Abstract
Catheter ablation of atrial fibrillation (AF) is a current therapeutic option but its efficacy for the treatment of long-lasting persistent AF (l-lpAF) remains suboptimal. We tested the laser method as an alternative for catheter ablation of l-lpAF by using an open-irrigated electrode laser mapping and ablation (ELMA) catheter. Laser ablation was attempted in 48 patients aged 50-81 years (69 ± 7.6 y, female = 28) with drug resistant (3.5 trials) l-lpAF (≥12 months). All of the patients had comorbidities: congestive heart failure NYHA II-III (100%), hypertension 29 (60%), coronary artery disease 19 (40%), and heart valve defect 17 (35%). None of the patients had diabetes or obstructive sleep apnea. All were in AF at the beginning of the procedure. Continuous wave (cw) 1064nm Nd:YAG laser applications at 15W/10-20s (14-26/patient) were applied via the ELMA catheter until local electrical activity displayed on the monitor in the bipolar focused local electrograms (LEG) recorded via the pin electrodes from the tip of the catheter was abolished permanently and sinus rhythm was achieved. Online monitoring of electrical potential amplitudes in the focused LEG recorded via the pin-electrodes of the ELMA catheter allowed for validation of ablation success. Procedure duration ranged from 82-175 min (118 ± 72 min), number of lesions were14-26 (19 ± 4) per patient and X-ray exposure times ranged from 15-82 min (23.2 ± 12 min). Interventions were without complications. After the ablation procedure all the patients were in sinus rhythm, off medication, however, 12 (25%) needed a repeat study for various arrhythmias. During followup of 9 months to 29.3 years (8.2 ± 6.5 years) patients’ quality of life improved significantly and during final follow-up control all except two were off medication still in sinus rhythm (lifelong success rate = 96%). As compared to other catheter ablation methods the laser method is an intriguing alternative for catheter ablation of l-lpAF.
Keywords: Long-lasting persistent atrial fibrillation, Laser catheter ablation, Bipolar focused local electrograms
Introduction
Atrial fibrillation (AF), the most frequent clinical arrhythmia, is associated with increased risk of stroke, myocardial infarction, heart failure, and death [1]. AF is generally considered a progressive disease, typically evolving from paroxysmal through persistent to permanent forms, a process attributed to electrical and structural remodeling related to both the underlying disease and AF itself. In 2010, the estimated numbers of men and women with AF worldwide were 20.9 million and 12.6 million, respectively, with higher incidence and prevalence rates in developed countries [2],[3]. AF prevalence increases with age; starting at the age of 50, this doubles every decade of life, corresponding to a 5% prevalence in the population older than 60, and a 13% prevalence in the population older than 80. This means that 70% of cases affect people who are between 65 and 85 years old [4]. AF treatment is a very active field of novel discoveries and research with strong translational potential, often in bedside–bench–bedside reiterative cycles. Unfortunately, available drugs for AF therapy have moderate success and important limitations, particularly increasing the risk of Lifethreatening proarrhythmic events and bleeding complications [5]. To cope with these limitations, catheter ablation with pulmonary vein isolation (PVI) by means of RF current has become the gold standard, the cornerstone treatment for paroxysmal AF [6]. Worldwide, thousands of procedures have been done, and hundreds of papers published. Given this history and knowledge, one would think the best technique to ablate AF would be agreed on. However, persistent AF suggests that the electrophysiology community isn't settled on the best technique to ablate AF. Up to now, the success rate of RF ablation in the more prevalent and highly heterogeneous l-lpAF populations has been disappointing [7]. Regardless of methods of catheter ablation applied with the aim to convert l-lpAF or permanent AF into stable sinus rhythm the success rate of the first ablation procedure, taking into account a 3-month blanking period, when measured as freedom from repeat catheter ablation, from need for cardioversion, and from need for the use of an antiarrhythmic drug was 40% at one year and 34% at two years [8]. The results of these ‘realworld’ experiences are remarkably consistent, indicating that the one-year success of l-lpAF ablation procedures at the centers actually delivering this therapy in Europe today is well under 50%, a success rate quite a lot lower than often stated. The question raises: is complete electrical isolation of pulmonary veins the cornerstone, alone? The same group the PVI procedure was initiated from showed, that for ablation of l-lpAF a stepwise approach combining PVI with complex fractionated atrial electrograms (CAFE) and linear ablation may improve the success rate of AF ablation [9].
Our early in-vivo animal experimental studies with laser catheter coagulation of atrial myocardium in dogs showed, that the method is safe and can be performed in a controllable manner [10]. Transmural laser lesions produced in the atrial walls achieved clear cut areas of coagulation necrosis without tissue vaporization and crater formation [Figure 1]. Lesions healed to dense fibrous scars without aneurysm formation, without compromising the anatomic integrity of the atrial wall [Figure 2]. Our initial attempts at laser catheter ablation of cardiac arrhythmias in humans were promising [11], [12], [13]. Based on these results laser catheter ablation of l-lpAF was attempted in patients.
Figure 1. Cross-sectional view of a 3h old HE stained lesion produced by laser application aimed at the endocardial side of the right atrial lateral wall of a dog, showing clear cut transmural coagulation necrosis with intramural hemorrhage and vacuolization. There is a slight dip in the central region of the lesion (thick arrow). Note: the endocardial layers are morphologically intact; there is no tissue vaporization with crater formation.
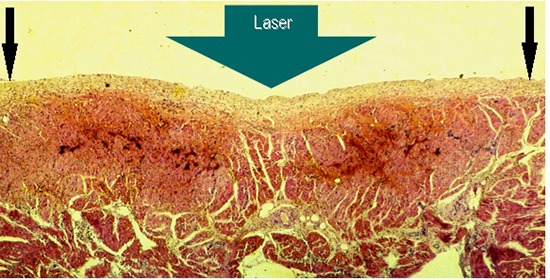
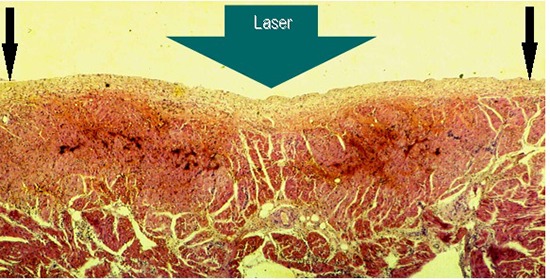
Figure 2. Gross pathology showing subacute 6 hours old atrial lesions produced by laser catheter applications at 15W/15s aimed at the endocardial surface of the posterior left atrial wall (LA2) A endocardial view: showing coagulation necrosis achieved without tissue vaporization with crater formation (black circle), the anatomic integrity of the atrial wall is preserved, and B Section through that transmural lesion (black oval), showing, a slight intramural hemorrhage at the margins of the lesion (black arrows), and, C and D showing two chronic, three months old transmural fibrous scars in the right atrial free wall, endocardial and epicardial view respectively (circles), and an acute, three hours old, transmural lesion of coagulation necrosis surrounded by a ring of hemorrhage.
Patients
Study participants were recruited from patients scheduled to undergo laser catheter ablation procedure for drug resistant (3.5 trials) l-lpAF. From 15.03.1988 to 31.07.2003 laser ablation was attempted in a series of 48 highly selected and severely symptomatic patients, aged 52-81 (69 ± 7.6) years, (f = 28), with long-lasting (≥12 months) persistent AF. They had multiple comorbidities and complex arrhythmias [Table 1].
Table 1. Two to 4 Comorbidities were present in all, and additional arrhythmias in 18 (37.5%) of the 48 patients candidates for laser catheter ablation of long persistent l-lpAF.
AVNRT = atrio-ventricular nodal reentrant tachycardia, AP = accessory atrio- entricular pathway,SSS = Sicksinus syndrome, AT = atrial tachycardia, nsRVOT = ventricular outflow tract tachycardia
| Comorbidities | No | Arrhythmias | No | |
|---|---|---|---|---|
| Congestive heart failure II-III | all | 100% | ||
| Coronary artery disease | 40% | |||
| Enlarged left atria | 38 | 79% | Left atrial flutter | 7 |
| Ø 46-52 mm | Common AVNRT | 3 | ||
| High blood pressure | 29 | 60% | Left AP | 3 |
| >160 / >95 mmHg | Atrial flutter | 2 | ||
| Mitral valve prolapse | 16 | RVOT | 1 | |
| insufficiency grade 1-2 | 35% | SSS | 1 | |
| Severe aortic valve stenosis / Gradient 60 mmHg at rest | 1 | (right atrial reentry) | 1 |
Congestive heart failure NYHA class II-III with decreased exercise capacity, fatigue, breathlessness and palpitations during emotional or physical stress were present in all of the patients. In their history one to 4 DC shock cardioversion failed to stabilize sinus rhythm. In addition, 4 had a permanent pacemaker implanted because of bradyarrhythmia or atrioventricular conduction disturbances, and anti-tachycardia pacing was not effective in one of the patients. Patients were anticoagulated with warfarin (CHA2DS2-VASc score before ablation = ≥2) that was discontinued and they were put on heparin prior to the laser ablation. Pre-interventional transthoracic 3D-Echo- Doppler and transesophageal echocardiography did not give evidence of intra-cardiac thrombus formation. Twenty nine (60%) of the patients were under treatment for hypertension, and 19 (40%) for coronary artery disease. These medications were not discontinued prior to the study. None of the patients had diabetes, obstructive sleep apnea, experienced stroke or other thromboembolic events. Their body mass index ranged from 22.2 to 28.4. One was alcohol addict that became evident first during follow-up. Five patients (10%) had an unsuccessful RF ablation attempt 13-16 months prior to the laser treatment. Antiarrhythmic drugs were discontinued prior to ablation and were not restarted after l-lpAF laser ablation.
Materials and Methods
Continuous wave 1064nm laser light from a cwMediLas 4060N fibertom, Dornier MedTech, provided with a light-guide protection system (LPS), was applied via an open-irrigated electrode laser mapping and ablation (ELMA) catheter, RytmoLas, LasCor GmbH. The proximal end of the optical fiber was connected to the laser via a Fiber Sub-Miniature Assembly (FSMA) connector [Figure 3]. The catheter tip was provided with three pin electrodes arranged symmetrically at the tip of the catheter, with interelectrode distances of ≤ 2.0 mm between each other. Each of the electrodes was connected via plugs to the manifold, for three bipolar focused local electrogram (LEG) recordings. The optical fiber had a conically shaped tip that produced a donut like illuminated spot on the targeted endocardial surface in front of the catheter end hole [Figure 4]. The development of the open-irrigated ELMA catheter I described in detail elsewhere [14].
Figure 3. Overview of the open-irrigated electrode-laser mapping and ablation (ELMA) catheter RytmoLas LasCor GmbH as used in this study for laser ablation of long persistent atrial fibrillation. Total length = 300 cm, working length (catheter) = 115 cm.
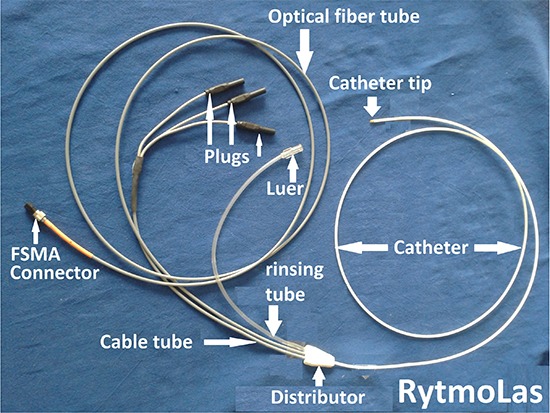
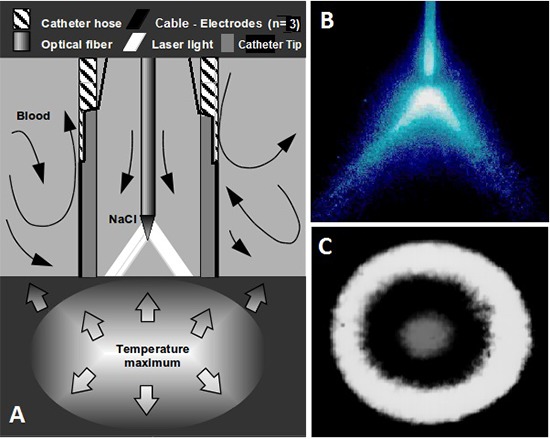
Figure 4. A Scheme, showing, the catheter tip provided with electrodes and an optical fiber mounted coaxially in its lumen at a given distance from the endhole of the catheter. Laser light is heating up the tissue with a maximum temperature intramurally 3-4 mm deep. B is showing the divergent laser beam emanated from the optical fiber tip, and C is showing the donut-like laser spot created on the targeted area in front of the catheter endhole.
In general, a slight sedation with phenobarbital was applied. After venous punctures in the groin (Seldinger technique) an 8F long preshaped sheath (RytmoGuide, LasCor GmbH) or a steerable introducer (AgilisTM NxT, St. Jude Medical) was inserted under X-ray control. Side selective transseptal puncture procedure was performed by using the TransLas laser puncture set [15]. The ELMA catheter was advanced via the transseptal access into the left atrium and was manipulated during continuous monitoring of electrical potential amplitudes and intermittent X-ray control of catheter position. LEGs were displayed simultaneously with 12-lead surface electrograms on the monitor. Prior to its insertion into the sheath the laser catheter was continuously rinsed with heparinized (5000 IU/L) saline at a rate of 15 mL/min. Saline flow increased automatically via the foot-switch to a rate of 30-35 mL/min during laser application.
Continuous variables are expressed as mean ± SD. The study was performed in the Laser and Applied Technologies Center, the Clinical Cardiac Electrophysiology Laboratory, 3d Medical Department, Cardiology, Hospital Harlaching, teaching hospital of the LM-University of Munich. Quality of life forms were completed prior to and following laser ablation. Clinical and electrocardiographic controls, and, only in case of need, redo procedures were performed during an intended life-long follow-up.
Study design / Ablation protocol
Study design, including the form of written informed consent, was granted a favorable ethical opinion and was approved by the Ethics Committee of the Board of Physicians of the Land of Bavaria (reference EK/h No: 95243). Written informed consent was obtained from all the 48 patients recruited to the study. In addition, patients completed a questionnaire for quality of life (QL-form) at baseline, 12-14 months after the study, and after ≥5.2 years, in regard of exercise capacity, angina, palpitations, breathlessness, and attacks of dizziness / syncope.
After advancement of the ELMA catheter beyond the endhole of the transseptal sheath manual catheter exploration of the left atrial cavity was performed systematically. Initially endocardial areas with relative regular rapid and sharp high frequency atrial potentials in the left atrial posterior wall were targeted. The catheter tip was brought in a stable intimate contact with the endocardial surface where the highest electrical potential amplitudes were recorded from. Laser application at 15W was aimed at that area until amplitudes of electrical potentials in the LEG displayed on the monitor were abolished permanently. The catheter was then moved to adjacent sites where electrical potentials were still present and the procedure was repeated. Stepwise the entire LA posterior wall around the pulmonary veins was rendered electrically inactive, devoid of electrical potentials. When sinus rhythm was achieved burst stimulation at pacing cycle lengths (PCL) of 150-200 ms was performed. If AF recurred laser applications were stepwise aimed at the LA roof, mitral isthmus, and around the margins of the left atrial appendage until local electrograms, including CFAE or torsade were abolished.
If AF persisted or was still inducible detailed analysis of the left atrium, inter-atrial septum in right atrium was performed to identify electrical activity of other areas that was then ablated. This process was considered as completed when sinus rhythm was restored and no residual CFAE or torsade like sites could be identified. Only split and blunt low amplitude potentials representing rather far potentials were left. Laser application inside the pulmonary veins, the atrial appendages, and the coronary sinus was avoided. After an observation time of 15-20 minutes final rapid burst stimulation was performed via the pinelectrodes of the ELMA catheter.
If other arrhythmogenic substrates were present these were also abolished. When the patient was in sinus rhythm 20--30 min after burst atrial stimulation the catheter was removed and the patient referred to the ward with Holter-monitor.
Acute Results and clinical outcome
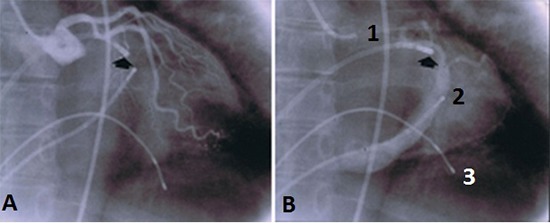
The very first l-lpAF laser ablation procedure was performed in April 13th, 1988 in the EP laboratory of the Cardiac Department of the Hospital Bogenhausen, Teaching hospital of the Technical University of Munich. The patient suffered from frequent attacks of palpitations and weekly syncope. Prior to l-lpAF ablation, an accessory atrioventricular pathway with bidirectional conduction properties was localized in left atrial lateral wall and was laser ablated March 3d, 1988 [Figure 5].
Figure 5. A shows RAO projection of a left coronary artery angiogram performed prior to the laser ablation attempt and B the coronary sinus filling with dye. The thick arrow shows the orientation of the catheter towards the location of a left sided accessory atrioventricular pathway. 1 = angiographic catheter positioned in the aortic root, 2 = 4polar electrode catheter in the coronary sinus, 3 = bipolar electrode catheter in the right ventricle.
All of the patients were in AF at the beginning of the ablation procedure. During catheter exploration of the atrial cavities various types of local electrical potentials including rapid relative regular high frequency sharp demarcated potentials, various types of CAFE and even torsade de point like electrograms were displayed in the focused LEG. In 5 of the 48 patients all these types of LEGs were found during catheter exploration in different sites of the atria. Torsades like tracings were seen in 5 patients in the right atrial LEGs [Figure 6]. During laser applications aimed at the areas with rapid firing as shown in LEG1 in [Figure 4], the potential amplitudes dwindled gradually and eventually were abolished permanently [Figure 7].
Figure 6. Examples of Intracardiac bipolar focused local electrograms (LEG) recorded from various sites of the left and right atrial walls recorded via the pin-electrodes of the open-irrigated electrode-laser mapping and ablation (ELMA) catheter RytmoLas during manual exploration of the atrial cavities in a patients with long-lasting persistent atrial fibrillation showing: LEG1 = sharp, high amplitude, regular electrical potentials (scar related tachycardia?), LEGT2 = complex atrial fragmented electrogram (CAFE potentials), and LEG3,4 = torsade like local electrograms. I and II = surface lead electrocardiograms.
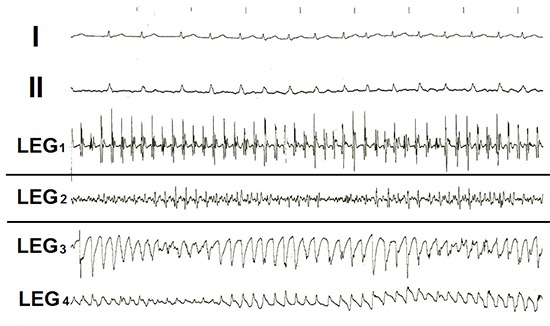
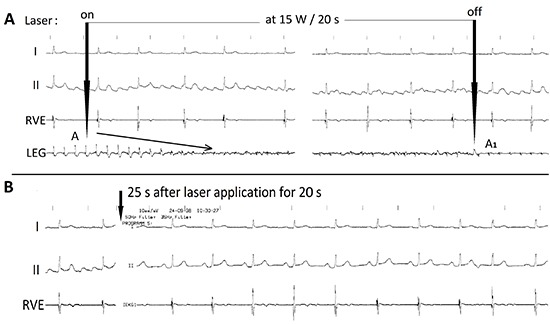
Figure 7. Bipolar focused local electrogram (LEG) recorded during redo laser ablation for atrial flutter in a patient 6 weeks after successful ablation of long-lasting persistent atrial fibrillation showing: A gradual abatement of local electrical potential amplitudes (oblique arrow) after the onset of laser application from A, last amplitude prior to, and A1 first potential amplitude after the laser impact, and B Sinus rhythm 55 bpm achieved 25s after laser application (vertical arrows). RVE = right ventricular electrogram, I and II = surface lead electrograms.
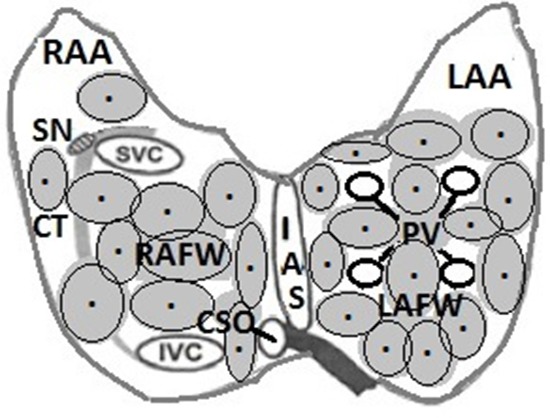
Initially in all of the patients the entire left atrial posterior wall was coagulated by creating an extensive contiguous lesion that rendered the area around the pulmonary veins electrically inactive. A series of 11 to 16 consecutive adjacent laser applications were needed to achieve that goal. Sixteen patients (33%) converted to sinus rhythm after LA posterior wall ablation alone. In 32 (67%) patients additional applications were needed for the abolishment of local potentials in the area of the left atrial isthmus and roof, and around the basis of the atrial appendage. In 12 (25%) patients sinus rhythm was achieved after additional 7-11 laser applications aimed at the right atrial posterior and/or lateral walls, or interatrial septum. In two of the patients 26 bi-atrial laser applications, the maximum of laser impacts per patient in this study, were needed to achieve sinus rhythm [Figure 8].
Figure 8. Scheme showing: approximate distribution of laser spots in the atrial cavities as stepwise applied during this study for electrically guided transmural coagulation of the atrial walls, beginning in the left atrial posterior wall around the pulmonary veins, and, if needed stepwise applications aimed at the region of the left atrial isthmus, around the left atrial appendage, and eventually the right atrial walls, until sinus rhythm was achieved. RAA and LAA = right and left atrial appendages, SN = sinus nodal area, CT = crista terminalis, RAFW and LAFW = right and left atrial free walls, IAS = interatrial septum, SVC= superior vena cava, IVC = inferior vena cava, CSO = coronary sinus ostium.
All of the patients were in sinus rhythm at the end of study. In the 4 patients with an implanted permanent pacemaker laser applications did not alter pacemaker functions but pacemaker probes hindered in some degree catheter exploration of the right atria. Laser ablation and transseptal laser puncture procedures were painless and without complications, except one bleeding of the venous puncture in the left groin. Total time of ablation procedure was 82-175 (118±72) min, and number of lesions produced per patient ranged from 14 - 26 (19 ± 4) [Table 2].
Table 2. AF Laser Ablation Procedures.
| Albation | Laser | ||
|---|---|---|---|
| Procedure duration (min ) | X-Ray Exposure time (min ) | 524 Applications No per patient | Radiation per patient (s) |
| 81-175 | 15-82 | 14-26 | 180-310 |
| (118 ±72) | (23 ± 12) | (19 ± 4) | (235 ± 75) |
Follow-up
Holter monitoring for 24-48 hours was performed immediately following, before released from the hospital, and, on a regular basis after a month, after 6 months for two years, and every year after ablation, and, whenever patients complained and AF recurrences were suspected. DC-cardioversion was needed in 3 of the 48 patients 2-5 days after ablation. Before leaving the hospital patients were learned to reduce/avoid increase in weight, nicotine abuse, to have regular moderate physical exercise, were learned to feel and control their peripheral radial or carotid pulse and in case of palpitations to consult medical services for ECG recordings; perhaps take Propafenon (”pill-in-the-pocket”) until recurrence of AF is ECG confirmed or is ruled out. After the final clinical checkup control of 2D-Doppler echo was carried out, that was repeated after 11-14 month of follow-up [Table 3].
Table 3. Left atrial dimensions at baseline and after laser ablation of atrial fibrillation.
NS = not statistically significant; NQS = not quite statistically significant VS = very statistically significant; ES = extremely statistically significant
| Left Atrial dimensions | Baseline 48 Patients | Post Ablation 5-11 days | P-values 46 patients | Post ablation 11-14 months | P-values 44 patients |
|---|---|---|---|---|---|
| Diameter(mm) | 44-59 /51.73 ± 3.28 | 45-56 /50.92±2.93 | P=0.2037 NS | 40-50 /44.73 | P <0.0001 ES |
| Major axis (mm) | 66-83 /75.92±4.41 | 65-82 /74.52±4.15 | P=0.1139 NS | 62-75 /67.18±1.17 | P<0.0001 ES |
| Area(cm²) | 25-46 /37.71±5.32 | 25-42 /35.92±4.84 | P =0.0877 NQS | 19-31 /26.48±5.18 | P<0.0001 ES |
| Volume(ml) | 71-86 /78.90±4.00 | 70-81 /76.69±2.81 | P=0.0023 VS | 68-77 /73.48±3.02 | P<0.0001 ES |
All the patients were in sinus rhythm and off antiarrhythmic medication when leaving the hospital but anticoagulation with Warfarin with INR target of 2-3 was continued for three-6 months in all of the patients but was discontinued when stable sinus rhythm persisted. Follow-up clinical controls and 12 lead ECG at rest were performed in an outpatient clinic or by house doctors. Continuous event monitoring was performed by means of a Wrist Recorder Plus, RalinMedical irregularly in case of suspected recurrences of AF.
Thirty three patients (69%) were arrhythmia free after a single study as described including ablation of accompanying arrhythmogenic substrates such as left accessory atrioventricular pathway (n = 3) and atrioventricular nodal reentrant tachycardia (n = 2). Patients were in sinus rhythm, off antiarrhythmic drugs and had a substantially improved exercise capacity [Table 4]. Sixteen patients needed continuous medication with diuretics, angiotensin converting enzymes, and beta- blocker (Carvedilol) because of hypertension, coronary artery disease and/or congestive heart failure NYHA I-II. In 5 patients Propafenon was used intermittently (“pill in-the-pocket”) for the control of transient episodes of palpitations suspected by the patient but not documented as arrhythmia recurrences.
Table 4. Quality of life after laser catheter ablation of long-persistent AF estimated with 5 points: 1 = best, 5 = worst; prior to (48 pts), after 12-14 months (45 pts), ≥ 5.2 years (39 pts).
| Not at all limited | A little | Moderate | Very strong | Extremely limited | |
|---|---|---|---|---|---|
| Marks: | 1 | 2 | 3 | 4 | 5 |
| Criteria evaluated | Prior to study in 48 patients | After 3-14 mo. in 45 patients | P values | ≥ 5.2 years in 39 patients | P values |
| Exercise / Sports | Mean = 4.48 SD ± 0.65 | Mean = 2.12 SD ± 0.97 / | P < 0.0001 | Mean=2.04 SD ± 0.89 / | P=0.7626 |
| Palpitations | Mean = 4.24 SD ± 0.78 | Mean =1.32 SD ± 0.56 | P < 0.0001 | Mean = 1.12 SD ± 0.33 | P=0.4507 |
| Angina | Mean = 3.0 SD ± 0.82 | Mean = 1.12 SD ± 0.33 | P < 0.0001 | Mean = 1.44 SD = 0.58 | P = 0.0211 |
| Breathlessness | Mean = 4.12 SD ± 0.78 | Mean = 1.76 SD ± 0.38 | P < 0.0001 | Mean = 1.60 SD ± 0.50 | P = 0.4134 |
| Dizziness/Syncope | Mean = 3.08 SD ± 1.19 | Mean = 1.20 SD ± 0.41 | P = 0.0001 | Mean = 1.16 SD ± 0.37 | P = 0.7196 |
During Follow-up the first patient in whom AF ablation was attempted in April 1988 no syncope but palpitations recurred after 6 months. She died in the ICU in another hospital after a MAZE operation she underwent 9 months after the attempted laser catheter ablation of l-lpAF. Another patient died in the ICU of another hospital after thoracic surgery because of hematothorax after a house accident 11 months, and a third patient died in hepatic coma 17 months after successful l-lpAF ablation.
Electrophysiological restudies were performed in 15 patients in whom reablation was needed for AF recurrences (n = 2) after 24 hours and 3 months respectively, and for other arrhythmias one week and up to 9 months after successful l-lpAF ablation [Table 5]. None of the patients with valvular defects had recurrences. Because of the mild valvular insufficiency we consider these patients also having a non-valvular AF.
Table 5. Long-term outcomes, 9.0 months to 9.2 (8.2 ± 6.5) years, in 48 patients after laser catheter ablation of long-lasting persistent atrial fibrillation (l-lpAF).
| OUTCOME | No of PATIENTS | % |
|---|---|---|
| In Sinus rhythm | 46 | 96% |
| off medication | 17 | 35% |
| under medication | 16 | 33% |
| after repeat study, for: | 15 | 31% |
| - left atrial flutter | 7 | |
| - recurrent atrial fibrillation | 2 | |
| - typical atrial flutter | 2 | |
| - AT (right atrial reentry) | 1 | |
| - Inappropriate sinus tachycardia | 1 | |
| - common AV nodal reentry | 1 | |
| - non sustained right ventricular | 1 | |
| outflow tract tachycardia | ||
| In permanent AF | 1 | 2% |
| Death | 1 after 9 months | 6% |
| 1 after 11 months | ||
| 1 after 17 months |
All of the patients, except one, were in sinus rhythm during their last control in an outpatient clinic or by cardiologist/general practitioners, in none of them ECG controls documented recurrences of l-lpAF or other arrhythmias. After a mean of 5.2 years, patients were gradually lost and further data no longer available because of noncardiac deaths (cancer, n = 9, pneumonia = 4, liver diseases = 3, traffic accident = 1), or relocation with unknown addresses (n = 5), or could not be contacted any longer (n = 24). Two patients are still alive and under observation. One was seen in October 2016 at the age of 69, the youngest patient in our study group, with permanent AF after a redo procedure for atrial flutter in 1998. He has an irregular heart beat 65 bpm at rest and 85 bpm during exercise (moderate walking, climbing 5 stairs); has congestive heart failure NYHA II-III, is anticoagulated with Dabigatranetexelat (Pradaxa), and is under medication with diuretics, ACE inhibitors, and beta-blocker (Carvedilol). He has no neurological deficit and is able to manage his every day needs. The second patient was contacted in January 30th 2017. She is 95 years of age, in sinus rhythm after successful l-lpAF ablation and after redo procedure for common atrioventricular nodal reentrant tachycardia 21 years ago. PQ-interval is 0.20ms. She is off antiarrhythmic medication but severely handicapped by arthrosis of her leg joints and spine and she needs home help. The last control scheduled for June this year could not be performed; she died at the age of 96 years.
Discussion
This study in a small heterogeneous group of patients after life-long follow-up suggests that laser catheter ablation of l-lpAF is safe and effective. It can achieve excellent long-term results when using the open-irrigated ELMA catheter RytmoLas. A lifelong success rate of 96% is unequalled by other ablation techniques in our knowledge so far. This can be explained by several reasons.
The LPS of the laser: in case of inadvertent overheating in front of the ELMA catheter endhole where the “cold” laser light is emanated from and hits the target, laser application is stopped automatically by the LPS prior to the possible occurrences of thermal damage to the endocardium or catheter tip. This is a crucial safety aspect of the laser method. Other important reasons represent:
The Nd:YAG laser
The Nd:YAG laser light at a wave length of 1064nm has a very low absorption in water [16]. Radiation of photons is scattered in the myocardium and can reach deep locations before ultimately being converted into a temperature rise through absorption, with impaired temperature gradient and distribution of heat. The heat generated during photon absorption will cause a gradual rise in temperature cumulative with exposure time and rate of absorption as a function of location [17],[18]. Heat is spreading concentrically in the myocardial wall and, besides of photon absorption is further distributed by heat convection. Continuous wave 1064nm Nd:YAG laser application can produce deep lesions of coagulation necrosis without thrombus or steam pop when using an open-irrigated ELMA laser catheter [19]. However, for safe laser application without collateral damages to adjacent structures of the heart, energy settings adapted to the thickness of the myocardial wall are the prerequisite [20].
The laser lesions
Laser application aimed at the thin structured atrial walls does not result in tissue vaporization with crater formation. There is no risk of myocardial wall perforation. Anatomic integrity of the irradiated atrial wall is always preserved. The gradually growing coagulation process intramurally results in a solid volume with well demarcated boundaries. Photons are absorbed intramurally whereas the catheter itself and the translucent endocardium are not heated up, and, in addition, are cooled by the saline flow of the catheter at room temperature (18ᵒC). Laser lesions are clear cut areas of homogenous coagulation necrosis healing to dense fibrous scars without aneurysm formation and are not arrhythmogenic. There are no remnants of vital myocardial cells inside the scars. In contrast to that RF current often cause tissue vaporization with crater formation and carbonization [21]. Even new second-generation open irrigated RF catheters produce pop and inhomogeneous lesions [22].
Laser lesions are not thrombogenic. As compared to RF current applications the estimation of D-dimer serum level in patients prior to and after laser application showed unhanged plasma D-dimer levels. Laser ablation is without thrombogenic effect neither immediately nor long term [23]. In contrast to that, RF ablation has a thrombogenic effect as reflected by elevated plasma D-dimer levels that persists through 48h after the procedure [24]. This effect needs to be taken into account when considering antithrombotic therapy in patients undergoing RF ablation. However, albeit under uninterrupted oral anticoagulation RF ablation is associated with a substantial risk of silent embolism [25]. This may represent a risk factor for significant cognitive decline [26], [27].
Also of importance for the success of the laser method are type and sizes of lesions achieved with the ELMA catheter. Transmural lesions at diameters of up to 10.0 mm and more can be created within seconds. Thus, extensive areas of coagulation can be achieved in the atrial walls with a relative small number of laser applications. The maximum of 26 laser applications in this study, totalized a radiation time of 310 s. Thereby, not points but spots, not lines but stripes of ≥5mm in width of the lesions were achieved. Width of lesions is of importance for the success of the laser method because conduction may occur over narrow lines even when transmural and contiguous [28].
The Catheter design
The open-irrigated ELMA catheter substantially contributes to both safety and efficacy of the laser method. It allows for non-contact laser light application because a fiber tip to endocardial surface contact is avoided and continuous saline irrigation through the catheter endhole avoids blood penetration into the catheter [29]. It creates a transparent medium for the laser light and cools the illuminated endocardial surface. The pin electrodes at the catheter tip with the interelectrode distances of ≤ 2.0 mm allow for registration of focused LEG from a very small area of the endocardial surface. Focused LEG recordings display electrical activity of a limited endocardial area that cannot be obtained by conventional electrode catheters with larger interelectrode distances [30], [31]. In contrast to the RF recordings, the coagulation process initiated by the laser light in the myocardial wall can be visualized online on the monitor by the gradual abatement of the electrical potentials amplitudes in the LEG. The gradual abatement of potentials amplitudes conspicuous in the LEG during radiation reflects the growths of the lesion, the spread of acute coagulation necrosis in the atrial wall. With the stop of laser application 2-3 second after abolishment of electrical potentials lesions are limited to the culprit tissue and collateral damages are avoided. This can be assumed by the follow-up of the patients which were all symptom free acutely and during long-term as well. Based on these experiences esophageal monitoring was not done and phrenic nerve captures was not confirmed neither during nor after the procedure in this study. More recently, we have tested successfully an esophageal light sensor that confirmed these assumptions [30].
A specific advantage of laser application for treatment of tachyarrhythmias is the ability to perform treatment under normothermic conditions while avoiding interfering with electrophysiologic monitoring principles. This immediate and real-time verification of the success is extremely beneficial. Electrophysiologically guided ablation allows for a systematic approach with simultaneous validation of initial success, and, it allows for laser mapping [32]. In this study we have systematically abolished local electrical activity rather than creating anatomically guided ablation lines in isthmus areas, atrial roofs, or free walls. It was mainly an electrically but not an anatomically guided procedure. Control of immediate success in this study was carried out by monitoring of the local electrical potentials amplitude in the LEGs displayed on the monitor. Catheter manipulation was performed also under short intermittent X-ray control. With a recently developed laser balloon catheter for PVI visual control of the laser beam inside of the heart is feasible during laser application [33]. As compared to the open-irrigated ELMA catheter the balloon technique show what you do, but you don’t see what you get. Efficacy similar to RF ablation is reported. It is of concern, that laser application into the stagnant blood that inevitably occurs in the occluded pulmonary veins in front of the inflated balloon bears a considerable risk for thrombus formation. In addition, it is a sophisticated and relative expensive approach. As compared to the other routinely used ablation methods manual laser catheter application with the open-irrigated ELMA catheter has short procedure duration times, shorter X-ray exposure and energy application times regardless of catheter technique used manual, robotic Hansen or Stereotaxis [34].
Further considerations
In patients with l-lpAF atrial myocardium is found to be predominantly fibrous. Extend of atrial wall fibrosis and ablation related scarring are major predictors of success in rhythm control of AF. Circumferential PV antral scarring predicts ablation success in mild left atrial fibrosis, while additional left atrial posterior wall and septal ablation is needed for moderate fibrosis [35]. Scarred tissue is insulating the viable myocardial fibers involved in the perpetuation of AF. The 1064nm laser light is penetrating through scarred tissue [36]. In the scar 1064nm laser light is mainly scattered and eventually absorbed in the darker atrial myocardium heating and coagulating the potentially arrhythmogenic still viable myocardial fibers contained and scattered as small or larger islets within the scars.
By manipulating the flexible and resilient ELMA catheter all over the endocardial areas in the atrial cavities stable positioning over the targeted sites could be achieved without pressure control. Pressure of the ELMA catheter tip on the endocardial surface with contact force measurement is not needed for lesion formation. Laser lesions can be achieved even without intimate endocardial catheter contact. Catheter contact force and catheter orientation towards the endocardial surface are not major determinants for laser lesion formation [29], [37], [38]. Albeit PVI is still considered the cornerstone for a successful AF ablation we did not target the pulmonary vein specifically, well-directed. Extensive coagulation of the posterior LA walls around the PV ostia was aimed at in our study by creating contiguous lesions by adjacent electrically guided laser applications. With the abolishment of potential amplitudes transmural scars around the pulmonary veins were produced, thereby inevitably resulting in effective PVI. After RF applications AF recurrences were observed despite isolated pulmonary veins [39]. In our study coronary sinus and atrial appendages were not targeted. Thereby avoiding mechanical damages to the appendages walls with the loss of their pumping function, and avoiding thrombus formation inside the appendages [40]; whereas ablation of substrates located around the basis of atrial appendages may have contributed to the success of the method [41].
As reflected by left atrial dimensions and improvements in QoL in our patients there was no indication for compromised left atrial blood transport attributable to the extensive laser induced scarring of the atrial walls. Left atrial dimensions decreased significantly in all the patients after AF ablation. Preserved pumping function of the atrial appendages might have been sufficient for a pulsatile blood flow to the ventricles, and, before all to avoid thrombus formation in the stagnant blood inside their lumen, a considerable risk of stroke. Shrinking of the atrial walls may have maintained unchanged or may have even reduced the volumes of atrial cavities. Possible laser effects on the ganglion plexi located behind the atrial posterior wall on the outcome of AF ablation cannot be ruled out but also not defined. Some decline in physical performance in this group of patients is attributed rather to their age. Metabolic effects, from diabetes, gout, obstructive sleep apnea or adiposity did not substantially influence outcome in this study group. Especially fibro-fatty infiltration of the subepicardium can contribute to the functional disorganization of the atrial myocardium [42]. Thus, maintenance of normal body weight may have substantially contributed to the success of l-lpAF ablation in this study population. Atrial metabolism and tissue perfusion are also determinants of electrical and structural remodeling in AF because rapid rates of electrical activity and contraction are enormous challenge to the energy balance of atrial myocytes [43].
Assessment of ablation-induced scarring in AF showed that catheter ablation of AF targeting PVs rarely achieves permanent encircling scar in the intended areas what is associated with recurrent arrhythmia [44]. In addition, it has been reported that PV reconnection is frequent in patients with heart failure, and, of great importance, patients presented arrhythmic recurrences even in the absence of PV reconnection, highlighting the importance of the underlying atrial substrate [45]. These facts are of special importance for evaluation of this study because all of the study patients were in heart failure NYHA II-III. Furthermore, the role of localized electrical rotors and focal impulse sources have been recognized in sustaining of AF, as well as the role of CAFE at the right atrium, where additional ablation provides an increment in efficacy when energy application addresses moving targets that changes frequently [46],[47]. All the above mentioned emphasizes that human l-lpAF is characterized by heterogeneous and unstable patterns of activation including wave fronts, transient rotational circuits, and disorganized activity [48]. Thus, PVI is far from being a cornerstone alone for successful ablation of l-lpAF.
Recently, first evidence was provided for asynchronous activation of the endo-epicardial wall during AF in humans. Endo-epicardial asynchrony as a major role in pathophysiology of AF may explain why in some patients therapy fails [49]. Rotating spiral waves have been observed also in a wide variety of nonlinear spatially distributed systems in physics, chemistry, and biology, called excitable media. In medicine, they are associated with cardiac arrhythmias. Based on these new insights, an ablation procedure has been clinically introduced that stops atrial fibrillation of the heart by destroying the electrical activity at the spiral core [50]. Some limitations and cause for concern during the follow-up of our patients are the patient compliance and feedback from surveillance centers. However, in an effort to cope with these limitations we have tried to keep close contact with patients and the surveilling physicians in order to minimize losses of information and to obtain valid results. Helpful in this regard would be an enhanced and prolonged Holter-electrocardiogram-monitoring for increased detection of atrial fibrillation [51].
Conclusions
This is the first study that qualifies the laser method as an intriguing technique superior for ablation of l-lpAF as compared to the hitherto routinely used AF ablation techniques, when using the open-irrigated ELMA catheter RytmoLas. With the laser very long-term excellent results can be achieved by including a preemptive strategy of taking out the electrical milieu substrate. The method has a low risk, a short procedure time, and reduces redo procedures. It comes down to a single catheter technique without the need for sophisticated mapping equipment. Manual exploration with the ELMA catheter under monitoring of local electrical potential amplitudes in the focused LEG are the essentials of the method, that is cost-effective and patient centered, and has the potential for becoming an all pervasive procedure. To achieve that goal further investigation, preferably multicenter study trials are warranted.
Acknowledgments
This study was supported in part by LasCor GmbH – Laser Medical Devices, Taufkirchen, Germany. The authors report no conflicts of interest for the published content.
Conflict Of Interests
None.
Disclosures
None.
References
- 1.Andrade Jason, Khairy Paul, Dobrev Dobromir, Nattel Stanley. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014 Apr 25;114 (9):1453–68. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 2.Chugh Sumeet S, Havmoeller Rasmus, Narayanan Kumar, Singh David, Rienstra Michiel, Benjamin Emelia J, Gillum Richard F, Kim Young-Hoon, McAnulty John H, Zheng Zhi-Jie, Forouzanfar Mohammad H, Naghavi Mohsen, Mensah George A, Ezzati Majid, Murray Christopher J L. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014 Feb 25;129 (8):837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colilla Susan, Crow Ann, Petkun William, Singer Daniel E, Simon Teresa, Liu Xianchen. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol. 2013 Oct 15;112 (8):1142–7. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 4.Fuster Valentin, Rydén Lars E, Cannom David S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Le Heuzey Jean-Yves, Kay G Neal, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann Samuel. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). Eur. Heart J. 2006 Aug;27 (16):1979–2030. doi: 10.1093/eurheartj/ehl176. [DOI] [PubMed] [Google Scholar]
- 5.Dobrev Dobromir, Nattel Stanley. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 2010 Apr 03;375 (9721):1212–23. doi: 10.1016/S0140-6736(10)60096-7. [DOI] [PubMed] [Google Scholar]
- 6.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 03;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 7.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Natale Andrea, Packer Douglas, Skanes Allan, Ambrogi Federico, Biganzoli Elia. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Feb;3 (1):32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 8.Weerasooriya Rukshen, Khairy Paul, Litalien Jean, Macle Laurent, Hocini Meleze, Sacher Frederic, Lellouche Nicolas, Knecht Sebastien, Wright Matthew, Nault Isabelle, Miyazaki Shinsuke, Scavee Christophe, Clementy Jacques, Haissaguerre Michel, Jais Pierre. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J. Am. Coll. Cardiol. 2011 Jan 11;57 (2):160–6. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 9.Haïssaguerre Michel, Sanders Prashanthan, Hocini Mélèze, Takahashi Yoshihide, Rotter Martin, Sacher Frederic, Rostock Thomas, Hsu Li-Fern, Bordachar Pierre, Reuter Sylvain, Roudaut Raymond, Clémenty Jacques, Jaïs Pierre. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1125–37. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 10.Weber H P, Heinze A, Enders S, Ruprecht L, Unsöld E. Catheter-directed laser coagulation of atrial myocardium in dogs. Eur. Heart J. 1994 Jul;15 (7):971–80. doi: 10.1093/oxfordjournals.eurheartj.a060618. [DOI] [PubMed] [Google Scholar]
- 11.Weber H, Heinze A. Laser catheter ablation of atrial flutter and of atrio-ventricular nodal reentrant tachycardia in a single session. Eur Heart J. 1994;15:1147–1149. doi: 10.1093/oxfordjournals.eurheartj.a060643. [DOI] [PubMed] [Google Scholar]
- 12.Weber H, Heinze A. Control of rapid ventricular response by laser catheter modification of atrioventricular node in a patient with medically refractory atrial fibrillation. Eur.J.C.P.E. 1995;5:215–217. [Google Scholar]
- 13.Weber H P, Kaltenbrunner W, Heinze A, Steinbach K. Laser catheter coagulation of atrial myocardium for ablation of atrioventricular nodal reentrant tachycardia. First clinical experience. Eur. Heart J. 1997 Mar;18 (3):487–95. doi: 10.1093/oxfordjournals.eurheartj.a015270. [DOI] [PubMed] [Google Scholar]
- 14.Weber H, Schmitz L, Heinze A, Ruprecht L, Sagerer-Gerhardt M. The development of a laser catheter with improved mapping resolution and online monitoring of lesion formation during arrhythmia ablation. In: Laser Ablation, C. Belucci (Ed). 2017 Nova Science Publishers, Inc. Chapter 2, pages: 39-86. ISBN. 1997;1:39–86. [Google Scholar]
- 15.Weber H, Sagerer-G M. Side-selective atrial transseptal laser puncture. The Journal of Innovations in Cardiac Rhythm Management. Innovative Techniques. 2013;4:1481–1485. [Google Scholar]
- 16.Boulnois JL. Photophysical processes in recent medical laser developments: a review. J Lasers Med Sci. 1986;1:47–66. [Google Scholar]
- 17.Weber H P, Heinze A, Hauptmann G, Ruprecht L, Unsöld E. In vivo temperature measurement during transcatheter endomyocardial Nd-YAG laser irradiation in dogs. Lasers Med Sci. 1997 Dec;12 (4):352–6. doi: 10.1007/BF02767159. [DOI] [PubMed] [Google Scholar]
- 18.Splinter R, Hooper B. Introduction in biomedical optics. Boca Raton: CRC Press/Taylor & Francis; 2007;0:0–0. [Google Scholar]
- 19.Ikeda A, Nakagawa H, Weber H, Sagerer-Gerhardt M, Weber D, Sharma T, Pitha JV, Lazzara R, Jackman WM. Open-irrigated laser catheter produces deep lesions without thrombus or steam pop. Heart Rhytm. 2011;8:262–0. [Google Scholar]
- 20.Weber Helmut, Sagerer-Gerhardt Michaela. Open-irrigated laser catheter ablation: relationship between the level of energy, myocardial thickness, and collateral damages in a dog model. Europace. 2014 Jan;16 (1):142–8. doi: 10.1093/europace/eut150. [DOI] [PubMed] [Google Scholar]
- 21.Weber H P, Heinze A, Enders S, Ruprecht L, Unsöld E. Laser versus radiofrequency catheter ablation of ventricular myocardium in dogs: a comparative test. Cardiology. 1997 Jul 1;88 (4):346–52. doi: 10.1159/000177358. [DOI] [PubMed] [Google Scholar]
- 22.Akca F, Hubay M, Zima E, Széplaki G, Végh EM, Skópal J, Lendvai Zs, Theuns D, Merkely B, Szili T. High-volume lesions using new second generation open-irrigated radiofrequency catheter are associated with the development of inhomogeneous lesions. PACE. 2014;37:864–873. doi: 10.1111/pace.12359. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang S, Weber H, Heinze A, Wanner G, Weiss L. D-Dimer serum levels after laser catheter ablation of tachyarrhythmias. PACE. 1999;22:196–0. [Google Scholar]
- 24.Manolis A S, Melita-Manolis H, Vassilikos V, Maounis T, Chiladakis J, Christopoulou-Cokkinou V, Cokkinos D V. Thrombogenicity of radiofrequency lesions: results with serial D-dimer determinations. J. Am. Coll. Cardiol. 1996 Nov 01;28 (5):1257–61. doi: 10.1016/S0735-1097(96)00324-5. [DOI] [PubMed] [Google Scholar]
- 25.Martinek Martin, Sigmund Elisabeth, Lemes Christine, Derndorfer Michael, Aichinger Josef, Winter Siegmund, Jauker Wolfgang, Gschwendtner Manfred, Nesser Hans-Joachim, Pürerfellner Helmut. Asymptomatic cerebral lesions during pulmonary vein isolation under uninterrupted oral anticoagulation. Europace. 2013 Mar;15 (3):325–31. doi: 10.1093/europace/eus329. [DOI] [PubMed] [Google Scholar]
- 26.Hui Dawn S, Morley John E, Mikolajczak Peter C, Lee Richard. Atrial fibrillation: A major risk factor for cognitive decline. Am. Heart J. 2015 Apr;169 (4):448–56. doi: 10.1016/j.ahj.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Piers Ryan J, Nishtala Arvind, Preis Sarah R, DeCarli Charles, Wolf Philip A, Benjamin Emelia J, Au Rhoda. Association between atrial fibrillation and volumetric magnetic resonance imaging brain measures: Framingham Offspring Study. Heart Rhythm. 2016 Oct;13 (10):2020–4. doi: 10.1016/j.hrthm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor G W, Walcott G P, Hall J A, Bishop S, Kay G N, Ideker R E. High-resolution mapping and histologic examination of long radiofrequency lesions in canine atria. J. Cardiovasc. Electrophysiol. 1999 Nov;10 (11):1467–77. doi: 10.1111/j.1540-8167.1999.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 29.Weber H, Sagerer-Gerhardt M. Open-irrigated laser catheter ablation: influence of catheter irrigation and contact and non-contact mode of laser application on lesion formation in bovine myocardium. Lasers Med Sci. 2014;29:1183–1187. doi: 10.1007/s10103-013-1505-0. [DOI] [PubMed] [Google Scholar]
- 30.Weber H, Sagerer-Gerhardt M. Electrocardiographic Monitoring of Myocardial Lesion Formation during Laser Catheter Ablation in A Dog Model. The Journal of Innovations in Cardiac Rhythm Management. 2014;5:1641–1649. [Google Scholar]
- 31.Price A, Leshen Z, Hansen J, Singh I, Arora P, Koblish J, Avitall B. Novel ablation catheter technology that improves mapping resolution and monitoring of lesion formation. The Journal of Innovations in Cardiac Rhythm Management. 2012;3:599–609. [Google Scholar]
- 32.Weber Helmut P, Sagerer-Gerhardt Michaela. Monitoring of laser effects on the conduction system by using an open-irrigated electrode-laser mapping and ablation catheter: laser catheter mapping. Europace. 2015 Apr;17 (4):664–70. doi: 10.1093/europace/euu328. [DOI] [PubMed] [Google Scholar]
- 33.Dukkipati Srinivas R, Kuck Karl-Heinz, Neuzil Petr, Woollett Ian, Kautzner Josef, McElderry H Thomas, Schmidt Boris, Gerstenfeld Edward P, Doshi Shephal K, Horton Rodney, Metzner Andreas, d'Avila Andre, Ruskin Jeremy N, Natale Andrea, Reddy Vivek Y. Pulmonary vein isolation using a visually guided laser balloon catheter: the first 200-patient multicenter clinical experience. Circ Arrhythm Electrophysiol. 2013 Jun;6 (3):467–72. doi: 10.1161/CIRCEP.113.000431. [DOI] [PubMed] [Google Scholar]
- 34.Di Biase L, Gilbert G, Mohanty P, Cunningham J, Metz T, Horton R, Gallinghouse GJ, Natale A. Procedural differences between manual, robotic and magnetic ablation of atrial fibrillation in consecutive patients. Europace. 2011;13:0–0. [Google Scholar]
- 35.Akoum Nazem, Daccarett Marcos, McGann Chris, Segerson Nathan, Vergara Gaston, Kuppahally Suman, Badger Troy, Burgon Nathan, Haslam Thomas, Kholmovski Eugene, Macleod Rob, Marrouche Nassir. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J. Cardiovasc. Electrophysiol. 2011 Jan;22 (1):16–22. doi: 10.1111/j.1540-8167.2010.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber H P, Heinze A, Enders S, Ruprecht L, Unsöld E. Laser catheter coagulation of normal and scarred ventricular myocardium in dogs. Lasers Surg Med. 1998;22 (2):109–19. doi: 10.1002/(sici)1096-9101(1998)22:2<109::aid-lsm7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 37.Sagerer-Gerhardt Michaela, Weber Helmut P. Open-irrigated laser catheter ablation: influence of catheter-tissue contact force on lesion formation. J Interv Card Electrophysiol. 2015 Mar;42 (2):77–81. doi: 10.1007/s10840-015-9977-4. [DOI] [PubMed] [Google Scholar]
- 38.Sagerer-Gerhardt Michaela, Weber Helmut P. Influence of catheter orientation on lesion formation in bovine myocardium by using an open-irrigated laser ablation catheter. Lasers Med Sci. 2016 Sep;31 (7):1333–8. doi: 10.1007/s10103-016-1980-1. [DOI] [PubMed] [Google Scholar]
- 39.Sultan Arian, Lüker Jakob, Hoffmann Boris, Servatius Helge, Schäffer Benjamin, Steven Daniel, Willems Stephan. Interventional management of recurrent paroxysmal atrial fibrillation despite isolated pulmonary veins: impact of an ablation strategy targeting inducible atrial tachyarrhythmias. Europace. 2016 Jul;18 (7):994–9. doi: 10.1093/europace/euv332. [DOI] [PubMed] [Google Scholar]
- 40.Rillig Andreas, Tilz Roland R, Lin Tina, Fink Thomas, Heeger Christian-H, Arya Anita, Metzner Andreas, Mathew Shibu, Wissner Erik, Makimoto Hisaki, Wohlmuth Peter, Kuck Karl-Heinz, Ouyang Feifan. Unexpectedly High Incidence of Stroke and Left Atrial Appendage Thrombus Formation After Electrical Isolation of the Left Atrial Appendage for the Treatment of Atrial Tachyarrhythmias. Circ Arrhythm Electrophysiol. 2016 May;9 (5) doi: 10.1161/CIRCEP.115.003461. [DOI] [PubMed] [Google Scholar]
- 41.Badhwar Nitish, Mittal Suneet, Rasekh Abdi, Vasaiwala Smit, Musat Dan, S Naeini Payam, Fang Qi, Nentwich Karin, Deneke Thomas, Chang Jie, Lakkireddy Dhanunjaya, Wilber David, Lee Randall J. Conversion of persistent atrial fibrillation to sinus rhythm after LAA ligation with the LARIAT device. Int. J. Cardiol. 2016 Dec 15;225 ():120–122. doi: 10.1016/j.ijcard.2016.09.099. [DOI] [PubMed] [Google Scholar]
- 42.Hatem Stéphane N, Redheuil Alban, Gandjbakhch Estelle. Cardiac adipose tissue and atrial fibrillation: the perils of adiposity. Cardiovasc. Res. 2016 Apr 01;109 (4):502–9. doi: 10.1093/cvr/cvw001. [DOI] [PubMed] [Google Scholar]
- 43.Opacic Dragan, van Bragt Kelly A, Nasrallah Hussein M, Schotten Ulrich, Verheule Sander. Atrial metabolism and tissue perfusion as determinants of electrical and structural remodelling in atrial fibrillation. Cardiovasc. Res. 2016 Apr 01;109 (4):527–41. doi: 10.1093/cvr/cvw007. [DOI] [PubMed] [Google Scholar]
- 44.Akoum Nazem, Wilber David, Hindricks Gerhard, Jais Pierre, Cates Josh, Marchlinski Francis, Kholmovski Eugene, Burgon Nathan, Hu Nan, Mont Lluis, Deneke Thomas, Duytschaever Mattias, Neumann Thomas, Mansour Moussa, Mahnkopf Christian, Hutchinson Mathew, Herweg Bengt, Daoud Emile, Wissner Erik, Brachmann Johannes, Marrouche Nassir F. MRI Assessment of Ablation-Induced Scarring in Atrial Fibrillation: Analysis from the DECAAF Study. J. Cardiovasc. Electrophysiol. 2015 May;26 (5):473–80. doi: 10.1111/jce.12650. [DOI] [PubMed] [Google Scholar]
- 45.Anselmino Matteo, Matta Mario, Bunch T Jared, Fiala Martin, Scaglione Marco, Nölker Georg, Qian Pierre, Neumann Thomas, Ferraris Federico, Gaita Fiorenzo. Conduction recovery following catheter ablation in patients with recurrent atrial fibrillation and heart failure. Int. J. Cardiol. 2017 Aug 01;240 ():240–245. doi: 10.1016/j.ijcard.2017.02.067. [DOI] [PubMed] [Google Scholar]
- 46.Narayan Sanjiv M, Krummen David E, Shivkumar Kalyanam, Clopton Paul, Rappel Wouter-Jan, Miller John M. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 2012 Aug 14;60 (7):628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tada Hiroshi, Yoshida Kentaro, Chugh Aman, Boonyapisit Warangkna, Crawford Thomas, Sarrazin Jean-Francois, Kuhne Michael, Chalfoun Nagib, Wells Darryl, Dey Sujoya, Veerareddy Srikar, Billakanty Sree, Wong Wai Shun, Kalra Dinesh, Kfahagi Ayman, Good Eric, Jongnarangsin Krit, Pelosi Frank, Bogun Frank, Morady Fred, Oral Hakan. Prevalence and characteristics of continuous electrical activity in patients with paroxysmal and persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008 Jun;19 (6):606–12. doi: 10.1111/j.1540-8167.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- 48.Lee Geoffrey, Kumar Saurabh, Teh Andrew, Madry Andrew, Spence Steven, Larobina Marco, Goldblatt John, Brown Robin, Atkinson Victoria, Moten Simon, Morton Joseph B, Sanders Prashanthan, Kistler Peter M, Kalman Jonathan M. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur. Heart J. 2014 Jan;35 (2):86–97. doi: 10.1093/eurheartj/eht267. [DOI] [PubMed] [Google Scholar]
- 49.de Groot Natasja, van der Does Lisette, Yaksh Ameeta, Lanters Eva, Teuwen Christophe, Knops Paul, van de Woestijne Pieter, Bekkers Jos, Kik Charles, Bogers Ad, Allessie Maurits. Direct Proof of Endo-Epicardial Asynchrony of the Atrial Wall During Atrial Fibrillation in Humans. Circ Arrhythm Electrophysiol. 2016 May;9 (5) doi: 10.1161/CIRCEP.115.003648. [DOI] [PubMed] [Google Scholar]
- 50.Zykov Vladimir, Krekhov Alexei, Bodenschatz Eberhard. Fast propagation regions cause self-sustained reentry in excitable media. Proc. Natl. Acad. Sci. U.S.A. 2017 Feb 07;114 (6):1281–1286. doi: 10.1073/pnas.1611475114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stahrenberg Raoul, Weber-Krüger Mark, Seegers Joachim, Edelmann Frank, Lahno Rosine, Haase Beatrice, Mende Meinhard, Wohlfahrt Janin, Kermer Pawel, Vollmann Dirk, Hasenfuss Gerd, Gröschel Klaus, Wachter Rolf. Enhanced detection of paroxysmal atrial fibrillation by early and prolonged continuous holter monitoring in patients with cerebral ischemia presenting in sinus rhythm. Stroke. 2010 Dec;41 (12):2884–8. doi: 10.1161/STROKEAHA.110.591958. [DOI] [PubMed] [Google Scholar]


