Abstract
Galantamine, a drug currently approved for treatment of Alzheimer's disease, has recently emerged as an effective pretreatment against the acute toxicity and delayed cognitive deficits induced by organophosphorus (OP) nerve agents, including soman. Since cognitive deficits can result from impaired glutamatergic transmission in the hippocampus, the present study was designed to test the hypothesis that hippocampal glutamatergic transmission declines following an acute exposure to soman and that this effect can be prevented by galantamine. To test this hypothesis, spontaneous excitatory postsynaptic currents (EPSCs) were recorded from CA1 pyramidal neurons in hippocampal slices obtained at 1 h, 24 h, or 6-9 days after guinea pigs were injected with: (i) 1xLD50 soman (26.3 μg/kg, s.c.); (ii) galantamine (8 mg/kg, i.m.) followed 30 min later by 1xLD50 soman, (iii) galantamine (8 mg/kg, i.m.), or (iv) saline (0.5 ml/kg, i.m.). In soman-injected guinea pigs that were not pretreated with galantamine, the frequency of EPSCs was significantly lower than that recorded from saline-injected animals. There was no correlation between the severity of soman-induced acute toxicity and the magnitude of soman-induced reduction of EPSC frequency. Pretreatment with galantamine prevented the reduction of EPSC frequency observed at 6-9 days after the soman challenge. Prevention of soman-induced long-lasting reduction of hippocampal glutamatergic synaptic transmission may be an important determinant of the ability of galantamine to counter cognitive deficits that develop long after an acute exposure to the nerve agent.
Keywords: Galantamine, glutamate, guinea pigs, hippocampus, organophosphorus, synaptic transmission
Introduction
Organophosphorus compounds (OPs) that are commonly referred to as nerve agents have been used as weapons of mass destruction in terrorist attacks against civilians in different parts of the world (Dolgin, 2013; Eason, 2013; Okumura et al., 1996). The acute toxicity of these agents, including soman, sarin, and VX, is attributed primarily to their action as irreversible inhibitors of acetylcholinesterase (AChE), the enzyme that hydrolyzes the neurotransmitter acetylcholine (ACh). Accumulation of ACh triggers an acute cholinergic syndrome as it induces overstimulation followed by desensitization of cholinergic receptors in the peripheral and central nervous systems and (Bajgar, 2004; Newmark, 2007). Consequently, treatment of the acute toxicity of nerve agents relies on the use of atropine to block the excessive activation of muscarinic receptors (mAChRs), oximes to reactivate nerve agent-inhibited AChE, and benzodiazepines to halt the progression of seizures (Dolgin, 2013). In 2002, the Food and Drug Administration also approved the use of pyridostigmine as a pretreatment for military personnel at risk of exposure to the nerve agent soman. As a reversible AChE inhibitor that does not cross the blood brain barrier, pyridostigmine protects a significant portion of AChE outside the CNS against the irreversible inhibition by soman (Haigh et al., 2010). The limited effectiveness of these treatments in preventing the development of neurological deficits following an acute exposure to nerve agents is recognized, and new, more effective therapies are sought.
Memory decline (Dahlgren et al., 2004; Hood, 2001; Loh et al., 2010; Murata et al., 1997; Nishiwaki et al., 2001), increased power of the slow electroencephalogram frequencies (Duffy et al., 1979), and structural damage in brain regions associated with cognitive processing, including the hippocampus (Mittal et al., 2011; Yamasue et al., 2007), are common clinical manifestations observed long after the exposure of humans to OP nerve agents and pesticides. Persistent neurological dysfunctions and structural brain damage that resemble those presented by humans exposed to nerve agents have been successfully reproduced in different animal models, including rats, mice, guinea pigs, and non-human primates. It is also noteworthy that these neurological deficits have been detected in animals that at the time of the OP exposure were either asymptomatic or presented only mild signs of acute toxicity (Kassa et al., 2002; Filliat et al., 2007; Mamczarz et al., 2011).
Cognitive processing relies heavily on synaptic transmission and plasticity (reviewed in Baudry et al., 2011). Yet, very little is known regarding the consequences of an in vivo exposure to OP nerve agents on synaptic transmission in different brain regions, particularly those known to play key roles in cognitive processing. A recent study from our laboratory revealed that within 1 h after an exposure to 1xLD50 soman, GABAergic synaptic activity in CA1 pyramidal neurons is increased in guinea pigs that present with mild signs intoxication and decreased in animals that develop severe signs of acute toxicity (Alexandrova et al., 2010). Approximately one week after the soman exposure, GABAergic synaptic transmission in CA1 pyramidal neurons is increased, regardless of the severity of the acute intoxication induced by soman (Alexandrova et al., 2010). Since modulation of the firing rate of CA1 pyramidal neurons, which is determined in part by the balance between excitatory and inhibitory synaptic inputs, is key to cellular mechanisms that support cognitive processing (Baudry et al., 2011), it becomes imperative to determine whether an exposure to soman also results in time-dependent changes in the excitatory synaptic activity in those neurons.
In recent years, galantamine emerged as an effective medical countermeasure to prevent both the acute and delayed toxicity of OP compounds (Albuquerque et al., 2006; Aracava et al., 2009; Gullapalli et al., 2010; Hilmas et al., 2009; Mamczarz et al., 2011; Pereira et al., 2010). Thus, the present study was designed to test the hypothesis that an acute in vivo exposure to soman suppresses glutamatergic synaptic transmission in CA1 pyramidal neurons and that this effect can be prevented by pretreatment with galantamine.
Results presented here demonstrate that an acute exposure of guinea pigs to soman induces significant, long-lasting suppression of glutamatergic synaptic activity in CA1 pyramidal neurons, an effect that is not seen in soman-challenged animals that are pretreated with galantamine. It is conceivable that the loss of excitatory drive allied to the increased inhibitory drive reported to occur 6-9 days after an exposure to soman reduces the excitability of CA1 pyramidal neurons, and, thereby, disrupts synaptic plasticity mechanisms that are essential to cognitive processing. The ability of galantamine to prevent both the sustained suppression of glutamatergic transmission (shown here) and the delayed increase of GABAergic transmission (Alexandrova et al., 2010) in CA1 pyramidal neurons of soman-challenged animals can be an important determinant of its effectiveness to counter the cognitive deficits that develop long after an acute exposure to the nerve agent.
Materials and Methods
Animal care and treatments
Female Hartley guinea pigs [Crl-(HA)Br] were purchased from Charles River Laboratories (Wilmington, MA) and were 30 to 33 days old on arrival at the animal-care facility. They were housed in groups of 3-4 per cage in climate- and light-controlled animal care unit and were acclimated for at least 48 h before any treatment. The study complied with the regulations and standards of the Animal Welfare Act and adhered to the principles of the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Guinea pigs were divided into four treatment groups. Group I received an injection of saline (0.9% NaCl; 0.5 ml/kg, i.m.). Group II received a subcutaneous injection of 1xLD50 soman (26.3 μg/kg, s.c., between the shoulder blades). Group III was treated with galantamine (8 mg/kg, i.m.) 30 min before the challenge with soman. This treatment regimen was selected because earlier studies demonstrated that pretreatment of guinea pigs with 8 mg galantamine/kg effectively prevents the lethality induced by 1xLD50 soman (Aracava et al., 2009). Group IV received only galantamine. Acute clinical signs were scored according to a modified Racine scale (Aracava et al., 2009) as described under Results. After injections, animals were monitored every 15 min during the first 2 h, hourly during the next 6 h, and daily subsequently. Whenever animals developed life-threatening signs of intoxication, including unremitting motor convulsions lasting longer than 10 min and respiratory distress manifested as gasping, they were euthanized according to the protocol approved by the Institutional Animal Care and Use Committee (IACUC).
Preparation of Hippocampal Slices
At 1 h, 24 h, and 6 to 9 days after the treatments, animals were euthanized by asphyxiation in a CO2 atmosphere immediately followed by decapitation. Brains were removed from the skulls and the hippocampi were dissected out in cold artificial cerebrospinal fluid (ACSF), which was composed of (in mM) 125 NaCl, 25 NaHCO3, 2.5 KCl, 2 CaCl2, 1.6 MgCl2, and 11 D-glucose. Using a vibratome (Leica VT1000S; Leica Microsystems Inc., Deerfield, IL), transverse slices (300 μm thick) were cut from the middle third section of each hippocampus in ice-cold ACSF bubbled with 95% O2/5% CO2. Hippocampal slices were maintained for at least 1 h at room temperature in the interface between air and ACSF that was continuously bubbled with 95% O2/5% CO2.
Electrophysiological Recordings
The researcher who performed the electrophysiological recordings was blind to the severity of the clinical signs of acute toxicity. Recordings were obtained from the soma of CA1 pyramidal neurons by using the conventional whole-cell patch-clamp technique and an LM-EPC7 amplifier (List Electronics, Darmstadt, Germany). After stabilization, hippocampal slices were transferred to the recording chamber and superfused with oxygenated ACSF at 2 ml/min. In the chamber, they were kept submerged in ACSF at room temperature (20-22°C). Patch pipettes were pulled from borosilicate glass capillaries (o.d. 1.2 mm; World Precision Instruments Inc., Sarasota, FL) with a P-97 Flaming-Brown puller (Sutter Instrument Company, Novato, CA). The internal pipette solution was composed of (in mM): 130 Cs methane sulfonate, 10 CsCl, 2 MgCl2, 10 EGTA, 10 HEPES, and 5 lidocaine N-ethyl bromide (QX-314, a Na+-channel blocker). The pH was adjusted to 7.3 with CsOH. When filled with internal solution, the patch pipettes had resistances between 3.5 and 6.5 M′Ω. Biocytin (final concentration 0.3 - 0.5%) was added to the pipette solution to label the neurons from which recordings were obtained.
Neurons were visualized by infrared-assisted video microscopy. Spontaneous EPSCs were recorded for 5 min at −50 mV, the reversal potential for GABAergic currents under the present experimental conditions. NMDA receptors were blocked by Mg2+ contained in the ACSF. At the end of the experiments, slices were superfused with ACSF containing the AMPA/kainate receptor antagonist CNQX (6-cyano-7-nitroquinoxaline-2,3-dione, 10 μM) to confirm that the synaptic currents under study were glutamatergic in nature. Currents were filtered at 3 kHz, digitized at 10 kHz, and recorded with the pClamp 9.2 software (Molecular Devices, Sunnyvale, CA). The series resistance, which ranged from 8 to 20 M′Ω, was not compensated but was continuously monitored during each experiment. Experiments were terminated if the series resistance changed by more than 20%.
Data analysis and statistics
The Mini Analysis 6.0.3 software (Synaptosoft Inc., Decatur, GA) was used to analyze the frequency, amplitude, rise time (10-90%), and decay time constant (τd) of synaptic events. The threshold amplitude for detecting EPSCs was set at twice the baseline noise (root mean square), and the EPSCs detected by the software were visually inspected to minimize errors. Events that did not show a typical synaptic waveform were rejected manually.
For kinetic analysis, only single events with a sharp rising phase and an exponential decay were chosen during visual inspection of the recordings. Rise times and τd were determined during the analysis of the averaged chosen single events aligned at half rise time in each cell. More than 95% of the synaptic events were fitted with a single exponential decay. To minimize potential sampling bias, a maximum of two cells per animal were studied. Data are expressed as mean ± S.E.M. of results obtained from CA1 pyramidal cells of various animals, and statistical significance was analyzed by using two-way ANOVA test.
Chemicals
A stock solution of soman (approximately 1.9 mg/ml) was obtained from the U.S. Army Edgewood Chemical Biological Center via an agreement with the U.S. Army Medical Research Institute of Chemical Defense. Soman (methylphosphonofluoridic acid 1,2,2-trimethylpropyl ester) was stored, handled, and disposed of according to the regulations set forth by the U.S. Army Medical Research Institute of Chemical Defense. Galantamine HBr was generously provided by Dr. Alfred Maelicke (Galantos Pharma, Mainz, Germany). (−)Bicuculline methiodide, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine (GYKI 52466), N-(2,6-Dimethylphenyl-carbamoylmethyl) triethylammonium bromide (QX-314) were purchased from Sigma Chemical Co. (St. Louis, MO).
Results
Acute effects of a single exposure of guinea pigs with 1xLD50 soman
The severity of the intoxication presented by guinea pigs challenged with 1xLD50 soman (26.3 μg/kg, s.c.) was evaluated using the qualitative staging system described in Aracava et al. (2009). Guinea pigs were considered mildly intoxicated if they had no abnormal gross behavior (stage 0) or showed facial twitches, chewing, and pawing at whiskers and mouth (stage 1). Moderately intoxicated animals presented head tremor and/or nodding, short periods of immobility (stage 2) along with forelimb clonuses (stage 3). Severely intoxicated guinea pigs showed intermittent tonic-clonic motor convulsions, strong grinding, gnashing or bruxism (stage 4) that progressed to loss of balance, unremitting convulsions, and respiratory distress characterized by gasping (stage 5). According to the IACUC-approved protocol, animals were euthanized as soon as clinical signs of intoxication became life-threatening. Therefore, only 16% of the severely intoxicated animals (stage 4) remained to be used in the electrophysiological experiments, and no animal that presented frank convulsions (stage 5) was alive at 24 hours after the exposure to soman. On the basis of the severity of intoxication, animals used in the electrophysiological experiments were divided into two groups: mildly intoxicated (stages 0-1) and moderately-to-severely intoxicated (stages 2-4). The clinical presentation of the animals used in this study is shown in Table 1.
Table 1. The severity of the acute toxicity exhibited by guinea pigs challenged with 1xLD50 soman.
| Stage of toxicity | Number of animals | Percentage of survival at 24 h* | |||
|---|---|---|---|---|---|
| Euthanized and used in experiments | Euthanized, not used | ||||
| 1h | 24h | 6-9 days | |||
| Mild (score 0-1) | 6 | 16 | 12 | 1 | 97% |
| Moderate-to-severe (score 2-5) | 9 | 8 | 13 | 45 | 32% |
Guinea pigs that were used in experiments at 1 h after soman challenge were not considered for calculation of the rate of survival.
At 24 h after the injection of soman, the guinea pigs had lost 10 to 15% of their initial body weight. Most of them did not show abnormal gross behavior. At 6 to 9 days, animals were gaining weight, albeit at a rate slower than control (saline-injected) guinea pigs, and could not be distinguished from controls on the basis of their gross behavior. Guinea pigs treated with galantamine (8 mg/kg, i.m.) only or challenged with soman 30 min after the treatment with galantamine survived with no apparent signs of acute toxicity and gained weight at the similar rate as control animals.
Characteristics of EPSCs recorded from CA1 pyramidal neurons in control conditions
Spontaneous postsynaptic currents were recorded from CA1 pyramidal neurons at -50 mV. This holding potential was chosen because, under the present experimental conditions, the null potential for Cl− determined by the Nernst equation was approximately -52 mV. Figure 1A shows that the spontaneous inward currents recorded at -50 mV were insensitive to the bath application of GABAA receptor antagonist bicuculline (10 μM). Instead, they were completely blocked by superfusion of the slices with ACSF containing the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM). In our experimental conditions, NMDA receptor-mediated currents were blocked by Mg2+ presented in the ACSF (Ascher and Nowak, 1988). The non-NMDA nature of the synaptic events recorded under the present experimental conditions was confirmed by the finding that these events were not affected by bath application of the selective NMDA antagonist 2-amino-5-phosphonopentanoic acid (APV, 50 μM) (data not shown). The frequency, amplitude, rise time, and decay time constant (τd) of EPSCs recorded in slices obtained from control animals at 1 h, 24 h or 6 to 9 days after their injection with saline were not significantly different (Figure 2A, 2B, Table 2). While the frequency of EPSCs recorded from CA1 pyramidal neurons did not change significantly in guinea pigs between the ages of 35 to 45 days, our previous study showed that the frequency of EPSCs recorded from CA1 stratum radiatum interneurons increased from age 11 to 18 days (Alkondon et al., 2013). These results suggest that glutamatergic synapses in CA1 region are developing in the guinea pig hippocampus during the first three postnatal weeks similar to those reported in rats. An age-dependent development of excitatory synapses onto CA1 pyramidal neurons has been shown in the rat hippocampus; both anatomical and functional studies have provided evidence that the number of glutamatergic synaptic connections onto CA1 pyramidal neurons in the rat hippocampus increases from neonatal (≤ 15 days) to young adult (40-60 days) ages and does not change significantly with subsequent aging up to 2 years (Harris et al., 1992; Hsia et al., 1998).
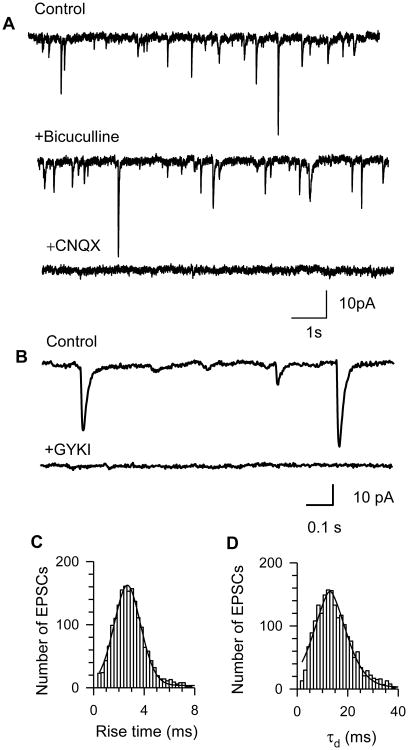
Figure 1. Characterization of EPSCs recorded from guinea pig CA1 pyramidal neurons.
A. Sample recordings of spontaneous EPSCs obtained from CA1 pyramidal neurons under control conditions (top trace), in the presence of the GABAA receptor antagonist 10 μM bicuculline (middle trace), and in the presence of the AMPA/kainate receptor antagonist 10 μM CNQX (bottom trace). B. Representative sample recordings of EPSCs obtained from CA1 pyramidal neurons in the slice of control guinea pig before (control) and during superfusion with selective AMPA receptor antagonist GYKI52466 (50 μM). C and D. Histogram of distribution of the rise time and decay-time constants (τd) of EPSCs recorded from CA1 pyramidal neurons in hippocampal slices of control animals. Both histograms were fit with a Gaussian distribution. To minimize potential sampling bias, a fixed number of EPSCs per neuron (the first 100 events) were pooled for constructing histograms. All recordings were obtained from neurons held at -50 mV.
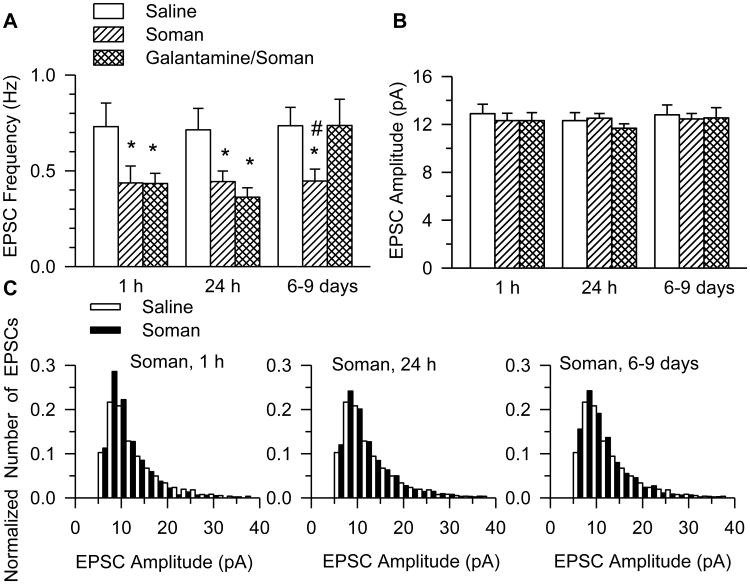
Figure 2. Frequency and amplitude of EPSCs recorded from CA1pyramidal neurons of galantamine-treated and untreated guinea pigs that were challenged with soman.
Graphs show the mean frequency (A) and amplitude (B) of EPSCs recorded from neurons in slices obtained at 1 h, 24 h, or 6-9 days after injection of saline, 1xLD50 soman, or galantamine (8 mg/kg) followed 30 min later by the injection of 1xLD50 soman. Graph and error bars represent mean and SEM, respectively, of results obtained from each of the three treatment groups. The total number of neurons from which recordings were obtained at 1 h, 24 h, and 6-9 days after the injections were, respectively, as follows: 8 (7 guinea pigs), 8 (7 guinea pigs), and 10 (10 guinea pigs) in the saline group; 10 (9 guinea pigs), 17 (15 guinea pigs), and 17 (14 guinea pigs) in the soman group; and 11 (8 guinea pigs), 9 (8 guinea pigs) and 8 (6 guinea pigs) in the galantamine/soman group. According to two-way ANOVA using treatment and testing time as factors, there was a significant main effect of treatment (p < 0.001), no significant main effect of testing time (p = 0.12), and no interaction treatment × testing time (p = 0.18) on the EPSC frequency. Post-hoc Fisher's LSD test for multi-group comparison revealed significant differences between treatments: *, p < 0.05 compared to saline, and #, p < 0.05 compared to galantamine/soman. C. Histograms show the distribution of amplitudes of EPSCs recorded from soman- and saline-injected guinea pigs. Challenge of the guinea pigs with soman did not alter the EPSC amplitude distribution.
Table 2. Kinetic properties of spontaneous EPSCs recorded from CA1 pyramidal neurons in hippocampal slices taken from guinea pigs in control and after treatments.
| Treatment | Time after treatments | Rise time (ms) | τd (ms) | # Neurons |
|---|---|---|---|---|
| Saline (control) | 1 h | 3.0 ± 0.2 | 13.7 ± 0.6 | 8 |
| 24 h | 2.9 ± 0.1 | 14.1 ± 0.4 | 8 | |
| 6-9 days | 2.7 ± 0.2 | 13.6 ± 0.6 | 10 | |
| Soman | 1h | 2.8 ± 0.2 | 13.4 ± 0.7 | 10 |
| 24 h | 3.0 ± 0.3 | 13.8 ± 1.1 | 17 | |
| 6-9 days | 2.9 ± 0.2 | 13.9 ± 1.4 | 17 | |
| Galantamine | 1h | 3.1 ± 0.3 | 12.5 ± 0.9 | 9 |
| 24 h | 3.1 ± 0.3 | 14.7 ± 1.6 | 10 | |
| 6-9 days | 2.9 ± 0.4 | 14.2 ± 0.6 | 8 | |
| Galantamine/Soman | 1h | 3.0 ± 0.1 | 14.0 ± 0.8 | 11 |
| 24 h | 3.2± 0.1 | 14.1 ± 0.7 | 9 | |
| 6-9 days | 3.0 ± 0.5 | 13.9 ± 1.1 | 8 |
Two types of non-NMDA receptors can be activated by the release of glutamate in the synaptic cleft: α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and the kainate (KA) receptors. While AMPA receptors (AMPARs) contribute substantially to glutamatergic postsynaptic responses recorded from most neurons, the contribution of KA receptors appears to be dependent on type of neuron and species/strain (Cossart et al., 2002; Goldin et al., 2007; Wondolowski et al., 2009). Here, kinetic and pharmacological analyses of EPSCs recorded from the CA1 pyramidal neurons in hippocampal slices from control guinea pigs led to the conclusion that these events were mediated primarily by AMPA receptors (AMPAR).
The mean rise time and τd of EPSCs recorded from CA1 pyramidal neurons of control animals (Table 1) are similar to those reported for AMPAR-mediated spontaneous EPSC recorded from CA1 pyramidal cells of rats (rise time: 2.8 ± 0.3 ms and τd: 11.6 ± 1.4 ms; Cossart et al., 2002). In addition, superfusion of the guinea pig hippocampal slices with ACSF containing the selective AMPAR antagonist GYKI 52466 (50 μM) abolished the EPSCs, supporting the contention that they were mediated by AMPARs (Figure 1B). Histograms of the distribution of the rise time and τd were well fitted to a single Gaussian distribution indicating that the population of EPSCs was homogeneous (Figure 1C, 1D). Thus, as in the rat and mouse hippocampus (Bureau et al., 1999; Cossart et al., 2002), EPSCs recorded from CA1 pyramidal neurons in the guinea pig hippocampus are mediated primarily by AMPARs.
Spontaneous EPSCs recorded from CA1 pyramidal neurons of guinea pigs exposed to soman with and without galantamine pre-treatment
The mean amplitude and frequency of EPSCs recorded from CA1 pyramidal neurons in slices cut from the hippocampi of untreated and galantamine-treated guinea pigs at 1 h, 24 h, and 6-9 days after the soman challenge were compared by two-way ANOVA using treatment (saline, soman, or soman/galantamine) and time after soman exposure as factors. The statistical analysis revealed a significant main effect of treatment (p < 0.001) on the EPSC frequency with no significant main effect of time (p = 0.12), and no significant interaction time × treatment (p = 0.18).
Post-hoc Fisher's LSD test for multi-group comparison revealed that at 1 h after the soman challenge, the mean frequency of EPSCs recorded from neurons in slices from soman-challenged animals without galantamine pre-treatment was significantly lower (p = 0.024) than that recorded from neurons of control animals (0.44 ± 0.09 Hz vs 0.73 ± 0.12 Hz; Figure 2A). Likewise, at 24 h and 6-9 days after soman, the mean EPSC frequencies were significantly lower than those recorded from neurons of control (saline-injected) animals (Figure 2A). The mean frequency of EPSCs recorded from soman-injected guinea pigs that were not pretreated with galantamine and from saline-injected guinea pigs were, respectively, 0.44 ± 0.06 Hz and 0.71 ± 0.11 Hz at 24 h, and 0.45 ± 0.06 Hz and 0.74 ± 0.10 Hz at 6-9 days post-injection.
The exposure to soman had no significant effect on the amplitude of the EPSCs. The mean EPSC amplitude and the distribution of EPSC amplitudes recorded from slices obtained at all three time points after the soman challenge were comparable to those recorded from slices of saline-injected animals (Figure 2B, 2C). In addition, the challenge of guinea pigs with soman had no significant effect on the rise time or τd of EPSCs (Table 2).
At 1 h and 24 h after the exposure of galantamine-pretreated guinea pigs to soman, the mean frequency of EPSCs was comparable to those recorded from untreated, soman-challenged guinea pigs (Figure 2A). However, at 6-9 days after the soman challenge of galantamine-treated guinea pigs, the mean frequency of EPSCs (0.74 ± 0.14 Hz) was comparable to saline-treated controls and was significantly higher than the frequency of the EPSCs recorded from soman-injected animals that did not receive galantamine (Figure 2A). The amplitude, rise time, and decay time constants of EPSCs recorded in slices from galantamine-treated, soman-challenged guinea pigs were comparable to those from controls (Figure 2B, Table 2).
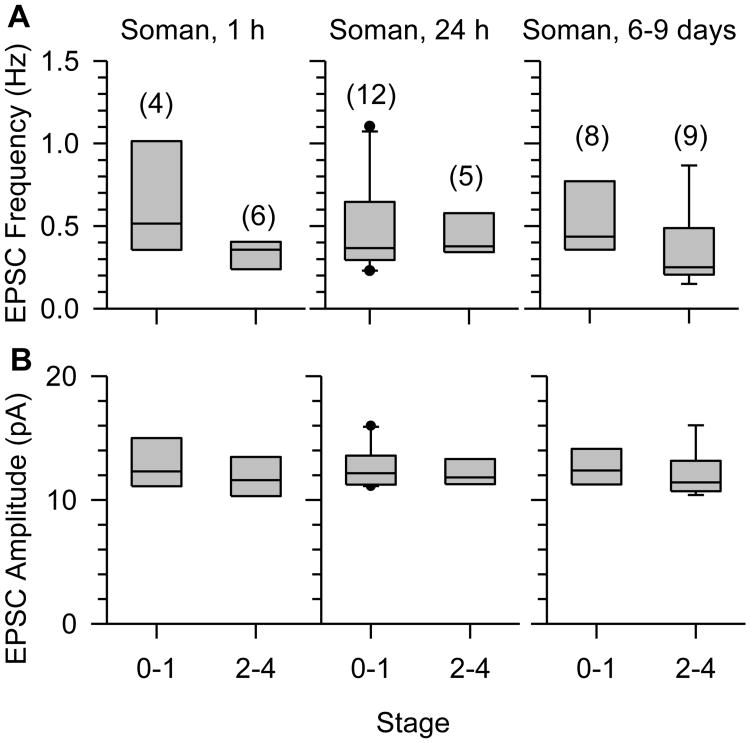
The mean frequency of EPSCs recorded from CA1 pyramidal neurons in hippocampal slices at different times after the exposure to soman were comparable between animals that had been classified as mildly intoxicated and those classified as moderately-to-severely intoxicated at the time of the exposure (Figure 3A). The same was true for the mean amplitude of the EPSCs recorded from pyramidal neurons (Figure 3B).
Figure 3. Relationship between the severity of acute toxicity and the EPSC frequency and amplitude recorded from CA1 pyramidal neurons in the hippocampi harvested from soman-exposed guinea pigs.
Box plots of toxicity scores versus EPSC frequency (A) or amplitude (B) in slices obtained at 1 h, 24 h, or 6 to 9 days after the injection of guinea pigs with 1xLD50 soman (26.3 μg/kg, s.c.). Scores refer to mild (0–1) and moderate-to-severe (2-4) signs of acute intoxication, as described in Results. Numbers of animals per group are shown in parentheses. The EPSC frequency or amplitude recorded in slices was not different among the different treatment groups, p > 0.05 according to one-way ANOVA.
Effects of galantamine on EPSCs recorded from CA1 pyramidal cells
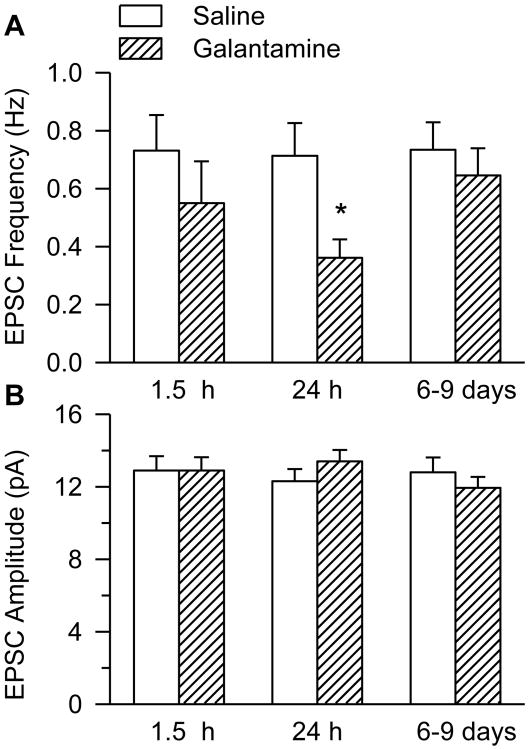
Pharmacologically relevant concentrations of galantamine remain present in brain tissue up to 3 h after intramuscular treatment of guinea pigs with the dose of 8mg/kg (Albuquerque et al., 2006). Therefore, during the first 3 h after the injection of galantamine, timing to obtain the hippocampal slices is an important determinant of the remaining tissue concentrations of the drug. Meaningful comparison of results was made by harvesting hippocampal slices at 1.5 h after treatment of naïve guinea pigs with galantamine, because they had also been harvested at 1.5 h after the galantamine treatment of soman-challenged animals.
The comparison of the mean EPSC frequency recorded from CA1 pyramidal neurons of galantamine- and saline-injected animals by two-way ANOVA using treatment (saline or galantamine) and time after injection as factors revealed a significant main effect of treatment (p = 0.02), no significant main effect of time (p = 0.45), and no significant interaction of treatment × time (p = 0.38). Post-hoc Fisher's LSD test for multi-group comparison indicated that at 1.5 h after the injection of galantamine, the frequency of EPSCs was not significantly different from control (Figure 4A, p = 0.26). However, at 24 h after the galantamine treatment, the mean EPSC frequency was significantly lower than control (Figure 4A, p = 0.013). The effect of galantamine on the EPSC frequency was transient and could no longer be detected at 6 to 9 days after the treatment with the drug (Figure 4A, p = 0.55). Treatment of naïve guinea pigs with galantamine had no significant effect on the amplitude, rise time, or τd of EPSCs (Figure 4B, Table 2).
Figure 4. Frequency and amplitude of EPSCs recorded from CA1 pyramidal neurons of guinea pigs treated with galantamine.
Graphs show mean frequency (A) and amplitude (B) of EPSCs recorded from slices obtained at 1.5 h, 24 h, or 6-9 days after the injection of guinea pigs with 8 mg/kg galantamine. Graph and error bars are mean and S.E.M., respectively, of results obtained at 1 h, 24 h and 6-9 days after treatments with saline or galantamine from the following number of CA1 pyramidal neurons: 8 (7 guinea pigs), 8 (7 guinea pigs) and 10 (10 guinea pigs) in the saline treatment group; and 9 (6 guinea pigs), 10 (9 guinea pigs), and 8 (7 guinea pigs) in the galantamine treatment group. Two-way ANOVA using testing time and treatment as factors revealed a significant main effect of treatment (p = 0.02), no significant main effect of testing time (p = 0.45), and no significant interaction treatment × testing time (p = 0.38) on the frequency of EPSCs. Post-hoc Fisher's LSD test for multi-group comparison revealed that the frequency of EPSCs recorded from neurons in slices obtained from guinea pigs 24 h after their treatment with galantamine was significantly lower than that recorded from animals that had been treated with saline (*, p < 0.05).
Discussion
The present study demonstrates that a single exposure of guinea pigs to 1xLD50 soman causes a sustained decline of glutamate transmission in CA1 pyramidal neurons, regardless of the severity of the acute signs of intoxication induced by the nerve agent. Specifically, the frequency of AMPAR-mediated EPSCs recorded from CA1 pyramidal neurons in hippocampal slices obtained from guinea pigs at 1 h, 24 h, and 6-9 days after a single injection of soman is significantly lower than that recorded from neurons in hippocampal slices of control animals. The apparent discrepancy between our results and those of earlier microdialysis studies reporting that an exposure of rodents to soman causes an increase in extracellular levels of glutamate in the hippocampus (Lallement et al., 1991; O'Donnell et al., 2011) can be reconciled by the fact that glutamate sampled from the brain by microdialysis is primarily of non-synaptic origin (van der Zeyden et al., 2008).
A sustained suppression of glutamatergic synaptic activity and the delayed increase in GABAergic synaptic activity (see Alexandrova et al., 2010) are likely to decrease the excitability of CA1 pyramidal neurons in the hippocampus of soman-exposed animals, and, consequently, disrupt synaptic plasticity mechanisms that are crucial for cognitive processing (Baudry et al., 2011). The ability of galantamine to prevent soman-induced sustained reduction of glutamatergic transmission in the hippocampus can be an important determinant of its effectiveness to prevent the cognitive deficits that develop long after an acute exposure of guinea pigs to soman (Mamczarz et al., 2011).
Potential mechanisms underlying soman-induced reduction of EPSC frequency
The net effects of soman exposure on the frequency of EPSCs at different time points of this study can be attributed to AChE-dependent and -independent mechanisms. The earliest neurochemical event triggered by an OP exposure is the inhibition of AChE that leads to an increase of ACh levels in the central and peripheral nervous systems (Fosbraey et al., 1990; McDonough and Shih, 1997). An earlier study reported that at 1 and 24 h after a single injection of guinea pigs with 1xLD50 soman, AChE activity in the hippocampus is inhibited by approximately 75% (Lintern et al., 1998). Given that the degree of cholinergic innervation and of AChE activity are layer specific within the hippocampus, being low in the stratum radiatum and high in the stratum oriens (Geneser-Jensen, 1972; Schäfer et al., 1998), ACh accumulation secondary to AChE inhibition may be of sufficient magnitude to increase the activation of muscarinic and nicotinic receptors (mAChRs and nAChRs, respectively) in some layers and to desensitize these receptors in others. In fact, a previous study from our laboratory provided evidence supporting the concept that α7 nAChRs in stratum oriens interneurons are desensitized 24 h after an acute exposure of guinea pigs to 1xLD50 soman, while, at the same time, α7 nAChRs in stratum radiatum interneurons are more sensitive to agonist-induced activation (Alkondon et al., 2009).
Activation of presynaptic mAChRs is known to reduce glutamate release from the Schaffer collateral terminals via inhibition of voltage-dependent Ca2+ channels (Fernández de Sevilla et al., 2002; Fernández de Sevilla and Buno, 2003; Qian and Saggau, 1997; Valentino and Dingledine, 1981). On the other hand, activation of postsynaptic mAChRs in CA1 and CA3 pyramidal neurons excites these neurons and enhances synaptic glutamate release in hippocampal slices (Madison et al., 1987; Sun and Kapur, 2012). Evidence exists that basal levels of ACh in hippocampal slices in vitro are sufficient to maintain mAChR and nAChR activation (Banerjee et al., 2013; Santos et al., 2003). Therefore, it is tempting to speculate that postsynaptic mAChR desensitization and/or presynaptic mAChR activation induced by excess ACh prevail and account for the reduction of synaptic glutamatergic transmission in CA1 pyramidal neurons observed between 1 and 24 h after an acute exposure to 1xLD50 soman.
Activation of presynaptic α7 nAChRs present on glutamatergic neurons/axons that synapse onto CA1 and CA3 pyramidal neurons is known to increase synaptic glutamatergic activity in these neurons, (Gray et al., 1996; Banerjee et al., 2013), and the frequency of spontaneous EPSCs recorded from CA1 pyramidal neurons is sustained in part by tonically active α7 nAChRs in hippocampal slices (Banerjee et al., 2012). Since α7 nAChRs desensitize very quickly in the continued presence of agonists (Alkondon and Albuquerque, 2005), it is conceivable that desensitization of α7 nAChRs caused by enhanced levels of ACh contributes to the reduction of EPSC frequency observed in CA1 pyramidal neurons in the hippocampi harvested 1 or 24 h after the injection of soman.
In a recent study from our laboratory, the frequency of EPSCs recorded from CA1 stratum radiatum interneurons at 24 h after the exposure of guinea pigs to 1xLD50 soman was shown to be higher than that recorded from the interneurons in slices from control guinea pigs (Alkondon et al., 2013). The apparent discrepancy between the results presented here and those reported in Alkondon et al. (2013) can be accounted for by the fact that different nAChR subtypes regulate spontaneous glutamatergic synaptic activity in CA1 pyramidal neurons and CA1 stratum radiatum interneurons. Synaptic glutamatergic activity in CA1 pyramidal neurons and in CA1 stratum radiatum interneurons is sustained in part by tonically active α7 nAChRs and non-α7 nAChRs, respectively (Alkondon et al., 2013; Banerjee et al., 2013). Thus, in soman-exposed animals, ACh build-up secondary to soman-induced AChE inhibition can lead to desensitization of α7 nAChRs and over-activation of non-α7 nAChRs. While α7 nAChR desensitization can contribute to the reduction of the EPSC frequency in CA1 pyramidal neurons reported here, non-α7 nAChR over-activation can lead to the increase in the EPSC frequency in CA1 stratum radiatum interneurons reported by Alkondon et al. (2013).
One week after an acute exposure of guinea pigs to 1xLD50 soman, hippocampal AChE activity recovers to a large extent (Lintern et al., 1998). However, the degree of suppression of glutamatergic transmission detected in the guinea pig hippocampus 6-9 days after the exposure to 1xLD50 soman was comparable to that seen at 1 and 24 h post-soman exposure. Therefore, it is likely that AChE-unrelated mechanisms contribute to the sustained reduction of glutamatergic synaptic activity in the pyramidal neurons.
Dabisch et al. (2007) reported that exposure of rats to soman causes mAChR desensitization that outlasts AChE inhibition in the eye. Long-lasting desensitization of M1 mAChRs in the guinea pig hippocampus could well explain the persistent suppression of glutamatergic transmission in CA1 pyramidal neurons of soman-exposed guinea pigs, because activation of these mAChRs has been shown to increase the frequency of EPSCs in CA1 pyramidal neurons (Bouron and Reuter, 1997).
Neuronal loss is a common feature observed in many brain regions of soman-exposed animals, including the CA1 area of hippocampus, amygdala, pyriform cortex, and striatum (Albuquerque et al., 2006; Carpentier et al., 2000; Filliat et al., 1999; Gullapalli et al., 2010). Because CA1 pyramidal neurons receive their inputs from other CA1 pyramidal neurons and from the amygdala (Somogyi and Klausberger, 2005), neuronal degeneration may contribute to the decrease in the frequency of spontaneous EPSCs seen in the hippocampi of soman-exposed guinea pigs. However, it is unlikely to be the only reason underlying the loss of glutamatergic synaptic activity in the CA1 pyramidal neurons of these animals, because neuronal loss and brain lesions increase proportionally to the severity of the acute toxicity induced by nerve agents (Carpentier et al., 2000, 2001; Gullapalli et al., 2010; Lallement et al., 1994; McDonough and Shih, 1993). Yet, in the present study, the frequency of glutamatergic synaptic events in CA1 pyramidal neurons was found to be independent of the severity of the acute toxicity induced by soman.
An in vitro study demonstrated that a distinct decline of pre- and postsynaptic markers occurs in the absence of neuronal loss in cultured hippocampal slices that are exposed to soman (Munirathinam and Bahr, 2004). A significant decrease in spine density on the basal dendrites of the hippocampal CA1 pyramidal neurons has also been observed in young rats that were repeatedly exposed to a dose of the OP pesticide paraoxon that was sufficient to inhibit 60% of AChE activity (Santos et al, 2004). Finally, the decrease in the frequency of miniature EPSCs observed in CA1 pyramidal neurons of mice at 3 months after their exposure to repeated subclinical doses of the OP pesticide chlorpyrifos has also been attributed to a reduction of the spine density in the pyramidal neurons (Speed et al., 2012). Therefore, it is conceivable that the decline of glutamatergic synaptic activity in CA1 pyramidal neurons results from a reduction of the number of dendritic spines in these neurons.
Galantamine effectively prevents long-lasting soman-induced suppression of excitatory synaptic transmission
The ability of galantamine to compete with soman and prevent the irreversible inhibition of AChE by the nerve agent may have contributed to the effectiveness of the pretreatment with the drug to prevent the long-lasting suppression of glutamatergic transmission induced by soman. The known neuroprotective actions of galantamine are also likely to have played an important role in its ability to counter the long-lasting suppressive effect of soman on glutamatergic transmission in CA1 pyramidal neurons. Several studies have provided evidence that galantamine, acting primarily as a nicotinic allosteric potentiating ligand, can minimize neuronal loss in different in vivo and in vitro models of neurodegeneration (Arias et al., 2005; Bhattacharya et al., 2014; Lorrio et al., 2007). We have also reported earlier that pre-treatment of soman-challenged guinea pigs with galantamine prevented neuronal loss and preserved the structural integrity of the hippocampus (Gullapalli et al., 2010).
The finding that at 24 h after the soman exposure of galantamine-pretreated guinea pigs glutamatergic synaptic transmission recorded from CA1 pyramidal neurons was still suppressed significantly could be accounted for by the effect of galantamine per se. As reported here, treatment of naïve guinea pigs with 8 mg galantamine/kg caused a transient reduction of the EPSC frequency recorded from CA1 pyramidal neurons. This effect, which became significant at 24 h after the treatment, was no longer detected at 6-9 days post-treatment and was unlikely due to over-activation/desensitization of cholinergic receptors secondary to accumulation of ACh due to galantamine-induced AChE inhibition. As reported in earlier studies, the half-life of galantamine in rodents is rather short (< 1.5 h). At 24 h after an injection of galantamine, the drug is cleared from the organism and AChE activity within and outside the CNS is comparable to normal (Albuquerque et al., 2006; Geerts et al., 2005; Goh et al., 2010; Mihailova and Yamboliev, 1986).
Numerous studies have reported that mAChRs are down-regulated in response to elevated levels of ACh. Li et al. (2003), for example, provided evidence that M1, M2, and M4 mAChRs are down-regulated in the hippocampus of mice lacking AChE, in part because of receptor internalization. Decossas et al. (2003) also demonstrated that trafficking of M2 mAChRs is highly dependent on the cholinergic tonus and that acute inhibition of AChE can lead to significant internalization of these receptors. Thus, it is conceivable that the transient reduction of the frequency of EPSCs in the CA1 pyramidal neurons of galantamine-treated naïve guinea pigs is secondary to a transient internalization of mAChRs that facilitate glutamatergic synaptic transmission in the CA1 field of the hippocampus.
In conclusion, the present study demonstrates that an acute exposure of guinea pigs to soman causes sustained suppression of synaptic glutamatergic activity in CA1 pyramidal neurons. This effect, whose magnitude is independent of the severity of the acute toxicity triggered by the nerve agent, can be effectively prevented by pretreatment of soman-exposed animals with a dose of galantamine that has been proposed to be compatible with human use (Albuquerque et al., 2006). Thus, the present data support the concept that galantamine is an effective medical countermeasure against both the immediate and the delayed toxicity of OP compounds.
Highlights.
Soman, an irreversible AChE inhibitor, is a potent neurotoxicant.
Soman suppresses glutamate synaptic transmission in CA1 pyramidal neurons.
Galantamine prevents soman's effect on glutamate synaptic transmission.
Galantamine is an effective antidote for soman-induced long term cognitive deficits.
Acknowledgments
The authors are indebted to Ms. Mabel A. Zelle and Ms. Bhagavathy Alkondon for their excellent technical assistance.
Grants: This study was supported by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke [Award UO1NS059344].
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- ACSF
artificial cerebrospinal fluid
- APV
2-amino-5-phosphonopentanoic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- EPSC
excitatory post synaptic currents
- GABA
gamma amino butyric acid
- GYKI 52466
1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine
- KA
kainate receptor
- LD50
dose which produces death in 50% of animals
- mAChRs
muscarinic acetylcholine receptors
- nAChR
nicotinic acetylcholine receptor
- NMDA
N-methyl-D-aspartate
- OP
organophosphorus
- QX-314
N-(2,6-Dimethylphenyl-carbamoylmethyl) triethylammonium bromide
- τd
time constant of decay
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Author Contributions: Participated in Research Design: Alexandrova, Alkondon, Aracava, Pereira, Albuquerque
Conducted Experiments: Alexandrova
Performed Data Analysis: Alexandrova, Pereira, Albuquerque
Contributed to the writing of the Manuscript: Alexandrova, Alkondon, Aracava, Pereira, Albuquerque
Other: Albuquerque acquired funding for the research
Part of the results of the present study was presented during the 2012 Annual Meeting of the Society for Neuroscience (Alexandrova E, Pereira EFR, Aracava Y, Albuquerque EX. Galantamine prevents long-lasting reduction of AMPA-mediated excitatory postsynaptic currents in CA1 pyramidal neurons of guinea pigs challenged with the organophosphorus poison soman. Program No. 537.17. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012. Online).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque EX, Pereira EFR, Aracava Y, Fawcett WP, Oliveira M, Randall WR, Hamilton TA, Kan RK, Romano JA, Jr, Adler M. Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc Natl Acad Sci USA. 2006;103:13220–5. doi: 10.1073/pnas.0605370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrova EA, Aracava Y, Pereira EFR, Albuquerque EX. Pretreatment of guinea pigs with galantamine prevents immediate and delayed effects of soman on inhibitory synaptic transmission in the hippocampus. J Pharmacol Exp Ther. 2010;334:1051–8. doi: 10.1124/jpet.110.167700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional up-regulation by nicotine and to block by bupropion. J Pharmacol Exp Ther. 2005;313:740–50. doi: 10.1124/jpet.104.081232. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Aracava Y, Pereira EFR, Albuquerque EX. A single in vivo application of cholinesterase inhibitors has neuron type-specific effects on nicotinic receptor activity in guinea pig hippocampus. J Pharmacol Exp Ther. 2009;328:69–82. doi: 10.1124/jpet.108.146068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracava Y, Pereira EFR, Akkerman M, Adler M, Albuquerque EX. Effectiveness of donepezil, rivastigmine, and (±)huperzine A in counteracting the acute toxicity of organophosphorus nerve agents: comparison with galantamine. J Pharmacol Exp Ther. 2009;331:1014–24. doi: 10.1124/jpet.109.160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E, Gallego-Sandín S, Villarroya M, García AG, López MG. Unequal neuroprotection afforded by the acetylcholinesterase inhibitors galantamine, donepezil, and rivastigmine in SH-SY5Y neuroblastoma cells: role of nicotinic receptors. J Pharmacol Exp Ther. 2005;315:1346–53. doi: 10.1124/jpet.105.090365. [DOI] [PubMed] [Google Scholar]
- Ascher D, Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurons in culture. J Physiol (Lond) 1988;399:247–66. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis and treatment. Adv Clin Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Alkondon M, Albuquerque EX. Kynurenic acid inhibits glutamatergic transmission to CA1 pyramidal neurons via α7 nAChR-dependent and -independent mechanisms. Biochem Pharmacol. 2012;84:1078–87. doi: 10.1016/j.bcp.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Alkondon M, Albuquerque EX, Pereira EFR. Contribution of CA3 and CA1 pyramidal neurons to the tonic α7 nAChR-dependent glutamatergic input to CA1 pyramidal neurons. Neurosci Lett. 2013;554:167–71. doi: 10.1016/j.neulet.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M, Bi X, Gall C, Lynch G. The biochemistry of memory: The 26-year journey of a ‘new and specific hypothesis’. Neurobiol Learn Mem. 2011;95:125–33. doi: 10.1016/j.nlm.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Haertel C, Maelicke A, Montag D. Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer's disease. PLoS One. 2014;9:e89454. doi: 10.1371/journal.pone.0089454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouron A, Reuter H. Muscarinic stimulation of synaptic activity by protein kinase C is inhibited by adenosine in cultured hippocampal neurons. Proc Natl Acad Sci USA. 1997;94:12224–9. doi: 10.1073/pnas.94.22.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Bischoff S, Heinemann SF, Mulle C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J Neurosci. 1999;19:653–63. doi: 10.1523/JNEUROSCI.19-02-00653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier P, Foquin A, Rondouin G, Lerner-Natoli M, deGroot DMG, Lallement G. Effects of atropine sulphate on seizure activity and brain damage produced by soman in guinea-pigs: ECoG correlates of neuropathology. Neurotoxicology. 2000;4:521–40. [PubMed] [Google Scholar]
- Carpentier P, Foquin A, Kamenka JM, Rondouin G, Lerner-Natoli M, de Groot DMG, Lallement G. Effects of thienylphencyclidine (TCP) on seizure activity and brain damage produced by soman in guinea-pigs: ECoG correlates of neurotoxicity. Neurotoxicology. 2001;22:13–28. doi: 10.1016/s0161-813x(00)00016-4. [DOI] [PubMed] [Google Scholar]
- Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben-Ari Y, Crépel V. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron. 2002;35:147–59. doi: 10.1016/s0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- Dabisch PA1, Horsmon MS, Muse WT, Mioduszewski RJ, Thomson S. Muscarinic receptor dysfunction induced by exposure to low levels of soman vapor. Toxicol Sci. 2007;100:281–9. doi: 10.1093/toxsci/kfm213. [DOI] [PubMed] [Google Scholar]
- Dahlgren JG, Takhar HS, Ruffalo CA, Zwass M. Health effects of diazinon on a family. J Toxicol Clin Toxicol. 2004;42:579–91. doi: 10.1081/clt-200026979. [DOI] [PubMed] [Google Scholar]
- Decossas M, Bloch B, Bernard V. Trafficking of the muscarinic m2 autoreceptor in cholinergic basalocortical neurons in vivo: differential regulation of plasma membrane receptor availability and intraneuronal localization in acetylcholinesterase-deficient and -inhibited mice. J Comp Neurol. 2003;462:302–14. doi: 10.1002/cne.10734. [DOI] [PubMed] [Google Scholar]
- Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med. 2013;19:1194–95. doi: 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- Duffy FH, Burchfiel JL, Bartels PH, Gaon M, Sim VM. Long-term effects of an organophosphate upon the human electroencephalogram. Toxicol Appl Pharmacol. 1979;47:161–76. doi: 10.1016/0041-008x(79)90083-8. [DOI] [PubMed] [Google Scholar]
- Eason MP. Sarin exposure: a simulation case scenario. South Med J. 2013;106:55–62. doi: 10.1097/SMJ.0b013e31827cd12d. [DOI] [PubMed] [Google Scholar]
- Fernández de Sevilla D, Buño W. Presynaptic inhibition of Schaffer collateral synapses by stimulation of hippocampal cholinergic afferent fibres. Eur J Neurosci. 2003;17:555–8. doi: 10.1046/j.1460-9568.2003.02490.x. [DOI] [PubMed] [Google Scholar]
- Fernández de Sevilla D, Cabezas C, de Prada AN, Sanchez-Jimenez A, Buño W. Selective muscarinic regulation of functional glutamatergic Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2002;545:51–63. doi: 10.1113/jphysiol.2002.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliat P, Baubichon D, Burckhart MF, Pernot-Marino I, Foquin A, Masqueliez C, et al. Memory impairment after soman intoxication in rat: correlation with central neuropathology. Improvement with anticholinergic and antiglutamatergic therapeutics. Neurotoxicology. 1999;20:535–49. [PubMed] [Google Scholar]
- Filliat P, Coubard S, Pierard C, Liscia P, Beracochea D, Four E, et al. Long-term behavioral consequences of soman poisoning in mice. Neurotoxicology. 2007;28:508–19. doi: 10.1016/j.neuro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Fosbraey P, Wetherell JR, French MC. Nenrotramsmitter changes in guinea-pig brain regions following soman intoxication. J Neurochem. 1990;54:72–9. doi: 10.1111/j.1471-4159.1990.tb13284.x. [DOI] [PubMed] [Google Scholar]
- Geerts H, Guillaumat PO, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005;1033:186–93. doi: 10.1016/j.brainres.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen FA. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. II. Subiculum and hippocampus. Z Zellforsch Mikrosk Anat. 1972;124:546–60. doi: 10.1007/BF00335257. [DOI] [PubMed] [Google Scholar]
- Goh CW, Aw CC, Lee JH, Chen CP, Browne ER. Pharmacokinetic and pharmacodynamic properties of cholinesterase inhibitors donepezil, tacrine, and galantamine in aged and young Lister hooded rats. Drug Metab Dispos. 2011;39:402–11. doi: 10.1124/dmd.110.035964. [DOI] [PubMed] [Google Scholar]
- Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crépel V, Cossart R. Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci. 2007;27:9560–72. doi: 10.1523/JNEUROSCI.1237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–6. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Gullapalli RP, Aracava Y, Zhuo J, Helal Neto E, Wang J, Makris G, Merchenthaler I, Pereira EFR, Albuquerque EX. Magnetic resonance imaging reveals that galantamine prevents structural brain damage induced by an acute exposure of guinea pigs to soman. Neurotoxicology. 2010;31:67–76. doi: 10.1016/j.neuro.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Haigh JR, Adler M, Apland JP, Deshpande SS, Barham CB, Desmond P, Koplovitz I, Lenz DE, Gordon RK. Protection by pyridostigmine bromide of marmoset hemi-diaphragm acetylcholinesterase activity after soman exposure. Chem Biol Interact. 2010;187:416–20. doi: 10.1016/j.cbi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmas CJ, Poole MJ, Finneran K, Clark MG, Williams PT. Galantamine is a novel post-exposure therapeutic against lethal VX challenge. Toxicol Appl Pharmacol. 2009;240:166–73. doi: 10.1016/j.taap.2009.07.029. [DOI] [PubMed] [Google Scholar]
- Hood E. The Tokyo attacks in retrospect: sarin leads to memory loss. Environ Health Perspect. 2001;109:A542. doi: 10.1289/ehp.109-a542a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol. 1998;79:2013–24. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- Kassa J, Krejcová G, Vachek J. The impairment of spatial memory following low-level sarin inhalation exposure and antidotal treatment in rats. Acta Medica (Hradec Kralove) 2002;45:149–53. [PubMed] [Google Scholar]
- Lallement G, Carpentier P, Collet A, Pemot-Marino I, Baubichon D, Blanchet G. Effects of soman-induced seizures on different extracellular amino acid levels and on glutamate uptake in rat hippocarnpus. Brain Res. 1991;563:234–40. doi: 10.1016/0006-8993(91)91539-d. [DOI] [PubMed] [Google Scholar]
- Lallement G, Pernot-Marino I, Baubichon D, Burckhart MF, Carpentier P, Blanchet G. Modulation of soman-induced neuropathology with an anticonvulsant regimen. Neuroreport. 1994;5:2265–8. doi: 10.1097/00001756-199411000-00015. [DOI] [PubMed] [Google Scholar]
- Li B, Duysen EG, Volpicelli-Daley LA, Levey AI, Lockridge O. Regulation of muscarinic acetylcholine receptor function in acetylcholinesterase knockout mice. Pharmacol Biochem Behav. 2003;74:977–86. doi: 10.1016/s0091-3057(03)00022-4. [DOI] [PubMed] [Google Scholar]
- Lintern MC, Wetherell JR, Smith ME. Differential recovery of acetylcholinesterase in guinea pig muscle and brain regions after soman treatment. Hum Exp Toxicol. 1998;17:157–62. doi: 10.1177/096032719801700306. [DOI] [PubMed] [Google Scholar]
- Loh Y, Swanberg MM, Ingram MV, Newmark J. Case report: Long-term cognitive sequelae of sarin exposure. Neurotoxicology. 2010;31:244–6. doi: 10.1016/j.neuro.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Lorrio S, Sobrado M, Arias E, Roda JM, García AG, López MG. Galantamine postischemia provides neuroprotection and memory recovery against transient global cerebral ischemia in gerbils. J Pharmacol Exp Ther. 2007;322:591–9. doi: 10.1124/jpet.107.122747. [DOI] [PubMed] [Google Scholar]
- Madison DV, Lancaster B, Nicoll RA. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987;7:733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamczarz J, Kulkarni GS, Pereira EFR, Albuquerque EX. Galantamine counteracts development of learning impairment in guinea pigs exposed to the organophosphorus poison soman: clinical significance. Neurotoxicology. 2011;32:785–98. doi: 10.1016/j.neuro.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. Pharmacological modulation of soman-induced seizures. Neurosci Biobehav Rev. 1993;17:203–15. doi: 10.1016/s0149-7634(05)80151-4. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–79. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Mihailova D, Yamboliev I. Pharmacokinetics of galanthamine hydrobromide (Nivalin) following single intravenous and oral administration in rats. Pharmacology. 1986;32:301–6. doi: 10.1159/000138184. [DOI] [PubMed] [Google Scholar]
- Mittal T, Gupta N, Kohli A, Bhalla A, Singh B, Singh S. Correlation of defects in regional cerebral blood flow determined by 99mTc SPECT with residual neurocognitive testing abnormalities during and 3 months post exposure in acutely poisoned patients with organophosphates. Clin Toxicol (Phila) 2011;49:464–70. doi: 10.3109/15563650.2011.591400. [DOI] [PubMed] [Google Scholar]
- Murata K, Araki S, Yokoyama K, Okumura T, Ishimatsu S, Takasu N, White RF. Asymptomatic sequelae to acute sarin poisoning in the central and autonomic nervous system 6 months after the Tokyo subway attack. J Neurol. 1997;244:601–6. doi: 10.1007/s004150050153. [DOI] [PubMed] [Google Scholar]
- Munirathinam S, Bahr BA. Repeated contact with subtoxic soman leads to synaptic vulnerability in hippocampus. J Neurosci Res. 2004;77:739–46. doi: 10.1002/jnr.20209. [DOI] [PubMed] [Google Scholar]
- Newmark J. Nerve agents. Neurologist. 2007;13:20–32. doi: 10.1097/01.nrl.0000252923.04894.53. [DOI] [PubMed] [Google Scholar]
- Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Minami M, Omae K Sarin Health Effects Study Group. Effects of sarin on the nervous system in rescue team staff members and police officers 3 years after the Tokyo subway sarin attack. Environ Health Perspect. 2001;109:1169–73. doi: 10.1289/ehp.011091169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell JC, McDonough JH, Shih TM. In vivo microdialysis and electroencephalographic activity in freely moving guinea pigs exposed to organophosphorus nerve agents sarin and VX: analysis of acetylcholine and glutamate. Arch Toxicol. 2011;85:1607–16. doi: 10.1007/s00204-011-0724-z. [DOI] [PubMed] [Google Scholar]
- Okumura T, Takasu N, Ishimatsu S, Miyanoki S, Mitsuhashi A, Kumada K, Tanaka K, Hinohara S. Report on 640 victims of the Tokyo subway sarin attack. Ann Emerg Med. 1996;28:129–35. doi: 10.1016/s0196-0644(96)70052-5. [DOI] [PubMed] [Google Scholar]
- Pereira EFR, Aracava Y, Alkondon M, Akkerman M, Merchenthaler I, Albuquerque EX. Molecular and cellular actions of galantamine: clinical implications for treatment of organophosphorus poisoning. J Mol Neurosci. 2010;40:196–203. doi: 10.1007/s12031-009-9234-3. [DOI] [PubMed] [Google Scholar]
- Qian J, Saggau P. Presynaptic inhibition of synaptic transmission in the rat hippocampus by activation of muscarinic receptors: involvement of presynaptic calcium influx. Br J Pharmacol. 1997;122:511–9. doi: 10.1038/sj.bjp.0701400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos HR, Cintra WM, Aracava Y, Maciel CM, Castro NG, Albuquerque EX. Spine density and dendritic branching pattern of hippocampal CA1 pyramidal neurons in neonatal rats chronically exposed to the organophosphate paraoxon. Neurotoxicology. 2004;25:481–94. doi: 10.1016/j.neuro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Santos MD, Pereira EFR, Aracava Y, Castro NG, Fawcett WP, Randall WR, Albuquerque EX. Low concentrations of pyridostigmine prevent soman-induced inhibition of GABAergic transmission in the central nervous system: involvement of muscarinic receptors. J Pharmacol Exp Ther. 2003;304:254–65. doi: 10.1124/jpet.102.043109. [DOI] [PubMed] [Google Scholar]
- Schäfer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998;84:361–76. doi: 10.1016/s0306-4522(97)80196-0. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurons structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed HE, Blaiss CA, Kim A, Haws ME, Melvin NR, Jennings M, Eisch AJ, Powell CM. Delayed reduction of hippocampal synaptic transmission and spines following exposure to repeated subclinical doses of organophosphorus pesticide in adult mice. Toxicol Sci. 2012;125:196–208. doi: 10.1093/toxsci/kfr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Kapur J. M-type potassium channels modulate Schaffer collateral-CA1 glutamatergic synaptic transmission. J Physiol (Lond) 2012;590:3953–64. doi: 10.1113/jphysiol.2012.235820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Dingledine R. Presynaptic inhibitory effect of acetylcholine in the hippocampus. J Neurosci. 1981;1:784–92. doi: 10.1523/JNEUROSCI.01-07-00784.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zeyden M, Oldenziel WH, Rea K, Cremers TI, Westerink BH. Microdialysis of GABA and glutamate: analysis, interpretation and comparison with microsensors. Pharmacol Biochem Behav. 2008;90:135–47. doi: 10.1016/j.pbb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Wondolowski J, Frerking M. Subunit-dependent postsynaptic expression of kainate receptors on hippocampal interneurons in area CA1. J Neurosci. 2009;29:563–74. doi: 10.1523/JNEUROSCI.4788-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Abe O, Kasai K, Suga M, Iwanami A, Yamada H, et al. Human brain structural change related to acute single exposure to sarin. Ann Neurol. 2007;61:37–46. doi: 10.1002/ana.21024. [DOI] [PubMed] [Google Scholar]