Abstract
Background:
Baroreflex activation therapy (BAT) exerts in severe heart failure sympathoinhibitory effects, improving clinical variables and reducing hospitalization rate. The current follow-up study was aimed at determining the long-term effects of BAT, assessing whether BAT in heart failure allows to restore physiological levels of sympathetic function.
Methods:
Seven patients out of the 11 heart failure patients aged 66.5 ± 3 years (mean ± SEM) in New York Heart Association Class III with left ventricular ejection fraction 40% or less and impaired functional capacity recruited in the study survived at the final follow-up (43.5 ± 2.1 months). Measurements included muscle sympathetic nerve activity (MSNA, microneurography) and spontaneous baroreflex-MSNA sensitivity together with hospitalization rate, echocardiography, Minnesota score, New York Heart Association class and standard clinical data. Measurements were collected before and at 6, 21 and 43 months following BAT. Data were compared with those collected in 17 age-matched healthy controls. All assessments were made with the heart failure patient on optimal active therapy.
Results:
In the seven patients, BAT maintained its beneficial effects over 43.5 ± 2.1 months of follow-up. MSNA values underwent a progressive significant reduction from baseline to 21 and 43 months follow-up following BAT (from 46.2 ± 2.4 to 31.3 ± 3.0 e 26.6 ± 2.0 bursts/min, P < 0.05 at least), becoming almost superimposable to the ones seen in healthy controls (25.5 ± 0.8 bursts/min). Baroreflex-MSNA sensitivity improved, without achieving, however, a full normalization. Blood pressure and heart rate did not change. Left ventricular ejection fraction improved significantly from 32.3 ± 2 to 36.7 ± 3% (P < 0.05). Hospitalization rate decreased substantially when measured as days/year/patients it decreased from 10.3 ± 2.5 preimplant to 1.01 ± 1.4 at the 43.5th month follow-up (P < 0.02). No side effects were reported in the long-term period.
Conclusion:
The current study provides evidence that BAT in heart failure with reduced ejection fraction allows not only to improve hemodynamic and clinical profile but also to exert profound sympathoinhibitory effects, allowing an almost complete restoration of physiological levels of the sympathetic neural function.
Keywords: baroreceptor activation therapy, baroreflex, congestive heart failure, sympathetic activity
INTRODUCTION
We have previously shown that in severe heart failure patients, baroreflex activation therapy (BAT) exerts sympathomodulatory effects, the decrease in muscle sympathetic nerve activity (MSNA) directly measured in the peroneal nerve via the microneurographic technique was associated with a reduction in the hospitalization rate as well as an improvement in the clinical status [1]. We have also shown, however, that the degree of the sympathetic deactivation obtained via the approach following a follow-up time of 21 months amounted to about 30%, leaving MSNA values well above the values historically reported in healthy patients [2]. The lack of normalization of sympathetic function, which is shared by the results of the studies aimed at determining the effects of pharmacological or nonpharmacological interventions commonly used in the treatment of heart failure on sympathetic function [3–12], allows investigators to advance the intriguing hypothesis that the therapeutic approaches employed in heart failure might be unable to restore to physiological levels the sympathetic neural function. The question is of clinical relevance given the prognostic importance of the sympathetic activation in heart failure. In the current study, we tested the above-mentioned hypothesis by extending the observation of the patients on BAT and examining whether a prolonged treatment (on average 43 months) would eventually allow to restore the physiological levels of sympathetic nerve traffic. Evaluation also included baroreflex modulation of sympathetic neural function, as baroreflex impairment is inversely related to survival in heart failure patients [13,14].
METHODS
Therapeutic intervention
Patients received chronic BAT administered by the Barostim neo System (CVRx, Inc., Minneapolis, Minnesota, USA). The system and implant procedure have been previously described [14]. Briefly, the device comprises a pulse generator similar to a conventional pacemaker implanted in a pectoral pocket. A unipolar electrode is affixed to the carotid sinus and the lead body is tunneled ipsilaterally to connect to the pulse generator. The system is preferentially implanted in the right side subclavicular pocket to avoid conflict with any preexisting or future cardiac rhythm management devices. Intensity of BAT is progressively up-titrated over the first 3 months of therapy, primarily by increasing electrical pulse amplitude. The pulse amplitude was set in the first month postimplantation and was currently checked approximately every 6 months. In the patients we followed in the present report period, it remained unchanged during the long-term.
Patients population
Patients’ population has been described already [1,2]. Patients needed to be in stable optimal medical therapy at full and stable daily dosage, which included diuretics, beta-blockers, Ace-inhibitors or angiotensin II receptor blockers, unless contraindicated or not tolerated. New York Heart Association (NYHA) Class III heart failure, left ventricular (LV) ejection fraction less than 40%, limited functional capacity (6-min walk distance of 150–450 m), heart rate (HR) comprised within 60–100 bpm, chronic kidney disease stage 3 or better (estimated glomerular filtration rate ≥30%) and freedom from dialysis with the expectation of remaining so for at least 1 year. Patients also needed to be appropriate surgical BAT implant candidates. This included carotid bifurcations below the level of the mandible and freedom from ulcerative carotid arterial plaques or atherosclerosis producing a reduction in diameter of the internal or distal common carotid artery of at least 50%.
Exclusion criteria included life expectancy less than 1 year, heart failure secondary to reversible cause or right ventricular failure, active resynchronization therapy, a heart failure state in NYHA Class IV within 30 days, episodes of angina, myocardial infarction, sudden cardiac arrest, appropriate defibrillator therapy, syncope or cerebrovascular accident within 3 months, implant of pacemaker or implantable cardioverter device within 3 months, baroreflex failure, autonomic neuropathy, severe chronic obstructive pulmonary disease, prior solid organ or hematologic transplant, prior surgery in the carotid sinus region limiting placement of system components, noncardiovascular conditions limiting assessment of functional capacity with the 6-min walk test and conditions which would adversely affect participation in the investigation. The control group consisted of 17 healthy patients matched for age and sex.
Study design
The investigation was a single-center, open-label evaluation of BAT in patients with congestive heart failure and reduced ejection fraction. The prospectively defined study duration was 6 months. Data presented here were collected during the long-term clinical follow-up as part of the IRCCS MultiMedica standard protocol of the heart failure clinic.
Measurements and endpoints
Study visits during which main results were previously collected occurred at 6 and 21 months following therapy activation. The 43rd month follow-up visit was added to the data collection schedule following completion of the prospective data acquisition. The primary study endpoint measure, MSNA, was collected via peroneal microneurography [1–3,6,9–12]. At the time of the microneurographic investigation, several other measurements were performed. These included an assessment of LV function via echocardiography, NYHA functional class, overall clinical status, occurrence of heart failure-related hospitalizations and ongoing therapy and any change of it from the previous follow-up visits and spontaneous MSNA baroreflex sensitivity, by using a variation of the method employed by Kienbaum and Peters [15,16]. The methods allow relating each spontaneous sympathetic burst to the DBP and the cardiac interval during which the burst was generated. All assessments were made with the patient on active therapy.
Data analysis
Effects of BAT on MSNA, spontaneous baroreflex sensitivity and echocardiographic parameters were evaluated by repeated measures analysis of variance comparing data from the previous assessments and the novel ones in the seven patients who survived over the years of the follow-up. Comparisons with respect the control group were made by using the unpaired t test. The Pearson correlation coefficient was used to determine the relationships between the changes in MSNA and the concomitant changes in MSNA baroreflex sensitivity or echocardiographic parameters. Statistical significance was identified by P values less than 0.05. Absolute measures are reported as means ± standard error, except for NYHA class belonging and number of drugs used by the patients, expressed as median values and range.
RESULTS
Four patients died during long-term follow-up. Three of the four deaths were of cardiovascular origin. The seven surviving patients (age: 66.5 ± 3.0 years, six men) suffered from a congestive heart failure state with reduced ejection fraction of ischemic origin in three of seven of the cases. Their clinical characteristics are shown in Table 1. Compared with the group described in the first report previously published [1], the seven patients had similar baseline values, the only exception being represented by a greater estimated glomerular filtration rate value due to the fact that the patients who died at the longer follow-up showed a reduced renal function at the study entry. Table 1 and Fig. 1 also show the effects of the procedure on the different clinical, hemodynamic, echocardiographic and neural variables measured during the prolonged follow-up. As overall result, BAT maintained its beneficial effects over a long-term follow-up of 43.5 ± 2.1 months (range 37–48 months). More specifically, the primary endpoint of the study, that is MSNA values, showed a significant reduction during the follow-up, although with a consistent interindividual variability in the responses, reaching at the latest evaluation a value almost superimposable to the one seen in a control group of 17 age-matched healthy individuals (age: 68.4 ± 2.9 years,13 men) (Fig. 1a). Concomitantly, baroreflex sensitivity showed a progressive improvement from the pre-BAT implantation to the 43 months follow-up, its average value observed in the seven patients at this time remaining, however, significantly lower than the one detected in the control group of age-matched healthy patients (Fig. 1b). The MSNA reduction observed during the follow-up after the procedure was not significantly related to the improvement in baroreflex MSNA sensitivity, although the correlation coefficient (r) and related P values were close to the minimal levels of statistical significance (r = −0.66, P = 0.07). It is worthy to note that LV ejection fraction trend toward an amelioration observed at the 6th and 21st month follow-up time achieved statistical significance at the latest follow-up (Table 1). Also, in the same seven patients, the hospitalization rate decreased substantially within 6 months of BAT. When expressed as days/year/patients, this variable significantly decreased from 10.3 ± 2.5 preimplant to 1.01 ± 1.4 over the whole 43 months follow-up (P < 0.02). Blood pressure (BP) and HR did not significantly change over the time of the follow-up.
TABLE 1.
Clinical data and muscle sympathetic nerve activity values of heart failure patients (CHF) underwent baroreflex activation therapy procedure and of healthy controls
| BAT–CHF patients, n = 7 | Healthy controls, n = 17 | ||||
| Variable | Baseline | 6th month | 21st month | 43rd month | Baseline |
| BMI (kg/m2) | 27.5 ± 2.1 | 27.2 ± 2.0 | 27.4 ± 2.1 | 27.6 ± 2.2 | 27.0 ± 2.5 |
| Clinic SBP (mmHg) | 115.0 ± 5.5 | 116.4 ± 4.7 | 110.2 ± 2.9 | 113.3 ± 2.0 | 119.4 ± 1.7 |
| Clinic DBP (mmHg) | 67.8 ± 2.4 | 64.3 ± 2.0 | 65.7 ± 2.0 | 64.1 ± 2.2 | 72.5 ± 1.5 |
| Heart rate (bpm) | 71.4 ± 2.0 | 72.0 ± 5.4 | 68.8 ± 3.9 | 68.7 ± 4.3 | 70.6 ± 2.8 |
| 6-min walking test (m) | 319 ± 19 | 400.0 ± 37 | 370.1 ± 48 | 425.8 ± 39* | n.a. |
| NYHA class (a.u.) | 3 (3–4) | 3 (3–4) | 2 (2–3)* | 2 (2–3)* | 0 |
| Minesota score (a.u.) | 24.6 ± 5 | 14.1 ± 3 | 8.3 ± 2 | 7.7 ± 2 | n.a. |
| 3D LVEF (%) | 32.3 ± 2 | 35.7 ± 3 | 33.2 ± 3 | 36.7 ± 3** | 56.3 ± 4 |
| 3D LVEDV (ml) | 159.6 ± 19 | 142.1 ± 17 | 134.3 ± 16 | 137.6 ± 18 | 109.4 ± 11 |
| EGFR (ml/min/1.73 m2) | 79.1 ± 9 | 86.8 ± 13 | 81.6 ± 15 | 88.7 ± 14 | 84.4 ± 10 |
| No. HF drugs (drugs/day) | 4 (3–5) | 4 (2–5) | 4 (2–5) | 4 (2–5) | 0 |
| MSNA (bursts/min) | 46.2 ± 2.4 | 30.6 ± 3.2** | 31.3 ± 3.0** | 26.6 ± 2.0* | 24.5 ± 0.8 |
| MSNA (bursts/100 hb) | 70.0 ± 4.3 | 44.1 ± 3.9** | 46.0 ± 3.6** | 36.9 ± 3.1* | 34.1 ± 1.4 |
In each group, data are shown before (baseline) and at the 6th, 21st and 43rd month after BAT implantation. Data are shown as means ± SEM, except for NYHA class and no. of heart failure (HF) drugs, shown as median values and range. 3D LVEDV, three-dimensional left ventricular end-diastolic volume; a.u., arbitrary units; bpm, beats per minute; CHF, congestive heart failure; EGFR, estimated glomerular filtration rate; hb, heart beats; LVEF, left ventricular ejection fraction; m, meters, MSNA, muscle sympathetic nerve activity; n.a., not assessed; no., number; NYHA, New York Heart Association.
*P < 0.02 vs. **P < 0.05 refer to the baseline vs. statistical significance values, respectively.
FIGURE 1.
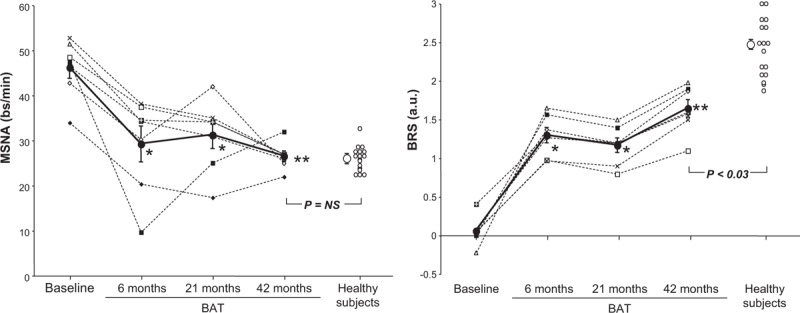
Effects of baroreflex activation therapy on muscle sympathetic nerve activity (a) and spontaneous baroreflex muscle sympathetic nerve activity sensitivity (BRS, b) in heart failure patients (closed circles). Individual and average (means ± SEM) data are shown and refer to values recorded before (baseline) and 6, 21 and 43 months following baroreflex activation therapy. In each panel, individual and average values of healthy controls are also shown (open circles). Asterisks (∗P < 0.05, ∗∗P < 0.02) refer to the statistical significance of the values recorded before and at various months after baroreflex activation therapy. Statistical significance between values recorded in heart failure patients at the 43rd month after baroreflex activation therapy and those obtained in controls are shown as P value. BRS, spontanoeus baroreflex sensitivity.
DISCUSSION
There are two major novel findings of the present investigation. First, the study results show for the first time that the sympathoinhibitory effects of BAT, as well as the concomitant improvement of the baroreflex function, do not vanish over time but appear to be well maintained at a follow-up close to 4 years of duration, that is the longest follow-up ever done with this procedure in heart failure patients. Second, the present data show, again for the first time, that the sustained sympathoinhibition detected during the follow-up and, more specifically following 43 months after BAT implantation, allows to achieve MSNA values almost superimposable to the ones found in age-matched healthy controls, although with a variability in the responses between the different patients. Given the adverse prognostic relevance the sympathetic activation has in heart failure [17–19] and the evidence that pharmacological interventions commonly used in heart failure treatment, or even cardiac resynchronization therapy, fail to achieve such a goal [3–12], our findings of a ‘normalization’ of sympathetic neural function in heart failure patients strongly support the clinical relevance of BAT in severe heart failure treatment.
Several other results of our study deserve to be discussed. In our study, we found that whereas MSNA values recorded at the 43rd month of the BAT implantation are similar to the ones detected in healthy controls, spontaneous baroreflex MSNA sensitivity, although improved after BAT, still remains below the values seen in healthy control patients. Several not mutually exclusive hypotheses can be advanced for explaining this finding. We can speculate that the sympathoinhibition observed after BAT depends not only on the baroreflex improvement and that other neural or nonneural mechanisms may participate in the phenomenon. These include, for example, an improvement in cardiopulmonary volume receptor control of MSNA, which is impaired in heart failure [20], or an inhibition of the renin–angiotensin system by BAT, with a reduction in the sympathoexcitatory effects exerted in chronic heart failure by elevated circulating angiotenin II levels at the level of the central and peripheral nervous system as well [21]. We can also speculate that at variance from carotid baroreceptor alteration (which is the target of BAT), aortic baroreceptors dysfunction, which is involved in MSNA regulation and thus in the estimation of baroreflex sensitivity [22], remains unaffected by the intervention. We can also advance the hypothesis that the time–course of the sympathetic and baroreflex responses to BAT is different from each other and that more time is needed to obtain a full restoration of the baroreflex function as compared with the one necessary to restore physiological MSNA values. Finally, we can speculate that whereas sympathetic activation can be fully reversed because of functional nature, baroreflex impairment might depend also on structural factors which are only in part reversed by BAT. This latter possibility appears to be supported by the recent finding provided by our group that BAT does not significantly improve the alterations in arterial stiffness characterizing heart failure patients [23]. As arterial stiffness is inversely related to baroreflex function in heart failure [24], the lack of favorable effects of BAT on vascular function may represent the structural factor responsible for the only partial reversibility of baroreflex dysfunction seen in heart failure patients.
A further result of our study needs to be emphasized. We found indeed that the sympathetic deactivation and the baroreflex improvement driven by BAT was accompanied during the whole follow-up period by a significant and marked decrease in the hospital readmissions, a pivotal marker of heart failure clinical status improvement. This evidence further strengthens the potential benefits of BAT once considering that the huge number of heart failure hospitalizations observed in the clinical history of these NYHA class III patients represents a powerful predictor of ominous outcome [25]. The improvement in the clinical heart failure status is also confirmed in our study by the evidence that LV ejection fraction was significantly ameliorated following 43 months of BAT, the distance during the 6-min walking test increased and a significant decrease in the Minnesota score and in the NYHA functional class belonging of our patients was also detected. The improvement in the above-mentioned clinical markers of heart failure severity should be interpreted with caution given the fact that some changes were not marked (see LV ejection fraction) or they were dependent on the investigator's judgment (NYHA class and Minnesota score). A further interesting finding of our study relates to the fact that baroreceptor stimulation elicited by BAT did not cause any tachycardia or BP reduction, documenting the favorable effect of the procedure on cardiac contractile function as opposed to any BP decrease.
Our results have some limitations but also a clinical implication. The limitations include the small study sample size, which however did not prevent us from detecting substantial improvements in a number of neural, hemodynamic and clinical variables achieving statistical significance during the prolonged follow-up of the present investigation. Another limitation is represented by fact that we investigated heart failure patients with a reduced ejection fraction. Whether and to what extent the results we found can be applied to the heart failure state with preserved ejection fraction needs to be investigated in future studies. Finally, in the current study, no data comparison with a control heart failure patient group was provided. This was because of the fact that it was impossible to keep the control heart failure patients under unmodified drug treatment for a time period lasting more than 3 years. However, in the above mentioned study aimed at investigating the effects of BAT in heart failure patients on sympathetic vascular function [23], we found that after a 3-month period of observation, no change in any measured variable was detected in a control group of heart failure patients with stable and unchanged drug treatment. The clinical implication refers to the fact that BAT is up-to-date the only available approach capable to restore physiological level of sympathetic neural function in severe congestive heart failure with favorable clinical and hemodynamic consequences. These latter have been confirmed by the results of the recently published large-scale trial on BAT in heart failure patients [26,27].
ACKNOWLEDGEMENTS
The trial reported in this article was sponsored by CVRx, Inc., Minneapolis, Minnesota, USA.
Conflicts of interest
E.G. and E.V. are paid consultants of CVRx, Inc.; G.G. and G.M. received speaking honoraria from CVRx.
Reviewers’ Summary Evaluation
Reviewer 2
The study indicates that normalization of the sympathetic drive might be a mechanism underlying previously reported beneficial effects of the long-term baroreflex activation therapy (BAT) in heart failure. The major strengths include complex assessment of sympathetic neural mechanisms and long-term follow-up. Importantly, the BAT was associated with dramatic reduction in hospitalization rate, and the method was safe as no side effects were reported. Potential limitations, acknowledged by the authors, include small number of the subjects and survival bias. Nevertheless, the cohort was unique and the study was powered enough to detect significant differences.
Footnotes
Abbreviations: BAT, baroreceptor activation therapy; HF, heart failure; MSNA, muscle sympathetic nerve activity
REFERENCES
- 1.Gronda E, Seravalle G, Brambilla G, Costantino G, Casini A, Alsheraei A, et al. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail 2014; 16:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gronda E, Seravalle G, Quarti-Trevano F, Costantino G, Casini A, Alsheraei A, et al. Long-term chronic baroreflex activation: persistent efficacy in patients with heart failure and reduced ejection fraction. J Hypertens 2015; 33:1704–1708. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson DW, Berg WJ, Sanders JS, Roach PJ, Kempf JS, Kienzle MG. Sympathoinhibitory responses to digitalis glycosides in heart failure patients. Direct evidence from sympathetic neural recordings. Circulation 1989; 80:65–77. [DOI] [PubMed] [Google Scholar]
- 4.Mark Al. Sympathetic dysregulation in heart failure: mechanisms and therapy. Clin Cardiol 1995; 18 (Suppl I):I3–I8. [DOI] [PubMed] [Google Scholar]
- 5.Newton GE, Tong JH, Schofield AM, Baines AD, Floras JS, Parker JD. Digoxin reduces cardiac sympathetic activity in severe heart failure. J Am Coll Cardiol 1996; 28:155–161. [DOI] [PubMed] [Google Scholar]
- 6.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Pozzi M, Morganti A, et al. Effects of ACE-inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation 1997; 96:1173–1179. [DOI] [PubMed] [Google Scholar]
- 7.Brunner-La Rocca HP, Vaddadi G, Esler MD. Recent insight into therapy of congestive heart failure: focus on ACE-inhibition and angiotensin II antagonism. J Am Coll Cardiol 1999; 33:1163–1173. [DOI] [PubMed] [Google Scholar]
- 8.Azevedo ER, Kubo T, Mak S, Al-Hesayen A, Schofield A, Allan R, et al. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation 2001; 104:2194–2199. [DOI] [PubMed] [Google Scholar]
- 9.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, et al. The effects of exercise training on sympathetic neural activation in advanced heart failure. J Am Coll Cardiol 2003; 42:854–860. [DOI] [PubMed] [Google Scholar]
- 10.Grassi G, Vincenti A, Brambilla R, Quarti-Trevano F, Dell’Oro R, Cirò A, et al. Sustained sympathoinhibitory effects of cardiac resynchronization therapy in severe heart failure. Hypertension 2004; 44:727–731. [DOI] [PubMed] [Google Scholar]
- 11.Usui K, Bradley TD, Spaak J, Ryan CM, Kubo T, Kaneko Y, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol 2005; 45:2008–2011. [DOI] [PubMed] [Google Scholar]
- 12.Ruzicka M, Floras JS, McReynolds AJ, Coletta E, Haddad H, Davies R, et al. Do high doses of AT(1)-receptor blockers attenuate central sympathetic outflow in chronic heart failure? Clin Sci (Lond) 2013; 124:589–595. [DOI] [PubMed] [Google Scholar]
- 13.Olivari MT, Levine TB, Cohn J. Abnormal neurohumoral responses to nitroprusside infusion in congestive heart failure. J Am Coll Cardiol 1983; 2:411–417. [DOI] [PubMed] [Google Scholar]
- 14.La Rovere MT, Pinna GD, Maestri R, Robbi E, Caporotondi A, Guazzotti G, et al. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol 2009; 53:193–199. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe UC, Brandt MC, Wachter R, Beige J, Rump LC, Kroon AA, et al. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens 2012; 6:270–276. [DOI] [PubMed] [Google Scholar]
- 16.Kienbaum P, Peters J. Muscle sympathetic baroreflex sensitivity is different at rest and during evoked hypotension. Basic Res Cardiol 2004; 99:152–158. [DOI] [PubMed] [Google Scholar]
- 17.Cohn JN, Leine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with congestive heart failure. N Engl J Med 1984; 311:819–823. [DOI] [PubMed] [Google Scholar]
- 18.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 1995; 26:1257–1263. [DOI] [PubMed] [Google Scholar]
- 19.Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 2009; 135:302–307. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson DW, Mark AL. Selective impairment of baroreflex-mediated vasoconstrictor responses in patients with ventricular dysfunction. Circulation 1984; 69:451–460. [DOI] [PubMed] [Google Scholar]
- 21.Zucher IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 2014; 126:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders JS, Mark AL, Ferguson Importance of aortic baroreflex regulation of sympathetic responses during hypotension. Evidence from direct sympathetic nerve recordings in humans. Circulation 1989; 79:83–92. [DOI] [PubMed] [Google Scholar]
- 23.Gronda E, Brambilla GM, Seravalle G, Maloberti A, Cairo M, Costantino G, et al. Effects of chronic baroreceptor activation on arterial stiffness in severe heart failure. Clin Res Cardiol 2016; 105:838–846. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Giannattasio C, Failla M, Pesenti A, Peretti G, Marinoni E, et al. Sympathetic modulation of radial artery compliance in congestive heart failure. Hypertension 1995; 26:348–354. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A, Allman RM, Fonarow GC, Love TE, Zannad F, Dell’Italia LJ, et al. Incident heart failure hospitalization and subsequent mortality in chronic heart failure: a propensity-matched study. J Card Fail 2008; 14:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zile MR, Abraham WT, Weaver FA, Butter C, Ducharme A, Halbach M, et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction: safety and efficacy in patients with and without cardiac resynchronization therapy. Eur J Heart Fail 2015; 17:1066–1074. [DOI] [PubMed] [Google Scholar]
- 27.Abraham WT, Zile MR, Weaver FA, Butter C, Ducharme A, Halbach M, et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. J Am Coll Cardiol Heart Fail 2015; 3:487–496. [DOI] [PubMed] [Google Scholar]


