Abstract
Background
Use of corticosteroids before and after atrial fibrillation (AF) ablation can decrease acute inflammation and reduce AF recurrence.
Purpose
To assess the efficacy of oral prednisone in improving the outcomes of pulmonary vein isolation with radiofrequency ablation and its effect on inflammatory cytokine.
Methods
A total of 60 patients with paroxysmal AF undergoing radiofrequency ablation were randomized (1:1) to receive either 3 doses of 60 mg daily of oral prednisone or a placebo. Inflammatory cytokine levels (TNF-α, IL-1, IL6, IL-8) were measured at baseline, prior to ablation, immediately after ablation, and 24 hours post ablation. Patients underwent 30 day event monitoring at 3 months, 6 months and 12 months post procedure.
Results
Immediate post ablation levels of inflammatory cytokines were lower in the steroid group when compared to the placebo group; IL-6: 9.0 ±7 vs 15.8 ±13 p=0.031; IL-8: 10.5 ±9 vs 15.3 ±8; p=0.047 respectively. Acute PV reconnection rates during the procedure (7/23% vs 10/36%; p = 0.39), and RF ablation time (51±13 vs 56±11 min, p = 0.11) trended to be lower in the placebo group than the steroid group. There was no difference in the incidence of early recurrence of AF during the blanking period and freedom from AF off AAD at 12 months between both groups (5/17% vs 8/27%; p = 0.347 and 21/70% vs 18/60%; p=0.417 in placebo and steroid groups respectively).
Conclusion
Although oral corticosteroids have significant effect in lowering certain cytokines, it did not impact the clinical outcomes of AF ablation.
Keywords: Atrial Fibrillation, Ablation, Pulmonary Vein Isolation, Interleukin, Tumor Necrosis Factor, Corticosteroid, Recurrence, Inflammatory cytokines, Prednisone
Introduction
Pulmonary vein isolation (PVI) is an effective treatment for symptomatic, drug refractory patients with paroxysmal atrial fibrillation (AF). Although the procedure decreases symptoms and AF burden in patients, the success rate of single procedure is only between 50%-75% [1]-[3] necessitating repeat procedures to improve the overall success rate [4]-[9]. Immediate post procedural atrial tissue inflammation can cause significant early recurrence of AF (ERAF). The long term recurrence of AF often is thought to be due to a persistent conduction gaps between the left atrium (LA) and pulmonary veins (PV).[10]-[13] The Inflammatory process associated with PVI can create significant local tissue edema resulting in transient loss of conduction in an area where permanent injury has not occurred. This can result in under ablation and subsequent reconnection of the PVs.
The anti-inflammatory effect of corticosteroids was studied extensively in cardiac surgery in the past. Corticosteroids have been shown to exert significant anti-inflammatory response as is evidenced by decreasing the levels of IL-6, IL-8, TNF, CRP, and oxygen free radicals after cardiac surgery [10]-[13]. Since the inception of our study, a few groups around the world have published data on the impact of steroids and AF ablation outcomes with significant variability. Koyama et al reported that the use of corticosteroid after AF ablation significantly decreases the immediate and late AF recurrence. However, 3 subsequent studies with different doses of IV corticosteroids found no effect in preventing early and late recurrence of AF [3], [13]-[15]. All of these studies attempted to understand the impact of only intra or post procedural steroid use. This approach may potentially suppress the immediate post ablation inflammation but may not have any impact on the intra-procedural reduction of tissue edema. We therefore attempted to study the impact of pre-treating at least 48 hours prior to the procedure to enable effective suppression of intra-procedural acute inflammatory response. This has not been addressed by any other study done so far. We aimed to systematically assess these effects through the measurement of inflammatory markers. The purpose of our study was to determine if the use of pre procedural corticosteroid can prevent early and late AF recurrence post ablation and evidence of reduction of inflammation through systemic cytokine assessment.
Methods
Study Population
We screened 105 patients of whom 60 patients with symptomatic drug refractory paroxysmal AF met inclusion and exclusion criteria and were enrolled in the study between September 2010 and November 2013. There were 30 patients in the steroid group and 30 patients in the placebo group. All patients were de novo AF ablation candidates. All patients failed at least 1 antiarrhythmic drug (AAD). AADs were discontinued 5 half-lives before the ablation procedure except for amiodarone. In those who were on AAD, it was continued for at least 8 weeks post ablation, and discontinued if no recurrence was found. The study was approved by the Institutional Review Board, and written consent was obtained from all participants. Patients were excluded due to history of corticosteroid use within 1 week of the study, use of non-steroidal anti- inflammatory drugs (NSAIDs), or colchicine within 1 week of the study,immunosuppressive disorders, chronic persistent AF, uncontrolled diabetes, or any other autoimmune disorders.
Study design
This is a prospective, randomized, double-blinded study. All patients were randomized for treatment with corticosteroid (corticosteroid group), or a placebo (placebo group) 1 day prior, on the day of ablation and 1 day after the procedure with the help of the investigational pharmacy staff to blind and dispense the drug and the placebo.
Steroid Administration
In the corticosteroid group, 60 mg of oral prednisone was administered one day prior, on the day of procedure and one day after the procedure. An oral lactose pill was administered to the placebo group with the same schedule.
Adverse effect monitoring
Fasting glucose levels were performed on all patients. Glucose levels were checked before each meal and before bed in patient with diabetes mellitus (DM). Hyperglycemia was defined as fasting glucose >110 mg/dl and post prandial glucose >180 mg/dl. Patients were also monitored for signs of fluid retention and infection.
Monitoring of AF Recurrence
Patients were monitored on telemetry during hospitalization. After discharge, patients underwent 1 month event monitoring at 3 months, 6 months and at 12 months post procedure. Any episode of AF lasting more than 30 seconds was considered as recurrence. Recurrence of any atrial arrhythmias (atrial tachycardia (AT) or AF) at 3 months, 6 months, and 12 months post ablation was recorded for the assessment of endpoints.
Inflammatory Cytokines Monitoring
A blood sample (5 ml) from antecubital vein was collected from all subjects at the time of before randomization which served as baseline sample. Blood samples were collected at the beginning and end of ablation procedure and at 24 hour post ablation for cytokine measurement (IL1, 6, 8, and TNF α). All blood samples were centrifuged to collect serum and froze at -70° C. Enzyme-linked immunosorbent assays (ELISA) were performed on the serum samples using kits (ELISA kit II, BD Bioscience, USA) for human-specific IL1β (Cat. No: 557966), IL6 (Cat. No: 550799), IL8 (Cat. No: 550799) and TNF-α (Cat. No: 550610), according to the manufacturer’s instructions.
Catheter Ablation and assessment of PVI
All patients underwent pre-procedural Cardiac CT to define Pulmonary vein anatomy and pre -procedural TEE to exclude thrombus. Three dimensional mapping of LA was reconstructed with CARTO (Biosense Webster, Inc, Diamond Bar, CA, USA) or Velocity (SJM, Minneapolis, MN,USA) electroanatomic mapping system. PVI was performed using 3.5/4.0 mm irrigated tip catheter (Thermocool, Biosense Webster, Inc or Coolpath Flex, SJM) with a maximum temperature of 50 º C and power output of 25-35 Watts using a roving Lasso technique at the antral level. A circular mapping catheter (Lasso, Biosense Webster, Inc) was used to confirm PVI. Bidirectional conduction block from the atrium to the PV and vice versa were confirmed. We performed induction testing using burst pacing or isoprotenolol and targeted if there were other non PV triggers only. If a patient had inducible right atrial flutter or a prior history of flutter, a cavo-tricuspid isthmus ablation was performed. No other lesions were allowed. Thirty minutes after the isolation of each PV, reconnection rates were assessed and re-ablated if necessary. Adenosine was not used for evaluation of dormant conduction. All patients had all PVs isolated at the end of the procedure.
Follow Up
Patient remained hospitalized under continuous rhythm monitoring for at least 24 hour after RFA with subsequent follow up in 3 months, 6 months and 12 month. 12 lead EKG was performed during every clinic visit with intensive questioning regarding any arrhythmia related symptoms. Additionally patients underwent 30 days event monitors at 3, 6 and 12 months post-ablation. All patients remained on antiarrhythmic drug for the first 2 months. In those who had symptomatic recurrences with in the first 2 months cardioversion and or AADs were changes; repeat ablation were performed if cardioversion and change in AAD did not alleviate AF symptoms.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation and categorical variables as proportions. Univariate analyses were performed using Chi-Square test (with or without Fisher’s exact correction) for categorical variables and Student t-test for continuous variables. Paired tests were performed when appropriate. McNemar test was used for evaluating paired samples. Pre and post ablation inflammatory markers were compared using paired t-test. Statistical analysis was considered significant at p values ≤ 0.05. Statistical analysis was performed using SPSS version 23.0 (IBM Inc., USA). Binary logistic regression was used for multivariable analysis. All known variables impacting the recurrence of AF were entered into the multivariate model. A two sided p-value ≤0.05 was considered statistically significant.
Results
Study population
We screened a total of 105 patients of whom 60 patients met inclusion criteria and were subsequently randomized to receive prednisone or placebo.
Baseline and clinical Characteristics
Baseline characteristics are shown in [Table 1]. Both groups comprised of 30 patients each. Baseline characteristics of mean age, gender, comorbidities, echocardiographic parameters, antiarrhythmic drugs and medications were not significantly different between 2 groups.
Table 1. Comparison of baseline characteristics, procedural variables and outcomes of atrial fibrillation after atrial fibrillation ablation between both the groups.
| Clinical characteristics | Placebo (n=30) | Corticosteroid (n=30) | p Value |
|---|---|---|---|
| Age | 63 ± 8.9 | 63 ± 8.7 | 0.65 |
| Body mass index | 28.5 ± 5.2 | 30.5 ± 5.9 | 0.21 |
| AF Duration (year) | 6.9 ± 6.7 | 4.1 ± 4.1 | 0.059 |
| LV Ejection Fraction (%) | 56.2 ± 8.4 | 57 ± 4.7 | 0.70 |
| LA Diameter (cm) | 4.2 ±0.64 | 4.6 ±0.7 | 0.12 |
| Procedure time (Minutes) | 166 ± 47 | 174 ± 38 | 052 |
| Male | 22 (73) | 24 (80) | 0.76 |
| Caucasian | 26 (87) | 26 (87) | 1.0 |
| Hypertension | 12 (40) | 18 (60) | 0.19 |
| Coronary Artery Disease | 9 (30) | 12 (40) | 0.58 |
| Obstructive Sleep Apnea | 9 (30) | 11 (36.7) | 0.78 |
| Diabetes | 2 (6.7) | 6 (20) | 0.25 |
| Chronic Kidney Disease | 3 (10) | 2 (6.7) | 1.0 |
| Tobacco use | 6 (20) | 6 (20) | 1.0 |
| Valvular Disease | 2 (6.7) | 1 (3.3) | 1.0 |
| COPD | 1 (3.3) | 2 (6.7) | 1.0 |
| Antiarrhythmic | 8 (26.6) | 4 (13.3) | 0.33 |
| Class 1 | |||
| Class 3 | 15 (50) | 17 (56.7) | 0.79 |
| Medication | 13 (43) | 12 (40) | 1.0 |
| Beta Blocker | |||
| Calcium channel blocker | 7 (23) | 6 (20) | 1.0 |
| Total Fluoroscopy Time (minutes) | 54.8 ± 17.2 | 54.7 ± 16.5 | 0.9 |
| Total RF tim (minutes) | 51 ±13.5 | 56 ±10.7 | 0.11 |
| Acute PV Reconnection rate | 7 (23) | 10 (36) | 0.39 |
| Early Recurrence (0-3 months); % | 5 (17) | 8 (27) | 0.347 |
| Recurrence after blanking period (3-12 months); % | 9 (30) | 12 (40) | 0.417 |
Procedural Characteristics
Catheter ablation parameters and intra-procedural recurrences are presented in [Table 1]. PVI was successfully performed in all patients and bidirectional block was achieved in all PVs. The catheter ablation parameters were comparable between the 2 groups. Total procedure and fluoroscopic times were not different between them. Acute PV reconnection rates during the procedure (23% vs 36%; p=0.39), and RF ablation time (51 ±13 vs 56 ±11 mins, p = 0.11) were lower in the placebo group than the steroid group although not statistically significant.
Complications
Procedure Related
There were 5 small to moderate sized groin hematomas noted after the procedure, 3 in the placebo group and 2 in the steroid group which resolved. None of these patients required blood transfusions or surgical repair. There were no strokes, atrio-esophageal injuries, diaphragmatic paralysis, PV stenosis, pericarditis or cardiac tamponade reported in both groups.
Steroid Related
There were 6 diabetic patients in the steroid group who needed slightly higher dose of insulin during peri-procedural period, however, no diabetic ketoacidosis or hyperglycemic hyperosmolar state induced by the steroid. Patients without DM did not experience hyperglycemia that required treatment.
AT/AF recurrence
There was no difference in the incidence of early recurrence of AT/AF during the blanking period and freedom from AT/AF off AAD at 12 months between both groups (5/17% vs 8/27%; p = 0.347 and 21/70% vs 18/60%; p = 0.417 in placebo and steroid groups respectively). A total of 18 (30%) patients underwent re-do ablation for recurrent AT/AF with no significant differences in between both the groups (7/23% vs. 11/37%; p= 0.267 in the placebo and steroid group respectively).
Levels of Inflammatory Cytokines
The Interleukin-6 (IL-6) and IL-8 post ablation was significantly lower in the steroid group than the control group (9.03 ± 7.026 vs 15.78 ± 13.19; p = 0.031) and (10.54 ± 9.63 vs 15.3 ±8.14; p = 0.047) respectively. However, no statistically significant difference noted in the level of TNF α, and IL 1 at all 4 time points between the two groups. See [Figure 1].
Figure 1. Comparison of inflammatory cytokines level at 4 different time points.
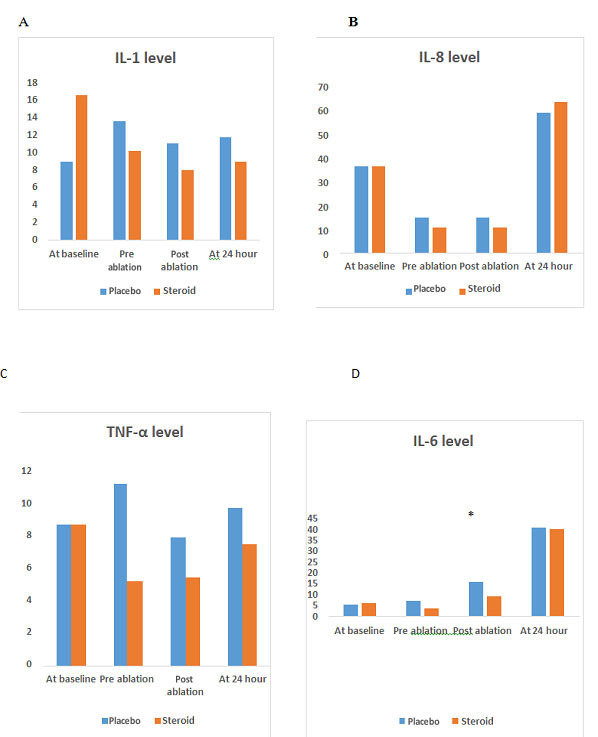
Discussion
Major Findings
The main findings of our study are - 1) Oral prednisone during peri-procedural period did not impact the outcome of AF ablation. 2) The levels of inflammatory cytokines, specifically the IL-6 and IL-8 immediately post- ablation were significantly lower in the steroid group after ablation suggestive of effective suppression of systemic anti-inflammatory response by steroids.
Role of Steroid in AF Recurrence post Ablation
Previous Studies
There were no prospective clinical studies performed at the time of conception of this study. However, multiple studies were published since and have variable results. The role of steroids in preventing AF recurrence post ablation was first studied by Koyama et al in 2010 and showed decreased AF recurrence rate in the immediate (0-3 days) and during long term follow up (14 months). This study used 2mg/kg IV hydrocortisone on the day of procedure, followed by oral prednisolone (0.5 mg/kg/day) for 3 days. In addition, cavo-tricuspid isthmus ablation was done in all patients and additional ablation consisting of linear ablation of LA roof line and SVC isolation was performed if AF was induced with coronary sinus burst pacing and isoproterenol.[15] Later Won et al (2013) performed a similar study using low dose hydrocortisone (IV 100 mg ) administered within 30 minutes post procedure with no difference in AF recurrence between steroid and placebo group. [13] Similarly, Andrade et al (2013) with 250 mg IV hydrocortisone immediately after transseptal puncture, Kim et al (2015) with low dose (100 mg IV hydrocortisone) and high dose (125 mg IV hydrocortisone) within 30 minutes post procedure did not show any difference in recurrence of AF. [14] Most of the clinical studies have been negative akin to our current study. This clearly points to the fact perhaps use of systemic corticosteroids and suppression of inflammation has no definitive impact on clinical outcomes in AF ablation.
Type and timing of Steroid Administration
The major difference between our study and the previous 4 studies described above was the timing of steroid administration and the type of steroid used. Prednisone has a half-life of 12-36 hours and it has 5 times more glucocorticoid potency than hydrocortisone. Based on pharmacologic properties, we chose prednisone which is an intermediate acting steroid and administered 3 doses prior to ablation to maximize the anti-inflammatory effect and prevent tissue edema which was thought to contribute to gaps in PV isolation. The 60 mg prednisone is equivalent to 300 mg IV hydrocortisone which has half-life of 8-12 hours. Contrary to our hypothesis, this strategy did not show any efficacy in preventing AF recurrence. Our study is in agreement with 3 previous studies. Positive effects reported by Koyama et al may be due to prolonged post procedural steroid therapy and possible suppression of regional and systemic inflammation in the setting of more aggressive ablation.[13]
Steroids and AF Recurrence
Although not statistically significant, our study found a trend towards higher recurrence rate in the steroid group. Studies have shown that acute administration of hydrocortisone can result in rapid concentration dependent cellular hyperpolarization in neural and cardiac tissue. [3], [16]-[18] This hyperpolarization is thought to counteract RF induced membrane depolarization in the surrounding tissue requiring prolonged ablation time and higher probability of conduction gaps or acute reconnections. [19]
Effects of Steroid on Pulmonary Reconnection Rates and Radiofrequency time
Similar to the study by Andrade et al, our study shows trend towards higher RF time and PV reconnection rate. Interestingly study Andrade et al. showed that pre-procedural steroids are associated with higher prevalence of dormant conduction and increased RF ablation time to accomplish PV isolation.[3] In addition, two large population based case control studies found an increased risk of AF during current use [20] or high dose [21] regimen of corticosteroid therapy.[22] Perhaps steroid inhibits effective scar formation from the ablation lesions, as a result of which partially injured atrial tissue has a higher propensity to recover due to the effect of the steroids and result in non-durable PV isolation.
Figure 2. Corticosteroid Comparison Chart.
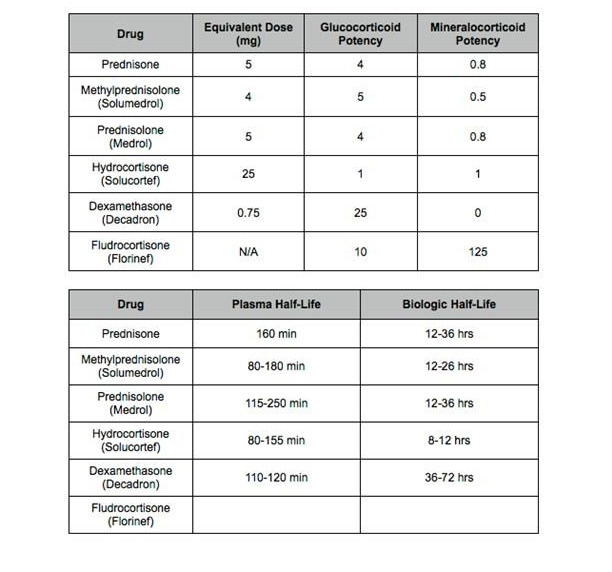
Inflammatory Cytokines and AF Recurrence
Our study showed a significantly lower IL-6 and IL-8 post procedure in the steroid group which reflected the anti-inflammatory effect of steroid;however, there was no association between these cytokine changes and AF recurrence. There is uncertainty whether the inflammation from ablation promotes AF, or if the inflammation is caused by the arrhythmia itself or both. Previous studies have shown CRP elevation after RFA which correlated with early arrhythmia recurrence suggesting that extensive tissue damage from ablation may be pro inflammatory. [23]-[25] However, animal studies by Nascimento et al using young healthy pigs without arrhythmia that was divided in to placebo, sham ablation, and ablation with 500 mg IV methylprednisolone showed no difference in CRP level and similar histological findings in all 3 groups suggestive of extensive tissue damage due to ablation suggesting that RF energy per se was not responsible for systemic inflammation and the rise in CRP was rather related to procedural stress.[25]
Study Limitation
It is a relatively small study with all the obvious limitations. We also realize that our study was probably underpowered to detect a difference in outcomes between the groups. However, this was a randomized double blind placebo control study with relevant inflammatory markers measured at various time lines clearly establishing the linear relationship between steroid use and anti-inflammatory effects. We did not measure CRP levels as we deemed it to be too non-specific and has not been shown to be very useful in the previous studies.
Conclusion
Use of oral corticosteroids resulted in significant anti-inflammatory effect as evidenced by reduction in inflammatory cytokine levels with no impact on the clinical outcomes of AF ablation. There is a trend towards higher incidence of AF recurrence, higher PV reconnection rate, and longer RF ablation time with administration of steroids.
Conflict Of Interests
None.
Disclosure
None.
References
- 1.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife Jose, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012 Apr;14 (4):528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 2.Santangeli Pasquale, Marchlinski Francis E. Pulmonary vein isolation for atrial fibrillation: forever young. J. Am. Coll. Cardiol. 2014 Dec 16;64 (23):2468–70. doi: 10.1016/j.jacc.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 3.Andrade Jason G, Khairy Paul, Nattel Stanley, Vanella Agustin, Rivard Léna, Guerra Peter G, Dubuc Marc, Dyrda Katia, Thibault Bernard, Talajic Mario, Mondesert Blandine, Roy Denis, Macle Laurent. Corticosteroid use during pulmonary vein isolation is associated with a higher prevalence of dormant pulmonary vein conduction. Heart Rhythm. 2013 Oct;10 (10):1569–75. doi: 10.1016/j.hrthm.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 4.Chen Michael S, Marrouche Nassir F, Khaykin Yaariv, Gillinov A Marc, Wazni Oussama, Martin David O, Rossillo Antonio, Verma Atul, Cummings Jennifer, Erciyes Demet, Saad Eduardo, Bhargava Mandeep, Bash Dianna, Schweikert Robert, Burkhardt David, Williams-Andrews Michelle, Perez-Lugones Alejandro, Abdul-Karim Ahmad, Saliba Walid, Natale Andrea. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J. Am. Coll. Cardiol. 2004 Mar 17;43 (6):1004–9. doi: 10.1016/j.jacc.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 5.Chen S A, Hsieh M H, Tai C T, Tsai C F, Prakash V S, Yu W C, Hsu T L, Ding Y A, Chang M S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999 Nov 02;100 (18):1879–86. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 6.Haïssaguerre M, Jaïs P, Shah D C, Garrigue S, Takahashi A, Lavergne T, Hocini M, Peng J T, Roudaut R, Clémenty J. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000 Mar 28;101 (12):1409–17. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 7.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 03;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 8.Marchlinski Francis E, Callans David, Dixit Sanjay, Gerstenfeld Edward P, Rho Robert, Ren Jian-Fang, Zado Erica. Efficacy and safety of targeted focal ablation versus PV isolation assisted by magnetic electroanatomic mapping. J. Cardiovasc. Electrophysiol. 2003 Apr;14 (4):358–65. doi: 10.1046/j.1540-8167.2003.02468.x. [DOI] [PubMed] [Google Scholar]
- 9.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000 Nov 21;102 (21):2619–28. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 10.Bourbon A, Vionnet M, Leprince P, Vaissier E, Copeland J, McDonagh P, Debré P, Gandjbakhch I. The effect of methylprednisolone treatment on the cardiopulmonary bypass-induced systemic inflammatory response. Eur J Cardiothorac Surg. 2004 Nov;26 (5):932–8. doi: 10.1016/j.ejcts.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Kilger Erich, Weis Florian, Briegel Josef, Frey Lorenz, Goetz Alwin E, Reuter Daniel, Nagy Andreas, Schuetz Albert, Lamm Peter, Knoll Anette, Peter Klaus. Stress doses of hydrocortisone reduce severe systemic inflammatory response syndrome and improve early outcome in a risk group of patients after cardiac surgery. Crit. Care Med. 2003 Apr;31 (4):1068–74. doi: 10.1097/01.CCM.0000059646.89546.98. [DOI] [PubMed] [Google Scholar]
- 12.Liakopoulos Oliver J, Schmitto Jan D, Kazmaier Stefan, Bräuer Anselm, Quintel Michael, Schoendube Friedrich A, Dörge Hilmar. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann. Thorac. Surg. 2007 Jul;84 (1):110–8. doi: 10.1016/j.athoracsur.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Won Hoyoun, Kim Jong-Youn, Shim Jaemin, Uhm Jae-Sun, Pak Hui-Nam, Lee Moon-Hyoung, Joung Boyoung. Effect of a single bolus injection of low-dose hydrocortisone for prevention of atrial fibrillation recurrence after radiofrequency catheter ablation. Circ. J. 2013;77 (1):53–9. doi: 10.1253/circj.cj-12-0728. [DOI] [PubMed] [Google Scholar]
- 14.Kim Da-Rae, Won Hoyoun, Uhm Jae-Sun, Kim Jong-Youn, Sung Jung-Hoon, Pak Hui-Nam, Lee Moon-Hyoung, Joung Boyoung. Comparison of two different doses of single bolus steroid injection to prevent atrial fibrillation recurrence after radiofrequency catheter ablation. Yonsei Med. J. 2015 Mar;56 (2):324–31. doi: 10.3349/ymj.2015.56.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyama Takashi, Tada Hiroshi, Sekiguchi Yukio, Arimoto Takanori, Yamasaki Hiro, Kuroki Kenji, Machino Takeshi, Tajiri Kazuko, Zhu Xu Dong, Kanemoto-Igarashi Miyako, Sugiyasu Aiko, Kuga Keisuke, Nakata Yoshio, Aonuma Kazutaka. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J. Am. Coll. Cardiol. 2010 Oct 26;56 (18):1463–72. doi: 10.1016/j.jacc.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 16.Hu Zhuang-li, Liu Hui, Hu Yan, Zhang De-yong, Sun Zong-quan, Jin Man-wen. [Effects of hydrocortisone sodium succinate on sodium current in human and guinea pig cardiac myocytes]. Yao Xue Xue Bao. 2004 Apr;39 (4):250–3. [PubMed] [Google Scholar]
- 17.Lovallo William R, Robinson Jennifer L, Glahn David C, Fox Peter T. Acute effects of hydrocortisone on the human brain: an fMRI study. Psychoneuroendocrinology. 2010 Jan;35 (1):15–20. doi: 10.1016/j.psyneuen.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pape H C. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu. Rev. Physiol. 1996;58 ():299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- 19.Datino Tomás, Macle Laurent, Qi Xiao-Yan, Maguy Ange, Comtois Philippe, Chartier Denis, Guerra Peter G, Arenal Angel, Fernández-Avilés Francisco, Nattel Stanley. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation. 2010 Mar 02;121 (8):963–72. doi: 10.1161/CIRCULATIONAHA.109.893107. [DOI] [PubMed] [Google Scholar]
- 20.Christiansen Christian Fynbo, Christensen Steffen, Mehnert Frank, Cummings Steven R, Chapurlat Roland D, Sørensen Henrik Toft. Glucocorticoid use and risk of atrial fibrillation or flutter: a population-based, case-control study. Arch. Intern. Med. 2009 Oct 12;169 (18):1677–83. doi: 10.1001/archinternmed.2009.297. [DOI] [PubMed] [Google Scholar]
- 21.van der Hooft Cornelis S, Heeringa Jan, Brusselle Guy G, Hofman Albert, Witteman Jacqueline C M, Kingma J Herre, Sturkenboom Miriam C J M, Stricker Bruno H Ch. Corticosteroids and the risk of atrial fibrillation. Arch. Intern. Med. 2006 May 08;166 (9):1016–20. doi: 10.1001/archinte.166.9.1016. [DOI] [PubMed] [Google Scholar]
- 22.Belhassen Bernard. Corticosteroid therapy after catheter ablation of atrial fibrillation for an authentic "blanking period". J. Am. Coll. Cardiol. 2010 Oct 26;56 (18):1473–5. doi: 10.1016/j.jacc.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 23.Kallergis E M, Manios E G, Kanoupakis E M, Mavrakis H E, Kolyvaki S G, Lyrarakis G M, Chlouverakis G I, Vardas P E. The role of the post-cardioversion time course of hs-CRP levels in clarifying the relationship between inflammation and persistence of atrial fibrillation. Heart. 2008 Feb;94 (2):200–4. doi: 10.1136/hrt.2006.108688. [DOI] [PubMed] [Google Scholar]
- 24.McCabe James M, Smith Lisa M, Tseng Zian H, Badhwar Nitish, Lee Byron K, Lee Randall J, Scheinman Melvin M, Olgin Jeffrey E, Marcus Gregory M. Protracted CRP elevation after atrial fibrillation ablation. Pacing Clin Electrophysiol. 2008 Sep;31 (9):1146–51. doi: 10.1111/j.1540-8159.2008.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nascimento Thais, Mota Fernanda, dos Santos Luis Felipe, de Araujo Sérgio, Okada Mieko, Franco Marcello, de Paola Angelo, Fenelon Guilherme. Impact of prophylactic corticosteroids on systemic inflammation after extensive atrial ablation in pigs. Europace. 2012 Jan;14 (1):138–45. doi: 10.1093/europace/eur259. [DOI] [PubMed] [Google Scholar]


