Abstract
Sotalol is a racemic mixture possessing beta-blocker and class III anti arrhythmic properties. Approved by US food and drug administration (FDA) since 2009 based on its bioequivalence with oral sotalol, clinicians are less familiar with the potential uses of the intravenous form despite its re-launch in United States in 2015. Available literature suggests that intravenous sotalol in recommended doses can be safely administered in adult and pediatric population achieving rapid reliable therapeutic plasma concentration and without additional proarrhythmic effects when compared to its oral form as well as other antiarrhythmic medications. Intravenous sotalol may have potential uses as an alternative agent for highly symptomatic atrial fibrillation post cardiac surgery as well as in life threatening ventricular arrhythmias. As with its oral form, judicious use with close attention to QTc and renal function is warranted. Further studies are needed to better understand the safety, efficacy and different dosing regimens of parenteral sotalol in adults and children.
Keywords: intravenous sotalol, sotalol hydrochloride, refractory ventricular arrhythmia, atrial fibrillation, post operative atrial arrhythmia, QT prolongation
Introduction
Sotalol is a racemic mixture that has beta blockade (conferred by the l enantiomer) and potassium channel blockade (conferred by the d enantiomer) properties. Recognized initially for its anti-anginal and anti-hypertensive properties as a non-cardioselective beta-blocker, sotalol became known for its anti-arrhythmic effects in the 1980s. While oral sotalol is commonly utilized to maintain sinus rhythm in patients with Atrial Fibrillation (AF), Atrial Flutter (AFL) and to suppress life threatening ventricular arrhythmias (VA), its intravenous formulation is less commonly used. The US Food and Drug Administration (FDA) approved intravenous sotalol originally in 2009 based on its bioequivalence to oral sotalol; however, the drug was not available in the USA until it was re-launched in 2015. The intravenous formulation has potential as an additional rapid onset medication to treat both supraventricular and ventricular arrhythmias particularly in an emergency setting. [1]
Electrophysiology and Mechanism of Action
The Class II electrophysiological effects of sotalol are manifested as an increase in sinus cycle length, decreased AV nodal conduction and increased refractoriness. Sotalol also exhibits class III antiarrhythmic effects through Ikr blockade resulting in prolongation of atrial and ventricular monophasic action potentials, and effective refractory periods in atria, ventricle, and accessory pathways.
The beta-blocker effect of oral sotalol is non-cardioselective, half maximal at 80mg/day dose and maximal at a dose of 320-640 mg/day. Compared to some other beta-blockers= sotalol does not have a partial agonist or membrane stabilizing activity. While studies have suggested that sotalol manifests its anti-arrhythmic and QT prolonging properties only with doses in excess of 160 mg, Somberg et al noted significant QT prolongation after administration of a single low dose of sotalol. [2]
Effect of sotalol therapy has been studied in both acute onset and persistent AF. Infusion at a dose of 1.5 mg/kg decreased the energy requirement for transvenous as well as transthoracic cardioversion to restore sinus rhythm. In a study of 18 patients with persistent AF, Lai et al reported a mean decrease in transthoracic cardioversion energy of 50 J. Sotalol infusion significantly increased the mean A-A (atrial local electrogram) intervals during AF in the patients needing lower energy for cardioversion.[4] Another study found the effect to be more evident in acute AF patients using transvenous atrial defibrillation as opposed to chronic AF.[5] Slowing of atrial rate and increase in R-R interval was observed in both studies.
D-sotalol has also been shown to lower defibrillation energy (DFT) for ventricular fibrillation up to 32 ± 27% with a statistically significant increase in ventricular effective refractory period and decrease in the incidence of hemodynamically significant and sustained ventricular arrhythmias. [6],[7] This effect was studied in comparison to amiodarone in the OPTIC trial where 94 patients were randomized to receive amiodarone, beta-blockers and/or sotalol therapy. While a 1.29 J statistically significant rise in DFT was observed in the amiodarone arm when compared to a slight decrease in DFT with beta-blockers and sotalol group, the overall effect was deemed clinically insignificant.[8]
Hemodynamic Effects of intravenous Sotalol in Humans
Intravenous sotalol causes a significant decrease in heart rate and cardiac output with little or no effect on mean stroke volume, right atrial and pulmonary capillary wedge pressure. These effects, evident within the first 10-15 minutes of the infusion, are seen in both healthy subjects and in patients with heart disease, at rest, with exercise and even at low doses. Thumala et al reported a significant decrease in HR, cardiac index and LV dp/dt at rest in patients with structural heart disease but with an increase in LVEDP and systemic vascular resistance. [9],[10]
Pharmacology, Safety & Dosing
Sotalol hydrochloride injection is FDA approved in the United States for life-threatening ventricular arrhythmias and maintenance of sinus rhythm in highly symptomatic, refractory AF or AFL. The principles applicable to oral sotalol in terms of safety are valid for intravenous sotalol administration as well. Per FDA labeling, intravenous sotalol is to be administered as a diluted infusion slowly over 5 hours.
Pharmacokinetics of Intravenous Sotalol
The bioavailability of oral sotalol is around 93% with peak plasma concentrations observed in 2.5-4 hours. It is not bound to plasma proteins and is excreted unchanged by the kidneys with an elimination half-life of 12-16 hours. In comparison, intravenous sotalol reaches therapeutic levels within minutes and the corresponding dose is slightly less than oral dose ([Table 1]).
Table 1. Dose Conversion between Oral and Intravenous Sotalol.
| Oral Sotalol | Intravenous Sotalol |
| 80 mg | 75 mg (5 mL) |
| 120 mg | 112.5 mg (7.5 mL) |
| 160 mg | 150 mg (10 mL) |
The maximum concentration (Cmax) of 75mg of intravenous sotalol is similar to an orally administered dose of 80mg when infused over 5 hours. The recommended infusion rate is based on evidence showing a large overshoot of maximum serum concentration with rapid administration and high risk of QT prolongation.[11]
Pharmacodynamic Effects of intravenous Sotalol
The beta-blocker effect is similar in both oral and intravenous sotalol and can be evaluated by decrease in HR and change in RR interval on EKG. The effect is most evident within the first half hour of drug administration, peaks at 1 hour and reaches a plateau thereafter. Further increase in RR interval may be counter acted by a reflex sympathetic activation in response to a fall in BP. The changes in RR interval are highly dose dependent and are evident at lower than anti-arrhythmic doses.[11]
In a bioequivalence study comparing oral and intravenous sotalol in 15 healthy volunteers, a strong correlation was found between serum sotalol concentration and QT prolongation with risk of Torsade de pointes (TdP) increasing when QTc exceeded 500 msec. QTc prolongation with intravenous administration occurs within 0.5 hours of infusion, reaches a maximum value at 2 hours, and shows a linear correlation with sotalol blood level.[11], [12]
Gender Differences in Response to Sotalol Therapy
Females are more likely to have drug induced excessive QTc prolongation and have a 2-3 times higher risk of developing TdP with intravenous sotalol as compared to males. The disparity in cardiac repolarization has been studied in females aged 18-45 years by Somberg et al. The mechanisms underlying this gender disparity is not completely understood but the fact that pre-pubertal males and females have no difference in QTc intervals points to some role for gonadal steroids. Therefore, close QTc monitoring is essential in females during intravenous sotalol use. [15]
Sotalol Therapy during Pregnancy and Lactation
Sotalol is classified as a category B drug with available human safety data. It does cross the placental barrier but in animal studies there was no increased incidence of congenital anomalies.[1] O’ Hare et al studied 6 healthy pregnant female volunteers between 32-36 weeks of gestation and up to 6 weeks post partum receiving 100 mg intravenous and 400 mg oral sotalol. The study showed altered pharmacokinetics of sotalol in antenatal patients with similar oral bioavailability, rapid plasma clearance (6.6 hours versus 9.3 hours in post natal period) and no change in volume of distribution.[13] Although data is inconclusive there is still suggestion that sotalol can be potentially teratogenic and hence, is not often the first choice in pregnant females. Close fetal monitoring is necessary when used. It is readily secreted in breast milk and infant may ingest as much as 20% of the maternal dose. Breast-feeding decision while on therapy should be made taking into account the importance of the drug to the mother and monitoring the baby for signs of toxicity. [1],[14]
Safety Profile, Proarrhythmic Effects & Adverse Reactions
Intravenous sotalol should be initiated in a monitored clinical setting with available resuscitation equipment. QT intervals, serum potassium and magnesium need to be checked periodically. Creatinine clearance should be calculated to establish dosing interval. It is generally recommended not to initiate sotalol therapy with baseline QTc more than 450 msec (Use JT >330 msec for QRS duration > 100 msec).
Contraindications to therapy include severe sinus bradycardia, sick sinus syndrome, second or third degree AV block unless functional pacemaker in place, congenital long QT syndrome, cardiogenic shock, uncontrolled heart failure, creatinine clearance <40 ml/min, serum potassium <4 meq/L, bronchospastic conditions or known hypersensitivity to sotalol.[16]
Proarrhythmia from oral sotalol is seen in 2-4% of patients. The estimated risk is similar or lower with intravenous sotalol. Interestingly, a meta-analysis of 962 patients, with the majority having underlying heart disease, showed the risk of TdP with intravenous sotalol to be <1%. Tissue accumulation has been postulated as a possible explanation for TdP with chronic oral therapy, which is not seen with short-term intravenous use. Alternative explanations for this low incidence of TdP in this study include administration during tachycardia in the acute setting and resultant shortened QT, possible reverse use-dependence, and heterogeneity in dosing and infusion duration.[17]
Hypotension is the most commonly reported side effect after intravenous sotalol, particularly when given in the early post-cardiac surgery setting. It is also of clinical significance in patients with VT with or without concomitant use of lidocaine. The incidence is lower in comparison to amiodarone and similar in groups randomized to lidocaine or sotalol.[1], [17], [20] Bradycardia, AV block and heart failure are also reported, especially in patients with low ejection fraction. Non-cardiac adverse effects from intravenous sotalol include non-specific gastrointestinal or neurological (headache, dizziness, malaise) complaints but the incidence is significantly lower than amiodarone. Non-allergic bronchospasm from beta-blocker properties of sotalol are reported in 1.8-2.4% cases.[1], [20]
FDA Approved Clinical Indications for Intravenous Sotalol
The current FDA approved indications for intravenous sotalol are:
As a substitution for oral formulation in patients. It can be particularly useful in the post-operative and critically ill patient group when reduced intestinal permeability and gastrointestinal absorption is insufficient to reach effective serum concentrations.
To prolong time in sinus rhythm and prevent atrial fibrillation/atrial flutter recurrence in highly symptomatic patients.
For the treatment of life threatening ventricular arrhythmia provided they are not associated with QT prolongation and TdP.
For the treatment of supraventricular and ventricular arrhythmia in pediatric population. [16]
IV Sotalol: Is there a potential role for another intravenous antiarrhythmic agent in 2016? ([Table 2])
Table 2. Potential Clinical Applications of Intravenous Sotalol.
| Clinical Applications of Intravenous Sotalol |
| Treatment of AF in post cardiac surgery patients (conversion rates similar to intravenous amiodarone with shorter loading time for Class III effects and shorter elimination half life upon withdrawal) |
| Prevention of early recurrence of atrial fibrillation post cardioversion and maintenance of SR |
| Potential use for reducing hospital time for sotalol initiation but further studies are needed |
| Life threatening ventricular tachycardia as an alternative agent to intravenous amiodarone or procainamide |
| Post congenital heart surgery in pediatric population |
| Pediatric supraventricular and ventricular tachycardia |
Post-Cardiac Surgery Atrial Arrhythmias
Atrial arrhythmias are common following cardiac surgery. These arrhythmias not only increase patient morbidity but also present a therapeutic challenge in terms of rate and rhythm control. Beta-blockers are first line therapy (class IA recommendation) followed by amiodarone (class IIA) and sotalol (class IIB) in perioperative period. [18] Randomized controlled trials have shown sotalol to be more effective than placebo in treating post-operative supraventricular tachycardia. Two clinical trials have shown a non-significant difference between sotalol and amiodarone in preventing post-operative AF. Sotalol was more effective than beta-blockers in reducing the incidence of AF in cardiac surgery patients. The combination of magnesium and sotalol had an augmented significant reduction in postoperative AF.[19], [20]
Intravenous sotalol may indeed prove to be useful for rate and rhythm control of atrial arrhythmias in post-cardiac surgery patients without contraindication to beta-blockers. The exact time for initiation of therapy remains a concern although it has shown similar efficacy before and after surgery. Hemodynamically significant hypotension and difficulty in maintaining sinus rhythm due to interaction with anesthesia (beta-blocker properties) are major issues when loaded before surgery. Given its advantage over amiodarone in terms of short duration of action as well as short loading time to achieve steady state, intravenous sotalol may prove to be a reliable alternative agent. [19]
Available data supports the cost-effectiveness of using intravenous sotalol, showing reduction in hospital stay by 0.5 days. Further studies are needed to provide definitive information in terms of benefit gained. [19]
Table 3. Recommendations for Use of Intravenous Class III Anti Arrhythmic Drugs in ACLS Guidelines.
| IV Sotalol | IV Amiodarone | IV Ibutilide | |
| Dosing | 1.0-1.5 mg/kg over 5-30 minutes | 1000 mg over first 24 hours: Initial Load:150 mg in 100 mL infused over 10 min; followed by 1mg/min for 6 hours; followed by 0.5 mg/min thereafter | Patients weighing ≥60 kg: 1 mg over 10 minutes. Patient weighing <60 kg: 0.1 mL/kg (0.01 mg/kg) over 10 minutes. A second 10-min infusion may be administered if arrhythmia does not terminate after first infusion. |
| Indications | Refractory VT/VF | Refractory VT/VF | AF Conversion |
| Major Warnings | QT prolongation and Torsades de Pointes | Boxed Warning for hypotension; bradycardia and AV block; hepatic injury, Pulmonary toxicity. | QT prolongation and Torsades de pointes |
Restoration and Maintenance of Sinus Rhythm in Atrial Fibrillation and Atrial Flutter
Sotalol has similar efficacy compared to class IA and IC anti-arrhythmics as well as amiodarone in conversion from AF to normal sinus rhythm. [20], [21] Thomas et al showed poor overall reversion rate in patients randomized to receive sotalol, amiodarone or digoxin but overall superior ventricular rate control (less than 100 beats per minute) with sotalol and amiodarone at 6 and 12 hours in comparison to digoxin. In the same study, patients receiving amiodarone infusion were more likely to have adverse reactions including hypotension. No additional benefit and increased risk of adverse effects were observed with higher doses of class III agents.[22] in another study of 120 patients with new onset AF randomized to digoxin, sotalol or amiodarone, a definite benefit was observed with amiodarone and sotalol in terms of reduction in time to reversion to normal sinus rhythm and ventricular rate control with minimal side effects. [23]
Analyzing pooled data from randomized studies, Somberg et al. reported similar conversion rates to NSR using either amiodarone or sotalol in new onset AF with the lowest success rates in persistent AF. Although patients on amiodarone reported more adverse reactions, those treated with sotalol had higher risk of AF recurrence.[20]
Only intravenous Ibutilide (given as 1-2 mg doses over 10 minutes) has been shown to be more effective than intravenous sotalol in converting AF/AFL to NSR. Similar conversion rate was observed for Dofetilide for AFL. The risk of sustained TdP with Ibutilide was around 2% when higher doses were used.[24]
In-Hospital Initiation of Sotalol for Atrial Fibrillation
Intravenous sotalol, offers significant flexibility in dosing compared to oral sotalol. It reaches therapeutic levels quicker and offers easier dose titration based on QTc and clinical response. These properties could be potentially useful in hospital initiation of sotalol and to facilitate transition to oral sotalol.
Further studies are needed and are being planned to look at dosing and duration for intravenous sotalol administered as a loading dose to initiate oral sotalol therapy in adult patients with AF.
Intravenous sotalol in prevention of early recurrence of atrial fibrillation after cardioversion
Early recurrence of atrial fibrillation (ERAF) is known to occur in 13-36% of the patients after electrical cardioversion. Repeat cardioversion without pharmacologic support is successful only in 10% of these patients. Atrial premature beats with shorter coupling interval and greater prematurity as well as sinus node suppression and bradycardia (long-short sequence) induced dispersion of refractoriness in atrial myocardium are postulated mechanisms. With the exception of profoundly bradycardiac patients, intravenous sotalol has been shown to suppress ERAF in up to 80% of patients. Sotalol confers benefits in these cases with its beta-blocking as well as anti-arrhythmic properties.[25]
Ventricular Arrhythmias
Intravenous sotalol is approved for life threatening ventricular arrhythmias with the initial dose being 75mg infused over 5 hours once or twice daily depending on renal clearance. Doses maybe titrated to therapeutic effect while monitoring hemodynamic parameters and QTc interval. Intravenous Sotalol rapidly increased ventricular effective refractory period and acutely terminated sustained VT in 69% as opposed to 18% given Lidocaine. It has been shown to be similar to Nifekalant and Dofetilide in suppressing inducible VT. While the SWORD trial with d-sotalol (no beta blocker effect) showed increased mortality in acute myocardial infarction, later studies suggest that racemic sotalol is effective and safe in ischemic cardiomyopathy and can be used in post infraction patients without added risk. [16], [26], [27]
Although amiodarone and sodium channel blockers remain the drugs of choice for treating VT, the availability of intravenous sotalol may provide an effective substitute particularly in young patients with structurally normal hearts. Studies have shown that the risk of TdP may be comparatively lower with intravenous dosing provided it is injected slowly and in the absence of significant renal dysfunction, hypomagnesemia or hypokalemia.
The acute advantage gained by terminating VA can be transitioned over to sustained response with continued oral therapy. In a prospective multicenter trial, Sotalol significantly reduced VT/VF seen in patients with implantable cardioverter defibrillators but the rate of discontinuation of the medication because of side effects remained high (35%). [28]
Utility in Arrhythmia therapy for Children
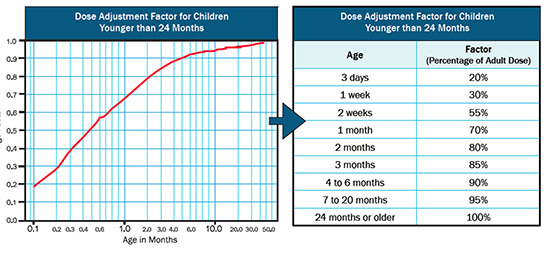
Sotalol is not the preferred first line of treatment in pediatric population due to lack of supporting evidence and safety concerns. It is however, used as a second line agent for incessant and refractory supraventricular and ventricular tachycardia in neonates, infants and children. High dose sotalol therapy (150 mg/m2) was shown to be safe and efficacious in achieving partial or complete suppression of SVT (including atrial tachycardia, AV reentrant tachycardia and junctional ectopic tachycardia) in 90% of the patients between ages of 7 to 728 days. No proarrhythmic effect or significant QTc prolongation requiring alteration of therapy was observed. [28] Similar results have been reported with intravenous sotalol with success rate of arrhythmia termination ranging from 60-70%. QT prolongation requiring treatment alteration, TdP, bradycardia, and AV block occurred only at very high doses (210 mg/m2). Intravenous sotalol may therefore be acceptable for use in children with resistant tachycardias, when initiated in hospital setting with doses normalized to body surface area ([Figure 1]). [1], [16]
Figure 1. Recommended Dose Adjustment for Intravenous Sotalol in Pediatric Patients.
Conclusion
Intravenous sotalol may provide a new therapeutic option to US physicians for effective treatment of supraventricular and ventricular arrhythmias in the pediatric and adult population particularly those with preserved ejection fraction and renal function. Moreover, by delivering a dose-dependent serum concentration independent of absorption and bioavailability, intravenous sotalol shortens time to reach therapeutically effective levels and could allow seamless transition to oral sotalol. As with its oral form, judicious use with close attention to QTc and renal function is warranted and further studies are needed to better understand the safety, efficacy and different dosing regimens for intravenous sotalol in adults and children.
Conflict Of Interests
None.
Disclosures
SAB-No relevant disclosures
References
- 1.Betapace and Betapace AF Prescribing Information February 2011. Available online at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm243980.htm. 0;0:0–0. [Google Scholar]
- 2.Somberg John C, Preston Richard A, Ranade Vasant, Molnar Janos. QT prolongation and serum sotalol concentration are highly correlated following intravenous and oral sotalol. Cardiology. 2010;116 (3):219–25. doi: 10.1159/000316050. [DOI] [PubMed] [Google Scholar]
- 3.Evidentiary Submission for Formulary Consideration of Sotalol hydrocholoride injection 15mg/mL Formular Dossier. AltaThera Pharmaceuticals. 2016;0:0–0. [Google Scholar]
- 4.Lai L P, Lin J L, Lien W P, Tseng Y Z, Huang S K. Intravenous sotalol decreases transthoracic cardioversion energy requirement for chronic atrial fibrillation in humans: assessment of the electrophysiological effects by biatrial basket electrodes. J. Am. Coll. Cardiol. 2000 May;35 (6):1434–41. doi: 10.1016/s0735-1097(00)00597-0. [DOI] [PubMed] [Google Scholar]
- 5.Lau C P, Lok N S. A comparison of transvenous atrial defibrillation of acute and chronic atrial fibrillation and the effect of intravenous sotalol on human atrial defibrillation threshold. Pacing Clin Electrophysiol. 1997 Oct;20 (10 Pt 1):2442–52. doi: 10.1111/j.1540-8159.1997.tb06084.x. [DOI] [PubMed] [Google Scholar]
- 6.Dorian P, Newman D. Effect of sotalol on ventricular fibrillation and defibrillation in humans. Am. J. Cardiol. 1993 Aug 12;72 (4):72A–79A. doi: 10.1016/0002-9149(93)90028-b. [DOI] [PubMed] [Google Scholar]
- 7.Dorian P, Newman D, Sheahan R, Tang A, Green M, Mitchell J. d-Sotalol decreases defibrillation energy requirements in humans: a novel indication for drug therapy. J. Cardiovasc. Electrophysiol. 1996 Oct;7 (10):952–61. doi: 10.1111/j.1540-8167.1996.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 8.Hohnloser Stefan H, Dorian Paul, Roberts Robin, Gent Michael, Israel Carsten W, Fain Eric, Champagne Jean, Connolly Stuart J. Effect of amiodarone and sotalol on ventricular defibrillation threshold: the optimal pharmacological therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation. 2006 Jul 11;114 (2):104–9. doi: 10.1161/CIRCULATIONAHA.106.618421. [DOI] [PubMed] [Google Scholar]
- 9.Mahmarian J J, Verani M S, Pratt C M. Hemodynamic effects of intravenous and oral sotalol. Am. J. Cardiol. 1990 Jan 02;65 (2):28A–34A. doi: 10.1016/0002-9149(90)90198-a. [DOI] [PubMed] [Google Scholar]
- 10.Thumala A, Hammermeister K E, Campbell W B, Pomerantz B, Overy H, Davies H. Hemodynamic studies with sotalol in man, performed at rest, during exercise, and during right ventricular pacing. Am. Heart J. 1971 Oct;82 (4):439–47. doi: 10.1016/0002-8703(71)90228-6. [DOI] [PubMed] [Google Scholar]
- 11.Somberg John C, Preston Richard A, Ranade Vasant, Molnar Janos. Developing a safe intravenous sotalol dosing regimen. Am J Ther. 2010 Jun 22;17 (4):365–72. doi: 10.1097/MJT.0b013e3181ea3184. [DOI] [PubMed] [Google Scholar]
- 12.Barbey J T, Sale M E, Woosley R L, Shi J, Melikian A P, Hinderling P H. Pharmacokinetic, pharmacodynamic, and safety evaluation of an accelerated dose titration regimen of sotalol in healthy middle-aged subjects. Clin. Pharmacol. Ther. 1999 Jul;66 (1):91–9. doi: 10.1016/S0009-9236(99)70058-5. [DOI] [PubMed] [Google Scholar]
- 13.O'Hare M F, Leahey W, Murnaghan G A, McDevitt D G. Pharmacokinetics of sotalol during pregnancy. Eur. J. Clin. Pharmacol. 1983;24 (4):521–4. doi: 10.1007/BF00609896. [DOI] [PubMed] [Google Scholar]
- 14.Cordina Rachael, McGuire Mark A. Maternal cardiac arrhythmias during pregnancy and lactation. Obstet Med. 2010 Mar;3 (1):8–16. doi: 10.1258/om.2009.090021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somberg John C, Preston Richard A, Ranade Vasant, Cvetanovic Ivana, Molnar Janos. Gender differences in cardiac repolarization following intravenous sotalol administration. J. Cardiovasc. Pharmacol. Ther. 2012 Mar;17 (1):86–92. doi: 10.1177/1074248411406505. [DOI] [PubMed] [Google Scholar]
- 16.Full Prescribing information sotalol hydrochloride available online at. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022306s000lbl.pdf. 0;0:0–0. [Google Scholar]
- 17.Marill K A, Runge T. Meta-analysis of the Risk of Torsades de Pointes in patients treated with intravenous racemic sotalol. Acad Emerg Med. 2001 Feb;8 (2):117–24. doi: 10.1111/j.1553-2712.2001.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 18.Fuster Valentin, Rydén Lars E, Cannom David S, Crijns Harry J, Curtis Anne B, Ellenbogen Kenneth A, Halperin Jonathan L, Le Heuzey Jean-Yves, Kay G Neal, Lowe James E, Olsson S Bertil, Prystowsky Eric N, Tamargo Juan Luis, Wann Samuel. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). Eur. Heart J. 2006 Aug;27 (16):1979–2030. doi: 10.1093/eurheartj/ehl176. [DOI] [PubMed] [Google Scholar]
- 19.Kerin Nicholas Z, Jacob Sony. The efficacy of sotalol in preventing postoperative atrial fibrillation: a meta-analysis. Am. J. Med. 2011 Sep;124 (9):875.e1–9. doi: 10.1016/j.amjmed.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Somberg John, Molnar Janos. Sotalol versus Amiodarone in Treatment of Atrial Fibrillation. J Atr Fibrillation. 2016 Dec 3;8 (5) doi: 10.4022/jafib.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milan David J, Saul J Philip, Somberg John C, Molnar Janos. Efficacy of Intravenous and Oral Sotalol in Pharmacologic Conversion of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Cardiology. 2017;136 (1):52–60. doi: 10.1159/000447237. [DOI] [PubMed] [Google Scholar]
- 22.Thomas Stuart P, Guy Duncan, Wallace Elisabeth, Crampton Roselyn, Kijvanit Pat, Eipper Vicki, Ross David L, Cooper Mark J. Rapid loading of sotalol or amiodarone for management of recent onset symptomatic atrial fibrillation: a randomized, digoxin-controlled trial. Am. Heart J. 2004 Jan;147 (1) doi: 10.1016/s0002-8703(03)00526-x. [DOI] [PubMed] [Google Scholar]
- 23.Joseph A P, Ward M R. A prospective, randomized controlled trial comparing the efficacy and safety of sotalol, amiodarone, and digoxin for the reversion of new-onset atrial fibrillation. Ann Emerg Med. 2000 Jul;36 (1):1–9. doi: 10.1067/mem.2000.107655. [DOI] [PubMed] [Google Scholar]
- 24.Vos M A, Golitsyn S R, Stangl K, Ruda M Y, Van Wijk L V, Harry J D, Perry K T, Touboul P, Steinbeck G, Wellens H J. Superiority of ibutilide (a new class III agent) over DL-sotalol in converting atrial flutter and atrial fibrillation. The Ibutilide/Sotalol Comparator Study Group. Heart. 1998 Jun;79 (6):568–75. doi: 10.1136/hrt.79.6.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tse H F, Lau C P, Ayers G M. Incidence and modes of onset of early reinitiation of atrial fibrillation after successful internal cardioversion, and its prevention by intravenous sotalol. Heart. 1999 Sep;82 (3):319–24. doi: 10.1136/hrt.82.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleifer J William, Sorajja Dan, Shen Win-Kuang. Advances in the pharmacologic treatment of ventricular arrhythmias. Expert Opin Pharmacother. 2015;16 (17):2637–51. doi: 10.1517/14656566.2015.1100170. [DOI] [PubMed] [Google Scholar]
- 27.Ho D S, Zecchin R P, Cooper M J, Richards D A, Uther J B, Ross D L. Rapid intravenous infusion of d-1 sotalol: time to onset of effects on ventricular refractoriness, and safety. Eur. Heart J. 1995 Jan;16 (1):81–6. doi: 10.1093/eurheartj/16.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Bunch T Jared, Anderson Jeffrey L. Adjuvant antiarrhythmic therapy in patients with implantable cardioverter defibrillators. Am J Cardiovasc Drugs. 2014 Apr;14 (2):89–100. doi: 10.1007/s40256-013-0056-x. [DOI] [PubMed] [Google Scholar]
- 29.Knudson Jarrod D, Cannon Bryan C, Kim Jeffrey J, Moffett Brady S. High-dose sotalol is safe and effective in neonates and infants with refractory supraventricular tachyarrhythmias. Pediatr Cardiol. 2011 Oct;32 (7):896–903. doi: 10.1007/s00246-011-0010-0. [DOI] [PubMed] [Google Scholar]
- 30.Yen Zhang, X Li, Z Xu, X Li. Intravenous sotalol for incessant tachyarrhythmias in children. Heart. 2012;98:260–0. [Google Scholar]


