Abstract
Premature ventricular contractions (PVCs) are usually regarded as benign in the absence of structural heart disease. However, frequent PVCs can lead to depressed LV function, called PVC-induced cardiomyopathy and can be reversible after suppression of PVCs. On the other hand, PVCs can be a part of underlying structural heart disease and may be linked to increased risk of sudden death. In this work, we reviewed the current literature on PVC-induced cardiomyopathy based on a case presentation.
Keywords: Ventricular premature contraction, PVC-induced cardiomyopathy, Tachycardia induced cardiomyopathy, Cardiac magnetic resonance
Introduction
Premature ventricular contractions (PVCs) are usually regarded as benign in the absence of structural heart disease. However, frequent PVCs can lead to depressed LV function, called PVC-induced cardiomyopathy (PVC CMP) and can be reversible after suppression of PVCs. On the other hand, PVCs can be a part of underlying structural heart disease and may be linked to increased risk of sudden death [1]-[6].
Differentiation of these two entities can be challenging. Late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) is the gold standard imaging modality for myocardial tissue characterization [7]. In patients with enlarged cardiac chambers, LGE CMR may have a role in the differentiation of PVC CMP from primary cardiomyopathies and in predicting the improvement of left ventricular function [8].
Figure 1.
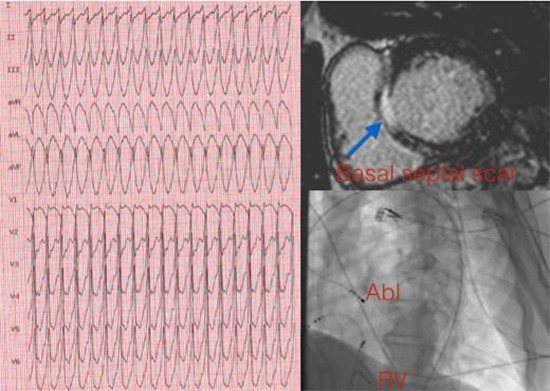
LGE CMR can facilitate VT ablations by providing detail about myocardial scar location and geometry. Here, we present the case of a patient with hemodynamically unstable sustained VT and frequent VPCs with left bundle branch block (LBBB) - inferior axis morphology, and review current methods used for differentiation of two entities.
Case
A 52-year-old man with a history of hypertension, frequent VPCs (34% in previous Holter) was referred to our hospital with hemodynamically unstable sustained VT (230 bpm). 12-lead ECG demonstrated LBBB and inferior axis morphology with early precordial transition. After electrical cardioversion, he had multiple episodes of nonsustained VT with the same morphology. His sinus ECG was unremarkable. Echocardiogram showed enlarged cardiac chambers, global hypokinesia with a LVEF of 30%. Coronary angiography revealed normal coronaries. Due to frequent VPCs, VPC CMP was suspected. For discrimination of VPC CMP from primary CMP a CMR study was planned.
CMR with a 1.5-T scanner (Siemens Essenza, Forchheim, Germany) was performed in multiple anatomic planes using T1-weighted and cine steady-state free precision sequences. Contrast-enhanced sequences to evaluate early myocardial perfusion and delayed myocardial enhancement were also performed, using 0.1 mL/kg gadobenate dimeglumine. LGE CMR demonstrated basal septal scar in the left ventricular outflow tract (%6 of LV myocardium), which raised the possibility of a substrate-mediated VT.
Programmed electrical stimulation performed at RV apex induced the clinical VT. Conventional activation mapping demonstrated earliest activation in the septal part of LVOT. The voltage during sinus rhythm at this site was <1.5 mV, suggesting scar consistent with delayed enhanced CMR image. Ablation at the superior border of the septal scar (30–50 W) resulted in noninducibility of the VT with programmed electrical stimulation of up to triple extrastimuli. Two days after the VT ablation, due to presence of septal scar the patient had an implantable cardioverter- defibrillator (ICD) implanted for secondary prevention. During a 12-month follow-up period despite the patient remained symptom free with no ventricular arrhythmia on Holter monitor and ICD interrogation, he had no reversal of depressed systolic function.
Discussion
Premature ventricular contractions occur frequently in the general population. Although in patients with structurally normal hearts, PVCs have previously been found to be benign, there is conflicting data suggesting that the presence of more than 30 PVCs per hour, PVC couplet, or the presence of R on T have been associated with a small absolute but statistically significant two-fold increase in mortality [9]. As frequent VPCs can be a part of a structural heart disease, and therefore frequent VPCs are a cause of cardiomyopathy. Discrimination of these two pathology is important, because their association with sudden cardiac death, and therapeutic options may be different. Primary LV cardiomyopathy in which LV scar is generally present, is associated with increased risk of sudden death and an ICD should be inserted, on the other hand systolic dysfunction caused by frequent PVCs may be reversible with catheter ablation.
Real prevalence of PVC CMP is unknown, due to challenges in the diagnosis of PVC CMP, which typically requires demonstration of the reversibility of the cardiomyopathy with suppression of PVCs. Among all patients with cardiomyopathy, those with PVC CMP likely constitute only a small percentage. In a community-based prospective study, 0.3% developed abnormal left ventricular ejection fraction (LVEF) over 5 years and the attributable risk of PVCs for congestive heart failure was estimated to be 8.1% [10]. High PVC burden increases the risk of PVC CMP. Baman et al. found that a PVC burden of ≥24% to have a sensitivity of 79% and a specificity of 78% for identifying patients with PVC CMP [11]. Other studies have found statistically discriminatory PVC burden thresholds of 16% (3) and 26% [12]. The clinical value of these proposed thresholds has not been further clarified, and a lower threshold may be desired to achieve a higher sensitivity when used to evaluate patients for potential treatment. A PVC burden of 10% or a PVC frequency of 10,000 PVCs/24h has been used as an inclusion criterion in a number of recent studies [12]-[14]. Besides frequency, there are several risk factors associated with PVC CMP development. The duration of the presence of frequent PVCs are linked to PVC CMP. The initial onset may occur sooner as PVC CMP in an experimental animal study was found after only 8 weeks of induced frequent PVCs [15]. Lack of palpitations, an epicardial origin of PVCs [16] and a longer PVC QRS duration [14] have been found to be predictors for the presence of PVC CMP. The PVC burden required to develop PVC CMP has been found to be lower in patients with QRS duration ≥ 150 ms [17].
Pathophysiology of PVC CMP is not fully described. PVC may be associated with increased wall stress, which may lead to LV remodeling and dysfunction. The increased wall stress may come from two different features associated with PVCs. Firstly, the propagation of the depolarization wave occurs independently of the conduction system and leads to a slower and dyssynchronous activation of the individual wall segments. Secondly, the abnormal coupling interval of PVCs may be followed by a compensatory pause, which leads to increased filling pressure from the long diastolic period. At the cellular level, in a canine model of PVC CMP, the calcium current, ICaL, the rapid component of delayed rectifier potassium current, IKr, and the inward rectifier potassium current, IK1, have been found to be decreased [18],[19]. Histological studies in PVC CMP model of canine myocardium showed no histopathologic abnormalities such as inflammation, fibrosis, increased apoptosis, or abnormal mitochondrial oxidative phosphorylation [20]. Fibrosis and scar deposition represents irreversible myocardial damage and generally consistent with underlying cardiomyopathic process. Imaging of fibrotic tissue may be key diagnostic modality to identify reversibility of systolic dysfunction after suppression of PVCs.
CMR imaging is able to visualize myocardial pathologies. LGE-MRI can identify scar in the LV. In this technique the signal of normal tissue is nulled and represented with black color. Due to delayed contrast enhancement scar tissue becomes bright. Scar size measurements typically based on signal intensity distribution, and the ratio of white to black regions. Therefore, delayed enhancement is only able to visualize localized scar not diffuse fibrosis.
Myocardial scar identified by CMR is associated with irreversible injury. Hasdemir et al [8]. investigated the prevalence of scar in patients with PVC CMP. They studied 298 consecutive patients with frequent PVCs and VT. Twenty-seven (9.1%) patients found to have LVEF ≤50% and diagnosed as presumptive PVC CMP. Improvement in LVEF after effective treatment of index ventricular arrhythmia was observed in 22 of 27 patients (PVC CMP group; mean PVC burden of 30.8 ± 9.9%). LVEF did not improve in five of 27 patients (primary cardiomyopathy group; mean PVC burden of 28.8 ± 10.1%). LGE-cardiac magnetic resonance (CMR) imaging was performed in 19 of 22 patients with PVC CMP and one patient (5%) had LGE. All five patients with primary cardiomyopathy underwent LGE-CMR imaging and four patients (80%) had LGE. Different studies have demonstrated that the prevalence of LGE range from 30% to 70%, in patients with different types of cardiomyopathy. For nonischemic cardiomyopathies, the type of LGE patterns are varied, including midwall patchy hyperenhancement, epicardial involvement, or global subendocardial enhancement aids in the diagnosis. They concluded that the presence of localized scar in those patients affects prognosis and can be used as a predictor of increased cardiac events. In patients with presumptive PVC CMP absence of LGE is a rule, and LGE CMR may have a role in the differentiation of PVC CMP from primary cardiomyopathies and in predicting the improvement of left ventricular function. In our case presence of basal septal located scar was considered as arrhythmic substrate of primary nonischemic cardiomyopathy.
Main goal of the treatment of PVC CMP is of suppression of PVCs pharmacologically or by catheter ablation. The initial treatment of frequent PVC, particularly with concurrent cardiomyopathy, is frequently beta-blockers, which is also part of guideline therapy for cardiomyopathies [21]. In patients with no more than mild LV dysfunction and absence of clinical heart failure, a non-dihydropyridine calcium channel blocker is a reasonable alternative that traditionally has been associated with good response to arrhythmias of outflow tract arrhythmia. Efficacy of beta-blockers and calcium channel blockers in suppression of PVCs are 10% and 15% respectively [22]. Class I and III antiarrhythmic drugs are more effective than beta-blockers and calcium-channel blockers. Among class I antiarrhythmics, flecainide and propafenone reduced PVC counts by 83% and 73%, respectively. The class III antiarrhythmic sotalol reduced PVC count by 70%. Highest efficacy was found for amiodarone with a reduction of PVC count by 84%. Despite the higher efficacy, use of antiarrhythmics are restricted due to potential side effects. Catheter ablation has emerged as an efficacious treatment option in the elimination of frequent PVCs. In a number of case series, acute success rate has been greater than 80-90% and long-term success has been greater than 80%. Reversal of LV dysfunction has been reported in more than 70% of patients in selected patient populations [18].
Although studies with catheter ablation have shown higher proportion of patients with PVC suppression and improvement of LV systolic function compared to studies with antiarrhythmic therapy, there are only very limited number of studies comparing the two treatment modalities directly. Two large studies have compared catheter ablation with antiarrhythmic drug therapy directly. In a randomized study, catheter ablation was associated with a significantly lower rate of PVC recurrence compared to antiarrhythmic therapy with either propafenone or metoprolol (19.4% vs. 88.6%) in patients with frequent PVCs from the RVOT [23]. The study was not designed to conclude whether there was a difference in the effect on patients with PVC CMP. The other study was non-randomized and catheter ablation was associated with a two-fold higher proportion of patients achieving normalization of LV systolic function compared with antiarrhythmic therapy only (47% vs. 21%) in patients with frequent PVCs [24]. After catheter ablation, LVEF improved by 13% in patients who achieved normalization of LV systolic function. Also, catheter ablation was associated with greater reduction in PVC frequency than antiarrhythmic therapy (94% vs. 49%). However, the patients in the antiarrhythmic group received a wide-variety of antiarrhythmic drugs. Also, relatively more patients with outflow tract PVCs were treated with ablation, confounding the results. The effect of the best available antiarrhythmic therapy may have been underestimated.
Although underlying pathology of PVC CMP is unclear, short-term animal experiments do not support a role for fibrosis [20], the study on PVC CMP by using electroanatomical mapping system showed a leftward shift in the unipolar voltage, but not in bipolar voltage distribution [25]. Bipolar voltage shows local events, where as unipolar mapping has wider field of view [26]. In the study of Tanaka and co-workers associated leftward shift in unipolar voltage distribution with diffuse interstitial fibrosis. CMR quantitation of global and regional interstitial fibrosis has recently become possible with the development of novel contrast-enhanced T1 mapping techniques. In this study, CMR data with T1 mapping was not available. Identification of diffuse fibrosis by T1 mapping in patients with PVC CMP has not been investigated yet. Diffuse fibrosis may be a reason for inadequate reversal of LV function after VPC ablation in some patients. Prevalence of diffuse fibrosis identified by T1 mapping and its relationship between reversibility after ablation should be an interest of future studies.
Although improvement in LVEF after PVC ablation was initially described in patients with suspected PVC-induced cardiomyopathy, a recent study showed a comparable benefit in patients with previously diagnosed cardiomyopathy and therefore considered to have a “PVC-worsened” cardiomyopathy [27]. El Kadiri M et al. investigated the impact of frequent VPCs on nonischemic cardiomyopathy. In this study all patients who underwent CMR imaging were found to have LGE suggesting a disease process other than PVC CMP. Successful ablation was found to improve LV function, but did not always normalize the EF [28].
Penela D, et al. assessed whether ablation might remove primary prevention implantable cardioverter-defibrillator (PP-ICD) indication in patients with frequent PVC. Sixty-six consecutive patients with PP-ICD indication and frequent PVC (17% ischemic heart disease) underwent PVC ablation. ICD was withheld and indication was re-evaluated at 6 and 12 months. LVEF progressively improved from 28±4% at baseline to 42±12% at 12 months (p<0.001). NYHA class improved from 2 (3%) patients with NYHA-1 at baseline to 35 (53%) at 12 months (p<0.001). The PP-ICD indication was removed from 42 (64%) patients during follow-up, 38 (92%) of them at 6 months, showing an independent association with baseline PVC burden and successful sustained ablation (SSA). In patients with SSA, a cut-off value of 13% PVC burden had 100% sensitivity and 93% specificity (AUC 99%) for removing ICD indication post-ablation. No sudden cardiac deaths or malignant ventricular arrhythmias were observed in this study. They concluded that in patients with frequent PVC and PP-ICD indication, ablation improves LVEF and in most cases allows removal of the indication [29]. CMR data of these patients is not available, CMR would be beneficial in selecting candidates in whom ICD indication would be removed. In addition to this, removal of ICD indication in two thirds of the patients seems to be high. Removal of the indication of ICD decision should be evaluated carefully, it should be kept in mind that late sudden cardiac death has been reported after apparent regression of tachycardia induced cardiomyopathy [30]. Larger randomized studies with CMR imaging are needed to identify patients whose ICD indication could be removed.
In our case, presence of localized basal septal scar and detection of earliest activation during VT on concomitant low voltage area supported the diagnosis of substrate related VT and primary LV cardiomyopathy. Despite ICD implantation for primary prevention in patients is seriously questioned in NICM after DANISH trial [31], we considered our patient as a high risk individual with more than 5% scar in LV myocardium [32]. Present case highlights the importance of scar imaging by CMR, a potentially useful technique for discrimination of these two entities.
Primary LV cardiomyopathy in which LV scar is generally present, is associated with increased risk of sudden death and an ICD should be inserted » this assumtion is no more valid since primary prevention ICD implantation is seriously questioned in non ischemic CM (see DANISH trial) and is only debatable in case of EF < 35%.
Conclusions
VPCs are commonly seen in general population. Identification of individuals who are at high risk of cardiomyopathy development is important. Furthermore, suppression of VPCs can improve LV ejection fraction in patients with depressed systolic function after catheter ablation. Presence of myocardial scar seen by CMR may identify patients who have irreversible primary cardiomyopathy and may not benefit from ablation therapy.
Conflict Of Interests
None.
Disclosures
None.
References
- 1.Lee Victor, Hemingway Harry, Harb Rami, Crake Tom, Lambiase Pier. The prognostic significance of premature ventricular complexes in adults without clinically apparent heart disease: a meta-analysis and systematic review. Heart. 2012 Sep;98 (17):1290–8. doi: 10.1136/heartjnl-2012-302005. [DOI] [PubMed] [Google Scholar]
- 2.Niwano S, Wakisaka Y, Niwano H, Fukaya H, Kurokawa S, Kiryu M, Hatakeyama Y, Izumi T. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart. 2009 Aug;95 (15):1230–7. doi: 10.1136/hrt.2008.159558. [DOI] [PubMed] [Google Scholar]
- 3.Hasdemir Can, Ulucan Cem, Yavuzgil Oguz, Yuksel Alper, Kartal Yildirim, Simsek Evrim, Musayev Oktay, Kayikcioglu Meral, Payzin Serdar, Kultursay Hakan, Aydin Mehmet, Can Levent H. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: the incidence, clinical and electrophysiologic characteristics, and the predictors. J. Cardiovasc. Electrophysiol. 2011 Jun;22 (6):663–8. doi: 10.1111/j.1540-8167.2010.01986.x. [DOI] [PubMed] [Google Scholar]
- 4.Sekiguchi Yukio, Aonuma Kazutaka, Yamauchi Yasuteru, Obayashi Tohru, Niwa Akihiro, Hachiya Hitoshi, Takahashi Atsushi, Nitta Junichi, Iesaka Yoshito, Isobe Mitsuaki. Chronic hemodynamic effects after radiofrequency catheter ablation of frequent monomorphic ventricular premature beats. J. Cardiovasc. Electrophysiol. 2005 Oct;16 (10):1057–63. doi: 10.1111/j.1540-8167.2005.40786.x. [DOI] [PubMed] [Google Scholar]
- 5.Yarlagadda Ravi K, Iwai Sei, Stein Kenneth M, Markowitz Steven M, Shah Bindi K, Cheung Jim W, Tan Vivian, Lerman Bruce B, Mittal Suneet. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005 Aug 23;112 (8):1092–7. doi: 10.1161/CIRCULATIONAHA.105.546432. [DOI] [PubMed] [Google Scholar]
- 6.Takemoto Masao, Yoshimura Hitoshi, Ohba Yurika, Matsumoto Yasuharu, Yamamoto Umpei, Mohri Masahiro, Yamamoto Hideo, Origuchi Hideki. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J. Am. Coll. Cardiol. 2005 Apr 19;45 (8):1259–65. doi: 10.1016/j.jacc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 7.Yalin Kivanc, Golcuk Ebru, Buyukbayrak Hakan, Yilmaz Ravza, Arslan Muhammet, Dursun Memduh, Bilge Ahmet Kaya, Adalet Kamil. Infarct characteristics by CMR identifies substrate for monomorphic VT in post-MI patients with relatively preserved systolic function and ns-VT. Pacing Clin Electrophysiol. 2014 Apr;37 (4):447–53. doi: 10.1111/pace.12289. [DOI] [PubMed] [Google Scholar]
- 8.Hasdemir Can, Yuksel Alper, Camli Dilsat, Kartal Yildirim, Simsek Evrim, Musayev Oktay, Isayev Elnur, Aydin Mehmet, Can Levent H. Late gadolinium enhancement CMR in patients with tachycardia-induced cardiomyopathy caused by idiopathic ventricular arrhythmias. Pacing Clin Electrophysiol. 2012 Apr;35 (4):465–70. doi: 10.1111/j.1540-8159.2011.03324.x. [DOI] [PubMed] [Google Scholar]
- 9.Bikkina M, Larson M G, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann. Intern. Med. 1992 Dec 15;117 (12):990–6. doi: 10.7326/0003-4819-117-12-990. [DOI] [PubMed] [Google Scholar]
- 10.Dukes Jonathan W, Dewland Thomas A, Vittinghoff Eric, Mandyam Mala C, Heckbert Susan R, Siscovick David S, Stein Phyllis K, Psaty Bruce M, Sotoodehnia Nona, Gottdiener John S, Marcus Gregory M. Ventricular Ectopy as a Predictor of Heart Failure and Death. J. Am. Coll. Cardiol. 2015 Jul 14;66 (2):101–9. doi: 10.1016/j.jacc.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baman Timir S, Lange Dave C, Ilg Karl J, Gupta Sanjaya K, Liu Tzu-Yu, Alguire Craig, Armstrong William, Good Eric, Chugh Aman, Jongnarangsin Krit, Pelosi Frank, Crawford Thomas, Ebinger Matthew, Oral Hakan, Morady Fred, Bogun Frank. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010 Jul;7 (7):865–9. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Ban Ji-Eun, Park Hwan-Cheol, Park Jae-Seok, Nagamoto Yasutsugu, Choi Jong-Il, Lim Hong-Euy, Park Sang-Weon, Kim Young-Hoon. Electrocardiographic and electrophysiological characteristics of premature ventricular complexes associated with left ventricular dysfunction in patients without structural heart disease. Europace. 2013 May;15 (5):735–41. doi: 10.1093/europace/eus371. [DOI] [PubMed] [Google Scholar]
- 13.Carballeira Pol Lidia, Deyell Marc W, Frankel David S, Benhayon Daniel, Squara Fabien, Chik William, Kohari Maria, Deo Rajat, Marchlinski Francis E. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014 Feb;11 (2):299–306. doi: 10.1016/j.hrthm.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 14.Deyell Marc W, Park Kyoung-Min, Han Yuchi, Frankel David S, Dixit Sanjay, Cooper Joshua M, Hutchinson Mathew D, Lin David, Garcia Fermin, Bala Rupa, Riley Michael P, Gerstenfeld Edward, Callans David J, Marchlinski Francis E. Predictors of recovery of left ventricular dysfunction after ablation of frequent ventricular premature depolarizations. Heart Rhythm. 2012 Sep;9 (9):1465–72. doi: 10.1016/j.hrthm.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Tan Alex Y, Hu Yuhning L, Potfay Jonathan, Kaszala Karoly, Howren Maureen, Sima Adam P, Shultz Michael, Koneru Jayanthi N, Ellenbogen Kenneth A, Huizar Jose F. Impact of ventricular ectopic burden in a premature ventricular contraction-induced cardiomyopathy animal model. Heart Rhythm. 2016 Mar;13 (3):755–61. doi: 10.1016/j.hrthm.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadron Blaye-Felice Marie, Hamon David, Sacher Frédéric, Pascale Patrizio, Rollin Anne, Duparc Alexandre, Mondoly Pierre, Derval Nicolas, Denis Arnaud, Cardin Christelle, Hocini Mélèze, Jaïs Pierre, Schlaepfer Jürg, Bongard Vanina, Carrié Didier, Galinier Michel, Pruvot Etienne, Lellouche Nicolas, Haïssaguerre Michel, Maury Philippe. Premature ventricular contraction-induced cardiomyopathy: Related clinical and electrophysiologic parameters. Heart Rhythm. 2016 Jan;13 (1):103–10. doi: 10.1016/j.hrthm.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Yokokawa Miki, Kim Hyungjin Myra, Good Eric, Crawford Thomas, Chugh Aman, Pelosi Frank, Jongnarangsin Krit, Latchamsetty Rakesh, Armstrong William, Alguire Craig, Oral Hakan, Morady Fred, Bogun Frank. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012 Sep;9 (9):1460–4. doi: 10.1016/j.hrthm.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Tran Cao Thach, Calkins Hugh. Premature ventricular contraction-induced cardiomyopathy: an emerging entity. Expert Rev Cardiovasc Ther. 2016 Nov;14 (11):1227–1234. doi: 10.1080/14779072.2016.1222901. [DOI] [PubMed] [Google Scholar]
- 19.Wang Yuhong, Eltit Jose M, Kaszala Karoly, Tan Alex, Jiang Min, Zhang Mei, Tseng Gea-Ny, Huizar Jose F. Cellular mechanism of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2014 Nov;11 (11):2064–72. doi: 10.1016/j.hrthm.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huizar Jose F, Kaszala Karoly, Potfay Jonathan, Minisi Anthony J, Lesnefsky Edward J, Abbate Antonio, Mezzaroma Eleonora, Chen Qun, Kukreja Rakesh C, Hoke Nicholas N, Thacker Leroy R, Ellenbogen Kenneth A, Wood Mark A. Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythm Electrophysiol. 2011 Aug;4 (4):543–9. doi: 10.1161/CIRCEP.111.962381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priori Silvia G, Blomström-Lundqvist Carina, Mazzanti Andrea, Blom Nico, Borggrefe Martin, Camm John, Elliott Perry Mark, Fitzsimons Donna, Hatala Robert, Hindricks Gerhard, Kirchhof Paulus, Kjeldsen Keld, Kuck Karl-Heinz, Hernandez-Madrid Antonio, Nikolaou Nikolaos, Norekvål Tone M, Spaulding Christian, Van Veldhuisen Dirk J. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015 Nov 01;36 (41):2793–867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 22.Stec Sebastian, Sikorska Agnieszka, Zaborska Beata, Kryński Tomasz, Szymot Joanna, Kułakowski Piotr. Benign symptomatic premature ventricular complexes: short- and long-term efficacy of antiarrhythmic drugs and radiofrequency ablation. Kardiol Pol. 2012;70 (4):351–8. [PubMed] [Google Scholar]
- 23.Ling Zhiyu, Liu Zengzhang, Su Li, Zipunnikov Vadim, Wu Jinjin, Du Huaan, Woo Kamsang, Chen Shaojie, Zhong Bin, Lan Xianbin, Fan Jinqi, Xu Yanping, Chen Weijie, Yin Yuehui, Nazarian Saman, Zrenner Bernhard. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: prospective randomized study. Circ Arrhythm Electrophysiol. 2014 Apr;7 (2):237–43. doi: 10.1161/CIRCEP.113.000805. [DOI] [PubMed] [Google Scholar]
- 24.Zhong Li, Lee Ying-Hsiang, Huang Xin-Miao, Asirvatham Samuel J, Shen Win-Kuang, Friedman Paul A, Hodge David O, Slusser Joshua P, Song Zhi-Yuan, Packer Douglas L, Cha Yong-Mei. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm. 2014 Feb;11 (2):187–93. doi: 10.1016/j.hrthm.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Yasuaki, Rahmutula Dolkun, Duggirala Srikant, Nazer Babak, Fang Qizhi, Olgin Jeffrey, Sievers Richard, Gerstenfeld Edward P. Diffuse fibrosis leads to a decrease in unipolar voltage: Validation in a swine model of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2016 Feb;13 (2):547–54. doi: 10.1016/j.hrthm.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Yalin Kivanc, Golcuk Ebru, Bilge Ahmet Kaya, Aksu Tolga, Buyukbayrak Hakan, Tiryakioglu Selma Kenar, Emet Samim, Adalet Kamil. Combined analysis of unipolar and bipolar voltage mapping identifies recurrences after unmappable scar-related ventricular tachycardia ablation. Europace. 2015 Oct;17 (10):1580–6. doi: 10.1093/europace/euv013. [DOI] [PubMed] [Google Scholar]
- 27.Penela Diego, Van Huls Van Taxis Carine, Van Huls Vans Taxis Carine, Aguinaga Luis, Fernández-Armenta Juan, Mont Lluis, Castel Maria Angels, Heras Magda, Tolosana Jose María, Sitges Marta, Ordóñez Augusto, Brugada Josep, Zeppenfeld Katja, Berruezo Antonio. Neurohormonal, structural, and functional recovery pattern after premature ventricular complex ablation is independent of structural heart disease status in patients with depressed left ventricular ejection fraction: a prospective multicenter study. J. Am. Coll. Cardiol. 2013 Sep 24;62 (13):1195–202. doi: 10.1016/j.jacc.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 28.El Kadri Moutaz, Yokokawa Miki, Labounty Troy, Mueller Gisela, Crawford Thomas, Good Eric, Jongnarangsin Krit, Chugh Aman, Ghanbari Hamid, Latchamsetty Rakesh, Oral Hakan, Pelosi Frank, Morady Fred, Bogun Frank. Effect of ablation of frequent premature ventricular complexes on left ventricular function in patients with nonischemic cardiomyopathy. Heart Rhythm. 2015 Apr;12 (4):706–13. doi: 10.1016/j.hrthm.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Penela Diego, Acosta Juan, Aguinaga Luis, Tercedor Luis, Ordoñez Augusto, Fernández-Armenta Juan, Andreu David, Sánchez-Millán Pablo J, Sánchez Pablo, Cabanelas Nuno, Tolosana Jose Maria, Vassanelli Francesca, Cabrera Mario, Korshunov Viatcheslav, Sitges Marta, Brugada Josep, Mont Lluis, Berruezo Antonio. Ablation of frequent PVC in patients meeting criteria for primary prevention ICD implant: Safety of withholding the implant. Heart Rhythm. 2015 Dec;12 (12):2434–42. doi: 10.1016/j.hrthm.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Nerheim Pamela, Birger-Botkin Sally, Piracha Lubna, Olshansky Brian. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004 Jul 20;110 (3):247–52. doi: 10.1161/01.CIR.0000135472.28234.CC. [DOI] [PubMed] [Google Scholar]
- 31.Køber Lars, Thune Jens J, Nielsen Jens C, Haarbo Jens, Videbæk Lars, Korup Eva, Jensen Gunnar, Hildebrandt Per, Steffensen Flemming H, Bruun Niels E, Eiskjær Hans, Brandes Axel, Thøgersen Anna M, Gustafsson Finn, Egstrup Kenneth, Videbæk Regitze, Hassager Christian, Svendsen Jesper H, Høfsten Dan E, Torp-Pedersen Christian, Pehrson Steen. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016 Sep 29;375 (13):1221–30. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 32.Klem Igor, Weinsaft Jonathan W, Bahnson Tristram D, Hegland Don, Kim Han W, Hayes Brenda, Parker Michele A, Judd Robert M, Kim Raymond J. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J. Am. Coll. Cardiol. 2012 Jul 31;60 (5):408–20. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]


