Abbreviations
- ALD
alcoholic liver disease
- ALR
autophagic lysosome reformation
- ATGL
adipose triglyceride lipase
- Dyn2
dynamin2
- EH
Eps15 Homology
- HSL
hormone‐sensitive lipase
- LC3
microtubule‐associated protein 1A/1B‐light chain 3
- LD
lipid droplet
- TFEB
transcription factor EB
Alcoholic liver disease (ALD) at early stages is characterized by hepatic steatosis, i.e., excessive accumulation of fat (e.g., triglyceride or triacylglycerol, esterified cholesterol) wrapped in lipid storage organelles called lipid droplets (LDs). While excessive hepatic steatosis can progress to more severe steatohepatitis or hepatocellular carcinoma in ALD, steatosis can be entirely reversible because hepatocytes have adopted several catabolic mechanisms to remove or break down the LDs.1 Therefore understanding the mechanisms of the catabolism of hepatic LDs may help to develop therapeutic interventions for ALD.
In the liver, there are two major pathways that are thought to play critical roles in LD catabolism: lipolysis through cytosolic neutral lipases, such as adipose triglyceride lipase (ATGL), and the autophagic/lysosomal pathway. In adipocytes, the most characterized mechanisms for lipase‐mediated LD catabolism is β‐adrenergic agonist‐mediated activation of cyclic adenosine monophosphate/protein kinase A, which phosphorylates and activates hormone‐sensitive lipase (HSL) and LD‐coating proteins perilipins. Phosphorylated perilipins then allow exposure of the stored lipids to HSL and ATGL, resulting in lipolysis. Compared to adipocytes, lipase‐mediated LD catabolism in hepatocytes is less studied. Genetic ablation or overexpression of ATGL in the liver exacerbates or alleviates steatosis in mouse livers, respectively. These findings suggest that cytosolic lipases are also important regulators in LD turnover in hepatocytes.2 Interestingly, a more recent study reported that hepatocytes isolated from chronic ethanol‐fed rats or acute ethanol‐treated hepatoma cells that can metabolize ethanol showed significantly perturbed β‐adrenergic stimuli‐induced LD breakdown.3 Mechanistically, the authors found that ethanol inhibited protein kinase A‐mediated phosphorylation of HSL and the recruitment of ATGL to the LD surface, resulting in the blockage of LD catabolism.3 In addition to the above lipase‐mediated LD breakdown, another emerging pathway to breakdown LD in hepatocytes involves the lipases and acidic hydrolases in lysosomes, a process known as lipophagy.4 During lipophagy, LDs are selectively taken up by autophagosomes likely through directly recruiting autophagy machinery proteins, and then autophagosome‐enwrapped LDs are further delivered to lysosomes where LDs are broken down to fatty acids. Although genetic deletion of autophagy‐related genes in mice leads to controversial results in hepatic steatosis and lipid metabolism likely due to the compensatory/secondary effects in these mice, studies that use pharmacological approaches to activate autophagy in the liver consistently demonstrate a beneficial role in protecting against the pathogenesis of both nonalcoholic fatty liver diseases and ALD.5, 6 Despite the emerging importance of lipophagy in the pathogenesis of ALD, it is still largely unknown how chronic alcohol consumption would impair lipophagy and more importantly how autophagy specifically and selectively recognizes LD during ALD.
In recent issues of Hepatology Communications, two back‐to‐back studies from McNiven and Casey's group from Mayo Clinic and University of Nebraska Medical Center studied the roles of Rab7 and dynamin2 (Dyn2) in lipophagy in the context of ethanol‐induced hepatic steatosis. In the first study, Schulze et al.7 investigated the role of Rab7 in hepatocyte lipophagy under chronic ethanol exposure. Rab7 belongs to the Rab family of guanosine triphosphate‐binding proteins that regulate intracellular vesicular trafficking. Rab7 is known to be required for transport of late endosomes, biogenesis of lysosomes, and fusion of autophagosomes with lysosomes. A previous study found that Rab7 mediated the docking of autophagosomes, multivesicular bodies, and lysosomes onto LDs during starvation‐induced lipophagy in hepatocytes.8 Furthermore, Rab7 depletion impaired starvation‐induced hepatocyte lipophagy,8 suggesting that Rab7 is an important regulator of hepatocyte lipophagy. To further elucidate the role of Rab7 in alcohol‐induced steatosis, Schulze et al.7 isolated primary hepatocytes from rats that were fed either with a Lieber‐Decarli ethanol diet or with a control diet for 6 weeks. They found that hepatocytes isolated from alcohol‐fed rats had increased LDs and were less sensitive to starvation‐induced LD turnover compared with hepatocytes from pair‐fed control mice. Intriguingly, ethanol did not alter the total protein levels of Rab7 but caused a marked 80% reduction of Rab7 activity in hepatocytes. Unlike the evenly spread distribution of lysosomes in normal hepatocytes, lysosomes displayed a juxtanuclear pattern in hepatocytes that were exposed to ethanol, suggesting that ethanol exposure altered lysosomal motility and dispersion. Decreased lysosomal mobility by alcohol exposure was further confirmed by a rhodamine‐labeled dextran‐loading assay and time‐lapse live‐cell imaging of LysoTracker‐stained lysosomes in hepatocytes. More importantly, pharmacological inhibition of Rab7 with CID1067700, a guanine nucleotide‐binding inhibitor, inhibited starvation‐induced LD breakdown. These observations implicate that ethanol may impair lipophagy through the inhibition of Rab7.
Autophagy is a highly dynamic process. In response to starvation conditions, autophagosomes carry the enveloped cargos, including cellular proteins and organelles, such as LDs, and eventually fuse with lysosomes to form autolysosomes where autophagic cargos are degraded. After the degradation and release of the contents, such as amino acids and fatty acids, to the cytosol as biological fuels for energy production, autolysosomes undergo a dynamic remodeling process called autophagic lysosome reformation (ALR). During ALR, autolysosomes form long tubular structures, and small vesicles can bud out from the tubules called protolysosomes, which eventually mature into functional lysosomes. ALR is thus considered as the terminal step of autophagy, which is essential for maintaining lysosome homeostasis. ALR is regulated by clathrin, phosphatidylinositol 4,5‐bisphosphate (PtdIns4, 5P2), Spinster (a lysosomal efflux transporter), and the kinesin motor protein KIF5B.9 Dyn2 is a 100‐kDa guanosine triphosphatase involved in scission of cell membranes and formation of microtubule bundles. During hepatocyte lipophagy, Schulze et al.10 showed that Dyn2 was also involved in vesiculation of autolysosomal tubules, which is required for LD breakdown. Dyn2 directly associated with the autolysosomal tubules, and genetic depletion or pharmacological inhibition of Dyn2 prevented lysosomal tubular scission, resulting in decreased lysosomal pools in hepatocytes. It is known that the activity of Dyn2 increases after it is phosphorylated by Src kinase, a nonreceptor protein tyrosine kinase. In the second study performed by McNiven and Casey's group, Rasineni et al.11 used similar models to determine the role of Dyn2 in lipophagy after alcohol exposure, as shown in the first study. They found that the phosphorylated (active) forms of Src and Dyn2 decreased in hepatocytes isolated from chronic alcohol‐fed rats compared with the hepatocytes from pair‐fed control rats. Pharmacological inhibition of Src or Dyn2 blocked starvation‐induced LD breakdown in hepatocytes from both alcohol and pair‐fed rats, but inhibition of Dyn2 seemed to be more potent than inhibition of Src. Although ALR was not determined in this study, the lysosomal‐associated membrane protein 1‐positive lysosome areas decreased in hepatocytes isolated from alcohol‐fed rats compared with hepatocytes from pair‐fed rats. The decreased lysosomal numbers by alcohol exposure is likely due to the impaired Dyn2‐mediated ALR and lysosomal biogenesis. Taken together, data from the studies by Schulze et al.7 and Rasineni et al.11 suggest that chronic alcohol consumption may impair lipophagy by affecting Rab7‐mediated trafficking of autophagic machinery to LDs and by reducing Dyn2‐mediated ALR (Fig. 1).
Figure 1.
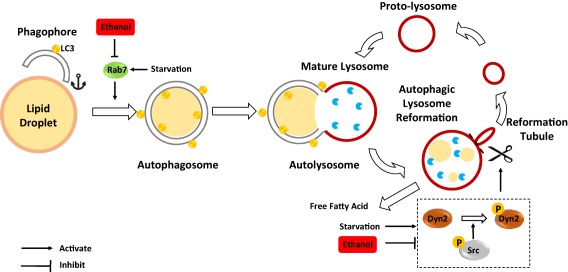
Schematic overview of the role of Rab7 and dynamin2 (Dyn2) in ethanol‐impaired lipophagy. Chronic ethanol exposure reduces Rab7 and Src/Dyn2 activity and thus may inhibit the docking of LDs to the autophagosome and impair ALR and lysosome biogenesis, respectively resulting in impaired lipophagy and promoting hepatic steatosis. Abbreviation: P, phosphorylation.
Although these two studies have further illustrated the important role of Rab7 and Dyn2 in chronic alcohol‐impaired lipophagy, there are many questions that remain to be answered. Perhaps one of the major limitations for these two studies is that these studies were only performed on primary cultured hepatocytes, and the relevance of these findings to in vivo liver is unclear. In addition to the liver, it is known that alcohol consumption also affects other organs, such as skeletal muscle, adipose tissue, and pancreas. Alcohol consumption affects the release of insulin from pancreas and adiponectin and fibroblast growth factor 21 from adipose tissue. Whether these circulating hormones or biomolecules also affect Rab7 and Dyn2 remain to be studied. While these studies nicely supported a correlative role of Src‐Dyn2 in chronic alcohol‐induced impairment of lipophagy, studies would be more convincing if the authors could test whether overexpression or activation of Src‐Dyn2 would rescue the impaired lipophagy induced by alcohol. To confirm the role of Rab7 and Dyn2 in lipophagy in vivo, it will also be interesting to determine lipophagy and ALR in alcohol‐induced liver steatosis and injury in Rab7 or Dyn2 knockout or knockdown mice. Lysosome homeostasis is also regulated by transcription factor EB (TFEB), a master regulator of lysosomal biogenesis. Unpublished data from our lab indicate that alcohol impairs TFEB in hepatocytes and that activation of TFEB protects against alcohol‐induced liver steatosis and injury in mice. It would be interesting to determine whether TFEB can also regulate Rab7 and Dyn2 activity. Finally, another important question that needs to be answered is how autophagy selectively removes excess LDs after alcohol exposure. Autophagy receptor proteins, such as p62/sequestosome 1, neighbor of BRCA1 gene 1 protein, nuclear dot protein 52, and optineurin, can directly interact with microtubule‐associated protein 1A/1B‐light chain 3 (LC3) through their LC3‐interacting region for selective mitophagy. However, it is unknown whether these canonical autophagy receptors are also important for lipophagy. More recently, a Rab10‐Eps15 homology (EH) domain binding protein 1 and the membrane‐deforming adenosine triphosphatase EH domain containing 2 complex has been proposed to selectively drive autophagy for LD engulfment in cultured cells.12 Whether this Rab10‐EH binding protein 1‐EH domain 2 complex is also important for lipophagy in vivo in mouse livers, in particular in alcoholic fatty liver disease, remains to be studied.
Author names in bold designate shared co‐first authorship.
Potential conflict of interest: Nothing to report.
Supported by NIH grants R01 AA020518‐06 (W.X.D.), R01 DK102142‐04 (W.X.D.), U01 AA024733‐01A1 (W.X.D.), P20GM103549‐10 (H.J.) and P30GM118247‐01 (H.J.). Y.L. is a recipient of a Biomedical Research Training Program Fellowship (2016‐2017), University of Kansas Medical Center.
REFERENCES
- 1. Williams JA, Manley S, Ding WX. New advances in molecular mechanisms and emerging therapeutic targets in alcoholic liver diseases. World J Gastroenterol 2014;20:12908‐12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mashek DG, Khan SA, Sathyanarayan A, Ploeger JM, Franklin MP. Hepatic lipid droplet biology: getting to the root of fatty liver. Hepatology 2015;62:964‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schott MB, Rasineni K, Weller SG, Schulze RJ, Sletten AC, Casey CA, et al. Beta‐adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure. J Biol Chem 2017; doi: 10.1074/jbc.M117.777748 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature 2009;458:1131‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, et al. Autophagy reduces acute ethanol‐induced hepatotoxicity and steatosis in mice. Gastroenterology 2010;139:1740‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med 2013;19:83‐92. [DOI] [PubMed] [Google Scholar]
- 7. Schulze RJ, Rasineni K, Weller SG, Schott MB, Schroeder B, Casey CA, et al. Ethanol exposure inhibits hepatocyte lipophagy by inactivating the small guanosine triphosphatase Rab7. Hepatol Commun 2017;1:140‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schroeder B, Schulze RJ, Weller SG, Sletten AC, Casey CA, McNiven MA. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology 2015;61:1896‐1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Yu L. Recent progress in autophagic lysosome reformation. Traffic 2017;18:358‐361. [DOI] [PubMed] [Google Scholar]
- 10. Schulze RJ, Weller SG, Schroeder B, Krueger EW, Chi S, Casey CA, et al. Lipid droplet breakdown requires dynamin 2 for vesiculation of autolysosomal tubules in hepatocytes. J Cell Biol 2013;203:315‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rasineni K, Donohue TM Jr, Thomes PG, Yang L, Tuma DJ, McNiven MA, et al. Ethanol‐induced steatosis involves impairement of lipohagy, associated with reduced dynamin2 activity. Hepatol Commun 2017; doi: 10.1002/hep4.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Z, Schulze RJ, Weller SG, Krueger EW, Schott MB, Zhang X, et al. A novel Rab10‐EHBP1‐EHD2 complex essential for the autophagic engulfment of lipid droplets. Sci Adv 2016;2:e1601470. [DOI] [PMC free article] [PubMed] [Google Scholar]


