend analysis, although baseline DBP was comparable among genotype groups, those homozygous for the minor allele G tended to have the greatest diastolic SSBP, followed by heterozygote minor allele carriers and major allele A homozygotes (10.1± 1.6 mm Hg vs 7.1 ± 0.7 vs 6.2 ± 0.3 mm Hg, P = 0.04, respectively) (Fig. 1). Furthermore, the significant association between rs10144225 three genotypes and changes in DBP was only observed in premenopausal women who are at the estrogen-replete state, but not in the postmenopausal women group or men with AA = 5.3 ± 0.6 mm Hg, AG = 7.6 ± 1.3 mm Hg, and GG = 12.0 ± 2.0 mm Hg, P = 0.003 (Fig. 2).
Figure 1.
Trend analysis of DBP changes (ΔDBP) according to ESR2 rs10144225 genotype (data are from the HyperPATH cohort). Participants with minor allele G homozygotes tended to have the highest diastolic SSBP and was followed by heterozygote allele carriers and major allele A homozygotes (P = 0.04). P values were obtained from trend analyses. Data are presented as mean ± SEM. An A indicates adenine and a G indicates guanine.
Figure 2.
Trend analysis of DBP changes (ΔDBP) according to ESR2 rs10144225 genotype stratified by estrogen state (data from the HyperPATH cohort). The premenopausal females (high estrogen state or estrogen-replete state) with minor allele G homozygotes showed the significant trend toward SSBP in the total cohort (P = 0.003) with low estrogen group or estrogen-deplete state (postmenopausal females and/or males) having a nonsignificant trend (P = 0.82). Data are presented as mean ± SEM. An A indicates adenine and a G indicates guanine. The P values were obtained from trend analyses.
Validation of primary association in HTN-IR cohort
To validate our findings, we assessed the association of rs10144225 with SSBP in a mixed hypertensive and normotensive Mexican American population from the HTN-IR cohort. In premenopausal women within this cohort, risk allele carrier status was significantly (P = 0.03) associated with systolic SSBP (4.3 ± 1.1 mm Hg) vs nonrisk allele carrier status (1.4 ± 0.6). There was no significant association between the risk allele and SSBP in postmenopausal women or in men. Of interest, although the minor allele carriers were similar between HTN-IR and HyperPATH, the number of subjects that were homozygotes was less in the HTN-IR.
Exploratory analysis for potential mechanisms of ESR2 polymorphism
To elucidate potential underlying mechanisms for SSBP, we assessed the activity of the RAAS during liberal salt intake (Table 3). Importantly, in the fully adjusted model, the ARR was significantly associated with genotype (P = 0.031); risk allele carriers had a higher ARR. Similar to the SSBP analyses, this result was driven by the premenopausal (estrogen-replete) women (β = +15.03 ng/dL per ng/mL/h per risk allele, 95% CI of 5.35 to 24.70; P = 0.003) and not by those in the estrogen-deplete or low-estrogen groups (postmenopausal women and/or men) (Table 3). However, the individual components of the ARR differed only marginally. The liberal salt serum aldosterone levels were not significantly different by genotype. Liberal salt, supine PRA was significantly lower in risk allele carriers vs noncarriers in univariate analysis (P = 0.01) but was only marginally significant when fully adjusted for age, sex, BMI, race, and underlying disease (P = 0.04). Furthermore, the relationship was driven by the estrogen-deplete state (postmenopausal women and/or men) (P = 0.05).
The relationship between ESR1 variants and SSBP
In participants carrying the minor allele C of rs3020317 in ESR1 there was no significant correlation with either systolic or diastolic SSBP (P = 0.51 and P = 0.53, respectively). Further analysis by estrogen state also did not demonstrate a significant association. Additionally, no significant correlation with SSBP was observed for any of the other seven ESR1 SNPs (rs1884054, rs1884049, rs2144025, rs3003925, rs726281, rs3020328, and rs3003924).
Discussion
The major findings from this study were: first, women with ESR2 rs10144225 minor alleles were more likely to have SSBP even when adjusted for age, sex, BMI, race, and underlying disease. Second, estrogen status (as reflected in women by age >51 years or age <51 or by male sex) displayed a crucial role in this relationship: a significant association between the ESR2 risk allele and SSBP was only seen in individuals who were estrogen replete (women < 51 years of age). The genetic impact on SSBP was substantial, with the estrogen-replete subgroup having an effect size of 4.4 mm Hg per allele. Indeed, the two estrogen-deplete groups (postmenopausal women and men) were indistinguishable in our analyses. Third, one potential mechanism underlying this association was the observation of an increased ARR in estrogen-replete risk allele carriers, suggesting that their aldosterone levels are inappropriate for the level of salt intake, which may be a mechanism for SSBP. The effect size for ARR was also substantial, with levels in the estrogen-replete group more than fourfold greater than in the estrogen-deplete group. Fourth, no associations were observed between SSBP and eight ESR1 gene polymorphic variants. Thus, variants in the ESR2 gene are associated with SSBP in individuals with an estrogen-replete but not an estrogen-deplete status, an association that may, in part, be mediated by increased aldosterone levels leading to sodium retention. There have been no cardiometabolic traits or hypertension that have been associated with ESR2 in GWAS analyses. Our results are similar: we found no association between hypertension state and ESR2, only with SSBP.
SSBP is an important risk factor that contributes to CVD and is also one of the heritable subgroups of hypertension (12). SSBP has been reported by us (17) and others (21) in normotensive individuals and can be a precursor for hypertension as well as a reflection of impaired vascular function (27, 28). There are data to support that normotensives with SSBP are at increased risk for early mortality as compared with those with salt-resistant BP (29).
Previous studies have demonstrated that ESR2 variants had a role in cardiac and vascular physiology in animal models and were consistent with human studies that supported this theory in both sexes as well (10, 30–32). Evidence that ESR2 can modify blood pressure is supported by several animal studies. In particular, Zhu et al. (9) demonstrated that in vascular smooth muscle cells isolated from ESR2-deficient mice, there were several abnormalities in ion channel function causing both male and female ESR2-deficient mice to develop sustained systolic and diastolic hypertension as they age. These data suggest that ESR2 may play a key role in the development of hypertension
More common ESR2 polymorphic variants such as rs1271572 and rs1256049 have been previously reported to be associated with an increased risk of CVD (myocardial infarction or stroke) in women (33). In a nested case control study, Rexrode et al. (33) examined the association between polymorphisms of ESR2 in 296 white women enrolled in the Women’s Health Study and 566 white men from the Physician’s Health Study with risk of CVD. They found that in women but not men with a variant within ESR2 rs1271572, there were increased odds of CVD (OR = 1.49, 95% CI of 1.10 to –2.01) and myocardial infarction (OR = 1.46, 95% CI of 0.96 to –2.23). In a haplotype analysis, women with a variant T allele of rs127152 had sevenfold higher odds of a myocardial infarction than did those without a haplotype that included this variant (33). In that study, blood pressure levels and SSBP were not reported and the influence of estrogen status (>40% of the women were on estrogen treatment) was not presented.
Peter et al. (31) showed that genetic variation within the ESR2 locus associated with left ventricular hypertrophy was sex-dependent. In this study, both ESR2 rs1256031 and rs1256059, in LD with each other, were associated with an increase in left ventricular mass (P = 0.047 and 0.03, respectively) and left ventricular wall thickness (P = 0.02 and 0.049, respectively), with the association being driven by those women who were hypertensive (31). However, the effect was not associated with variation in blood pressure. Additionally, another study by the same group demonstrated no significant relationship between ESR2 SNPs (rs1256031 rs1256059, rs944460, and rs1256034) and blood pressure levels (2). Our results are in agreement, as ESR2 SNPs in our study also were not associated with hypertension or blood pressure per se, only with SSBP.
Comparable to the preceding studies, we demonstrated that there is an estrogen status–specific association between ESR2 rs10144225 and SSBP in premenopausal women (estrogen-replete state) but not in postmenopausal women and/or men (estrogen-deplete state). In premenopausal women with the major allele, estrogen may bind to ESR2, leading to vasodilation and acting to protect against SSBP. However, in the women with the risk allele, estrogen may decrease binding affinity and therefore is unable to cause vasodilation, so SSBP will manifest. It is also possible that estrogen may bind effectively to ESR2 containing the risk alleles, but its effect on postreceptor action and ligand receptor binding is altered (acting as a cofactor to modify ESR2 gene function or expression predisposing them to SSBP). Further studies are warranted to investigate these possibilities.
Intriguingly, from the trend analysis, our results raised the potential that there may be an allele dosage–dependent relationship to SSBP. Subjects who were homozygous for the G allele at rs10144225 had the greatest changes in blood pressure after salt loading, whereas those who were homozygous for the A allele had the least changes in blood pressure response. This dosage dependence was observed only in estrogen-replete women and not in estrogen-deplete states (postmenopausal women and/or men). Ogawa et al. (32) studied elderly women (61 to 91 years old) that were free of diseases that could modify blood pressure. They reported that those who possessed two alleles of an ESR2 CA repeat polymorphism had significantly higher SBP than did subjects possessing fewer copies of the high-risk alleles. However, there was no effect on DBP and SSBP.
For ESR1, no SNPs in our cohort had high LD (r2 > 0.8) with SNPs in preceding studies that examined the association regarding SSBP and ESR1 (2, 34). However, associations of rs3020317 with fasting glucose levels and type 2 diabetes have been reported (35, 36). In contrast to ESR2, the SNPs in ESR1 showed no significant association with SSBP, consistent with the findings of Zhu et al. (9) in an animal study. They provided evidence that ESR2 has an essential role in attenuation of vascular contraction by inducing nitric oxide synthase expression, but ESR1 does not. Unlike our study, the GenSalt study of a Han Chinese population documented a significant relationship with SSBP in men carrying minor alleles of ESR1 rs9397453, rs9383951, plus other markers rs9340844, rs9371562, and rs9397459, but not in women (34). It is possible that the differing results between that study and the present one could be due to ethnic differences between the studies or to differences in the ESR1 SNPs selected for study. The SNPs used in GenSalt study had no strong LD (r2 > 0.8) with our ESR1 SNPs. Further study is needed to elucidate the ESR1 polymorphisms and their correlation with blood pressure.
The mechanism by which a change in ESR2 would affect salt sensitivity is not known. Although several studies have demonstrated an interaction between estrogen and aldosterone (10, 37), the data related to ER subtype specificity are controversial. We have documented that the ER inhibits mineralocorticoid receptor transcriptional regulatory function (38), but only the effect of ESR1 was assessed. Other studies have reported that both ESR1 and ESR2 prevent aldosterone-induced oxidative stress in vascular smooth muscle cells via increased reduced NAD phosphate bioavailability and attenuate cardiovascular remodeling in aldosterone salt-treated rats (39). Other studies suggest that only ESR2 modulates aldosterone synthesis and protects against aldosterone/salt-induced hypertension in female rats (10, 40, 41). Finally, other studies have suggested that the relationship is indirect: estrogens activate Rac-1 that in turn activates the mineralocorticoid receptor (42, 43). Because these studies did not provide any insight into ER specificity, we investigated whether the association between ESR2 polymorphisms and SSBP was mediated by alteration in the RAAS. We observed that rs10144225 risk allele carrier status, driven particularly by estrogen-replete, premenopausal women, was strongly associated with an elevated ARR on a liberal salt diet, suggesting that aldosterone was not appropriately suppressed for the level of PRA and salt intake. Both PRA and aldosterone were both low, as expected, as they were determined when the subjects were in balance on a 200 mmol/d diet and urine sodium was >150 mmol/d. The results were not due to lower PRA levels, falsely elevating the ARR, because in the estrogen-replete group there was no association between liberal salt PRA levels and genotype. Interestingly, the estrogen-deplete group had a marginally significant association between PRA, liberal salt intake, and genotype with the risk allele being associated with lower PRAs. However, the ARR in the estrogen-deplete group was not associated with genotype, suggesting that in the estrogen-deplete group, the relationship between the RAAS and salt intake was intact even in risk allele carriers.
What could be the potential mechanism for these findings? Krishnamurthi et al. (44) documented that administering estradiol to ovariectomized rats significantly attenuated type 1 angiotensin II (AT1) receptor expression in adrenal glands. The decrease in AT1 receptor was modulated by cytosolic RNA binding proteins (44). Therefore, estrogens reduced the key receptor modulating aldosterone secretion in response to changes in angiotensin II levels. Thus, one explanation for our findings is that ESR2 risk allele carriers no longer can mediate this negative effect on the AT1 receptor, thereby leading to increased aldosterone secretion on the liberal salt diet.
Taken together, the results support the hypothesis that there is a sexual dimorphism in the effect of ESR2 gene polymorphic variants, specifically those at rs10144225. This dimorphism is likely related to an interaction between estrogen and polymorphic variants in ESR2, as young (age <51 years) and presumably mostly premenopausal, estrogen-replete state, risk (minor) allele carriers have SSBP as documented in two cohorts of women. This suggests that the effect may be driven by estrogen occupation of the ER-β. This effect may be, in part, mediated by suboptimal suppression of aldosterone and potentially angiotensin II in the face of increased salt intake. Importantly, these associations are present in both hypertensive and normotensive individuals, suggesting that this polymorphic variant may be useful to define individuals who will be responsive to specific preventive strategies.
Strengths of this study include: (1) the uniqueness of the HyperPATH cohort in terms of the precision and quality of the phenotypic data; (2) confirmation of our primary finding in a second cohort; (3) large sample sizes in the two cohorts; (4) extensive control of known environmental factors that influence blood pressure, for example, dietary salt intake, position, and time of day of measurements; (5) the study of almost all subjects off antihypertensives; and (6) comparison of outcomes between ESR1 and ESR2 variants.
There are also limitations to our study. We did not have estrogen levels on all subjects, so we could not evaluate direct association between estrogen levels and risk allele status. We had to use age as a surrogate of menopausal status and divide the women into probable estrogen-deplete and estrogen-replete states. However, in the subset in whom hormonal levels (FSH, estradiol) were measured, the results were as expected for premenopausal and postmenopausal groups of women, supporting the validity of this approach. Additionally, it is likely that potential misclassification would have limited our likelihood of finding the significance difference between groups. Additionally, in the HTN-IR cohort, hormonal treatment use was not documented for postmenopausal women; however, if anything, this lack of specific data would underestimate the significance of our findings. The subgroup sample size of postmenopausal women was relatively small, and thus we may not have had enough power to see a significant association of rs10144225 status with SSBP in this group. The cohort used for the ESR1 did not completely overlap with the ESR2 cohort, and the number of subjects was small, which may have limited the ability to detect a significant association of ESR1 with SSBP. It is possible that low renin status is associated with the GG genotype, as this group was enriched with Africans. As posture studies were not performed in low-salt balance, we are unable to determine whether such a relationship exists based on current data. Because the number of subjects with the GG genotype was small, low renin status and/or race may have influenced the findings and require future studies for further clarification.
In summary, premenopausal women (estrogen-replete state) with risk allele carriers of ESR2 rs10144225 often have SSBP whether they are hypertensive or normotensive. The mechanism for their SSBP may be in part explained by increased ARRs on a liberal salt diet. These findings are not present in estrogen-deplete subjects (postmenopausal women and/or men), suggesting that the effects may be secondary to a receptor/estrogen interaction. These findings provide physiologic insight as to why a subset of premenopausal women may have susceptibility to hypertension.
Acknowledgments
We thank the Center for Clinical Investigation and Brigham and Women’s Hospital’s staff as well as other investigators and staff of the HyperPath protocol and participants at each protocol site, including the Clinical Investigation Center, INSERM CIC 9201, Hôpital Européen Georges Pompidou (Paris, France), University of Utah Medical Center (Salt Lake City, UT), Vanderbilt University (Nashville, TN), and University of La Sapiena (Rome, Italy).
Financial Support: This work was supported by the Brigham and Women’s Hospital, Division of Endocrinology, Diabetes, and Hypertension, and Harvard Medical School, in addition to National Institutes of Health Grants P50HL055000 (HyperPATH cohort), Genetics of Hypertension Grants 5R01HL086907-05 and K24HL096141 (to E.W.S.), and National Institutes of Health T32 Training Grant T32HL007609-27, HL67974 (to J.I.R.), P50-HL55005 (to J.I.R.), NCATS UL1TR001881 (Cedars-Sinai Medical Center), M01-RR000425 (Cedars-Sinai Medical Center), M01-RR000043 (University of Southern California), and P30-DK063491 (NIDDK Diabetes Research Center).
Author Contributions: W.M. analyzed the data and wrote the manuscript. J.W.T., B.S., J.L., and J.C. analyzed the data. C.M.R. acquired the data on HyperPATH cohort. M.O.G. supervised studies in the HTN-IR cohort and wrote the manuscript. A.S. and J.S.W. acquired the data on HyperPATH cohort. X.G., K.D.T., Y.I.C., A.H.X., W.A.H., L.J.R., T.A.B., and J.I.R. acquired the data on HTN-IR cohort. G.H.W. is the director of HyperPATH cohort and wrote the manuscript. E.W.S. created the concept of the study, interpreted data, and wrote and edited manuscript.
Acknowledgments
Disclosure Summary: J.S. is a consultant for Metabolon, Inc. The remaining authors have nothing to disclose.
Footnotes
- ARR
- aldosterone/renin ratio
- AT1
- type 1 angiotensin II
- BMI
- body mass index
- CI
- confidence interval
- CVD
- cardiovascular disease
- DBP
- diastolic blood pressure
- ER
- estrogen receptor
- FSH
- follicle-stimulating hormone
- HTN-IR
- Hypertension Insulin–Resistance Study
- HyperPATH
- International Hypertensive Pathotype
- LD
- linkage disequilibrium
- OR
- odds ratio
- PRA
- plasma renin activity
- RAAS
- renin–angiotensin–aldosterone system
- SBP
- systolic blood pressure
- SEM
- standard error of the mean
- SNP
- single nucleotide polymorphism
- SSBP
- salt sensitivity of blood pressure
- ΔDBP
- change in diastolic blood pressure
- ΔSBP
- change in systolic blood pressure.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics 2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peter I, Shearman AM, Zucker DR, Schmid CH, Demissie S, Cupples LA, Larson MG, Vasan RS, D’Agostino RB, Karas RH, Mendelsohn ME, Housman DE, Levy D. Variation in estrogen-related genes and cross-sectional and longitudinal blood pressure in the Framingham Heart Study. J Hypertens. 2005;23(12):2193–2200. [DOI] [PubMed] [Google Scholar]
- 3.Bateman BT, Shaw KM, Kuklina EV, Callaghan WM, Seely EW, Hernández-Díaz S. Hypertension in women of reproductive age in the United States: NHANES 1999–2008. PLoS One. 2012;7(4):e36171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daviglus ML, Stamler J, Pirzada A, Yan LL, Garside DB, Liu K, Wang R, Dyer AR, Lloyd-Jones DM, Greenland P. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA. 2004;292(13):1588–1592. [DOI] [PubMed] [Google Scholar]
- 5.Rokach A, Pollak A, Rosen L, Friedlander Y, Blumenfeld A, Reznik L, Dresner-Pollak R. Estrogen receptor alpha gene polymorphisms are associated with the angiographic extent of coronary artery disease. J Clin Endocrinol Metab. 2005;90(12):6556–6560. [DOI] [PubMed] [Google Scholar]
- 6.Figtree GA, Kindmark A, Lind L, Grundberg E, Speller B, Robinson BG, Channon KM, Watkins H. Novel estrogen receptor alpha promoter polymorphism increases ventricular hypertrophic response to hypertension. J Steroid Biochem Mol Biol. 2007;103(2):110–118. [DOI] [PubMed] [Google Scholar]
- 7.Shearman AM, Cooper JA, Kotwinski PJ, Humphries SE, Mendelsohn ME, Housman DE, Miller GJ. Estrogen receptor α gene variation and the risk of stroke. Stroke. 2005;36(10):2281–2282. [DOI] [PubMed] [Google Scholar]
- 8.Jazbutyte V, Arias-Loza PA, Hu K, Widder J, Govindaraj V, von Poser-Klein C, Bauersachs J, Fritzemeier KH, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T. Ligand-dependent activation of ERβ lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc Res. 2008;77(4):774–781. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor β. Science. 2002;295(5554):505–508. [DOI] [PubMed] [Google Scholar]
- 10.Arias-Loza PA, Hu K, Dienesch C, Mehlich AM, König S, Jazbutyte V, Neyses L, Hegele-Hartung C, Heinrich Fritzemeier K, Pelzer T. Both estrogen receptor subtypes, α and β, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension. 2007;50(2):432–438. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Li Y, Chen F, Pan H, Shen H, Sun Z, Wu Y, Zhou J, Ba L, Zhao J. Estrogen receptor β genetic variants and combined oral contraceptive use as relates to the risk of hypertension in Chinese women. Arch Med Res. 2010;41(8):599–605. [DOI] [PubMed] [Google Scholar]
- 12.Williams GH, Hollenberg NK. Sodium-sensitive essential hypertension: emerging insights into an old entity. J Am Coll Nutr. 1989;8(6):490–494. [DOI] [PubMed] [Google Scholar]
- 13.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23(4):531–550. [DOI] [PubMed] [Google Scholar]
- 14.Rao AD, Sun B, Saxena A, Hopkins PN, Jeunemaitre X, Brown NJ, Adler GK, Williams JS. Polymorphisms in the serum- and glucocorticoid-inducible kinase 1 gene are associated with blood pressure and renin response to dietary salt intake. J Hum Hypertens. 2013;27(3):176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang AH, Azen SP, Raffel LJ, Tan S, Cheng LS, Diaz J, Toscano E, Henderson PC, Hodis HN, Hsueh WA, Rotter JI, Buchanan TA. Evidence for joint genetic control of insulin sensitivity and systolic blood pressure in hispanic families with a hypertensive proband. Circulation. 2001;103(1):78–83. [DOI] [PubMed] [Google Scholar]
- 16.Garza AE, Rariy CM, Sun B, Williams J, Lasky-Su J, Baudrand R, Yao T, Moize B, Hafiz WM, Romero JR, Adler GK, Ferri C, Hopkins PN, Pojoga LH, Williams GH. Variants in striatin gene are associated with salt-sensitive blood pressure in mice and humans. Hypertension. 2015;65(1):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurwitz S, Fisher ND, Ferri C, Hopkins PN, Williams GH, Hollenberg NK. Controlled analysis of blood pressure sensitivity to sodium intake: interactions with hypertension type. J Hypertens. 2003;21(5):951–959. [DOI] [PubMed] [Google Scholar]
- 18.Pojoga LH, Underwood PC, Goodarzi MO, Williams JS, Adler GK, Jeunemaitre X, Hopkins PN, Raby BA, Lasky-Su J, Sun B, Cui J, Guo X, Taylor KD, Chen YD, Xiang A, Raffel LJ, Buchanan TA, Rotter JI, Williams GH. Variants of the caveolin-1 gene: a translational investigation linking insulin resistance and hypertension. J Clin Endocrinol Metab. 2011;96(8):E1288–E1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric. 2010;13(5):419–428. [DOI] [PubMed] [Google Scholar]
- 20.Guo X, Cui J, Jones MR, Haritunians T, Xiang AH, Chen YD, Taylor KD, Buchanan TA, Davis RC, Hsueh WA, Raffel LJ, Rotter JI, Goodarzi MO. Insulin clearance: confirmation as a highly heritable trait, and genome-wide linkage analysis. Diabetologia. 2012;55(8):2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grim CE, Luft FC, Fineberg NS, Weinberger MH. Responses to volume expansion and contraction in categorized hypertensive and normotensive man. Hypertension. 1979;1(5):476–485. [DOI] [PubMed] [Google Scholar]
- 22.International HapMap Consortium The International HapMap Project. Nature. 2003;426(6968):789–796. [DOI] [PubMed] [Google Scholar]
- 23.Palmer ND, Goodarzi MO, Langefeld CD, Wang N, Guo X, Taylor KD, Fingerlin TE, Norris JM, Buchanan TA, Xiang AH, Haritunians T, Ziegler JT, Williams AH, Stefanovski D, Cui J, Mackay AW, Henkin LF, Bergman RN, Gao X, Gauderman J, Varma R, Hanis CL, Cox NJ, Highland HM, Below JE, Williams AL, Burtt NP, Aguilar-Salinas CA, Huerta-Chagoya A, Gonzalez-Villalpando C, Orozco L, Haiman CA, Tsai MY, Johnson WC, Yao J, Rasmussen-Torvik L, Pankow J, Snively B, Jackson RD, Liu S, Nadler JL, Kandeel F, Chen YD, Bowden DW, Rich SS, Raffel LJ, Rotter JI, Watanabe RM, Wagenknecht LE. Genetic variants associated with quantitative glucose homeostasis traits translate to type 2 diabetes in Mexican Americans: the GUARDIAN (genetics underlying diabetes in hispanics) consortium. Diabetes. 2015;64(5):1853–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. [DOI] [PubMed] [Google Scholar]
- 25.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen MH, Yang Q. GWAF: an R package for genome-wide association analyses with family data. Bioinformatics. 2010;26(4):580–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laffer CL, Scott RC III, Titze JM, Luft FC, Elijovich F. Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension. 2016;68(1):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu FQ, Mu JJ, Liu ZQ, Shi DC, Huang Q, Yuan ZY, Lian QF, Zheng SH. Endothelial dysfunction in normotensive salt-sensitive subjects. J Hum Hypertens. 2012;26(4):247–252. [DOI] [PubMed] [Google Scholar]
- 29.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(2 Pt 2):429–432. [DOI] [PubMed] [Google Scholar]
- 30.Gürgen D, Hegner B, Kusch A, Catar R, Chaykovska L, Hoff U, Gross V, Slowinski T, da Costa Goncalves AC, Kintscher U, Gustafsson JÅ, Luft FC, Dragun D. Estrogen receptor-β signals left ventricular hypertrophy sex differences in normotensive deoxycorticosterone acetate-salt mice. Hypertension. 2011;57(3):648–654. [DOI] [PubMed] [Google Scholar]
- 31.Peter I, Shearman AM, Vasan RS, Zucker DR, Schmid CH, Demissie S, Cupples LA, Kuvin JT, Karas RH, Mendelsohn ME, Housman DE, Benjamin EJ. Association of estrogen receptor β gene polymorphisms with left ventricular mass and wall thickness in women. Am J Hypertens. 2005;18(11):1388–1395. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa S, Emi M, Shiraki M, Hosoi T, Ouchi Y, Inoue S. Association of estrogen receptor β (ESR2) gene polymorphism with blood pressure. J Hum Genet. 2000;45(6):327–330. [DOI] [PubMed] [Google Scholar]
- 33.Rexrode KM, Ridker PM, Hegener HH, Buring JE, Manson JE, Zee RY. Polymorphisms and haplotypes of the estrogen receptor-β gene (ESR2) and cardiovascular disease in men and women. Clin Chem. 2007;53(10):1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly TN, Rebholz CM, Gu D, Hixson JE, Rice TK, Cao J, Chen J, Li J, Lu F, Ma J, Mu J, Whelton PK, He J. Analysis of sex hormone genes reveals gender differences in the genetic etiology of blood pressure salt sensitivity: the GenSalt study. Am J Hypertens. 2013;26(2):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keene KL, Mychaleckyj JC, Smith SG, Leak TS, Perlegas PS, Langefeld CD, Herrington DM, Freedman BI, Rich SS, Bowden DW, Sale MM. Comprehensive evaluation of the estrogen receptor α gene reveals further evidence for association with type 2 diabetes enriched for nephropathy in an African American population. Hum Genet. 2008;123(4):333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahlman I, Vaxillaire M, Nilsson M, Lecoeur C, Gu HF, Cavalcanti-Proença C, Efendic S, Ostenson CG, Brismar K, Charpentier G, Gustafsson JA, Froguel P, Dahlman-Wright K, Steffensen KR. Estrogen receptor α gene variants associate with type 2 diabetes and fasting plasma glucose. Pharmacogenet Genomics. 2008;18(11):967–975. [DOI] [PubMed] [Google Scholar]
- 37.Pedram A, Razandi M, Korach KS, Narayanan R, Dalton JT, Levin ER. ERβ selective agonist inhibits angiotensin-induced cardiovascular pathology in female mice. Endocrinology. 2013;154(11):4352–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrett Mueller K, Lu Q, Mohammad NN, Luu V, McCurley A, Williams GH, Adler GK, Karas RH, Jaffe IZ. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155(11):4461–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muehlfelder M, Arias-Loza PA, Fritzemeier KH, Pelzer T. Both estrogen receptor subtypes, ERα and ERβ, prevent aldosterone-induced oxidative stress in VSMC via increased NADPH bioavailability. Biochem Biophys Res Commun. 2012;423(4):850–856. [DOI] [PubMed] [Google Scholar]
- 40.Xue B, Zhang Z, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. Estrogen receptor-β in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension. 2013;61(6):1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caroccia B, Seccia TM, Campos AG, Gioco F, Kuppusamy M, Ceolotto G, Guerzoni E, Simonato F, Mareso S, Lenzini L, Fassina A, Rossi GP. GPER-1 and estrogen receptor-β ligands modulate aldosterone synthesis. Endocrinology. 2014;155(11):4296–4304. [DOI] [PubMed] [Google Scholar]
- 42.Ando K, and Fujita T. Pathophysiology of salt sensitivity hypertension. Ann Med. 2012;44(Suppl 1):S119–S126. [DOI] [PubMed] [Google Scholar]
- 43.Kawarazaki W, Nagase M, Yoshida S, Takeuchi M, Ishizawa K, Ayuzawa N, Ueda K, Fujita T. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol. 2012;23(6):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnamurthi K, Verbalis JG, Zheng W, Wu Z, Clerch LB, Sandberg K. Estrogen regulates angiotensin AT1 receptor expression via cytosolic proteins that bind to the 5′ leader sequence of the receptor mRNA. Endocrinology. 1999;140(11):5435–5438. [DOI] [PubMed] [Google Scholar]