Abstract
Vitamin D deficiency during pregnancy is linked to adverse perinatal outcomes such as small for gestational age infants. Recent evidence suggests that changes in placental amino acid transport contribute to altered fetal growth. We tested the hypothesis that 1,25-dihydroxy vitamin D3 increases the gene expression of System A and L amino acid transporter isoforms and stimulates placental amino acid transport activity in cultured primary human trophoblast cells mediated by mTOR signalling. Treatment with 1,25-dihydroxy vitamin D3 significantly increased mRNA expression of the System A isoform SNAT2 and System A activity, but had no effect on System L and did not affect mTOR signaling. siRNA silencing of the vitamin D receptor prevented 1,25-dihydroxy vitamin D3-stimulated System A transport. In conclusion, 1,25-dihydroxy vitamin D3 regulates System A activity through increased mRNA expression of SNAT2 transporters. Effects on placental amino acid transport may be the mechanism underlying the association between maternal vitamin D status and fetal growth.
Keywords: Placenta, nutrient transport, fetal growth, maternal-fetal exchange
1. Introduction
Vitamin D deficiency has emerged as a global public health issue (Holick and Chen, 2008) due to inadequate sunlight exposure and intake. In the United States, the incidence of vitamin D deficiency continues to rise and women of reproductive age are particularly at risk of deficiency (Looker et al., 2011). Besides the well-established function of vitamin D in calcium homeostasis and bone mineralization, vitamin D deficiency during pregnancy is associated with a range of poor perinatal outcomes, including preterm birth, pre-eclampsia, small-for-gestational-age infants, insulin resistance and gestational diabetes mellitus (Bodnar et al., 2014; Gernand et al., 2014). The mechanisms underlying these associations are not fully understood, however the placenta, which is the interface between maternal and fetal circulations, is likely to be involved.
The bioactive form of vitamin D, 1,25-dihydroxy vitamin D3, exerts its function via genomic and non-genomic pathways. Transcriptional regulation is initiated though the vitamin D binding receptor (VDR) to alter gene expression. The rapid non-transcriptional responses involve stimulation of secondary messenger systems (Ca2+ and cyclic AMP) and activation of signaling molecules, such as phospholipase C, phospholipase A2, protein kinase C and mitogen-activated protein kinases (Haussler et al., 2011; Hii and Ferrante, 2016; Omdahl et al., 2002). The placenta is the primary site for the exchange of nutrients, gases and waste products and in concert with decidua has an important role in vitamin D metabolism during pregnancy (Murthi et al., 2016). The human placenta not only expresses all components of vitamin D signaling, including VDR, retinoid X receptor (RXR), and vitamin D hydroxylase but also synthesizes 1,25-dihydroxy vitamin D3 (Shin et al., 2010; Weisman et al., 1979), suggesting a potential link between maternal vitamin D levels and placental function.
Placental amino acid transfer is pivotal for fetal growth and more than 20 amino acid transport systems have been identified in human placenta (Cleal and Lewis, 2008; Jansson, 2001). System A amino acid transporters mediate sodium-dependent uptake of non-essential amino acids such as alanine, serine and glutamine (Mackenzie and Erickson, 2004). The system A isoforms expressed in the human placenta are Sodium-coupled Neutral Amino acid Transporter 1 (SNAT1), SNAT2 and SNAT4 which are encoded by the genes Slc38a1, Slc38a2 and Slc38a4 respectively (Broer, 2014). System L is a Na+-independent transporter for mediating the transport of essential amino acids such as leucine across the placenta (Jansson, 2001). It is a heterodimer consisting of a light chain, typically L-type amino acid transporter 1 (LAT1) or LAT2, covalently attached to a heavy chain (CD98/4F2hc) (Jansson, 2001). Both LAT1 and LAT2 mRNA are highly expressed in the placenta (Gaccioli et al., 2015; Pineda et al., 1999; Prasad et al., 1999).
Recently, a study from the Southampton Women's survey demonstrated that maternal 25-hydroxyvitamin D and vitamin D binding protein levels were positively associated with placental expression of several amino acid transporter genes, suggesting placental amino acid transport may be regulated by maternal vitamin D and/or vitamin D-binding protein (Cleal et al., 2015). Previous studies in murine skeletal myotubes have shown that vitamin D enhances protein synthesis through mechanistic target of rapamycin (mTOR) signaling pathway (Salles et al., 2013) and we have reported that mTOR signalling is a positive regulator of amino acid transport in cultured primary human trophoblast (PHT) cells (Rosario et al., 2013). We therefore hypothesized that 1,25-dihydroxy vitamin D3 increases the gene expression of system A and L amino acid transporter isoforms and stimulates placental amino acid transport activity in cultured PHT cells mediated by mTOR signalling.
2. Materials and Methods
2.1. Study subjects and tissue collection
Term placental tissue was collected after written informed consent from thirteen healthy pregnant women undergoing elective Cesarean section at 37 to 40 weeks of gestation. Samples and medical information were added to a tissue repository approved by the Colorado Multiple Institutional Review Board (COMIRB-14-1073) and subsequently study personnel were provided anonymized placental tissue and clinical information used in this study.
2.2. Primary human trophoblast cell culture and treatments
Placental tissue was collected within 15 minutes of delivery and processed immediately. PHT cells were isolated as originally described (Kliman et al., 1986) with modifications (Aye et al., 2014; Aye et al., 2013; Roos et al., 2009). Briefly, approximately 35 g of villous tissue was dissected free of decidua and blood vessels and washed in warm phosphate buffered saline (PBS) to remove excess blood. Cells were transferred to digestion buffer with trypsin (0.25 %, Invitrogen, Carlsbad, CA) and Deoxyribonuclease I (Sigma-Aldrich, St. Louis, MO). Cytotrophoblast cells were separated and collected through a discontinuous 10 – 70 % Percoll gradient centrifugation. Cells were then cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM, Sigma-Aldrich) and Ham's F-12 nutrient mixture (Invitrogen) containing 10 % fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA), 50 μg/ml gentamicin, 60 μg/ml benzyl penicillin and 100 μg/ml streptomycin (Sigma-Aldrich), and incubated in a 5 % CO2 humidified atmosphere at 37 °C. Cells were plated in 35 mm dishes at 1.2 million cells per well for amino acid uptake or at 2.75 million per well for RNA and protein analyses. Following 18 h of incubation, attached PHT cells were washed twice with warmed Dulbecco's PBS and culture media was changed daily.
At 66 h (total culture time), PHT cells were treated with increasing concentrations of 1,25-dihydroxy vitamin D3 (0, 0.1, 1 and 10 nM, Sigma-Aldrich) in culture media containing 1 % FBS. All experiments were terminated at 90 h of culture. At this time, cell lysates were processed for RNA extraction or protein lysates, and amino acid uptake or cell viability assays were performed. The viability and differentiation of PHTs following any treatment was determined by daily human chorionic gonadotropin (hCG) secretion in the culture media (from 18 to 90 hours of culture) and hCG secretion was not altered by any treatment (Supplemental Figure 1). In addition, syncytin protein expression increased over the culture period (18-90 hours, Supplemental Figure 2), confirming syncytialization.
2.3. Small interfering RNA (siRNA) transfection
After 18 hours of culture, PHT cells were transfected with 10μM siRNA targeting VDR (1,25-dihydroxy vitamin D3 receptor, ThermoFisher, AM51331) or non-targeting Scrambled (Scr) siRNA (SIC001, Sigma-Aldrich) using Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's protocol and as previously reported (Aye et al., 2015).
2.4. Measurement of amino acid transport activity
System A and L amino acid transport activity were determined by measuring Na+-dependent uptake of 14C-methyl-aminoisobutyric acid (MeAIB) and 2-amino-2-norbornane-carboxylic acid (BCH)-inhibitable uptake of 3H-leucine (Leu), respectively, as previously described (Roos et al., 2009). Following treatment of PHT cells as indicated above, cells plated in triplicate were washed 3 times with 4 ml 37°C Tyrode's salt solution with or without Na+ (iso-osmotic choline replacement) and then incubated with Tyrode's salt solution (Na+ or Na+-free with addition of 1 mM BCH) containing 14C-MeAIB (final concentration 20 μM) and 3H-Leu (final concentration 12.5 nM) for 8 minutes, a time point on the initial linear phase of uptake (Rosario et al., 2013). The uptake was terminated by washing cells 3 times with ice-cold Na+ free Tyrode's salt solution. Cells were then lysed for 2 hours in distilled water and the water was counted in a liquid scintillation counter. Protein content of lysed cells was determined using the Lowry method (Lowry et al., 1951). Transporter-mediated uptakes were calculated by subtracting uptake in Na+-free/BCH buffer (non-mediated uptake) from uptake in Na+-containing buffer (total uptake) and transport activity is expressed as pmol per mg of protein per minute (pmol/mg/min).
2.5. Reverse transcription and quantitative polymerase chain reaction (Q-PCR)
Extraction of total RNA was performed using TRIzol Reagent (Thermo Fisher Scientific) and followed by cDNA synthesis using the High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific). Q-PCR for SNAT1, SNAT2, SNAT4, LAT1, LAT2, SDHA and TBP was performed in triplicate with 0.2 μg of total RNA reverse transcribed into cDNA using SYBR Select Master Mix (Thermo Fisher Scientific). PCR amplification and detection were performed on a QuantStudio 6 Flex Real-Time PCR system (Thermo Fisher Scientific) using the primers as shown in supplemental table. Amplification of a single product was confirmed by melting curve analysis. The amplified transcripts were quantified using the relative standard curve method and normalized to the geometric mean of SDHA and TBP.
2.6. Western blot analyses
Cells were harvested in radioimmunoprecipitation (RIPA) buffer (50 mM Tris HCl, pH 7.4; 150 mM NaCl; 0.1 % SDS; 0.5 % Na-deoxycholate and 1 % Triton X100) containing protease inhibitors and phosphatase inhibitor cocktail 1 and 2 (1:100, Sigma-Aldrich). Protein concentrations were determined using the bicinchoninic acid assay, as per manufacturer's instructions using bovine serum albumin as the standard (Thermo Fisher).
Protein (2 μg total protein/well) was separated on Any KD Mini-Protean Tris Glycine Pre-cast gels (BioRad, Hercules CA) and then transferred onto polyvinylidene difluoride membranes (Thermo Scientific). After blocking with 5 % blotting-grade blocker (BioRad) for 1 h, membranes were incubated in primary antibodies overnight at 4 °C and followed by incubation in corresponding secondary antibody for 1 hour at room temperature. Antibodies against eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), and protein kinase B (Akt), and VDR were purchased from Cell Signaling Technology (Boston, MA, USA). After washing, bands were visualized using enhanced chemiluminescence detection reagents (Pierce Biotechnology, Rockford, IL, USA) and images obtained by G:Box ChemiXL1.4 (Syngene, Cambridge, UK). Where indicated in the figure legend, blots were stripped and re-probed. Target protein expression was normalized to β-actin (Sigma-Aldrich) expression. Blots were analyzed by using ImageJ software (Schneider et al., 2012). For each target, the mean density of control sample bands was arbitrarily assigned a value of 1.0 and all individual density values were expressed relative to this mean.
2.7. Data presentation and statistical analysis
Data are presented as mean ± SEM. Statistical significance was determined either by Student's T-test or by repeated measures ANOVA followed by Bonferroni post-hoc test. A P value < 0.05 was considered significant. Statistical analysis and graph plotting were performed using Prism 6 software (Graph Pad, La Jolla, CA, USA).
3. Results
3.1. 1,25-dihydroxy vitamin D3 stimulates system A amino acid transport
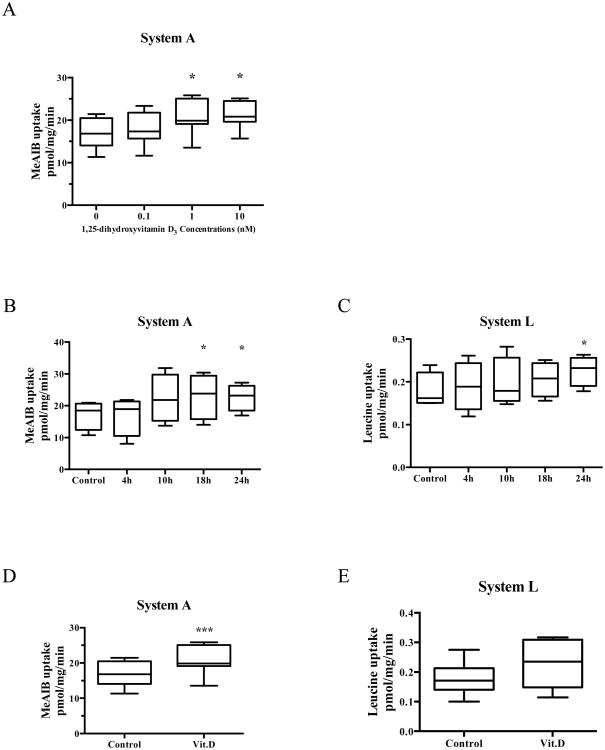
We first determined the dose response of amino acid transport activity to 1,25-dihydroxy vitamin D3. In cultured PHT cells (Figure 1A), system A amino acid uptake increased following incubation in 1 nM and 10 nM 1,25-dihydroxy vitamin D3 for 24 hours (23 % and 23 %, respectively, P < 0.05). We also determined the optimal time for stimulation of placental amino acid transport by 1,25-dihydroxy vitamin D3 from 4 to 24 hours. As shown in Figure 1B and 1C, system A amino acid transport activity increased after 18h of 1,25-dihydroxy vitamin D3 treatment whereas system L transport activity increased after 24h of 1,25-dihydroxy vitamin D3 treatment. Based on these findings, we used 1nM 1,25-dihydroxy vitamin D3 treatment for 24h in a new set of PHT cells to determine the effect of 1,25-dihydroxy vitamin D3 on the amino acid transport activity. System A activity (Fig. 1D) was increased by 23 % (P < 0.001) following stimulation with 1 nM 1,25-dihydroxy vitamin D3. System L-mediated uptake of leucine on the other hand was not significantly affected by 1 nM 1,25-dihydroxy vitamin D3 (Fig. 1E).
Figure 1. Effect of 1,25-dihydroxy vitamin D3 on amino acid transport activity in PHT cells.
(A) Dose response of system A amino acid transport activity, n = 7. (B) Time course of system A amino acid transport activity, n = 4. (C) Time course of system L amino acid transport activity, n = 4. (D) System A and (E) System L transport after exposure to 1,25-dihydroxy vitamin D3 (1 nM) for 24 h, n = 7. Data represent mean ± SEM; *P < 0.05; ***P < 0.001 vs control. Vit.D is 1,25-dihydroxy vitamin D3.
3.2. Vitamin D3 receptor silencing inhibits 1,25-dihydroxy vitamin D3 stimulation of system A aminoacid transporter activity
To test the hypothesis that the effect of 1,25-dihydroxy vitamin D3 on amino acid transporter activity is mediated by VDR, we silenced VDR expression using RNA interference. Compared to transfection with scrambled siRNA, transfection with VDR siRNA significantly reduced VDR protein expression by 75 % (Figure 2A). Incubation with 1,25-dihydroxy vitamin D3 stimulated system A transport activity in PHT cells transfected with scrambled siRNA, which was completely prevented by VDR silencing (Figure 2B). In contrast, 1,25-dihydroxy vitamin D3 did not stimulate System L amino acid transporter activity in either PHT cells transfected with VDR or scrambled siRNA (Figure 2C).
Figure 2. Effects of VDR silencing on amino acid transport activity.
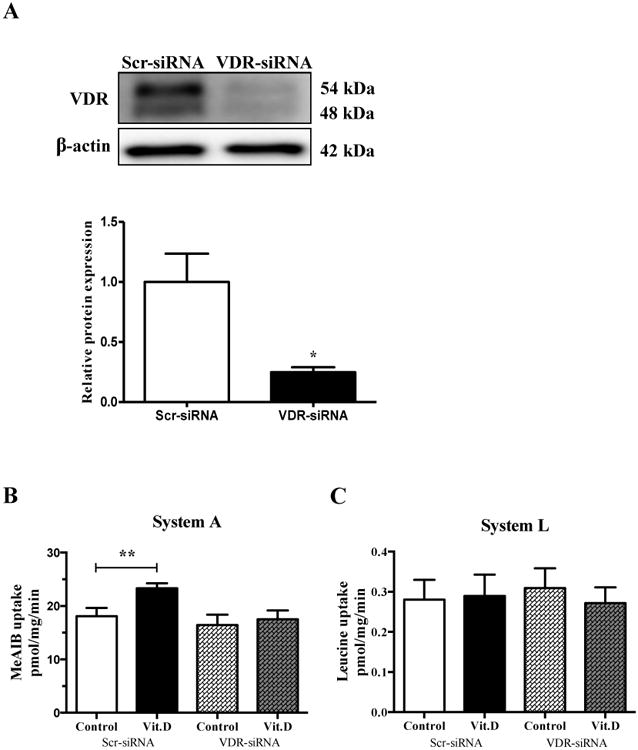
Primary human trophoblast cells were transfected with VDR siRNA or scrambled siRNA. (A) VDR protein expression in cells transfected with scramble or VDR siRNA, n = 4. System A (B) and System L (C) activity measured in cells transfected with Scramble or VDR siRNA with or without treatment with 1,25-dihydroxy vitamin D3 (1 nM, 24h), n = 6. Data represent mean ± SEM; *P < 0.05; **P < 0.01 vs. control. Scr, scramble; VDR, vitamin D3 receptor; Vit.D is 1,25-dihydroxy vitamin D3.
3.3. Regulation of system A and system L amino acid transporter mRNA by 1,25-dihydroxy vitamin D3
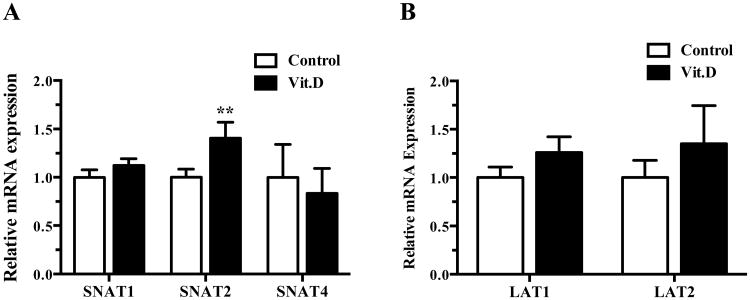
In order to identify which amino acid transporter isoform is regulated by 1,25-dihydroxy vitamin D3, we determined the effect of 1,25-dihydroxy vitamin D3 treatment on specific amino acid transporter mRNA expression. Treatment with 1,25-dihydroxy vitamin D3 significantly increased SNAT2 mRNA expression (41 %, P < 0.01), but not SNAT1 or SNAT4 (Figure 3A). 1,25-dihydroxy vitamin D3 did not influence the expression of LAT1 or LAT2 mRNA in cultured PHT cells (Figure 3B).
Figure 3. Regulation of system A and system L amino acid transporter mRNA by 1,25-dihydroxy vitamin D3.
(A) mRNA expression of system A amino acid transporter isoforms after exposure to 1,25-dihydroxy vitamin D3 (1 nM) for 24 h. (B) mRNA expression of system L amino acid transporter isoforms after exposure to 1,25-dihydroxy vitamin D3 (1 nM) for 24 h. Data represent mean ± SEM, n = 6; *P < 0.05 vs. control.
3.4. 1,25-dihydroxy vitamin D3 treatment does not affect mTOR signaling in cultured PHT cells
We used the phosphorylation of mTOR downstream targets, 4E-BP1 and Akt, to determine the effect of 1,25-dihydroxy vitamin D3 treatment on mTOR signaling. As shown in Figure 4A and 4B, 1,25-dihydroxy vitamin D3 treatment did not affect the expression of total or phosphorylated 4E-BP1 Thr-37/46 or Akt Ser-473 which are downstream readouts of mTORC1 and mTORC2 respectively.
Figure 4. Effect of 1,25-dihydroxy vitamin D3 on mTOR signalling in PHT cells.
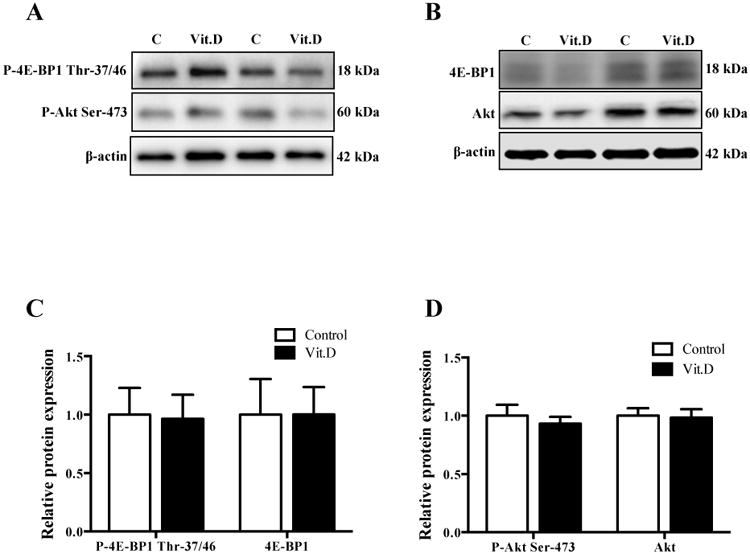
(A) Representative Western blots for P-4E-BP1 Thr-37/46 and P-Akt Ser-473. (B) Representative Western blots for 4E-BP1 and Akt. (C) Histogram summarizing the Western blot data of P-4E-BP1 Thr-37/46 and 4E-BP1. (D) Histogram summarizing the Western blot data of P-Akt Ser-473 and Akt. Exposure to 1,25-dihydroxy vitamin D3 (1 nM) for 24 h. Data represent mean ± SEM, n = 6. Vit.D is 1,25-dihydroxy vitamin D3.
4. Discussion
Vitamin D deficiency in pregnancy is associated to fetal growth restriction (FGR) (Gernand et al., 2014; Gernand et al., 2013), however the underlying mechanisms remain unknown. In the current study, we demonstrate for the first time that 1,25-dihydroxy vitamin D3 promotes amino acid transport in cultured PHT cells. Given that fetal growth has been suggested to be linked to amino acid availability (Gaccioli et al., 2013), these observations implicate effects on placental amino acid transport as one possible mechanism underlying the association between maternal vitamin D status and fetal growth.
Vitamin D is, directly or indirectly, related to many diverse functions of placenta (Olmos-Ortiz et al., 2015). 1,25-dihydroxy vitamin D3 promotes extravillous trophoblast invasion and regulates the synthesis of several hormones including estradiol, human chorionic gonadotropin and placental lactogen (Barrera et al., 2007; Barrera et al., 2008; Chan et al., 2015; Stephanou et al., 1994). 1,25-dihydroxy vitamin D3 also plays a role in balancing innate immune response and exaggerated inflammation to ensure the success of pregnancy. 1,25-dihydroxy vitamin D3 achieves this coordination by inducing innate antibacterial responses in human trophoblasts and inhibiting pro-inflammatory cytokines such as TNF-α and IL-6 (Liu et al., 2009; Noyola-Martinez et al., 2013). Although Cleal and colleagues recently identified an association between maternal serum 25-hydroxy vitamin D3 concentrations and placental gene expression of amino acid transporters (Cleal et al., 2015), to the best of our knowledge vitamin D regulation of placental amino acid transporter activity has not previously been reported.
Fetal amino acid availability, which is closely linked to placental transport, constitutes an important determinant of fetal growth and FGR is associated with decreased placental amino acid transporter activity (Glazier et al., 1997; Jansson et al., 1998; Jansson et al., 2002; Mahendran et al., 1993). In addition, animal studies have shown that decreased placental amino acid transport precedes the development of FGR in cases of maternal nutrient deprivation (Jansson et al., 2006). Understanding the mechanisms of how placental amino acid transport is regulated will help us to elucidate the pathogenesis of FGR. Therefore, our study was designed to specifically evaluate the mechanisms of 1,25-dihydroxy vitamin D3 action on placental amino acid transport system.
1,25-dihydroxy vitamin D3 modulates cell functions by genomic mechanisms mediated by the VDR or via non-genomic pathways. In the present study, placental amino acid transport activity increased after 18 hours of treatment, suggesting that regulation of placental amino acid transporter activity by 1,25-dihydroxy vitamin D3 is likely to be mediated by transcriptional regulation via genomic actions rather than the rapid non-transcriptional actions. Consistent with these observations, the expression of SNAT2 mRNA was significantly increased after 1,25-dihydroxy vitamin D3 treatment in our study. It is noteworthy that only SNAT2 mRNA expression was increased but not the gene expression of the other two system A amino acid transporter isoforms. This finding is consistent with observations reported in the literature that SNAT2 is the most often regulated system A isoform in human placenta. For example, our previous studies showed pro-inflammatory cytokines stimulated SNTA2 expression in cultured PHT cells (Aye et al., 2015; Jones et al., 2009). In addition, placental SNAT2 down regulation has been demonstrated in animal models and human FGR (Chen et al., 2015; Jansson et al., 2006; Mando et al., 2013) suggesting that SNAT2 plays a crucial role in the control of amino acid transport and fetal growth.
The genomic action is initiated by 1,25-dihydroxy vitamin D3 binding to VDR, a member of the nuclear hormone receptor superfamily that acts as a ligand-inducible transcription regulator (Omdahl et al., 2002). Activated VDR binds to RXR to form a stable protein-DNA heterodimeric complex, and then it binds to a specific vitamin D response element (VDRE) in the promoter region of target genes. In the present study, 1,25-dihydroxy vitamin D3-stimulated amino acid transport activity was prevented by the silencing of VDR, confirming that 1,25-dihydroxy vitamin D3 stimulates system A amino acid transporter activity mediated by VDR. Whether this transcriptional regulation is direct (binding to SNAT2 gene) or indirect (activation of other genes) was, however, not addressed in this study and further studies are needed.
Although mTOR signaling has been shown to mediate some of the effects of vitamin D on cell functions such as stimulation of protein synthesis (Salles et al., 2013), we did not find increased phosphorylation of mTOR downstream targets in 1,25-dihydroxy vitamin D3-treated PHT cells. This finding could be explained by observations that the effect of 1,25-dihydroxy vitamin D3 on mTOR signaling is cell-specific. For example, VDR-bound 1,25-dihydroxy vitamin D3 activates the mTOR pathway in human dendritic cells (Ferreira et al., 2015) but suppresses mTOR signaling in osteoblast and cancer cells (Li et al., 2015; Lisse et al., 2011).
Although the concentration of 1,25-dihydroxy vitamin D3 (1 nM) used in this study is somewhat higher than the serum levels reported in pregnant women at term (Kumar et al., 1979; Papapetrou, 2010), they are much lower than the concentrations (10 - 100 nM) typically used to investigate hormone actions in cell culture studies. It is highly likely that the local concentrations may be much higher due to placental vitamin D production. We have elected not to study higher 1,25-dihydroxyvitmain D3 concentrations than 10 nM because high doses may induce apoptosis (Simboli-Campbell et al., 1996) and decrease cell proliferation (Pande et al., 2015), which may affect placental amino acid transport activity. The effect of 1,25-dihydroxy vitamin D3 treatment on system L amino acid transporter activity was inconsistent in our study, suggesting that 1,25-dihydroxy vitamin D3 is not involved in the regulation of system L amino acid transport in PHT cells.
In summary, our study shows that 1,25-dihydroxy vitamin D3 increases placental amino acid transport mediated by VDR and increased SNAT2 mRNA expression. Although the exact mechanism by which 1,25-dihydroxy vitamin D3 promotes System A activity remains to be defined, our results are consistent with the possibility that that improved vitamin D status through supplementation may be a potential strategy for reducing the risk of FGR by increasing amino acid transport across the placenta.
Supplementary Material
Supplemental Figure 1. hCG secretion following siRNA transfection and 1,25-dihydroxy vitamin D3 treatment. Primary human trophoblasts were transfected with VDR siRNA or scrambled siRNA controls with/without 1,25-dihydroxy vitamin D3 (1 nM) for 24 h. Data represent mean ± SEM, n = 4. Scr, scramble; VDR, vitamin D3 receptor; Vit.D is 1,25-dihydroxy vitamin D3.
Supplemental Figure 2. Protein expression of syncytin in PHT cells in culture. PHT cell lysates were prepared at 18, 42, 60 and 90 hours in culture and protein expression of syncytin was measured by immunoblotting. A representative blot is shown. Data represent mean ± SEM, n = 2; due to the small sample number statistics was not performed.
Appendix A: Supplemental Table. Primers used for reverse transcription and quantitative polymerase chain reaction
Appendix B: Supplemental Figure 1.
Appendix C: Supplemental Figure 2.
Highlights.
Effects of vitamin D on primary human trophoblast amino acid transport were studied
1,25-dihydroxy vitamin D3 increased mRNA expression of the System A isoform SNAT2
1,25-dihydroxy vitamin D3 stimulated system A amino acid transport activity
Trophoblast mTOR signaling was not regulated by1,25-dihydroxy vitamin D3
Altered placental amino acid transport may link maternal vitamin D to fetal growth
Acknowledgments
The authors would like to acknowledge the help of Anita Kramer, the University of Colorado Perinatal Clinical Translational Research Center (CTRC) staff and Labor and Delivery Unit at the University of Colorado Hospital for their assistance with placental collections.
Funding Sources: This work was supported by the National Institutes of Health [HD068370].
Abbreviations
- 4E-BP1
eukaryotic translation initiation factor 4E-binding protein 1
- Akt
protein kinase B
- FGR
fetal growth restriction
- LAT
L-type amino acid transporter
- Leu
3H-leucine
- MeAIB
14C-methyl-aminoisobutyric acid
- mTOR
mechanistic target of rapamycin
- PHT
primary human trophoblast
- RXR
retinoid X receptor
- SNAT
sodium-coupled neutral amino acid transporter
- VDR
vitamin D binding receptor
- VDRE
vitamin D response element
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aye IL, Gao X, Weintraub ST, Jansson T, Powell TL. Adiponectin inhibits insulin function in primary trophoblasts by PPARalpha-mediated ceramide synthesis. Mol Endocrinol. 2014;28:512–24. doi: 10.1210/me.2013-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aye IL, Jansson T, Powell TL. Interleukin-1beta inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts. Mol Cell Endocrinol. 2013;381:46–55. doi: 10.1016/j.mce.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aye IL, Jansson T, Powell TL. TNF-alpha stimulates System A amino acid transport in primary human trophoblast cells mediated byp38 MAPK signaling. Physiol Rep. 2015;3:e12594. doi: 10.14814/phy2.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera D, Avila E, Hernandez G, Halhali A, Biruete B, Larrea F, Diaz L. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol. 2007;103:529–32. doi: 10.1016/j.jsbmb.2006.12.097. [DOI] [PubMed] [Google Scholar]
- Barrera D, Avila E, Hernandez G, Mendez I, Gonzalez L, Halhali A, Larrea F, Morales A, Diaz L. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod Biol Endocrinol. 2008;6:3. doi: 10.1186/1477-7827-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Klebanoff MA, Gernand AD, Platt RW, Parks WT, Catov JM, Simhan HN. Maternal vitamin D status and spontaneous preterm birth by placental histology in the US Collaborative Perinatal Project. Am J Epidemiol. 2014;179:168–76. doi: 10.1093/aje/kwt237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S. The SLC38 family of sodium-amino acid co-transporters. Pflugers Arch. 2014;466:155–72. doi: 10.1007/s00424-013-1393-y. [DOI] [PubMed] [Google Scholar]
- Chan SY, Susarla R, Canovas D, Vasilopoulou E, Ohizua O, McCabe CJ, Hewison M, Kilby MD. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta. 2015;36:403–9. doi: 10.1016/j.placenta.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Chen YY, Rosario FJ, Shehab MA, Powell TL, Gupta MB, Jansson T. Increased ubiquitination and reduced plasma membrane trafficking of placental amino acid transporter SNAT-2 in human IUGR. Clin Sci (Lond) 2015;129:1131–41. doi: 10.1042/CS20150511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal JK, Day PE, Simner CL, Barton SJ, Mahon PA, Inskip HM, Godfrey KM, Hanson MA, Cooper C, Lewis RM, Harvey NC, Group SWSS. Placental amino acid transport may be regulated by maternal vitamin D and vitamin D-binding protein: results from the Southampton Women's Survey. Br J Nutr. 2015;113:1903–10. doi: 10.1017/S0007114515001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20:419–26. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- Ferreira GB, Vanherwegen AS, Eelen G, Gutierrez AC, Van Lommel L, Marchal K, Verlinden L, Verstuyf A, Nogueira T, Georgiadou M, Schuit F, Eizirik DL, Gysemans C, Carmeliet P, Overbergh L, Mathieu C. Vitamin D3 Induces Tolerance in Human Dendritic Cells by Activation of Intracellular Metabolic Pathways. Cell Rep. 2015;10:711–25. doi: 10.1016/j.celrep.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Gaccioli F, Aye IL, Roos S, Lager S, Ramirez VI, Kanai Y, Powell TL, Jansson T. Expression and functional characterisation of System L amino acid transporters in the human term placenta. Reprod Biol Endocrinol. 2015;13:57. doi: 10.1186/s12958-015-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaccioli F, Lager S, Powell TL, Jansson T. Placental transport in response to altered maternal nutrition. J Dev Orig Health Dis. 2013;4:101–15. doi: 10.1017/S2040174412000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand AD, Simhan HN, Caritis S, Bodnar LM. Maternal vitamin D status and small-for-gestational-age offspring in women at high risk for preeclampsia. Obstet Gynecol. 2014;123:40–8. doi: 10.1097/AOG.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernand AD, Simhan HN, Klebanoff MA, Bodnar LM. Maternal serum 25-hydroxyvitamin D and measures of newborn and placental weight in a U.S. multicenter cohort study. J Clin Endocrinol Metab. 2013;98:398–404. doi: 10.1210/jc.2012-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–9. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha, 25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–59. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Hii CS, Ferrante A. The Non-Genomic Actions of Vitamin D. Nutrients. 2016;8:135. doi: 10.3390/nu8030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–46. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T. Amino acid transporters in the human placenta. Pediatr Res. 2001;49:141–7. doi: 10.1203/00006450-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–7. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–9. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol. 2009;297:C1228–35. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification characterization and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Kumar R, Cohen WR, Silva P, Epstein FH. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J Clin Invest. 1979;63:342–4. doi: 10.1172/JCI109308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HX, Gao JM, Liang JQ, Xi JM, Fu M, Wu YJ. Vitamin D3 potentiates the growth inhibitory effects of metformin in DU145 human prostate cancer cells mediated by AMPK/mTOR signalling pathway. Clin Exp Pharmacol Physiol. 2015;42:711–7. doi: 10.1111/1440-1681.12409. [DOI] [PubMed] [Google Scholar]
- Lisse TS, Liu T, Irmler M, Beckers J, Chen H, Adams JS, Hewison M. Gene targeting by the vitamin D response element binding protein reveals a role for vitamin D in osteoblast mTOR signaling. FASEB J. 2011;25:937–47. doi: 10.1096/fj.10-172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod. 2009;80:398–406. doi: 10.1095/biolreprod.108.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001-2006. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–95. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34:661–5. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Mando C, Tabano S, Pileri P, Colapietro P, Marino MA, Avagliano L, Doi P, Bulfamante G, Miozzo M, Cetin I. SNAT2 expression and regulation in human growth-restricted placentas. Pediatr Res. 2013;74:104–10. doi: 10.1038/pr.2013.83. [DOI] [PubMed] [Google Scholar]
- Murthi P, Yong HE, Ngyuen TP, Ellery S, Singh H, Rahman R, Dickinson H, Walker DW, Davies-Tuck M, Wallace EM, Ebeling PR. Role of the Placental Vitamin D Receptor in Modulating Feto-Placental Growth in Fetal Growth Restriction and Preeclampsia-Affected Pregnancies. Front Physiol. 2016;7:43. doi: 10.3389/fphys.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyola-Martinez N, Diaz L, Avila E, Halhali A, Larrea F, Barrera D. Calcitriol downregulates TNF-alpha and IL-6 expression in cultured placental cells from preeclamptic women. Cytokine. 2013;61:245–50. doi: 10.1016/j.cyto.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Olmos-Ortiz A, Avila E, Durand-Carbajal M, Diaz L. Regulation of calcitriol biosynthesis and activity: focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients. 2015;7:443–80. doi: 10.3390/nu7010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omdahl JL, Morris HA, May BK. Hydroxylase enzymes of the vitamin D pathway: expression function, and regulation. Annu Rev Nutr. 2002;22:139–66. doi: 10.1146/annurev.nutr.22.120501.150216. [DOI] [PubMed] [Google Scholar]
- Pande VV, Chousalkar KC, Bhanugopan MS, Quinn JC. Super pharmacological levels of calcitriol (1,25-(OH)2D3) inhibits mineral deposition and decreases cell proliferation in a strain dependent manner in chicken mesenchymal stem cells undergoing osteogenic differentiation in vitro. Poult Sci. 2015;94:2784–96. doi: 10.3382/ps/pev284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou PD. The interrelationship of serum 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D in pregnancy at term: a meta-analysis. Hormones (Athens) 2010;9:136–44. doi: 10.14310/horm.2002.1263. [DOI] [PubMed] [Google Scholar]
- Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738–44. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Wang H, Huang W, Kekuda R, Rajan DP, Leibach FH, Ganapathy V. Human LAT1, a subunit of system L amino acid transporter: molecular cloning and transport function. Biochem Biophys Res Commun. 1999;255:283–8. doi: 10.1006/bbrc.1999.0206. [DOI] [PubMed] [Google Scholar]
- Roos S, Lagerlof O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol. 2009;297:C723–31. doi: 10.1152/ajpcell.00191.2009. [DOI] [PubMed] [Google Scholar]
- Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol. 2013;591:609–25. doi: 10.1113/jphysiol.2012.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles J, Chanet A, Giraudet C, Patrac V, Pierre P, Jourdan M, Luiking YC, Verlaan S, Migne C, Boirie Y, Walrand S. 1,25(OH)2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol Nutr Food Res. 2013;57:2137–46. doi: 10.1002/mnfr.201300074. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31:1027–34. doi: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367–76. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- Stephanou A, Ross R, Handwerger S. Regulation of human placental lactogen expression by 1,25-dihydroxyvitamin D3. Endocrinology. 1994;135:2651–6. doi: 10.1210/endo.135.6.7988455. [DOI] [PubMed] [Google Scholar]
- Weisman Y, Harell A, Edelstein S, David M, Spirer Z, Golander A. 1 alpha, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature. 1979;281:317–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. hCG secretion following siRNA transfection and 1,25-dihydroxy vitamin D3 treatment. Primary human trophoblasts were transfected with VDR siRNA or scrambled siRNA controls with/without 1,25-dihydroxy vitamin D3 (1 nM) for 24 h. Data represent mean ± SEM, n = 4. Scr, scramble; VDR, vitamin D3 receptor; Vit.D is 1,25-dihydroxy vitamin D3.
Supplemental Figure 2. Protein expression of syncytin in PHT cells in culture. PHT cell lysates were prepared at 18, 42, 60 and 90 hours in culture and protein expression of syncytin was measured by immunoblotting. A representative blot is shown. Data represent mean ± SEM, n = 2; due to the small sample number statistics was not performed.
Appendix A: Supplemental Table. Primers used for reverse transcription and quantitative polymerase chain reaction
Appendix B: Supplemental Figure 1.
Appendix C: Supplemental Figure 2.