Abstract
Background
Adenosine can unmask dormant conduction during pulmonary vein isolation (PVI) for atrial fibrillation (AF). Studies of adenosine use in radiofrequency PVI show high reconnection rates and conflicting results for long-term success, however there is limited data with cryoballoon ablation (CBA).
Methods
A prospectively maintained database of patients undergoing first CBA at a single institution was analyzed. Adenosine use was at the discretion of the primary operator. Additional freezes were delivered for reconnected veins until dormant conduction was eliminated. The primary endpoint, time to AF recurrence defined as any episode < 30 seconds after a 3-month blanking period, was assessed by Kaplan-Meier analysis.
Results
From 2011 to 2015, 406 patients underwent CBA, 361 of whom had > 3 months follow-up. The mean age was 61.7 years, 69% were male, and the prevalence of paroxysmal AF was 79% with no significant difference between those that did and did not receive adenosine (77% vs 86%, respectively, p = 0.23). Adenosine testing was performed in 78 patients (21.6%) with a mean dose of 10.6 mg/vein. Of the 306 veins evaluated, 17 (6%) demonstrated dormant conduction. Over a median 14.4 months follow-up, there was no significant difference in freedom from AF with adenosine use (p= 0.86).
Conclusions
Dormant conduction with adenosine is uncommon following CBA and its use does not improve long-term success rates.
Keywords: atrial fibrillation, cryoballoon ablation, adenosine, pulmonary vein isolation Abbreviations, Atrial fibrillation (AF), pulmonary vein isolation (PVI), cryoballoon ablation (CBA), radiofrequency ablation (RFA), intracardiac echocardiography (ICE)
Introduction
Pulmonary vein isolation (PVI) is the primary method of catheter ablation for atrial fibrillation (AF), however its long term efficacy is limited, in part, by electrical reconnection of the veins to the left atrium. Dormant conduction has been shown to be a predictor of late reconnection and recurrence of AF[1]. If discovered at the time of ablation, additional lesions can be delivered to the implicated vein to achieve complete isolation. Adenosine has previously been demonstrated to reveal dormant conduction by activating adenosine-sensitive potassium channels leading to hyperpolarization of the resting membrane potential [2]. Large trials of adenosine usage with radiofrequency ablation (RFA) have shown conflicting results with regards to long term benefit on recurrence of AF [3], [4]. Unlike radiofrequency ablation, cryoballoon ablation (CBA) provides a circumferential lesion which theoretically can result in a more complete isolation of the pulmonary veins, however overall effectiveness is similar between radiofrequency and CBA [5]. As reconnection rates in general are lower with CBA than RFA, the utility of adenosine in this setting is unclear [3], [4], [6], [7].
MATERIALS AND METHODS
Data Source
A prospectively maintained database of patients undergoing first CBA at Northwestern Memorial Hospital was analyzed. Patients were included who underwent CBA between 2011 and 2015. Both patients with paroxysmal and persistent AF were included. This study was approved by the institutional review board at Northwestern University.
Ablation Procedure
A decapolar catheter was advanced through a left femoral venous sheath and positioned in the coronary sinus. An intracardiac echocardiography (ICE) catheter (AcuNav, Biosense Webster, Diamond Bar, California) was then advanced through a left femoral sheath into the right atrium. Baseline ICE imaging was done to survey left atrial and pulmonary vein (PV) anatomy and evaluate for thrombus. Transseptal catheterization was performed from the right femoral vein using a transseptal sheath (SL1, St. Jude Medical, St. Paul, Minnesota or Preface, Biosense Webster) with fluoroscopy and ICE for guidance. Intravenous heparin was administered with goal activated clotting time < 270 seconds (i-STAT 1, Abbott Point of Care, Princeton, New Jersey). A comprehensive EP study was performed in patients with a history of suspected supraventricular tachycardia. In some cases, chamber reconstruction of the left atrium was performed using a mapping system (NavX, St. Jude Medical). The transseptal sheath was exchanged for a larger steerable, 15F outer diameter sheath (Flexcath, Medtronic, Minneapolis, Minnesota) to accommodate the cryoballoon (CB) (Arctic Front or Arctic Front Advance, Medtronic) and circular mapping catheter (Achieve, Medtronic). The circular mapping catheter was advanced through the CB catheter into the pulmonary veins. Ablation was performed with the CB catheter. The choice of 23mm vs. 28mm CB was based on pre-procedural computed tomography or magnetic resonance imaging. Prior to each ablation, a pulmonary venogram was performed to assess balloon occlusion of the PV ostium. In cases where pre-procedure imaging was not performed, pulmonary venography was utilized to guide selection of CB size. A minimum of two cryoablations were made per PV, with each ablation lasting 3-4 minutes. During isolation of the right-sided PVs, a catheter was positioned in the superior vena cava to perform high-output pacing to monitor for phrenic nerve injury. Monitoring of compound motor action potential amplitude was added to the protocol early in the CBA experience [8]. Acute PVI was defined as entrance block [9], [10]. If PVI could not be achieved with the CB, focal ablation was performed using a conventional cryocatheter (Cryocath, Medtronic) or radiofrequency catheter to achieve PVI. Following PVI, reconnection was assessed after a mandatory 30-minute waiting period. The decision to use adenosine during the procedure was at the discretion of the primary operator. Adenosine was administered 30 minutes after attempted PV isolation starting in increments of 6 mg and increasing until transient complete heart block was achieved. Additional freezes and/or RF lesions were delivered for reconnected vein until dormant conduction was eliminated.
Post-Procedure Care and Follow-up
Oral anticoagulation was resumed within 6-24 hours of the procedure per the patient’s prior regimen of warfarin or other oral anticoagulant. In the case of warfarin, unfractionated heparin was administered by intravenous infusion starting 6 hours following removal of sheath unless the procedure was performed with a therapeutic INR. This regimen was transitioned to subcutaneous low molecular weight heparin the following morning to continue until therapeutic international normalized ratio was achieved. The first 3 months following the procedure were considered a blanking period during which time arrhythmic events were not classified as treatment failures. All antiarrhythmic medications were stopped after the blanking period. Rhythm follow-up included, at minimum, a 3-week AF monitor at 3 months post-procedure, and 24 to 48-hour Holter monitors thereafter at 6-month intervals up to two years, or downloads from implanted devices when available. Additional monitoring was performed in response to patient symptoms. Surface ECGs were obtained at each clinic visit.
Statistical Analysis
The primary endpoint, time to AF recurrence without the need for antiarrhythmic drugs, was defined as any episode >30 seconds after a 3-month blanking period and was assessed by Kaplan-Meier analysis. These designations are consistent with the HRS/EHRA/ECAS definition of recurrent AF.[9] Continuous variables were summarized by mean and standard deviation. Categorical variables were summarized by frequencies and proportions.
RESULTS
From 2011 to 2015, 406 patients underwent CBA, 361 of whom had greater than 3 months follow-up. The mean age was 61.7±10.0 years and 69% were male. There was no significant difference in duration of AF, prior use of anti-arrhythmic drugs, or CHADS2 score between those that did and did not receive adenosine ([Table 1]). In the adenosine group, 14% had persistent AF, compared with 23% in the no-adenosine group; the remainder were classified as paroxysmal AF except for one patient in the no-adenosine group with long-standing persistent AF. In the adenosine group, 31% of ablations were done with the first generation cryoballoon, compared with 13% of the no-adenosine group (p < 0.001). Fifteen percent of the ablations in the adenosine group were performed with the 23mm cryoballoon, compared with 10% of the no-adenosine group (p = 0.21).
Table 1. Baseline Characteristics.
| Variable | No Adenosine (N=283) | Adenosine (N=78) | P-value |
| Age (years) | 61.3 +- 10.4 | 62.9 +- 8.4 | 0.20 |
| Female, No. (%) | 94 (33%) | 18 (23%) | 0.09 |
| Diabetes, No. (%) | 28 (10%) | 7 (9%) | 0.83 |
| Hypertension, No. (%) | 116 (41%) | 37 (48%) | 0.27 |
| Structural Heart Disease, No. (%) | 44 (16%) | 6 (8%) | 0.07 |
| CHADS2 score, No. (%) | 0.37 | ||
| 0 | 141 (50%) | 37 (47%) | |
| 1 | 98 (35%) | 30 (38%) | |
| 2 | 36 (13%) | 7 (9%) | |
| 3 | 7 (2%) | 3 (4%) | |
| 4 | 0 (0%) | 1 (1%) | |
| 5 | 1 (0%) | 0 (0%) | |
| AF Duration (Years) | 5.8 +- 5.9 | 4.8 +- 3.8 | 0.14 |
| Antiarrhythmic Drug Use | 1.0 +- 0.7 | 0.9 +- 0.7 | 0.30 |
| Left Ventricular Ejection Fraction | 56.2 +- 10.7 | 59.2 +- 5.5 | 0.08 |
| Left Atrial Diameter (cm) | 3.8 +- 0.7 | 3.9 +- 0.6 | 0.60 |
| Redo, No. (%) | 56 (20%) | 16 (21%) | 0.90 |
| Device, No. (%) | 30 (11%) | 7 (9%) | 0.67 |
| Baseline Rhythm, No. (%) | 0.45 | ||
| AF | 86 (30%) | 22 (28%) | |
| Atypical Atrial Flutter | 2 (1%) | 0 (0%) | |
| Other | 1 (0%) | 1 (1%) | |
| Sinus | 187 (66%) | 55 (71%) | |
| Typical Atrial Flutter | 7 (2%) | 0 (0%) |
Adenosine testing was performed in 78 patients (21.6%) with a mean dose of 10.6 mg/vein. Of the 306 veins evaluated, 17 (6%) demonstrated dormant conduction with adenosine. Reconnection of pulmonary veins is summarized in [Figure 1]. The most commonly reconnected vein was the left inferior (6 occurrences), then right superior (4 occurrences), followed by left common (with 3). Left superior and right inferior were the least common veins to reconnect.
Figure 1. Distribution of reconnection of pulmonary veins with adenosine.
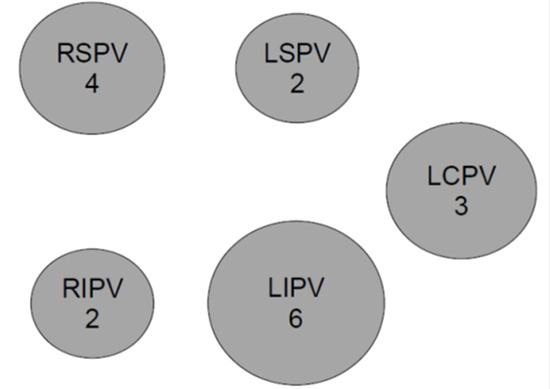
There was no significant difference between the mean dose of adenosine that resulted in dormant conduction (11.2 ± 3.1) and the mean dose that did not lead to reconnection (10.5 ± 3.7), p = 0.50. There were 8 veins (2.6%) that spontaneously reconnected during the 30-minute waiting period prior to the administration of adenosine. A common left pulmonary vein was present in 19% and 20% of the no-adenosine and adenosine groups, respectively. One patient had a third right pulmonary vein and received 2 CBA lesions to that vein. Over a median follow-up period of 14.4 months, there was no significant difference in freedom from AF between those that did and did not receive adenosine (p = 0.86) ([Figure 2]). Results were similar when stratified by paroxysmal and persistent AF ([Figure 3]). There was no significant difference in freedom from AF in 1st generation compared to 2nd generation cryoballoon (p = 0.91). Nine patients (2%) experienced complications, (7/283 in no-adenosine group and 2/78 in adenosine group, p = 0.96) with the most common complications being phrenic nerve injury (including transient injury) and bleeding events ([Table 2]). RFA was also used, primarily for additional lesion sets, in 8 patients (10%) in the adenosine group and 43 patients (15%) of the no-adenosine group (p = 0.65).
Figure 2. Freedom from AF by adenosine group; there was no significant difference in freedom from AF by adenosine group overall or by type of AF.
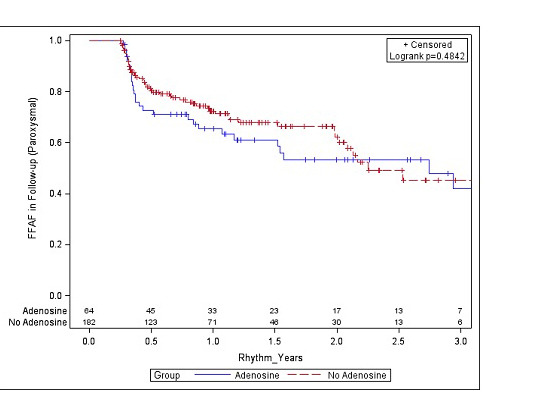
Figure 3. Figure 3.
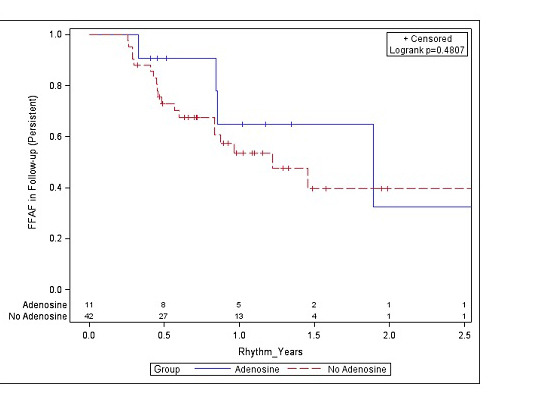
Table 2. Procedural Complications.
| No adenosine(N = 283) | Adenosine(N = 78) | P-value | |
| Procedural Complications, No. (%) | 7 (2%) | 2 (3%) | 0.96 |
| Bleeding, No. (%) | 1 (0%) | 1 (1%) | 0.33 |
| Perforation, No. (%) | 0 (0%) | 1 (1%) | 0.06 |
| Stroke, No. (%) | 0 (0%) | 0 (0%) | . |
| Pneumothorax, No. (%) | 0 (0%) | 0 (0%) | . |
| Phrenic nerve injury, No. (%) | 3 (1%) | 0 (0%) | 0.36 |
| Death, No. (%) | 0 (0%) | 0 (0%) | . |
| Other complication, No. (%) | 4 (1%) | 0 (0%) | 0.29 |
Discussion
In this study of 361 patients who underwent CBA, adenosine usage during CBA for AF did not improve freedom from AF. Notably, of those patients who received adenosine, the rate of reconnection was quite low at 6% of veins that were tested. This is similar to the rate noted by Ciconte et al. who found that just 4% of veins demonstrated reconnection after CBA [6].
Table 3. Additional lesion sets.
*Because some patient had multiple lesion sets performed, categories may add up to more than 100
| Type of lesion set, No. (%) | No-adenosine (N=41)* | Adenosine (N=5)* |
| Cavotricuspid linear lesion | 32 (78) | 4 (80) |
| Ablation at fractionated electrogram sites in left atrium | 6 (14) | 1 (20) |
| Left atrial linear roof line | 6 (14) | 0 |
| Left atrial linear mitral isthmus line | 6 (14) | 0 |
| Other left atrial linear lesion | 3 (7) | 0 |
| Isolation of superior vena cava | 3 (7) | 0 |
| Ablation of autonomic ganglion plexus | 1 (2) | 0 |
| Ablation at fractionated electrogram sites in right atrium | 1 (2) | 0 |
Reconnection rates are higher in RFA with three large studies finding rates of 21% (ADVICE), 27% (UNDER-ATP) and 34% [3], [4], [7]. With far fewer veins reconnecting with adenosine during CBA, the lack of difference in recurrence of AF over time is not surprising. With improvement in rates of complete isolation of the pulmonary veins, the additional effect of adenosine is considerably lessened. As a result, we found that routine use of adenosine in CBA does not improve long-term outcomes in AF. Results from the randomized trials of adenosine with RFA have been mixed. Interestingly, Ghanbari et al. noted that although adenosine did reveal dormant conduction, this difference did not translate to improvement in long-term outcomes. It is speculated that adenosine identifies acute pulmonary vein reconnection but is not predictive of long term reconnection [7].
In contrast to our findings, a study by Kumar et al. involving 90 patients (45 of whom received adenosine) and a study by Van Belle et al. of 99 patients (34 receiving adenosine) did find an improvement in clinical success rates for AF with CBA, despite similarly low rates of vein reconnection [11], [12]. A second study also by Kumar et al. investigated this question as well in 40 patients using CBA but did not find any difference in long-term success with 337 ± 92 days of follow-up [13]. The differing results obtained in the present study are likely due to the larger sample size. Additionally, the present study includes the longest follow-up to date of any CBA study with adenosine. Differences in effectiveness of adenosine in previously published studies may in part be due to variation in incorporation of a waiting period after ablation prior to giving adenosine or pursuing further ablation. Incorporation of a thirty minute waiting period after CBA has previously been shown to increase the incidence of dormant conduction in a study by Compier et al [14].
Although the decision to use adenosine was at the discretion of the primary operator, the majority of adenosine cases were performed by a single operator who routinely used adenosine in all cases. The dose of adenosine used was not significantly different for the veins that reconnected and those that did not; in each case, this dose was based on the minimum amount necessary to achieve AV block, a strategy supported by a study by Kapa et al [15].
The most common veins to demonstrate dormant conduction with adenosine were the LIPV and RSPV in our study. In comparison, UNDER-ATP reported that LSPV and RSPV were the most common to reconnect with RFA [3].
Limitations of this study include the lack of a randomized study design. Additionally, rhythm evaluation at follow-up was determined by routine ECGs and event monitors at 3-month intervals or if symptoms necessitated additional evaluations, however it is possible that asymptomatic recurrence of AF in between evaluations was not detected. The AchieveTM catheter was used to detect PV potentials in all cases; though this catheter may detect more farfield signals than the typical lasso used in RF, the sensitivity of the AchieveTM for detecting dormant potentials has not yet been determined [16].
Conclusion
Dormant conduction with adenosine is uncommon during CBA compared to RFA and use of adenosine does not improve freedom from AF.
Conflict Of Interests
None.
Disclosures
Kaplan – none; Dandamudi – none; Bohn – none; Verma – none; Tomson – none; Arora – none; Chicos – none; Goldberger – none; Kim – none; Knight – speaker’s bureau, compensation for services, research grants from Medtronic; Lin – none; Passman – speaker’s bureau, compensation for services, research grants from Medtronic
This research was presented during an oral abstract session at Heart Rhythm Society Scientific Sessions, May 2016, San Francisco, CA.
References
- 1.Cappato Riccardo, Negroni Silvia, Pecora Domenico, Bentivegna Stefano, Lupo Pier Paolo, Carolei Adriana, Esposito Cristina, Furlanello Francesco, De Ambroggi Luigi. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003 Sep 30;108 (13):1599–604. doi: 10.1161/01.CIR.0000091081.19465.F1. [DOI] [PubMed] [Google Scholar]
- 2.Datino Tomás, Macle Laurent, Qi Xiao-Yan, Maguy Ange, Comtois Philippe, Chartier Denis, Guerra Peter G, Arenal Angel, Fernández-Avilés Francisco, Nattel Stanley. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation. 2010 Mar 02;121 (8):963–72. doi: 10.1161/CIRCULATIONAHA.109.893107. [DOI] [PubMed] [Google Scholar]
- 3.Macle Laurent, Khairy Paul, Weerasooriya Rukshen, Novak Paul, Verma Atul, Willems Stephan, Arentz Thomas, Deisenhofer Isabel, Veenhuyzen George, Scavée Christophe, Jaïs Pierre, Puererfellner Helmut, Levesque Sylvie, Andrade Jason G, Rivard Lena, Guerra Peter G, Dubuc Marc, Thibault Bernard, Talajic Mario, Roy Denis, Nattel Stanley. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet. 2015 Aug 15;386 (9994):672–9. doi: 10.1016/S0140-6736(15)60026-5. [DOI] [PubMed] [Google Scholar]
- 4.Kobori Atsushi, Shizuta Satoshi, Inoue Koichi, Kaitani Kazuaki, Morimoto Takeshi, Nakazawa Yuko, Ozawa Tomoya, Kurotobi Toshiya, Morishima Itsuro, Miura Fumiharu, Watanabe Tetsuya, Masuda Masaharu, Naito Masaki, Fujimoto Hajime, Nishida Taku, Furukawa Yoshio, Shirayama Takeshi, Tanaka Mariko, Okajima Katsunori, Yao Takenori, Egami Yasuyuki, Satomi Kazuhiro, Noda Takashi, Miyamoto Koji, Haruna Tetsuya, Kawaji Tetsuma, Yoshizawa Takashi, Toyota Toshiaki, Yahata Mitsuhiko, Nakai Kentaro, Sugiyama Hiroaki, Higashi Yukei, Ito Makoto, Horie Minoru, Kusano Kengo F, Shimizu Wataru, Kamakura Shiro, Kimura Takeshi. Adenosine triphosphate-guided pulmonary vein isolation for atrial fibrillation: the UNmasking Dormant Electrical Reconduction by Adenosine TriPhosphate (UNDER-ATP) trial. Eur. Heart J. 2015 Dec 07;36 (46):3276–87. doi: 10.1093/eurheartj/ehv457. [DOI] [PubMed] [Google Scholar]
- 5.Kuck Karl-Heinz, Brugada Josep, Fürnkranz Alexander, Metzner Andreas, Ouyang Feifan, Chun K R Julian, Elvan Arif, Arentz Thomas, Bestehorn Kurt, Pocock Stuart J, Albenque Jean-Paul, Tondo Claudio. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016 Jun 09;374 (23):2235–45. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 6.Ciconte Giuseppe, Chierchia Gian-Battista, DE Asmundis Carlo, Sieira Juan, Conte Giulio, Juliá Justo, DI Giovanni Giacomo, Wauters Kristel, Baltogiannis Giannis, Saitoh Yukio, Mugnai Giacomo, Catanzariti Domenico, Tondo Claudio, Brugada Pedro. Spontaneous and adenosine-induced pulmonary vein reconnection after cryoballoon ablation with the second-generation device. J. Cardiovasc. Electrophysiol. 2014 Aug;25 (8):845–51. doi: 10.1111/jce.12421. [DOI] [PubMed] [Google Scholar]
- 7.Ghanbari Hamid, Jani Ronak, Hussain-Amin Atheer, Al-Assad Wassim, Huether Elizabeth, Ansari Sardar, Jongnarangsin Krit, Crawford Thomas, Latchamsetty Rakesh, Bogun Frank, Morady Fred, Oral Hakan, Chugh Aman. Role of adenosine after antral pulmonary vein isolation of paroxysmal atrial fibrillation: A randomized controlled trial. Heart Rhythm. 2016 Feb;13 (2):407–15. doi: 10.1016/j.hrthm.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi Frédéric, Dubuc Marc, Guerra Peter G, Delisle Stéphane, Romeo Philippe, Landry Evelyn, Koutbi Linda, Rivard Léna, Macle Laurent, Thibault Bernard, Talajic Mario, Roy Denis, Khairy Paul. Diaphragmatic electromyography during cryoballoon ablation: a novel concept in the prevention of phrenic nerve palsy. Heart Rhythm. 2011 Jun;8 (6):885–91. doi: 10.1016/j.hrthm.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife José, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9 (4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Andrade Jason, Khairy Paul, Dubuc Marc, Deyell Marc W, Roy Denis, Talajic Mario, Thibault Bernard, Guerra Peter G, Rivard Léna, Macle Laurent. The time course of exit and entrance block during cryoballoon pulmonary vein isolation. Europace. 2014 Apr;16 (4):500–4. doi: 10.1093/europace/eut231. [DOI] [PubMed] [Google Scholar]
- 11.Kumar Narendra, Dinh Trang, Phan Kevin, Timmermans Carl, Philippens Suzanne, Dassen Willem, Vranken Nousjka, Pison Laurent, Maessen Jos, Crijns Harry J. Adenosine testing after second-generation cryoballoon ablation (ATSCA) study improves clinical success rate for atrial fibrillation. Europace. 2015 Jun;17 (6):871–6. doi: 10.1093/europace/euu352. [DOI] [PubMed] [Google Scholar]
- 12.Van Belle Y L E, Janse P A, de Groot N M S, Anné W, Theuns D A M J, Jordaens L J. Adenosine testing after cryoballoon pulmonary vein isolation improves long-term clinical outcome. Neth Heart J. 2012 Nov;20 (11):447–55. doi: 10.1007/s12471-012-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar Narendra, Blaauw Yuri, Timmermans Carl, Pison Laurent, Vernooy Kevin, Crijns Harry. Adenosine testing after second-generation balloon devices (cryothermal and laser) mediated pulmonary vein ablation for atrial fibrillation. J Interv Card Electrophysiol. 2014 Oct;41 (1):91–7. doi: 10.1007/s10840-014-9921-z. [DOI] [PubMed] [Google Scholar]
- 14.Compier Marieke G, De Riva Marta, Dyrda Katia, Zeppenfeld Katja, Schalij Martin J, Trines Serge A. Incidence and predictors of dormant conduction after cryoballoon ablation incorporating a 30-min waiting period. Europace. 2015 Sep;17 (9):1383–90. doi: 10.1093/europace/euu411. [DOI] [PubMed] [Google Scholar]
- 15.Kapa Suraj, Killu Ammar, Deshmukh Abhishek, Mulpuru Siva K, Asirvatham Samuel J. Dose-dependent pulmonary vein reconnection in response to adenosine: relevance of atrioventricular block during infusion. J Interv Card Electrophysiol. 2016 Oct;47 (1):117–123. doi: 10.1007/s10840-016-0149-y. [DOI] [PubMed] [Google Scholar]
- 16.Andrade Jason G, Dubuc Marc, Collet Daina, Khairy Paul, Macle Laurent. Pulmonary vein signal interpretation during cryoballoon ablation for atrial fibrillation. Heart Rhythm. 2015 Jun;12 (6):1387–94. doi: 10.1016/j.hrthm.2015.02.027. [DOI] [PubMed] [Google Scholar]


