Abstract
Atrial fibrillation (AF) is the most common serious heart rhythm disorder, with a lifetime incidence of 1 in 4 for patients >40 years of age[1]. AF is a major cause of death and disability, as it is associated with a 4-5 fold increase in the risk of ischemic stroke[2]. In patients with AF, oral anticoagulation (OAC) therapy can reduce the risk of stroke by about two-thirds and the risk of all-cause mortality by approximately one-quarter, but is associated with an increased risk of bleeding[3], [4]. Atrial fibrillation (AF) is the most common serious heart rhythm disorder and is associated with an increased risk of ischemic stroke. This risk can be moderated with oral anticoagulation therapy, but the decision to do so must be balanced against the risks of bleeding. Herein, we discuss three emerging areas where more high-quality evidence is required to guide risk stratification: 1) the relationships between the pattern and burden of AF and stroke 2) the risk conferred by short episodes of device-detected “sub-clinical” atrial fibrillation (SCAF) and 3) the significance of AF that occurs transiently with stress (AFOTS), as is often detected during medical illness or after surgery. Risk stratification is important to identify patients with AF who can benefit from OAC therapy. There are, however, several common clinical scenarios where guidelines do not yet provide direction for stroke prevention; or do so based on limited high-quality evidence.
Keywords: Atrial fibrillation, subclinical, stress, stroke
Introduction
Atrial fibrillation (AF) is the most common serious heart rhythm disorder, with a lifetime incidence of 1 in 4 for patients >40 years of age[1]. AF is a major cause of death and disability, as it is associated with a 4-5 fold increase in the risk of ischemic stroke[2]. In patients with AF, oral anticoagulation (OAC) therapy can reduce the risk of stroke by about two-thirds and the risk of all-cause mortality by approximately one-quarter, but is associated with an increased risk of bleeding[3], [4]. Risk stratification is important to identify patients with AF who can benefit from OAC therapy. There are, however, several common clinical scenarios where guidelines do not yet provide direction for stroke prevention; or do so based on limited high-quality evidence.
AF Burden and the Risk of Stroke
Current AF guidelines recommend risk stratification, to estimate the risks of stroke and bleeding and offering OAC to patients who have a favorable risk-benefit profile[5]-[7]. The risk of stroke and bleeding are estimated based on the patient’s age and comorbidities; typically using the CHA2DS2-VASc score for stroke[8]-[12], and the HAS-BLED score for major bleeding[13]-[15]. Current guidelines do not consider the pattern of AF (paroxysmal, persistent and permanent), nor the burden of time that a patient spends in AF when estimating the risk of stroke and whether or not to offer patients OAC [5]-[7].
Emerging evidence suggests that the pattern, frequency and duration of episodes of AF (also known as arrhythmia burden) may influence stroke risk. A large analysis of more than 6500 aspirin-treated patients from the ACTIVE-A and AVERROES trials suggested a clear gradient of increasing risk of stroke/systemic embolism (SE) from paroxysmal to persistent to permanent AF. In this study, which included rigorously adjudicated outcomes, annualized ischemic rates of stroke/SE rates were 2.1, 3.0, and 4.2% respectively, with an adjusted hazard ratio (HR) of 1.83 (95% CI 1.43-2.35; P < 0.001) for permanent vs. paroxysmal AF and 1.44 (95% CI 1.05-1.98 P = 0.02) for persistent vs. paroxysmal AF[16]. The concept of differing risk according to AF pattern is further supported by a report from the Fushimi AF registry that demonstrated that sustained (permanent or persistent) AF was independently associated with a higher incidence of stroke/SE as compared to paroxysmal AF (non-OAC users: HR 2.2, 95% CI 1.3–3.7; P<0.01 and OAC users: HR 1.7, 95% CI 1.1-2.9; P=0.03)[17]. Owing to observations of increasing risk of stroke/SE with increasing AF burden, some authors have proposed that embolic risk be estimated on the basis of refined algorithms that consider both the burden of AF and patient characteristics[18], [19]. Such schemata represent an emerging area of research, but require further prospective validation before they can be used clinically.
In the meantime, it should be stressed that the presence of paroxysmal AF does NOT obviate the need for OAC in patients with additional stroke risk factors. In the pooled ACTIVE-A and AVERROES analysis, the 5 year rate of stroke among patients with paroxysmal AF was approximately 10% - this is well above our current threshold to consider treatment with OAC[16]. Another group of patients of interest are young patients without additional stroke risk factors who have persistent or permanent AF, which may place them at increased risk of stroke and other adverse neurological outcomes[20], [21]. Approximately 10-15% of patients with AF may not have any additional stroke risk factors, but still have some risk of stroke[22]. Such patients are currently being randomized to receive aspirin or 15 mg of rivaroxaban once daily in the BRAIN-AF trial (NCT02387229) to determine if stroke (clinical and covert) as well as cognitive decline can be prevented through the use of OAC.
Subclinical Atrial Fibrillation (SCAF)
Given the current widespread use of continuous long-term cardiac monitoring, it is now recognized that many patients have evidence of short-lasting AF, without recognizable symptoms. This phenomenon has been termed subclinical atrial fibrillation (SCAF), and was first described in studies of pacemaker patients, such as ASSERT, TRENDS and MOST, where it was initially given the more descriptive term “atrial high-rate episodes (AHRE)”[23]-[25]. SCAF does not simply mean asymptomatic AF, which could encompass AF that is permanent. Arrhythmias referred to as SCAF must also be short-lasting, be detected only with long-term continuous monitoring, and not captured on routine surface ECG[23], [26]-[29]. The concept is that short-lasting AF detected after many weeks of monitoring represents a low overall burden of AF, which appears to convey an increased risk of stroke/SE, albeit lower than would be expected in otherwise similar patients with clinical AF[23], [25].
The management of patients with SCAF is much less clear. High quality evidence for treatment benefit is lacking. The Relationship Between Daily Atrial Tachyarrhythmia Burden From Implantable Device Diagnostics and Stroke Risk (TRENDS) study was among the first to suggest that SCAF was associated with an increased thromboembolic risk, although these were prospective observational study included some patients with clinical AF[24], [25]. The increased stroke risk associated with SCAF was confirmed in The Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT), which reported exclusively on patients without a known history of clinical AF[23]. In this study, SCAF was associated with an increased risk of new clinical AF (HR 5.56, 95%CI 3.78-8.17; P<0.001) and of ischemic stroke/SE (HR 2.49, 95%CI 1.28-4.85; P=0.007). However, among patients average CHADS2 score of 2, the annual risk of stroke was only 1.3%; far lower than would be expected in similar patients with clinical AF [Table 1][9].
Table 1. Comparison of annualized event rates in patients with clinical AF, according to Gage et al., and SCAF, according to Healey et al.[9], [23].
| Rate of Ischemic Stroke/ Systemic Embolism (%/year) | ||
| CHADS2 Score | Clinical AF | SCAF |
| 1 | 1.9 | 0.56 |
| 2 | 2.8 | 1.29 |
| >2 | 4.0-12.5 | 3.78 |
Where it is generally accepted that SCAF is associated with an increased risk of stroke, the decision to anti-coagulate patients with SCAF is not straightforward. Despite the assertion of some experts that patients with SCAF and additional risk factors for stroke are high risk and merit OAC, clinical practice varies[23], [30]-[36].
There are several reasons why patients with SCAF might not derive the same risk-benefit from OAC as similar patients with clinical AF. First, the reported thromboembolic rates in patients with SCAF are low compared with patients with clinical AF who have otherwise similar risk profiles (Table 1)[9], [23], [25]. Second, the Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices (IMPACT) was stopped for futility[37]. This study was designed to test the hypothesis that initiation and withdrawal of OAC guided by continuous ambulatory monitoring of the atrial electrogram would improve clinical outcomes by reducing the combined rate of stroke, systemic embolism, and major bleeding as compared with conventional clinical management[33]. Third, it is important to recognize that observational data have failed to show a temporal relationship between SCAF and stroke in the majority of patients[25], [29], [38], [39]. For example, in an analysis of the ASSERT trial, only 8% of patients had SCAF detected in the 30 days prior to their stroke/SE. Furthermore, 16% of patients with SCAF and ischemic stroke/SE did not have any SCAF detected prior to their stroke/SE[29]. Similarly, in the TRENDS study, 73% of patients who experienced a stroke/SE had zero AF burden in the 30 days prior to their event[40]. These findings, in particular, raise the possibility that stroke/SE in patients with AF may occur secondary to pathophysiologic mechanisms other than the classically hypothesized construct of a minimum of 24 to 48 hours AF leading to atrial stasis, clot formation and subsequent SE[41]. Fourth, the trials that established the benefit of OAC for stroke prevention in AF were comprised predominantly of patients with sustained AF or high burdens of paroxysmal AF [3], [4], and data suggest that patients with paroxysmal AF may be at lower (albeit still significant) risk of stroke, as discussed in the previous section. The burden of SCAF has also been identified as a possible risk factor for stroke. In the TRENDS study, as compared to patients without AF, patients with <5.5 hours of SCAF were not at increased risk of stroke/TIA/SE (HR 0.98, 95% CI 0.34-2.82; p =0.97, but there was a trend towards increased risk in patients with >5.5 hours of SCAF (HR 2.2, 95% CI 0.96-5.05; p=0.06)[25]. In an analysis of data from the ASSERT study, the risk of ischemic stroke/SE was found to be increased with episodes of SCAF as short as 6 minutes in duration (RR 1.77, 95% CI 1.01-3.10; p=0.047) and the relative risk reached as high as 4.96 (95% CI 2.39-10.3; p<0.01) with episodes >24 hours in duration[23]. It is conceivable that on a spectrum of risk, SCAF could fall lower than paroxysmal AF and below a threshold at which the benefits of stroke prevention from OAC are outweighed by the risks of bleeding. Even assuming that OAC confers the same relative risk reduction for stroke in patients with SCAF as compared to those clinical AF, the lower absolute risk of stroke may have an important impact on the risk-benefit ratio and cost-effectiveness of OAC. As a result, clinical practice remains divided regarding the treatment of SCAF. At least two randomized clinical trials are underway to address the use of OAC for patients with SCAF, including Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA, NCT01938248) and Non-vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes (NOAH, NCT02618577).
Atrial Fibrillation Occurring Transiently with Stress (AFOTS)
Uncertainly surrounds the approach to stroke prevention for patients who experience AF occurring transiently with stress (AFOTS). In patients without a history of the arrhythmia, AF is often observed for the first time in the setting of an acute stressor, such as medical illness or surgery, typically while the patient is undergoing continuous surface ECG monitoring. Physicians frequently do not prescribe OAC therapy when it is judged that AF may have occurred due to a potentially reversible precipitant[6], [42]-[45].
It is not known whether a presentation of AFOTS occurs secondary to a reversible cause and is ultimately benign, or is simply the first documentation of paroxysmal AF and is therefore associated with an increased risk of stroke[46]. Major guidelines do not currently make recommendations for OAC or for post-discharge screening for recurrent AF in patients who experience AFOTS[5]-[7]. Moreover, more recent guidelines have acknowledged that we are lacking in data to direct the long-term management of patients with AFOTS[6].
It can be conceptualized that during an episode of AFOTS, there is interplay between fixed and transient arrhythmogenic factors [Figure 1][44], [47]-[57]. Traditional risk factors for AF are present in many acutely ill patients, and may have led to the development of AF-promoting electro-anatomical alterations in atrial tissue[48], [50]. This substrate has been termed atrial myopathy[57], [58]. In the setting of acute stress, such as accompanies acute medical illness or surgery, multiple additional and potentially provoking acute factors come into play[51]-[56]. What remains unknown is to what degree an episode of AFOTS represents a predisposition for recurrence of AF (and therefore a risk of stroke, heart failure and death).
Figure 1. Pathophysiological Factors implicated in Atrial Fibrillation Occurring Transiently with Stress (AFOTS).
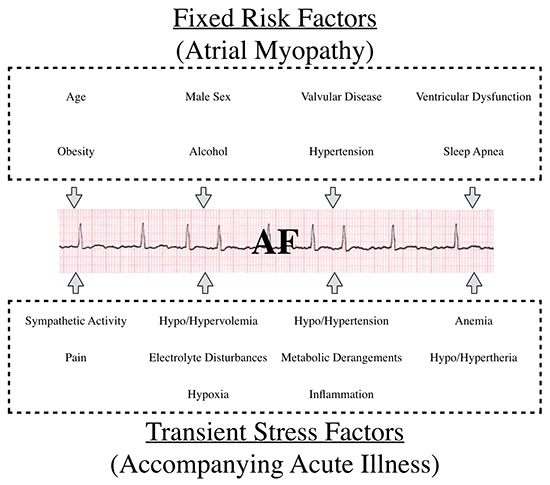
AFOTS occurs frequently (incidence 4-44%) in the setting of acute illness[56], [59]-[83]. AFOTS has been shown to occur with many medical illnesses, including local and systemic infections, myocardial infarction, hyperthyroidism, lung disease and venous thromboembolic disease. In patients admitted to general medical wards and intensive care units, AFOTS is common across a wide variety of conditions. The incidence of AFOTS has been reported to range from 5-44% in sepsis[60], [66]-[74], 4-18% in acute pulmonary syndromes (e.g. pneumonia, exacerbation of chronic obstructive pulmonary disease and pulmonary embolism)[75]-[80] and 10-25% in hyperthyroidism[81], [82]. AFOTS is also common in post-surgical patients. Here, the incidence of AFOTS is approximately 1% for all surgery, 8-10% in vascular surgery, 9-14% in colorectal surgery, 10-35% in thoracic surgery and 18-50% in cardiac surgery[84]-[96]. Across the literature, the incidence of AFOTS tends to be higher in prospective studies. This likely originates from the fact that detection of AFOTS usually requires continuous monitoring and active surveillance facilitates recognition of this intermittent arrhythmia. The incidence also tends to be higher in critically ill patients. This could be a reflection of an increased propensity for AFOTS with more severe illness or simply a reflection of more intensive rhythm monitoring.
Some data exist on the recurrence of AF following AFOTS, but these are limited to retrospective study designs and rely on opportunistic diagnosis of AF through non-systematic follow-up methods. A recent publication from the Framingham Heart Study investigated long-term AF outcomes after diagnosis during a secondary precipitant (i.e. AFOTS). AFOTS precipitants included surgery, acute myocardial infarction, acute infection, acute alcohol consumption, thyrotoxicosis, acute pericardial disease and acute pulmonary syndromes. In this study, patients with AFOTS had an AF recurrence rate of 42% at 5 years. This was similar to the 59% recurrence rate of AF for patients in the cohort whose first presentation of AF was not in the setting of AFOTS (i.e. incident paroxysmal AF). Stroke risk (HR 1.13, 95%CI; 0.82-1.57; P=0.45) and mortality (HR 1.00, 95%CI 0.87-1.1.5; P=0.95) did not differ between those with AFOTS and those with incident paroxysmal AF[97]. A retrospective study using a United States Medicare 5% sample investigated long-term outcomes following development of AFOTS during sepsis. Recurrence of AF after discharge was ascertained through health care claims using International Classification of Diseases (ICD-9) codes. In this study, incidence of AF recurrence at one-year following sepsis hospitalizations was 44% in AFOTS patients. This was significantly higher than patients who did not have AFOTS during sepsis (7.7%, p<0.001). Compared with patients with no AF during sepsis, those with AFOTS during sepsis had greater 5-year risks of ischemic stroke (5.3% vs. 4.7%, HR 1.22, 95%CI 1.10-1.36), and death (74.8% vs. 72.1%, HR 1.04; 95%CI 1.01-1.07)[98]. Gialdini et al. reported on a 1,729,360-person retrospective cohort study of surgical patients who experienced AFOTS. Recurrence of AF after discharge was ascertained through health care claims using ICD-9 codes. Even though IC-9D coding may lack sensitivity for AF detection, the investigators found the one-year rate of recurrent AF after an episode of AFOTS associated with non-cardiac surgery to be 37%. This was higher than the rate of new AF diagnosis in patients without AFOTS (1.5%). At 1 year after hospitalization for non-cardiac surgery, cumulative rates of stroke were 1.47% in those with perioperative AF and 0.36% in those without AF (HR for all stroke = 2.0, 95%CI 1.7-2.3; HR for embolic stroke = 4.9, 95%CI 3.5-6.7)[84].
Where the three above studies provide a strong signal that AFOTS is associated with a risk of recurrent AF and stroke, they have important limitations that greatly impair their sensitivity for ascertaining recurrent AF. Thus, they have likely underestimated the rate of AF recurrence after AFOTS. First, none of these studies systematically investigated AF recurrence. Not all participants in these studies would have been subject to the same post-AFOTS monitoring strategy and it is likely that many were not subject to any monitoring at all. Therefore, study populations are heterogeneous and subject to bias towards lower rates of AF recurrence. Second, by relying on opportunistic diagnosis of AF, these studies are more likely to miss a substantial proportion of asymptomatic or unrecognized AF. Consequently, where the specificity for the diagnosis of recurrent AF is reasonably high, the ability to rule out AF is much more limited. Third, the most sensitive technology that would have been employed in either study would have been a 48-hour Holter monitor – a tool that is less sensitive as compared to the best technologies that are available currently, which includes patch ECG monitors that can be worn for 14 or more days and the implantable loop recorder[99], [100]. It is also important to note that because these studies did not employ a prospective and systematic strategy for monitoring for recurrent AF, they are therefore unable to offer clinicians any guidance on post-discharge rhythm monitoring for patients who manifest AFOTS. Prospective studies with systematic and sensitive screening are required to better define the recurrence rate of AF after AFOTS.
Take home messages
• Clinical guidelines for stroke prevention in patients with atrial fibrillation do not currently take pattern and duration of arrhythmia into account as part of risk stratification. More recent studies show that these factors may be important and further research is required to develop risk stratification schemata that incorporate clinical characteristics and arrhythmia burden.
• Where subclinical atrial fibrillation (SCAF) is associated with a risk of stroke and systemic embolism, the benefit of oral anti-coagulation in this patient group is not established and the results of ongoing clinical trials are awaited to help direct their management.
• Atrial fibrillation is often detected transiently in the setting of an acute, reversible stressor, such as a medical illness or surgery (AFOTS). AF recurs by 5 years in about half of these patients. However; the true rate may be under-estimated, as most studies used relatively insensitive methods to detect recurrent AF. Further studies employing prospective screening strategies that are both systematic and sensitive are required to better define recurrence rates and to guide management with respect to strategies for detection of recurrent AF and/or provision of prophylaxis against stroke.
Conflict Of Interests
None.
Disclosures
Dr Healey has received a personnel award from the Heart and Stroke Foundation, Ontario Provincial office (MC7450). Dr McIntyre has received fellowship support from the Canadian Stroke Prevention Intervention Network (C-SPIN) and the McMaster Cooper Award.
References
- 1.Lloyd-Jones Donald M, Wang Thomas J, Leip Eric P, Larson Martin G, Levy Daniel, Vasan Ramachandran S, D'Agostino Ralph B, Massaro Joseph M, Beiser Alexa, Wolf Philip A, Benjamin Emelia J. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004 Aug 31;110 (9):1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Wolf P A, Abbott R D, Kannel W B. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch. Intern. Med. 1987 Sep;147 (9):1561–4. [PubMed] [Google Scholar]
- 3.Ruff Christian T, Giugliano Robert P, Braunwald Eugene, Hoffman Elaine B, Deenadayalu Naveen, Ezekowitz Michael D, Camm A John, Weitz Jeffrey I, Lewis Basil S, Parkhomenko Alexander, Yamashita Takeshi, Antman Elliott M. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014 Mar 15;383 (9921):955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 4.Hart Robert G, Pearce Lesly A, Aguilar Maria I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007 Jun 19;146 (12):857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 5.Verma Atul, Cairns John A, Mitchell L Brent, Macle Laurent, Stiell Ian G, Gladstone David, McMurtry Michael Sean, Connolly Stuart, Cox Jafna L, Dorian Paul, Ivers Noah, Leblanc Kori, Nattel Stanley, Healey Jeff S. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014 Oct;30 (10):1114–30. doi: 10.1016/j.cjca.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 6.January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cigarroa Joaquin E, Cleveland Joseph C, Conti Jamie B, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Murray Katherine T, Sacco Ralph L, Stevenson William G, Tchou Patrick J, Tracy Cynthia M, Yancy Clyde W. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014 Dec 02;64 (21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Vardas P, Al-Attar N, Alfieri O, Angelini A, Blömstrom-Lundqvist C, Colonna P, De Sutter J, Ernst S, Goette A, Gorenek B, Hatala R, Heidbüchel H, Heldal M, Kristensen SD, Kolh P, Le Heuzey J-Y, Mavrakis H, Mont L, Filardi PP, Ponikowski P, Prendergast B, Rutten FH, Schotten U, Van Gelder IC, Verheugt FWA. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 8.8. The efficacy of aspirin in patients with atrial fibrillation: Analysis of pooled data from 3 randomized trials. Arch Intern Med. 1997;157:1237–1240. [PubMed] [Google Scholar]
- 9.Gage B F, Waterman A D, Shannon W, Boechler M, Rich M W, Radford M J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001 Jun 13;285 (22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 10.Hart R G, Pearce L A, McBride R, Rothbart R M, Asinger R W. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke. 1999 Jun;30 (6):1223–9. doi: 10.1161/01.str.30.6.1223. [DOI] [PubMed] [Google Scholar]
- 11.Lip Gregory Y H, Nieuwlaat Robby, Pisters Ron, Lane Deirdre A, Crijns Harry J G M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010 Feb;137 (2):263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 12.Wang Thomas J, Massaro Joseph M, Levy Daniel, Vasan Ramachandran S, Wolf Philip A, D'Agostino Ralph B, Larson Martin G, Kannel William B, Benjamin Emelia J. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003 Aug 27;290 (8):1049–56. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 13.Fang Margaret C, Go Alan S, Chang Yuchiao, Borowsky Leila H, Pomernacki Niela K, Udaltsova Natalia, Singer Daniel E. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J. Am. Coll. Cardiol. 2011 Jul 19;58 (4):395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gage Brian F, Yan Yan, Milligan Paul E, Waterman Amy D, Culverhouse Robert, Rich Michael W, Radford Martha J. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am. Heart J. 2006 Mar;151 (3):713–9. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Pisters Ron, Lane Deirdre A, Nieuwlaat Robby, de Vos Cees B, Crijns Harry J G M, Lip Gregory Y H. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010 Nov;138 (5):1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 16.Vanassche Thomas, Lauw Mandy N, Eikelboom John W, Healey Jeff S, Hart Robert G, Alings Marco, Avezum Alvaro, Díaz Rafael, Hohnloser Stefan H, Lewis Basil S, Shestakovska Olga, Wang Jia, Connolly Stuart J. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur. Heart J. 2015 Feb 01;36 (5):281–7a. doi: 10.1093/eurheartj/ehu307. [DOI] [PubMed] [Google Scholar]
- 17.Takabayashi Kensuke, Hamatani Yasuhiro, Yamashita Yugo, Takagi Daisuke, Unoki Takashi, Ishii Mitsuru, Iguchi Moritake, Masunaga Nobutoyo, Ogawa Hisashi, Esato Masahiro, Chun Yeong-Hwa, Tsuji Hikari, Wada Hiromichi, Hasegawa Koji, Abe Mitsuru, Lip Gregory Y H, Akao Masaharu. Incidence of Stroke or Systemic Embolism in Paroxysmal Versus Sustained Atrial Fibrillation: The Fushimi Atrial Fibrillation Registry. Stroke. 2015 Dec;46 (12):3354–61. doi: 10.1161/STROKEAHA.115.010947. [DOI] [PubMed] [Google Scholar]
- 18.Botto Giovanni L, Padeletti Luigi, Santini Massimo, Capucci Alessandro, Gulizia Michele, Zolezzi Francesco, Favale Stefano, Molon Giulio, Ricci Renato, Biffi Mauro, Russo Giovanni, Vimercati Marco, Corbucci Giorgio, Boriani Giuseppe. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J. Cardiovasc. Electrophysiol. 2009 Mar;20 (3):241–8. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 19.Seet Raymond C S, Friedman Paul A, Rabinstein Alejandro A. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation. 2011 Jul 26;124 (4):477–86. doi: 10.1161/CIRCULATIONAHA.111.029801. [DOI] [PubMed] [Google Scholar]
- 20.Gaita Fiorenzo, Corsinovi Laura, Anselmino Matteo, Raimondo Cristina, Pianelli Martina, Toso Elisabetta, Bergamasco Laura, Boffano Carlo, Valentini Maria Consuelo, Cesarani Federico, Scaglione Marco. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J. Am. Coll. Cardiol. 2013 Nov 19;62 (21):1990–7. doi: 10.1016/j.jacc.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 21.de Bruijn Renée F A G, Heeringa Jan, Wolters Frank J, Franco Oscar H, Stricker Bruno H C, Hofman Albert, Koudstaal Peter J, Ikram M Arfan. Association Between Atrial Fibrillation and Dementia in the General Population. JAMA Neurol. 2015 Nov;72 (11):1288–94. doi: 10.1001/jamaneurol.2015.2161. [DOI] [PubMed] [Google Scholar]
- 22.Oldgren Jonas, Healey Jeff S, Ezekowitz Michael, Commerford Patrick, Avezum Alvaro, Pais Prem, Zhu Jun, Jansky Petr, Sigamani Alben, Morillo Carlos A, Liu Lisheng, Damasceno Albertino, Grinvalds Alex, Nakamya Juliet, Reilly Paul A, Keltai Katalin, Van Gelder Isabelle C, Yusufali Afzal Hussein, Watanabe Eiichi, Wallentin Lars, Connolly Stuart J, Yusuf Salim. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation. 2014 Apr 15;129 (15):1568–76. doi: 10.1161/CIRCULATIONAHA.113.005451. [DOI] [PubMed] [Google Scholar]
- 23.Healey Jeff S, Connolly Stuart J, Gold Michael R, Israel Carsten W, Van Gelder Isabelle C, Capucci Alessandro, C.P Lau, Fain Eric, Yang Sean, Bailleul Christophe, Morillo Carlos A, Carlson Mark, Themeles Ellison, Kaufman Elizabeth S, Hohnloser Stefan H. Subclinical Atrial Fibrillation and the Risk of Stroke. New England Journal of Medicine. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 24.Glotzer Taya V, Hellkamp Anne S, Zimmerman John, Sweeney Michael O, Yee Raymond, Marinchak Roger, Cook James, Paraschos Alexander, Love John, Radoslovich Glauco, Lee Kerry L, Lamas Gervasio A. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003 Apr 01;107 (12):1614–9. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 25.Glotzer Taya V, Daoud Emile G, Wyse D George, Singer Daniel E, Ezekowitz Michael D, Hilker Christopher, Miller Clayton, Qi Dongfeng, Ziegler Paul D. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009 Oct;2 (5):474–80. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 26.Flaker Greg C, Belew Kathy, Beckman Karen, Vidaillet Humberto, Kron Jack, Safford Robert, Mickel Mary, Barrell Patrick. Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am. Heart J. 2005 Apr;149 (4):657–63. doi: 10.1016/j.ahj.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler Paul D, Glotzer Taya V, Daoud Emile G, Wyse D George, Singer Daniel E, Ezekowitz Michael D, Koehler Jodi L, Hilker Christopher E. Incidence of newly detected atrial arrhythmias via implantable devices in patients with a history of thromboembolic events. Stroke. 2010 Feb;41 (2):256–60. doi: 10.1161/STROKEAHA.109.571455. [DOI] [PubMed] [Google Scholar]
- 28.Andrade Jason G, Field Thalia, Khairy Paul. Detection of occult atrial fibrillation in patients with embolic stroke of uncertain source: a work in progress. Front Physiol. 2015;6 () doi: 10.3389/fphys.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brambatti Michela, Connolly Stuart J, Gold Michael R, Morillo Carlos A, Capucci Alessandro, Muto Carmine, Lau Chu P, Van Gelder Isabelle C, Hohnloser Stefan H, Carlson Mark, Fain Eric, Nakamya Juliet, Mairesse Georges H, Halytska Marta, Deng Wei Q, Israel Carsten W, Healey Jeff S. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014 May 27;129 (21):2094–9. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 30.Healey Jeff S, Martin Jason L, Duncan Andrew, Connolly Stuart J, Ha Andrew H, Morillo Carlos A, Nair Girish M, Eikelboom John, Divakaramenon Syamkumar, Dokainish Hisham. Pacemaker-detected atrial fibrillation in patients with pacemakers: prevalence, predictors, and current use of oral anticoagulation. Can J Cardiol. 2013 Feb;29 (2):224–8. doi: 10.1016/j.cjca.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Lip Gregory Y H. How to Manage Occult Atrial Fibrillation Detected on Long-Term Monitoring. Circulation. 2016 Mar 29;133 (13):1290–5. doi: 10.1161/CIRCULATIONAHA.115.016797. [DOI] [PubMed] [Google Scholar]
- 32.Lamas Gervasio. How much atrial fibrillation is too much atrial fibrillation? N. Engl. J. Med. 2012 Jan 12;366 (2):178–80. doi: 10.1056/NEJMe1111948. [DOI] [PubMed] [Google Scholar]
- 33.Ip John, Waldo Albert L, Lip Gregory Y H, Rothwell Peter M, Martin David T, Bersohn Malcolm M, Choucair Wassim K, Akar Joseph G, Wathen Mark S, Rohani Pooyan, Halperin Jonathan L. Multicenter randomized study of anticoagulation guided by remote rhythm monitoring in patients with implantable cardioverter-defibrillator and CRT-D devices: Rationale, design, and clinical characteristics of the initially enrolled cohort The IMPACT study. Am. Heart J. 2009 Sep;158 (3):364–370.e1. doi: 10.1016/j.ahj.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Anderson K, BS B. Do patients with pacemaker-detected atrial brillation receive appropriate anticoagulation? Europace. 2011;13:519–0. [Google Scholar]
- 35.Cloutier J, Khoo C, Hiebert B, Wassef A and C S. Physician Decisions Making in Anticoagulating Atrial Fibrillation: A Prospective Survey Evaluating a Physician Alert System for Arial Fibrillaiton Detected on Cardiac Implantable Electronic Devices. J Am Coll Cardiol. 2016;67:837–0. [Google Scholar]
- 36.Hess Paul L, Lopes Renato D, Healey Jeff S. Letter by Hess et al Regarding Article, "How to Manage Occult Atrial Fibrillation Detected on Long-Term Monitoring". Circulation. 2016 Aug 02;134 (5):e28–9. doi: 10.1161/CIRCULATIONAHA.116.023088. [DOI] [PubMed] [Google Scholar]
- 37.Martin David T, Bersohn Malcolm M, Waldo Albert L, Wathen Mark S, Choucair Wassim K, Lip Gregory Y H, Ip John, Holcomb Richard, Akar Joseph G, Halperin Jonathan L. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur. Heart J. 2015 Jul 07;36 (26):1660–8. doi: 10.1093/eurheartj/ehv115. [DOI] [PubMed] [Google Scholar]
- 38.Reddy M, Moustapha Atoui, Rajarajeswari Swarna, Mamatha Vodapally, Maruthsakhi Molugu, Quratulain Javed, Anirudh Gone, Karthik Gangu, Ravali Neerumalla, Sudharani Bommana, Sandia Iskandar, Mohit Turagam, Madhav Lavu, Donita Atkins, Luigi Di Biase, Andrea Natale, Lakkireddy D. Temporal relationship between episodes of atrial fibrillation and incident stroke in patients with an implantable cardiac device. J Am Coll Cardiol. 2016;67:687–0. [Google Scholar]
- 39.Shanmugam Nesan, Boerdlein Annegret, Proff Jochen, Ong Peter, Valencia Oswaldo, Maier Sebastian K G, Bauer Wolfgang R, Paul Vince, Sack Stefan. Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Europace. 2012 Feb;14 (2):230–7. doi: 10.1093/europace/eur293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daoud Emile G, Glotzer Taya V, Wyse D George, Ezekowitz Michael D, Hilker Christopher, Koehler Jodi, Ziegler Paul D. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011 Sep;8 (9):1416–23. doi: 10.1016/j.hrthm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Watson Timothy, Shantsila Eduard, Lip Gregory Y H. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009 Jan 10;373 (9658):155–66. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez Cecilia, Blanchard Daniel G. Diagnosis and Treatment of Atrial Fibrillation. Am Fam Physician. 2016 Sep 15;94 (6):442–52. [PubMed] [Google Scholar]
- 43.Healey Jeff S, Parkash Ratika, Pollak Tim, Tsang Teresa, Dorian Paul. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: etiology and initial investigations. Can J Cardiol. 2011 Feb 19;27 (1):31–7. doi: 10.1016/j.cjca.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Prystowsky Eric N, Padanilam Benzy J, Fogel Richard I. Treatment of Atrial Fibrillation. JAMA. 2015 Jul 21;314 (3):278–88. doi: 10.1001/jama.2015.7505. [DOI] [PubMed] [Google Scholar]
- 45.Fuster Valentin, Rydén Lars E, Cannom Davis S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Kay G Neal, Le Huezey Jean-Yves, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann L Samuel. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2011 Mar 15;57 (11):e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Walkey Allan J, Hogarth D Kyle, Lip Gregory Y H. Optimizing atrial fibrillation management: from ICU and beyond. Chest. 2015 Oct;148 (4):859–864. doi: 10.1378/chest.15-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen Mark J, Choi Eue-Keun, Tan Alex Y, Lin Shien-Fong, Fishbein Michael C, Chen Lan S, Chen Peng-Sheng. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol. 2011 Sep 27;9 (1):30–9. doi: 10.1038/nrcardio.2011.139. [DOI] [PubMed] [Google Scholar]
- 48.Andrade Jason, Khairy Paul, Dobrev Dobromir, Nattel Stanley. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014 Apr 25;114 (9):1453–68. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 49.Iwasaki Yu-ki, Nishida Kunihiro, Kato Takeshi, Nattel Stanley. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011 Nov 15;124 (20):2264–74. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 50.Krahn A D, Manfreda J, Tate R B, Mathewson F A, Cuddy T E. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am. J. Med. 1995 May;98 (5):476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 51.Danelich Ilya M, Lose Jennifer M, Wright Sampaguita S, Asirvatham Samuel J, Ballinger Beth A, Larson David W, Lovely Jenna K. Practical management of postoperative atrial fibrillation after noncardiac surgery. J. Am. Coll. Surg. 2014 Oct;219 (4):831–41. doi: 10.1016/j.jamcollsurg.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 52.Bessissow A, Khan J, Devereaux P J, Alvarez-Garcia J, Alonso-Coello P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J. Thromb. Haemost. 2015 Jun;13 Suppl 1 ():S304–12. doi: 10.1111/jth.12974. [DOI] [PubMed] [Google Scholar]
- 53.Kanji Salmaan, Williamson David R, Yaghchi Behrooz Mohammadzadeh, Albert Martin, McIntyre Lauralyn. Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J Crit Care. 2012 Jun;27 (3):326.e1–8. doi: 10.1016/j.jcrc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Chelazzi C, Villa G, De Gaudio A R. Postoperative atrial fibrillation. ISRN Cardiol. 2011;2011 () doi: 10.5402/2011/203179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darghosian Leon, Free Marcia, Li Jie, Gebretsadik Tebeb, Bian Aihua, Shintani Ayumi, McBride Brian F, Solus Joseph, Milne Ginger, Crossley George H, Thompson David, Vidaillet Humberto, Okafor Henry, Darbar Dawood, Murray Katherine T, Stein C Michael. Effect of omega-three polyunsaturated fatty acids on inflammation, oxidative stress, and recurrence of atrial fibrillation. Am. J. Cardiol. 2015 Jan 15;115 (2):196–201. doi: 10.1016/j.amjcard.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Artucio H, Pereira M. Cardiac arrhythmias in critically ill patients: epidemiologic study. Crit. Care Med. 1990 Dec;18 (12):1383–8. doi: 10.1097/00003246-199012000-00015. [DOI] [PubMed] [Google Scholar]
- 57.Goldberger Jeffrey J, Arora Rishi, Green David, Greenland Philip, Lee Daniel C, Lloyd-Jones Donald M, Markl Michael, Ng Jason, Shah Sanjiv J. Evaluating the Atrial Myopathy Underlying Atrial Fibrillation: Identifying the Arrhythmogenic and Thrombogenic Substrate. Circulation. 2015 Jul 28;132 (4):278–91. doi: 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calenda Brandon W, Fuster Valentin, Halperin Jonathan L, Granger Christopher B. Stroke risk assessment in atrial fibrillation: risk factors and markers of atrial myopathy. Nat Rev Cardiol. 2016 Sep;13 (9):549–59. doi: 10.1038/nrcardio.2016.106. [DOI] [PubMed] [Google Scholar]
- 59.Annane Djillali, Sébille Véronique, Duboc Denis, Le Heuzey Jean-Yves, Sadoul Nicolas, Bouvier Erik, Bellissant Eric. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am. J. Respir. Crit. Care Med. 2008 Jul 01;178 (1):20–5. doi: 10.1164/rccm.200701-031OC. [DOI] [PubMed] [Google Scholar]
- 60.Walkey Allan J, Wiener Renda Soylemez, Ghobrial Joanna M, Curtis Lesley H, Benjamin Emelia J. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011 Nov 23;306 (20):2248–54. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ambrus Daniel B, Benjamin Emelia J, Bajwa Ednan K, Hibbert Kathryn A, Walkey Allan J. Risk factors and outcomes associated with new-onset atrial fibrillation during acute respiratory distress syndrome. J Crit Care. 2015 Oct;30 (5):994–7. doi: 10.1016/j.jcrc.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baumfeld Yael, Novack Victor, Almog Yaniv. [Atrial fibrillation in medical intensive care unit patients: characteristics and consequences]. Harefuah. 2013 Sep;152 (9):520–3, 564. [PubMed] [Google Scholar]
- 63.Chen Alyssa Y, Sokol Sarah S, Kress John P, Lat Ishaq. New-onset atrial fibrillation is an independent predictor of mortality in medical intensive care unit patients. Ann Pharmacother. 2015 May;49 (5):523–7. doi: 10.1177/1060028015574726. [DOI] [PubMed] [Google Scholar]
- 64.Tongyoo S, Permpikul C. The correlation of daily caloric intake, route of nutrition supplement and outcomes of critically ill medical patients. Intensive Care Med. 2013;39:0–0. [Google Scholar]
- 65.Tongyoo Surat, Permpikul Chairat, Haemin Ratchada, Epichath Nantawan. Predicting factors, incidence and prognosis of cardiac arrhythmia in medical, non-acute coronary syndrome, critically ill patients. J Med Assoc Thai. 2013 Feb;96 Suppl 2 ():S238–45. [PubMed] [Google Scholar]
- 66.Guenancia Charles, Binquet Christine, Laurent Gabriel, Vinault Sandrine, Bruyère Rémi, Prin Sébastien, Pavon Arnaud, Charles Pierre-Emmanuel, Quenot Jean-Pierre. Incidence and Predictors of New-Onset Atrial Fibrillation in Septic Shock Patients in a Medical ICU: Data from 7-Day Holter ECG Monitoring. PLoS ONE. 2015;10 (5) doi: 10.1371/journal.pone.0127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, Curtis LH, Emelia J, Benjamin M. Atrial Fibrillation Among Medicare Beneficiaries Hospitalized With Sepsis: Incidence and Risk Factors. Am Heart J. 2013;165:649955–0. doi: 10.1016/j.ahj.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asfar Pierre, Meziani Ferhat, Hamel Jean-François, Grelon Fabien, Megarbane Bruno, Anguel Nadia, Mira Jean-Paul, Dequin Pierre-François, Gergaud Soizic, Weiss Nicolas, Legay François, Le Tulzo Yves, Conrad Marie, Robert René, Gonzalez Frédéric, Guitton Christophe, Tamion Fabienne, Tonnelier Jean-Marie, Guezennec Pierre, Van Der Linden Thierry, Vieillard-Baron Antoine, Mariotte Eric, Pradel Gaël, Lesieur Olivier, Ricard Jean-Damien, Hervé Fabien, du Cheyron Damien, Guerin Claude, Mercat Alain, Teboul Jean-Louis, Radermacher Peter. High versus low blood-pressure target in patients with septic shock. N. Engl. J. Med. 2014 Apr 24;370 (17):1583–93. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 69.Christian Sangita-Ann, Schorr Christa, Ferchau Lynn, Jarbrink Maria E, Parrillo Joseph E, Gerber David R. Clinical characteristics and outcomes of septic patients with new-onset atrial fibrillation. J Crit Care. 2008 Dec;23 (4):532–6. doi: 10.1016/j.jcrc.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 70.Kuipers Sanne, Klein Klouwenberg Peter M C, Cremer Olaf L. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care. 2014 Dec 15;18 (6) doi: 10.1186/s13054-014-0688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koyfman Leonid, Brotfain Evgeni, Kutz Ruslan, Frenkel Amit, Schwartz Andrei, Boniel Avi, Zlotnik Alexander, Klein Moti. Epidemiology of new-onset paroxysmal atrial fibrillation in the General Intensive Care Unit population and after discharge from ICU. A retrospective epidemiological study. Anaesthesiol Intensive Ther. 2015;47 (4):309–14. doi: 10.5603/AIT.a2015.0040. [DOI] [PubMed] [Google Scholar]
- 72.Salman Salam, Bajwa Abubakr, Gajic Ognjen, Afessa Bekele. Paroxysmal atrial fibrillation in critically ill patients with sepsis. J Intensive Care Med. 2008 Apr 30;23 (3):178–83. doi: 10.1177/0885066608315838. [DOI] [PubMed] [Google Scholar]
- 73.Seguin Philippe, Signouret Thomas, Laviolle Bruno, Branger Bernard, Mallédant Yannick. Incidence and risk factors of atrial fibrillation in a surgical intensive care unit. Crit. Care Med. 2004 Mar;32 (3):722–6. doi: 10.1097/01.ccm.0000114579.56430.e0. [DOI] [PubMed] [Google Scholar]
- 74.Wells Gretchen L, Morris Peter E. Incidence and Prognosis of Atrial Fibrillation in Patients With Sepsis. Cardiol Res. 2011 Dec;2 (6):293–297. doi: 10.4021/cr108w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bajaj Navin, Bozarth Andrew L, Guillot Juan, Kojokittah Joseph, Appalaneni Sri Ram, Cestero Cesar, Amankona Raymond Kofi, Pippim James A. Clinical features in patients with pulmonary embolism at a community hospital: analysis of 4 years of data. J. Thromb. Thrombolysis. 2014 Apr;37 (3):287–92. doi: 10.1007/s11239-013-0942-8. [DOI] [PubMed] [Google Scholar]
- 76.Boey Elaine, Teo Swee-Guan, Poh Kian-Keong. Electrocardiographic findings in pulmonary embolism. Singapore Med J. 2015 Oct;56 (10):533–7. doi: 10.11622/smedj.2015147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cangemi Roberto, Calvieri Camilla, Falcone Marco, Bucci Tommaso, Bertazzoni Giuliano, Scarpellini Maria G, Barillà Francesco, Taliani Gloria, Violi Francesco. Relation of Cardiac Complications in the Early Phase of Community-Acquired Pneumonia to Long-Term Mortality and Cardiovascular Events. Am. J. Cardiol. 2015 Aug 15;116 (4):647–51. doi: 10.1016/j.amjcard.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 78.Mandal P, Chalmers J D, Choudhury G, Akram A R, Hill A T. Vascular complications are associated with poor outcome in community-acquired pneumonia. QJM. 2011 Jun;104 (6):489–95. doi: 10.1093/qjmed/hcq247. [DOI] [PubMed] [Google Scholar]
- 79.Short PM, Chalmers JD, Akram AR, Singanayagam A, Schembri S, Williamson PA. Impact of tachycardia and new onset atrial fibrillation in acute exacerbations of COPD. Thorax. 2012;67:0–0. [Google Scholar]
- 80.Violi Francesco, Carnevale Roberto, Calvieri Camilla, Nocella Cristina, Falcone Marco, Farcomeni Alessio, Taliani Gloria, Cangemi Roberto. Nox2 up-regulation is associated with an enhanced risk of atrial fibrillation in patients with pneumonia. Thorax. 2015 Oct;70 (10):961–6. doi: 10.1136/thoraxjnl-2015-207178. [DOI] [PubMed] [Google Scholar]
- 81.Bielecka-Dabrowa Agata, Mikhailidis Dimitri P, Rysz Jacek, Banach Maciej. The mechanisms of atrial fibrillation in hyperthyroidism. Thyroid Res. 2009 Apr 02;2 (1) doi: 10.1186/1756-6614-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ertek Sibel, Cicero Arrigo F. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch Med Sci. 2013 Oct 31;9 (5):944–52. doi: 10.5114/aoms.2013.38685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arora S, Lang I, Nayyar V, Stachowski E, Ross D L. Atrial fibrillation in a tertiary care multidisciplinary intensive care unit--incidence and risk factors. Anaesth Intensive Care. 2007 Oct;35 (5):707–13. doi: 10.1177/0310057X0703500508. [DOI] [PubMed] [Google Scholar]
- 84.Gialdini Gino, Nearing Katherine, Bhave Prashant D, Bonuccelli Ubaldo, Iadecola Costantino, Healey Jeff S, Kamel Hooman. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014 Aug 13;312 (6):616–22. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amar D, Roistacher N, Burt M, Reinsel R A, Ginsberg R J, Wilson R S. Clinical and echocardiographic correlates of symptomatic tachydysrhythmias after noncardiac thoracic surgery. Chest. 1995 Aug;108 (2):349–54. doi: 10.1378/chest.108.2.349. [DOI] [PubMed] [Google Scholar]
- 86.Curtis J J, Parker B M, McKenney C A, Wagner-Mann C C, Walls J T, Demmy T L, Schmaltz R A. Incidence and predictors of supraventricular dysrhythmias after pulmonary resection. Ann. Thorac. Surg. 1998 Nov;66 (5):1766–71. doi: 10.1016/s0003-4975(98)00942-4. [DOI] [PubMed] [Google Scholar]
- 87.Krowka M J, Pairolero P C, Trastek V F, Payne W S, Bernatz P E. Cardiac dysrhythmia following pneumonectomy. Clinical correlates and prognostic significance. Chest. 1987 Apr;91 (4):490–5. doi: 10.1378/chest.91.4.490. [DOI] [PubMed] [Google Scholar]
- 88.Materazzo Carlo, Piotti Patrizia, Mantovani Costanza, Miceli Rosalba, Villani Fabrizio. Atrial fibrillation after non-cardiac surgery: P-wave characteristics and Holter monitoring in risk assessment. Eur J Cardiothorac Surg. 2007 May;31 (5):812–6. doi: 10.1016/j.ejcts.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 89.Noorani Ayesha, Walsh Stewart R, Tang Tjun Y, Sadat Umar, Cooper David G, Callaghan Christopher J, Varty Kevin, Gaunt Michael E. Atrial fibrillation following elective open abdominal aortic aneurysm repair. Int J Surg. 2009 Feb;7 (1):24–7. doi: 10.1016/j.ijsu.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 90.Raghavan Deepa, Gao Ang, Ahn Chul, Torres Fernando, Mohanka Manish, Bollineni Srinivas, Peltz Matthias, Wait Michael, Ring Steve, Kaza Vaidehi. Contemporary analysis of incidence of post-operative atrial fibrillation, its predictors, and association with clinical outcomes in lung transplantation. J. Heart Lung Transplant. 2015 Apr;34 (4):563–70. doi: 10.1016/j.healun.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 91.Camm A John, Kirchhof Paulus, Lip Gregory Y H, Schotten Ulrich, Savelieva Irene, Ernst Sabine, Van Gelder Isabelle C, Al-Attar Nawwar, Hindricks Gerhard, Prendergast Bernard, Heidbuchel Hein, Alfieri Ottavio, Angelini Annalisa, Atar Dan, Colonna Paolo, De Caterina Raffaele, De Sutter Johan, Goette Andreas, Gorenek Bulent, Heldal Magnus, Hohloser Stefan H, Kolh Philippe, Le Heuzey Jean-Yves, Ponikowski Piotr, Rutten Frans H. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010 Oct;31 (19):2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 92.Rubin D, Nieminski K, Reed G, Herman M. Predictors, prevention, and longterm prognosis of atrial fibrillation after coronary artery bypass graft operations. J Thorac Cardiovasc Surg. 1987;94:331–335. [PubMed] [Google Scholar]
- 93.Stamou S C, Hill P C, Dangas G, Pfister A J, Boyce S W, Dullum M K, Bafi A S, Corso P J. Stroke after coronary artery bypass: incidence, predictors, and clinical outcome. Stroke. 2001 Jul;32 (7):1508–13. doi: 10.1161/01.str.32.7.1508. [DOI] [PubMed] [Google Scholar]
- 94.Walsh S R, Oates J E, Anderson J A, Blair S D, Makin C A, Walsh C J. Postoperative arrhythmias in colorectal surgical patients: incidence and clinical correlates. Colorectal Dis. 2006 Mar;8 (3):212–6. doi: 10.1111/j.1463-1318.2005.00881.x. [DOI] [PubMed] [Google Scholar]
- 95.Batra G S, Molyneux J, Scott N A. Colorectal patients and cardiac arrhythmias detected on the surgical high dependency unit. Ann R Coll Surg Engl. 2001 May;83 (3):174–6. [PMC free article] [PubMed] [Google Scholar]
- 96.Polanczyk C A, Goldman L, Marcantonio E R, Orav E J, Lee T H. Supraventricular arrhythmia in patients having noncardiac surgery: clinical correlates and effect on length of stay. Ann. Intern. Med. 1998 Aug 15;129 (4):279–85. doi: 10.7326/0003-4819-129-4-199808150-00003. [DOI] [PubMed] [Google Scholar]
- 97.Lubitz Steven A, Yin Xiaoyan, Rienstra Michiel, Schnabel Renate B, Walkey Allan J, Magnani Jared W, Rahman Faisal, McManus David D, Tadros Thomas M, Levy Daniel, Vasan Ramachandran S, Larson Martin G, Ellinor Patrick T, Benjamin Emelia J. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015 May 12;131 (19):1648–55. doi: 10.1161/CIRCULATIONAHA.114.014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walkey Allan J, Hammill Bradley G, Curtis Lesley H, Benjamin Emelia J. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014 Nov;146 (5):1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheung Christopher C, Kerr Charles R, Krahn Andrew D. Comparing 14-day adhesive patch with 24-h Holter monitoring. Future Cardiol. 2014 May;10 (3):319–22. doi: 10.2217/fca.14.24. [DOI] [PubMed] [Google Scholar]
- 100.Sanna Tommaso, Diener Hans-Christoph, Passman Rod S, Di Lazzaro Vincenzo, Bernstein Richard A, Morillo Carlos A, Rymer Marilyn Mollman, Thijs Vincent, Rogers Tyson, Beckers Frank, Lindborg Kate, Brachmann Johannes. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014 Jun 26;370 (26):2478–86. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]


