Abstract
In this article we provide nurses with information on the importance of studying environmental exposures during fetal, infant, and childhood development in the National Children's Study. Nurses should be aware of this study to aid in mitigating the complex health problems that arise from environment–health interactions. Nurses may help to educate the public, patients, and caregivers and are in an ideal position to be strong advocates for policy change and regulatory monitoring and enforcement.
Keywords: environmental exposure, reproductive epidemiology, National Children's Study
Agrowing recognition that children may be more susceptible than adults to exposures to toxins has led to the development of the large scale National Children's Study (NCS) to further understand the effects of environmental exposure. The NCS uses a longitudinal cohort to evaluate the effects of environmental exposure on the health and development of 100,000 children enrolled from birth to age 21 (Landrigan, Trasande, & Thorpe, 2006). The main objective is to learn about environmental and genetic factors influencing the risk for conditions such as asthma, autism, diabetes, obesity, and attention and learning disabilities (NCS, 2012). Organizations involved in this study are The National Institute of Child Health and Human Development (NICHD), the Centers for Disease Control and Prevention (CDC), and the U.S. Environmental Protection Agency (EPA).
The need for a children's study of this magnitude was recognized in the 1990s with growing evidence that fetuses, infants, and children are particularly vulnerable to environmental exposure, with health effects appearing years later (Wascham, 2009). Exposure is defined as contact between an agent and a target; contact takes place at an exposure surface over an exposure period (World Health Organization, 2002; Zartarian, Ott, & Dunn, 1997). The ability of children to metabolize and excrete certain agents is different than adults and therefore may result in more biological disruptions. More specifically, we know children are being exposed to >80,000 synthetic chemicals, the majority developed since the 1950s (Landrigan et al., 2006). These chemicals include plastics, pesticides, antibiotics, flame retardants, and synthetic hormones (Landrigan et al.). The U.S. Department of Health and Human Services (USDHHS) has elevated environmental health to a national priority and urged health promotion for all through a healthy environment that includes outdoor air and water quality, healthy homes and communities, and global environmental health (National Institute of Environmental Health and Safety [NIEHS], 2006; USDHHS, 2000). The NCS will be the largest and most comprehensive study of children's health ever conducted in the United States, and the ultimate goal is to develop prevention strategies and appropriate public health guidelines to improve the health of families and children (NCS, 2012).
Researchers in the NCS will use a national probability sampling approach based on counties within the United States (Landrigan et al., 2006; Landrigan, Raugh, & Galvez, 2010). Environmental exposure histories and biological data will be obtained during pregnancy from each mother enrolled in the study. Newborns will be assessed at birth, and samples of cord blood and placenta will be collected. The children in the study will be regularly examined as they grow (Landrigan et al., 2010). The NCS will form the basis of child-health guidance and policy in the United States, as it addresses critical questions in the area of environmental health and the long-term health effects of environmental exposure early in life (Landrigan et al., 2010).
Researchers in the NCS will investigate the impact of environmental exposures during pregnancy on children's health. The purpose of this article is to provide nurses with information on the NCS and how this study may affect infant and childhood health.
Environmental Exposure
According to the EPA (2011), environmental exposure is defined as the exposure to potentially harmful chemical, physical, or biological agents in the environment or to environmental factors that may include ionizing radiation, pathogenic organisms, or toxic chemicals. Chronic exposure can be broken down into continuous, intermittent, or episodic. Continuous exposure is a similar exposure repeated over time. Intermittent exposure is a small or large exposure that occurs more than once over a period of time at defined intervals. Episodic exposure is typically large and occurs more than once over a long period of time but at unknown intervals (e.g., dust particles released during building demolitions) (EPA).
A broad model depicting the pathway from an environmental exposure to a biological response, manifested by measurable health outcomes, is illustrated in Figure 1. The response begins first with contact between a target (individual) and an agent (chemical, biological, physical, radiological), in one or more media (air, water, soil, dust, fluids), by one or more pathways (inhalation, dermal, ingestion), at one time (acute) or over a period of time (chronic). Although the model may appear simple, large gaps of knowledge exist due to the multidimensional aspects of exposure to environmental agents. Such unknowns include the following: What dosages of the various agents create toxicity? What are the latency periods from exposure to the agent and type of media to exposure response or manifestation of disease? Through the NCS, the investigation of environmental exposure in the context of fetuses, infants, and children promises to advance science, including nursing science. Translation of research findings to evidence-based perinatal and pediatric nursing interventions will be important.
Figure 1.
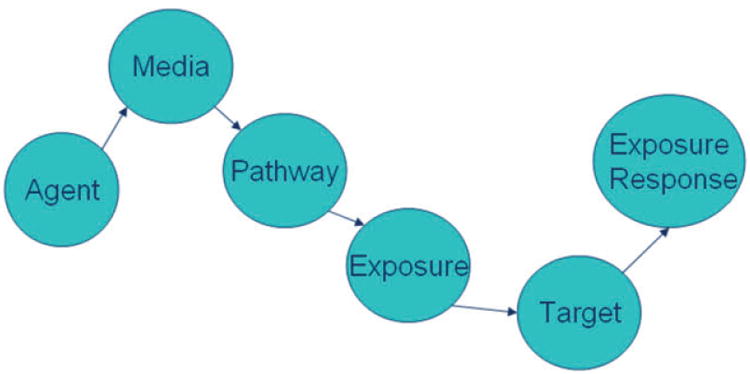
Pathway from environmental agent exposure to environmental health response.
Environmental Reproductive Epidemiology: Exposures in Utero and Suspected Teratogens
The field of reproductive environmental epidemiology expanded tremendously since 1961 when thalidomide, a drug used to treat morning sickness, was shown to cause birth defects (Selevan, 1991). Seeking to elucidate the mechanism of action in thalidomide teratogenesis has led to the publication of 2,000 papers, yet the exact mechanism of action remains unknown (Stephens, Bundle, & Fillmore, 2000). Thus, environmental epidemiology has evolved into an important branch of epidemiology that investigates why and how environmental factors, such as biological agents (pathogens), physical and chemical (radiation, lead and mercury, volatile organic compounds, and pesticides) affect health (Merrill, 2010).
Research evidence demonstrates that environmental exposure beginning in utero can affect infant, childhood, and adult health conditions (Burton, Barker, Moffett, & Thornburg, 2011; Merrill, 2010). Infants defined as younger than age 2, neonates defined as younger than 4 weeks, and children ages 6 to 12 are particularly susceptible to the toxic effects of environmental exposure (Au, 2002). Despite this growing area of concern, incomplete scientific evidence about the long-term consequences of exposure to environmental agents exists. Therefore, the NCS will be useful to guide primary prevention activities that reduce the opportunity for exposure, which is the first and most important line of defense to protect children's health.
Chemical Exposures
The effects of environmental chemicals on reproductive health are largely unknown (Yang, Needham, & Barr, 2005). Several environmental chemicals are known to affect hormonal activity, and their effects have been demonstrated in animal studies (Goldman, Laws, Balchak, Cooper, & Kavlock, 2000; NIEHS, 2011; Stoker, Parks, Gray, & Cooper, 2000). The NCS will include the study of a wide variety of chemicals, including suspected eco-estrogens such as perfluorochemicals (PFCs), phytoestrogens, bisphenol A (BPA), and phthalates (Hellerstedt, McGovern, Fontaine, Oberg, & Cordes, 2008).
Perfluorinated chemicals (PFCs) are compounds used in products such as nonstick cookware and fabrics that are resistant to heat, oil, grease, and water (Lau et al., 2007). Evidence that PFCs are associated with adverse reproductive health is inconclusive (Apelberg et al., 2007; Inoue, Okada, & Ito, 2004). However, animal studies have demonstrated that the fetus and neonate are sensitive to PFC exposures, and further research is needed to explore the impact on the developing fetus and children (EPA, 2012).
Phytoestrogens are naturally derived from plant sources commonly found in soy products. These chemicals have notable estrogenic activity and have been investigated for their association with altered time of onset of puberty (Yang et al., 2005). Combined nutritional status, genetic predisposition, and environmental chemicals are associated with altered age at puberty (Yang et al.). The early onset of puberty is clinically and socially important to the population, as the early onset of thelarche is associated with an early diagnosis of breast cancer in susceptible populations (Hamilton & Mack, 2003; Yang et al.).
Bisphenal A (BPA) is used to make polycarbonate plastic products clear and shatter-proof (Hellerstedt, McGovern, Fontaine, Oberg, & Cordes, 2008). Adverse reproductive effects associated with BPA exposure include abnormalities in the female reproductive tract, early puberty, and alterations in mammary gland and uterus (Honma et al., 2002; Howdeshell, Hotchkiss, Thayer, Vandenbergh, & vom Saal, 1999; Markey, Luque, Munoz De Toro, Sonnenschein, & Soto, 2001).
Phthalates are a group of chemicals that provide the soft and flexible characteristics of plastics and are found in many common consumer products such as perfume, hairspray, lotion, pacifiers, and food packaging (Pak & McCauley, 2007). As a result of their widespread use in common consumer products, exposure to these chemicals are ubiquitous in human populations (CDC, 2005). Birth defects have been associated with exposure to phthalates, particularly in male offspring; these defects include testicular atrophy, decreased anogenital distance, defects of penile structure, and reduced sperm count (Environmental Working Group, 2006; Swan et al., 2005; Toppari et al., 1996).
Additional research is required to determine the long-term effects of chronic, low-dose exposures to the aforementioned chemicals. Researchers in the NCS will study the interactions of these exposures along with individual susceptibility factors that underlie disease (Landrigan et al., 2006).
Gene–Environment Interactions
Understanding the impact of environmental exposure on human genetics and subsequent fetal and childhood health is an important first step in developing innovative nursing care to promote health in women and families. As almost all diseases result from complex interactions between an individual's genetic make-up and environmental agents. Subtle differences in genetic factors may cause people to respond differently to the same environmental exposure (Khoury, Davis, Gwinn, Lindegren, & Yoon, 2005). Exposure to environmental factors can have far-reaching, even cellular, effects on the developing fetus.
One of the most essential aspects of our environment is the accessibility of and the demands for energy (Wallace, 2010a). The flow of energy, mediated by mitochondria in the cell, is exclusively maternally inherited in humans. Only the mother provides the genetic information for mitochondria and the production of energy for her fetus through her oocyte (Giles, Blanc, Cann, & Wallace, 1980). At the moment of conception, the mother and the interuterine environment she creates will have critical impact on the developing fetus. The fetus emerges from the interplay between energy, structure (anatomy), and genetic information (Wallace, 2010a). Gene–environment interaction is the interface between genetic and environmental factors (Dempfle et al., 2008).
The study of gene–environment interaction is important for improving accuracy and precision in the assessment of genetic and environmental influences. The NCS will explore gene–environment interaction with protocols to acquire biological samples from family members across multiple generations. This investigation will provide scientists with unique opportunities for linkage of genetic data with environmental measures from across generations (Landrigan et al., 2006). Toxic exposures may have specific effects on the fetus, infant, and child by epigenetic mechanisms.
Epigenetics
Epigenetics is a term from the 1940s used to describe how genes might interact with their surroundings to produce observable characteristics of an individual (Waddington, 1942). Epigenetics now refers to heritable traits that may occur over multiple rounds of cell division and sometimes transgenerational that do not involve changes to the underlying deoxyribonucleic acid (DNA) sequence (Cassidy & Allanson, 2010). The prefix epi or on top of helps explain that the epigenetic activity does not change a gene but rather covers it up or reveals it. The patterns of gene expression are governed by the epigenome, which sits on top of the genome. Epigenetics is a new field of emerging science in which researchers explore gene expression caused by mechanisms other than changes in the underlying DNA sequence (Cassidy & Allanson).
Extensive layers of cellular regulation occur for a gene to be transcribed, resulting in a protein product (Wallace, 2010b). These regulation activities are viewed as turning on and off of genes and have become the study of epigenetics. Human DNA is nuclear (nDNA) and mitochondrial (mtDNA) (Wallace, 2010b). Environmental factors can alter the heritable transcription states of nDNA and mtDNA and give rise to epigenetic effects (Wallace, 2010b). These heritable transcription states may be passed on from one generation to the next. For example, a pregnant woman may be exposed to a toxin, which can affect her, her fetus, and future offspring (Wallace, 2010b).
Epigenetic Systems
Several kinds of epigenetic systems have been identified (Chandler, 2007; Russo, Martienssen, & Riggs, 1996). The NCS, by design, incorporates the possible epigenetic influences on maternal, fetal, infant, child, and family health. Chromatin remodeling, methylation, RNA transcripts, and prions are a few of the multiple epigenetic mechanisms that can alter phenotype and cause disease in humans as discussed below.
Chromatin Remodeling
One way that genes are regulated is through chromatin remodeling (Chandler, 2007; Russo et al., 1996). Chromatin comprises DNA strands that wrap around structures called histones (Bird, 1986; Eberharter & Becker, 2002). Histone proteins are small spheres that connect to form a backbone that the DNA wraps around forming the structure of the chromosome. If the shape or size of the histones is altered, DNA may either be more available for transcription or fold upon or kink resulting in less available for transcription. Thus, chromatin remodeling alters gene expression. Additionally, histones can be acetylated (acetyl molecules bind to histones), and this process is thought to improve access to genes and increase gene expression (Bird; Chandler; Eberharter & Becker; Russo et al.).
Methylation
Another epigenetic mechanism is methylation or the addition of methyl groups to DNA. Methyl molecules bind to DNA and block access to genes. Methyl groups may permanently activate or deactivate genes (Bird, 1986; Eberharter & Becker, 2002). Some areas of the genome are methylated more deeply than others, and heavily methylated stretches of DNA tend to be less transcriptionally active (Eberharter & Becker). Methylation patterns can also persist from the germ line of one of the parents into the zygote. This process marks the chromosome as being inherited from the mother or father and has been termed “genetic imprinting” (Cassidy & Allanson, 2010). The combined effects of histone modification and DNA methylation on gene expression has been described as the epigenetic code (Bird; Holliday, 1994). The epigenetic code may play a role in short-term adaptations of species to their environments by allowing for reversible phenotype variability (Rando & Verstrepen, 2007). For example, maternal methyl dietary supplements in mice affect epigenetic variation in the color, weight, and propensity for cancer in their offspring (Cooney, Dave, & Wolff, 2002; Waterland & Jirtle, 2003).
RNA Transcripts
Transcription is the process of creating a complementary Ribonucleic acid (RNA) copy of a sequence of DNA (Cassidy & Allanson, 2010). RNA and DNA are nucleic acids that use base pairs of nucleotides as a complementary language that can be converted back and forth from DNA to RNA by the action of the enzymes (Cassidy & Allanson). During transcription, a DNA sequence is read by RNA polymerase, which produces a complementary, antiparallel RNA strand. Transcription involves the unzipping of DNA, adding RNA nucleotides that are paired with complementary DNA and then untwisting the RNA+DNA helix and freeing the newly synthesized RNA strand (Cassidy & Allanson). Some epigenetic changes are mediated by the production of different forms of RNA or by the creation of a double helix RNA. Ribonucleic acid can be spread through diffusion from one cell to another. A large amount of RNA and protein is contributed to the zygote by the mother during oogenesis and results in maternal effect phenotypes (Mattick, Amaral, Dinger, Mercer, & Mehler, 2009).
Prions
Prions are infectious forms of protein that are capable of converting other native state versions of the same protein into a conformational infectious state (Yool & Edmunds, 1998). These prions or infectious proteins do not function like native proteins. They can alter phenotypes. Prions are considered epigenetic because they can be inherited but do not change the underlying DNA (Yool & Edmunds). Prion diseases or transmissible spongiform encephalopathies are a group of rare progressive neurodegenerative disorders that affect both humans and animals. They are differentiated by long incubation periods, and characteristic spongiform changes are associated with neuronal loss and a failure to induce inflammatory response. Human prion diseases are Creutzfeldt-Jakob disease, Gerstmann-Straussler-Scheinker syndrome, fatal familial insomnia, and kuru (CDC, 2011).
Implications for Practice
The time lag between initial environmental exposures and potential disease make it difficult to develop the extensive body of evidence needed to regulate many environmental hazards (Ashford & Miller, 1998). Observable adverse effects can occur years after exposure. As a result, the NCS will be useful in elucidating the effects of these exposures. At the same time, the NCS highlights the emerging science surrounding chronic low-level environmental exposure and the need for a new way of thinking about environmental contaminants and health.
Precautionary Principle
The adoption of the Precautionary Principle, a concept that has been popular in the European Union, is warranted. The concept can be stated as follows: when an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause-and-effect relationships are not fully established scientifically (Kriebel & Tickner, 2001). For instance, given the complex nature of endocrine disrupting compounds, the uncertainty of the impact of low-dose chronic exposures to chemicals, and the availability of certain alternatives (e.g., BPA or phthalate-free products), a precautionary approach on the part of the public is needed, particularly for children who are more susceptible to the effects of exposure.
The promotion of precautionary actions and primary prevention methods is one of the key responsibilities of nurses. As researchers help to investigate the role of environmental exposure in childhood disease, findings from the NCS may help to inform practice (Landrigan et al., 2006). For instance, sources and exposure agents may be identified through a pediatric environmental history-taking process (National Environmental Education and Training Foundation, 2006) or a pediatric environmental health assessment conducted during a home visit (National Center for Healthy Housing, 2007). To understand the exposure of a fetus/ infant/child, nurses may question how the exposure occurs. They may also consider attributes of the concentration of the hazardous agent in the environment of the mother and child and individual exposure factors. Table 1 lists reputable websites for current information on potential agents to which women may be exposed to during pregnancy and lactation. Clinicians should use Organization of Teratology Information Specialists (OTIS) and LactMed on a regular basis to assess safety of particular agents (e.g., drugs, pesticides, cosmetics, cleaning agents). Knowledge exists on some agents, but much more knowledge is needed so health care providers can translate the information about exposures in the environment into practice aimed at promoting optimal maternal and infant health outcomes.
Table 1. Reputable Source of Information for the Most Current Information of Potential Exposures during Pregnancy and Lactation.
| Source | Function |
|---|---|
| Organization of Teratology Information Specialists (OTIS) http://www.otispregnancy.org/ | OTIS provides accurate, evidence-based, clinical information to patients and health care professionals about exposures during pregnancy and lactation. |
| U.S. National Library of Medicine (LactMed) http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT | LactMed is a peer-reviewed database of drugs that provides information to health care providers and breastfeeding women or those considering breastfeeding regarding: drug levels, possible effects to the neonate, and possible drug alternatives. |
Nurses have the opportunity to facilitate the identification of particularly susceptible individuals and populations for exposure and the development of interventions to reduce these exposures. For instance, when research suggested that phthalates in intravenous (IV) tubing were leaching into the fluids delivered to infants in the neonatal intensive care (NICU), well-informed nurses implemented a phthalate-free policy in their units to reduce exposure for susceptible infants (Pak, Briscoe, & McCauley, 2006; Pak, Nailon, & McCauley, 2007). Nurses play an important role informing pregnant women about how to reduce in utero exposure. For example, many pregnant women may not be aware of the dangers from endocrine-disrupting chemicals (Pak & McCauley, 2007) and commonly used pesticides (Raugh et al., 2011; Whyatt et al., 2007). Nurses should educate women on the availability of phthalate-free personal care products and BPA-free alternatives and safe methods to control insects to reduce these exposures.
In addition, it is important for nurses to be cognizant of racial and ethnic disparities regarding environmental exposure. Low-income, disadvantaged, inner-city, marginalized minorities are at high risk for adverse birth outcomes and also are more likely to be exposed to environmental contaminants (Landrigan et al., 2010).
Recruiting and retaining approximately 1,000 to 1,250 families per study center will be important, and nurses are positioned to help the research team build relationships with families and encourage participation (Hellerstedt et al., 2008). Therefore, it is important that nurses are aware of NCS. Perinatal nurses are poised to provide strong leadership in environmental health for women and their families. Nursing professionals may help to educate the public, patients, and caregivers and are in an ideal position to advocate for policy change and regulatory monitoring and enforcement. Through the collaboration of nurses, physicians, and families, the NCS will help to improve the health and welfare of future generations.
Acknowledgments
The views expressed in this article are the responsibility of the authors and do not represent the position of the National Children's Study, the National Institutes of Health, or the U.S. Department of Health and Human Services.
Footnotes
The authors and planners for this activity report no conflict of interest or relevant financial relationships. The article includes no discussion of off-label drug or device use. No commercial support was received for this educational activity.
Contributor Information
Victoria Pak, Postdoctoral fellow in the Department of Circadian Neurobiology, Division of Sleep Medicine, University of Pennsylvania, Philadelphia, PA.
Margaret C. Souders, Assistant professor of human genetics in the School of Nursing, University of Pennsylvania, Philadelphia, PA.
References
- Apelberg B, Goldman L, Calafat A, Herbstman J, Kuklenyik Z, Heidler J, et al. Witter F. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environmental Science & Technology. 2007;41(11):3891–3897. doi: 10.1021/es0700911. [DOI] [PubMed] [Google Scholar]
- Ashford N, Miller C, editors. Chemical exposures: Low levels and high stakes. New York, NY: Wiley & Sons; 1998. [Google Scholar]
- Au WW. Susceptibility of children to environmental toxic substances. International Journal of Hygiene and Environmental Health. 2002;505(6):501–503. doi: 10.1078/1438-4639-00179. [DOI] [PubMed] [Google Scholar]
- Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Barker DJ, Moffett A, Thornburg A. The placenta and human programming. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- Cassidy SB, Allanson JE. Management of genetic syndromes. 3rd. Hoboken, NJ: Wiley-Blackwell; 2010. Introduction. [Google Scholar]
- Centers for Disease Control and Prevention. Third national report on human exposure to environmental chemicals. 2005 Retrieved from http://www.cdc.gov/exposurereport/
- Chandler VA. Paramutation: From maize to mice. Cell. 2007;128(4):641–645. doi: 10.1016/j.cell.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice: Affect, epigenetic variation and DNA methylation of offspring. Journal of Nutrition. 2002;132(8):2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- Dempfle A, Scherag A, Hein R, Beckmann L, Chang-Claude J, Schafer H. Gene-environment interactions for complex traits: Definitions, methodological requirements and challenges. European Journal of Human Genetics. 2008;16:1164–1172. doi: 10.1038/ejhg.2008.106. [DOI] [PubMed] [Google Scholar]
- Eberharter A, Becker PB. Histone acetylation: A switch between repressive and permissive chromatin. European Molecular Biology Organization. 2002;3(3):224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Working Group. Industry spin vs. fact. 2006 Retrieved from http://www.ewg.org/node/8174.
- Giles RE, Blanc H, Cann HM, Wallace DC. Maternal inheritance of human mitochondrial DNA. Proceedings of the National Academy of Sciences. 1980;77:6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Laws C, Balchak SK, Cooper RL, Kavlock RJ. Endocrine-disrupting chemicals: Prepubertal exposures and effects on sexual maturation and thyroid activity in the female rat. A focus on the EOSTAC recommendations. Critical Reviews Toxicology. 2000;30:135–196. doi: 10.1080/10408440091159185. [DOI] [PubMed] [Google Scholar]
- Hamilton AS, Mack TM. Puberty and genetic susceptibility to breast cancer in a case-control study in twins. New England Journal of Medicine. 2003;348:2313–2322. doi: 10.1056/NEJMoa021293. [DOI] [PubMed] [Google Scholar]
- Hellerstedt W, McGovern P, Fontaine P, Oberg C, Cordes J. Prenatal environmental exposures and child health. Minnesota's role in the National Children's Study. Clinical and Health Affairs. 2008;91(9):40–43. Retrieved from http://www.minnesotamedicine.com/PastIssues/PastIssues2008/September2008/ClinicalHellerstedtSeptember2008.aspx. [PubMed] [Google Scholar]
- Holliday R. Epigenetics: An overview. Developmental Genetics. 1994;15:453–457. doi: 10.1002/dvg.1020150602. [DOI] [PubMed] [Google Scholar]
- Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reproductive Toxicology. 2002;15:117–122. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Howdeshell K, Hotchkiss A, Thayer K, Vandenbergh J, vom Saal F. Environmental toxins: Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Inoue K, Okada F, Kato S, Sasaki S, Nakajima S, Uno A, et al. Nakazawa H. Perflurooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: Assessment of PFOS exposure in a susceptible population during pregnancy. Environmental Health Perspectives. 2004;112(11):1204–1207. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury MJ, Davis R, Gwinn M, Lindegren ML, Yoon P. Do we need genomic research for the prevention of common diseases with environmental causes? American Journal of Epidemiology. 2005;161:799–805. doi: 10.1093/aje/kwi113. [DOI] [PubMed] [Google Scholar]
- Kriebel D, Tickner J. Reenergizing public health through precaution. American Journal of Public Health. 2001;91(9):1351–1355. doi: 10.2105/ajph.91.9.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Raugh VA, Galvez MP. Environmental justice and the health of children. Mount Sinai Journal of Medicine. 2010;77(2):178–187. doi: 10.1002/msj.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Trasande L, Thorpe LE. The National Children's Study: A 21-year prospective study of 100,000 American children. Pediatrics. 2006;118(5):2173–2186. doi: 10.1542/peds.2006-0360. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicology Science. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biology of Reproduction. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- Mattick JS, Amaral PP, Dinger ME, Mercer TR, Mehler MF. RNA regulation of epigenetic processes. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. 2009;31(1):51–59. doi: 10.1002/bies.080099. [DOI] [PubMed] [Google Scholar]
- Merrill RM. Reproductive epidemiology. Sudbury, MA: Jones and Bartlett; 2010. [Google Scholar]
- National Center for Healthy Housing. Pediatric environmental home assessment on-line training for public health and visiting nurses. 2007 Retrieved from http://www.healthyhomestraining.org/Nurse/PEHA_Start.htm.
- National Children's Study. About the study. 2012 Retrieved from http://www.nationalchildrensstudy.gov/about/Pages/default.aspx.
- National Environmental Education and Training Foundation. Pediatric environmental history initiative. 2006 Retrieved from http://www.neefusa.org/programs/index.htm.
- National Institute of Environmental Health and Safety. Strategic Plan: New frontiers in environmental science and human health. 2006:2006–2011. (NIH Publ. No. 2006-18). Retrieved from http://www.niehs.nih.gov/about/od/strategicplan/strategicplan2006/niehs_20062011_strategic_plan.pdf.
- National Institute of Environmental Health and Safety. Endocrine disruptors. 2011 Retrieved from http://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm.
- Pak VM, Briscoe V, McCauley LA. How to reduce DEHP in your NICU: A plan of simple steps to promote change. Neonatal Network. 2006;25(6):447–449. doi: 10.1891/0730-0832.25.6.447. [DOI] [PubMed] [Google Scholar]
- Pak VM, McCauley LA. Risks of phthalate exposure among the general population: Implications for occupational health nurses. American Association of Health Nurses. 2007;55(1):12–17. doi: 10.1177/216507990705500102. [DOI] [PubMed] [Google Scholar]
- Pak VM, Nailon RE, McCauley LA. Controversy: Neonatal exposure to plasticizers in the NICU. American Journal of Maternal Child Nursing. 2007;32(4):244–249. doi: 10.1097/01.NMC.0000281965.45905.c0. [DOI] [PubMed] [Google Scholar]
- Rando OJ, Verstrepen KJ. Timescales of genetic and epigenetic inheritance. Cell. 2007;128(40):655–668. doi: 10.1016/j.cell.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Raugh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whatt R. Seven-year neurodevelopment scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environmental Health Perspectives. 2011;119(8):1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo VEA, Martienssen RA, Riggs AD. Epigenetic mechanisms of gene regulation. Plainview, NY: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- Selevan SG. Environmental exposures and reproduction. In: Kiely M, editor. Reproductive and perinatal epidemiology. Boca Raton, FL: CRC Press; 1991. pp. 115–130. [Google Scholar]
- Stephens TD, Bundle CJ, Fillmore BJ. Mechanism of action of thalidomide teratogenesis. Biochemical Pharmacology. 2000;59:1489–1499. doi: 10.1016/s0006-2952(99)00388-3. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Parks LG, Gray LE, Cooper RL. Endocrine-disrupting chemicals: Prepubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC recommendations. Critical Review Toxicology. 2000;30(2):197–252. doi: 10.1080/10408440091159194. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, et al. Calafat AM. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental Health Perspectives. 2005;113(8):1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ. Male reproductive health and environmental xenoestrogens. Environmental Health Perspectives. 1996;104(4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Healthy People 2010. Washington, DC: Author; 2000. [Google Scholar]
- United States Environmental Protection Agency. Toxic and exposure assessment for children's health. 2011 Retrieved from http://www.epa.gov/teach/
- United States Environmental Protection Agency. Long-chain perfluorinated chemicals (PFCs) action plan summary. 2012 Retrieved from http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/pfcs.html.
- Waddington C. The epigenotype. Endeavour. 1942;1:18–20. [Google Scholar]
- Wallace DC. Bioenergetics and the epigenome: Interface between the environment and genes in common diseases. Developmental Disabilities. 2010a;16(2):114–119. doi: 10.1002/ddrr.113. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA mutations in disease and aging. Environmental and Molecular Mutagenisis. 2010b;51(5):440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- Wascham C. Children's health: The National Children's Study begins recruitment. Environmental Health Perspectives. 2009;117(1):A19. doi: 10.1289/ehp.117-a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Molecular and Cellular Biology. 2003;23(15):5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Garfinkel R, Hoepner LA, Holmes D, Borjas M, Williams MK, Camann DE. Within and between-home variability in indoor-air insecticide levels during pregnancy among innercity cohort from New York City. Environmental Health Perspectives. 2007;115(3):383–389. doi: 10.1289/ehp.9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. IPCS risk assessment terminology. Geneva, Switzerland: Author; 2002. Harmonization Project Document No. 1. [Google Scholar]
- Yang RY, Needham LL, Barr DB. Effects of environmental agents on the attainment of puberty: Considerations when assessing exposure to environmental chemicals in the National Children's Study. Environmental Health Perspectives. 2005;113(8):1100–1107. doi: 10.1289/ehp.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool A, Edmunds WJ. Epigenetic inheritance and prions. Journal of Evolutionary Biology. 1998;11(2):241–242. [Google Scholar]
- Zartarian VG, Ott WR, Duan N. A quantitative definition of exposure and related concepts. Journal of Exposure and Analytic Environmental Epidemiology. 1997;7:411–437. [PubMed] [Google Scholar]


