Abstract
Exposure of the developing brain to chlorpyrifos (CPF), an organophosphorus (OP) pesticide used extensively in agriculture worldwide, has been associated with increased prevalence of cognitive deficits in children, particularly boys. The present study was designed to test the hypothesis that cognitive deficits induced by prenatal exposure to sub-acute doses of CPF can be reproduced in precocial small species. To address this hypothesis, pregnant guinea pigs were injected daily with CPF (25 mg/kg, s.c.) or vehicle (peanut oil) for 10 days starting on presumed gestation day (GD) 53–55. Offspring were born around GD 65, weaned on postnatal day (PND) 20, and subjected to behavioral tests starting around PND 30. On the day of birth, butyrylcholinesterase (BuChE), an OP bioscavenger used as a biomarker of OP exposures, and acetylcholinesterase (AChE), a major molecular target of OP compounds, were significantly inhibited in the blood of CPF-exposed offspring. In their brains, BuChE, but not AChE, was significantly inhibited. Prenatal CPF exposure had no significant effect on locomotor activity or on locomotor habituation, a form of non-associative memory assessed in open fields. Spatial navigation in the Morris water maze (MWM) was found to be sexually dimorphic among guinea pigs, with males outperforming females. Prenatal CPF exposure impaired spatial learning more significantly among male than female guinea pigs and, consequently, reduced the sexual dimorphism of the task. The results presented here, which strongly support the test hypothesis, reveal that the guinea pig is a valuable animal model for preclinical assessment of the developmental neurotoxicity of OP pesticides. These findings are far reaching as they lay the groundwork for future studies aimed at identifying therapeutic interventions to treat and/or prevent the neurotoxic effects of CPF in the developing brain.
Keywords: Acetylcholinesterase, Butyrylcholinesterase, Guinea pig, Learning, Pesticide, Organophosphorus
1. Introduction
Chlorpyrifos (CPF) is the most commonly used organophosphorus (OP) pesticide in agriculture worldwide and continues to be the top-selling OP pesticide for residential use in various countries. It is anticipated that, in part because of the broad-spectrum pest control effectiveness and low cost of this pesticide, the global market of CPF will grow steadily between 2015 and 2022 (Grand View Research, 2015). Upon recognizing the detrimental effects of CPF to the developing brain, the United Stated (U.S.) Environmental Protection Agency banned in 2001 the residential use of this pesticide in the U.S. (Israel, 2012). Nevertheless, CPF could still be detected in 78% of American households surveyed between 2005 and 2006 (Stout et al., 2009). Thus, exposure to CPF will remain for years to come a serious public health concern in both agricultural and residential settings in the U.S. and throughout the world.
Epidemiological studies have provided evidence that OP pesticides are toxic to the developing human brain (Rauh et al., 2012; Rosas and Eskenazi, 2008). Specifically, low birth weight and length in addition to increased prevalence of psychomotor and cognitive deficits among children ages 2–7 have been associated with prenatal exposure to CPF levels that produce measurable concentrations of the pesticide in maternal and/or umbilical cord blood but do not trigger overt signs of maternal intoxication (Perera et al., 2003; Rauh et al., 2006; Whyatt et al., 2004). Working memory deficits have also been observed among 7-year-old children prenatally exposed to sub-acute levels of CPF, with boys presenting more severe deficits than girls (Horton et al., 2012).
The acute toxicity of CPF results primarily from the irreversible inhibition of acetylcholinesterase (AChE), the enzyme that hydrolyzes the neurotransmitter acetylcholine (ACh), and is characterized by a classical cholinergic crisis defined by miosis, profuse secretions, diarrhea, diuresis, muscle fasciculation, tremors, motor convulsions, and respiratory distress that can lead to death. However, mounting evidence supports the notion that AChE inhibition alone does not explain the neurotoxic effects of sub-acute doses of OP pesticides (reviewed in Terry, 2012), particularly in the developing CNS (Slotkin and Seidler, 2008).
The translational capacity of data generated in preclinical toxicological studies is contingent upon several factors, including the appropriateness of the animal model. To date all developmental studies with sub-acute doses of CPF have been conducted on altricial species, specifically rats and mice. However, striking differences exist between their CNS development and that of humans making it difficult to extrapolate sensitive gestational periods from these rodents to humans (Byrnes et al., 2004; Dobbing and Sands, 1970, 1979). In addition, the toxicokinetics of CPF is likely to be differently influenced by pregnancy in humans and rats or mice, because, compared to humans, rats and mice have remarkably different placental structure and relatively higher levels of circulating carboxylesterases, the enzymes that metabolize and inactivate OP compounds (Carter, 2007; de Jong et al., 1993). The rodent that has relatively lower levels of circulating carboxylesterases and more closely resembles humans and primates in terms of brain development and placental structure is the guinea pig (Dobbing and Sands, 1979; Carter, 2007; de Jong et al., 1993). Therefore, the present study was designed to test the hypothesis that guinea pigs prenatally exposed to sub-acute doses of CPF develop cognitive deficits that bear resemblance to those observed in humans prenatally exposed to this pesticide.
In this study, guinea pigs were exposed in utero to sub-acute doses of CPF during the gestational period spanning from the time of brain growth spurt, which peaks around gestation day (GD) 50, to the time of rapid brain myelination, which peaks around GD 60 (Dobbing and Sands, 1970). When offspring reached prepubertal ages, locomotor activity and locomotor habituation, a form of non-associative memory were assessed in open fields, while spatial learning was assessed in the classic version of the Morris water maze (MWM). Data presented here support the hypothesis as they reveal that, similar to humans, guinea pigs prenatally exposed to sub-acute doses of CPF develop learning deficits, with males being more affected than females. Based on the results of this study, the guinea pig emerges as a valuable preclinical model of developmental neurotoxicity of OP pesticides.
2. Material and methods
2.1. Animal care and treatments
Pregnant Hartley guinea pigs [Crl(HA)Br; Charles River Laboratories, Wilmington, MA] were delivered to the animal facility in groups of four on presumed gestation day (GD) 33–35. There were 13 shipments. Dams were singly housed in stainless steel cages in climate-controlled rooms (21 ± 0.5 °C; 12-h light/dark cycle). Food and water were available ad libitum.
Starting on approximate GD 53–55 and lasting 10 consecutive days, pregnant guinea pigs received a daily subcutaneous (s.c.) injection of 25 mg/kg CPF (dissolved in peanut oil) or peanut oil. This CPF dose regimen was selected to reproduce a number of salient features associated with occupational exposures of humans to pesticides. First, the daily injections were intended to mimic the repetitive nature of these exposures (Farahat et al., 2011). Second, the s.c. route of exposure allows for a slow sustained release of the pesticide in the systemic circulation and, thereby, approximates human exposures via the dermal route, one of the most relevant routes of exposure to CPF (Cattani et al., 2001; Fenske et al., 2012). Third, the daily dose of CPF was selected to be below doses that induce overt signs of acute toxicity. The oral LD50 of CPF in guinea pigs is 504 mg/kg, and, in general, oral and s.c. LD50 s of OP compounds are very similar (McCollister et al., 1974). As a result, the cumulative dose of CPF used here would be well below 0.5xLD50, which is lower than the threshold for OP-induced acute toxicity (Shih and McDonough, 1997). The intention was to model a scenario in which occupational human exposure may be presumed safe.
From each delivery two mothers were injected with peanut oil and two with CPF. On rare occasions, pregnant dams died after delivery or during injections; therefore, experimental groups had offspring born from different numbers of dams (see Table 1) from different numbers of shipments (8–10). Offspring were born around GD 65–67, weaned on PND 20, and, then, housed according to their sexes in groups of 2–6 per cage. All investigators complied with the regulations and standards of the Animal Welfare Act and adhered to the principles of the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Table 1.
Number of maternal deaths, miscarriages, litters with perinatal deaths, and offspring that died perinatally, and litter size per experimental group.
| Peanut Oil | Chlorpyrifos | |
|---|---|---|
| Maternal deathsa | 1/15 | 1/18 |
| Miscarriagesb | 0/14 | 1/17 |
| Litters with perinatal deathsc | 7/14 | 5/16 |
| Perinatal deathsd | 14/70 | 10/73 |
| Litter sizee | 5.10 ± 0.55 | 4.41 ± 0.42 |
| Total number of viable female pups (range/litter) | 31 (0–4) | 24 (0–4) |
| Total number of viable male pups (range/litter) | 25 (0–4) | 38 (0–4) |
Number of pregnant guinea pigs that died during injections/total number of injected guinea pigs.
Number of pregnant guinea pigs that miscarried after beginning of injections/total number of pregnant guinea pigs that survived the injections.
Number of litters with offspring that died within 24 h after birth/total number of viable litters.
Number of offspring that died within 24 h after birth/total number of offspring.
Litter size is presented as mean ± SEM of total number of offspring per litter per group.
2.2. Measurements of cholinesterase activity
The colorimetric assay described in Fawcett et al. (2009) was used to measure AChE and butyrylcholinesterase (BuChE) activities in individual samples of red blood cells (RBCs) and plasma, respectively, collected from dams and offspring on the day of birth. Enzyme activities were also measured in extracts of different brain regions (hippocampus, thalamus, cerebral cortex, and cerebellum) harvested from offspring on the day of birth. Protein concentrations, determined using the bicinchoninic acid assay (Pierce Chemical, Rockford, IL), were used to normalize results from tissue samples. Conversion factors, 26 and 32 nmoles/AU, were used to convert rates of change in absorbance (AU/min) to rates of change in substrate (nmoles/min) at 412 nm and 436 nm, respectively. These values were determined empirically using the reaction of L-cysteine standards with DTNB as described by the manufacturer [https://tools.thermofisher.com/content/sfs/manuals/MAN0011216_Ellmans_Reag_UG.pdf].
2.3. Behavioral apparatuses
2.3.1. Open fields
The open field apparatus consisted of a black, non-glare Starboard plastic box (120 cm × 120 cm × 60 cm) in which the floor was covered with the same paper bedding as used for housing. Four incandescent lamps hung over the apparatus generated evenly distributed light with intensity of 20 lux on the floor of the field. Conair sound machines were set to generate “white noise” on one side and “running stream” sound on the opposite side of the apparatus. For the purpose of data analysis, a virtual center zone (40 cm × 40 cm) was delineated 40 cm from the walls of the open fields. For the novel object exploration test, a cylindrical glass jar (height: 8.8 cm; diameter: 7.2 cm) with a black plastic lid was placed in the center of the arena.
2.3.2. Water maze
The apparatus consisted of a gray galvanized metal circular tank (diameter: 198 cm; height: 60 cm) filled to a depth of 40 cm with water that was made opaque with non-toxic black tempera paint. A two-level, round, black-painted Plexiglas escape platform (diameter: 20 cm) was submerged in the center of one of the quadrants 3–4 cm below the water level.
As described in Mamczarz et al. (2011), during the acquisition phase the water temperature was progressively decreased from 30.5 ± 0.25 °C on the 1st training day to 24 ± 0.25 °C on the 5th training day. This protocol was used because the swimming performance of guinea pigs is very sensitive to water temperature (Wilber, 1959). Starting training guinea pigs in warm water temperatures that minimize the animals’ exhaustion and progressively dropping the water temperature in subsequent days to create an aversive environment from which the animals are motivated to escape nearly eliminates potential drowning of animals and produces a consistent learning behavior. The water temperature of 24 ± 0.25 °C was maintained during the probe test and platform relocation tests. In the room there were four Conair sound machines placed in each corner, which were set to generate “white noise” on one diagonal, and “running stream” sound on the other diagonal.
2.4. Behavioral testing
2.4.1. Open field and novel object exploration
Evaluation of behavior in the open field started when animals were approximately 30 days old. Four days before testing begun, animals were taken to the testing room for 3–4 h of acclimation on each of two consecutive days. Immediately before testing started, animals were weighed and placed in waiting cages adjacent to the open field apparatus for 1–3 min. Then, animals were placed in the center of the arena and allowed to explore the open field for 10 min. The test was repeated 24 h later. Immediately after the second test, all animals were placed in the waiting cage adjacent to the open field for 3–5 min while a novel object was positioned in the center of the open field. Animals were then placed in the corner of the apparatus and allowed to explore for an additional 10 min. The Any-Maze software (Stoelting Co., Wood Dale, IL) was used to record and analyze the behavior of the animals. Locomotor activity as well as time and distance traveled in different zones of the open field were analyzed. Distance, number of entries, and time traveled in the center zone were taken as measures of novel object exploration.
2.4.2. Water maze training and testing
Training in the MWM started when animals were approximately 38 days old. First, cognitive performance was evaluated in a reference memory paradigm in which animals were trained to escape onto a hidden platform in a fixed position for five consecutive days.
Animals were placed in the water facing the wall, with the start locations varying pseudo-randomly (N, S, E, or W) and were permitted to swim until they reached the escape platform. A maximum of 90 s was allowed for the animal to escape onto the platform and remain on it for 15 s before beginning of a new trial. Animals that failed to locate the platform within 90 s were gently guided by an experimenter and left on the platform for 15 s. After finishing the block of four trials in each training day, guinea pigs were dried and transferred to their home cages.
After the end of the training phase, the animals were subjected to two probe tests for assessment of memory retention. One was performed 72 h and the other 96 h after the end of the last training trial. During the probe trials, the platform was removed and animals were allowed to freely explore the pool for 90 s. Starting 24 h after completion of the second probe test, animals were subjected to two consecutive platform relocation tests. The primary purpose of these tests was to evaluate spatial learning after the animals had the opportunity to master the contextual and procedural requirements of the task during the acquisition phase. On the first relocation test, the platform was positioned in the center of the quadrant opposite to that where the platform was located during the reference memory training. On the second test, the platform was placed in the center of the quadrant adjacent to both previous locations. On each of the two days, the animals were placed on the hidden platform for 15 s before the first trial and were subsequently given 4 trials (each lasting 120 s) interspaced by 1-h inter-trial intervals (ITI).
Data were collected using the Any-Maze software, which was set to turn off tracking at 90 s (during the acquisition phase) or 120 s (during the relocation tests) after the beginning of each trial or when the animal stopped on the platform for at least 2 s.
2.5. Statistical analysis
In developmental studies, statistical analysis based on individual offspring as the experimental unit has the potential to overestimate the statistical significance of an observed effect because siblings share the same genetic characteristics and receive the same maternal care. Results obtained from offspring within a litter are likely to be highly correlated and, therefore, violate the assumption of statistical independence of observations. To overcome this issue, offspring-related data throughout the manuscript were statistically analyzed using a random effect model similar to that originally described by Laird and Ware (1982). In this model, results from individual offspring are nested under the offspring dam. The robustness of this model as it applies to preclinical developmental studies was discussed recently by Wainwright et al. (2007).
Differences between groups were analyzed using a random effect ANOVA model with two or three fixed factors and one random factor (mothers which were nested under prenatal exposure). Fixed factors included prenatal exposure and animal sex. Time intervals and water maze zones were used as repeated measure factors in the analysis of learning curves and of the probe test, respectively. The analysis was performed using PROC Mixed of SAS version 9.2, whose outputs include the significance of main effects, simple main effects, and interactions. The Tukey-Kramer post-hoc test was used for pairwise comparisons of multiple groups.
In addition to analyzing differences among groups, preference of the target platform zone (platform area or quadrant) was analyzed within each tested group as a distribution of time or distance each animal swam in the four virtual quadrants of the water maze. This analysis was performed for the probe test using a random effect one-way ANOVA followed by the Dunnett’s post-hoc test.
2.6. Chemicals
Chlorpyrifos (CPF, analytical-grade, purity, 99.8%) and peanut oil were purchased from Sigma-Aldrich (St. Louis, MO).
3. Results
3.1. Pregnancy outcome
Pregnancy outcomes were analyzed for pregnant guinea pigs that were subjected to daily injections of peanut oil or CPF (25 mg/kg, s.c.) for 10 consecutive days and whose offspring were subsequently tested behaviorally (Table 1). The number of pregnant guinea pigs that died during the injection period, number of miscarriages, number of perinatal deaths (i.e., number of offspring that died within the first 24 h after birth), number of litters with perinatal deaths, and mean litter size were comparable between both groups (Table 1). Repeated measures ANOVA revealed that throughout the period of injections daily body weights of CPF-injected pregnant guinea pigs were not significantly different from those of peanut oil-injected dams (Fig. 1A). While there was a main effect of day on the body weight of the pregnant guinea pigs [F(9,243) = 96.229; p < 0.0001], there was no significant main effect of treatment [F(1,29) = 0.320, p = 0.576] and no significant day × treatment interaction [F(9,243) = 0.756, p = 0.649].
Fig. 1.
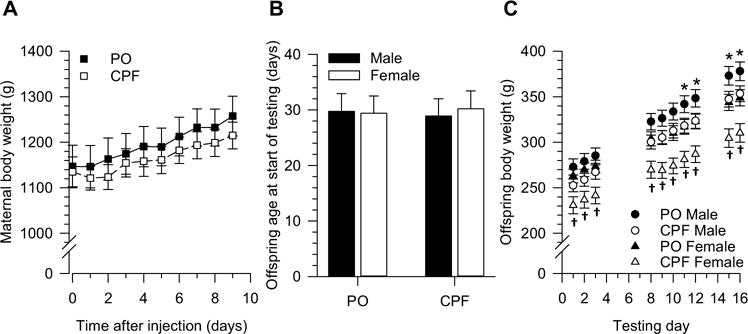
Effect of gestational exposure to chlorpyrifos (CPF) on maternal and offspring body weight gain. A. Body weight of pregnant guinea pigs measured immediately before their daily s.c. injection with peanut oil (PO, n = 13) or CPF (25 mg/kg/day, n = 15) starting on approximate GD53-55. B. Age of offspring at the beginning of behavioral testing. Data analyzed using the random ANOVA model originated from 26 male offspring (1–4/litter) and 29 female offspring (2–4/litter) of 13 PO-exposed sows and from 37 male offspring (1–4/litter) and 25 female offspring (0–4/litter) of 15 CPF-exposed sows. C. Body weight of offspring during open field testing (days 1–3), water maze training (days 8–13), and probe tests (days 15–16). Data in graph C excluded 7 offspring that could not swim in the MWM (2 PO-exposed males, 4 CPF-exposed males, and 1 CPF-exposed female). In all graphs, data are presented as mean ± SEM. According to the post-hoc Tukey-Kramer test: * p < 0.05 PO males vs. PO females; †p < 0.05 CPF males vs. CPF females.
3.2. Body weight of offspring during testing
At the start of the behavioral tests, there were no significant differences in the ages of animals that had been prenatally exposed to peanut oil or CPF (Fig. 1B). The body weights of these animals were recorded daily over the course of open field testing (days 1–3), water maze training (days 8–13), and water maze probe tests (days 15–16). Seven animals that were not able to swim in the water maze were not included in this analysis.
Although testing day, animal sex, and prenatal exposure had significant main effects on body weight [F(9, 1033) = 145.48, p < 0.0001; F(1,1033) = 180.83, p < 0.0001; and F(1,27) = 4.65, p = 0.0401, respectively], interpretation of the results was complicated by the significant testing day × sex and prenatal exposure × sex interactions [F(9, 1033) = 2.10, p = 0.027 and F(9, 1033) = 6.67, p = 0.010, respectively]. Analysis of the simple main effect of each factor in the significant interactions revealed that body weight of male and female offspring increased significantly with time [males: F(9, 1033) = 97.51, p < 0.0001; females: F(9, 1033) = 53.07, p < 0.0001]. It also revealed that prenatal exposure to CPF significantly reduced the body weight gain of female offspring [F(9, 1033) = 6.16, p = 0.0132], while having no significant effect on the body weight of males [F(9, 1033) = 3.20, p = 0.0738].
At the beginning of behavioral testing, animals were approximately 30 days old (Fig. 1B). Pairwise comparisons revealed that during the first 10 days of testing, i.e. between approximately 30 and 40 days of age, control male and female offspring had comparable body weights (Fig. 1C). Between the 11th and the 16th day of testing, when animals were approximately 41–46 days old, control male offspring became significantly heavier than their female counterparts (Fig. 1C). These findings are in agreement with a previous report that male Dunkin-Hartley guinea pigs gain weight at faster rate that their female counterparts, with the body weight of males becoming considerably larger than that of females after approximately 40 days of age (Slob et al., 1973). In contrast, since the first testing day, male offspring that had been prenatally exposed to CPF were significantly heavier than their female counterparts (Fig. 1C).
3.3. Cholinesterase inhibition
Repeated exposure of the pregnant guinea pigs to the dose of CPF used in the present study induced no clinical signs of acute toxicity in the dams or their offspring. To ascertain that the offspring were exposed to CPF in utero, the activities of BuChE and AChE were measured in different blood compartments and brain regions of male and female offspring at time of birth.
The activity of plasma and brain BuChE was approximately 80–90% lower among male and female offspring born to CPF-injected dams than among those born to peanut oil-injected dams (Fig. 2A, C and E). There was a significant main effect of maternal exposure to CPF on plasma BuChE activity [F(1,3) = 1575.77; p < 0.0001]. There was no significant main effect of sex [F(1,13) = 0.14; p = 0.710] and no significant maternal exposure × sex interaction [F (1,13) = 0.16; p = 0.691] on plasma BuChE activity. BuChE is generally taken as a sensitive biomarker of OP exposure because it is inhibited more effectively than AChE by most OP compounds, including CPF (Farahat et al., 2011). Thus, the present results indicate that offspring were exposed to CPF in utero. The degree of BuChE inhibition observed in the plasma of CPF-exposed offspring was similar to that seen in the plasma of CPF-exposed dams (mean SE: 90.2 1.97%, n = 3).
Fig. 2.
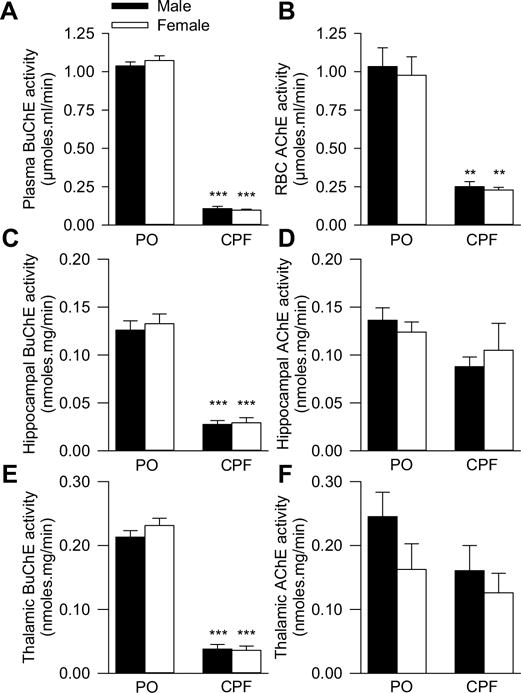
Effect of prenatal exposure to chlorpyrifos (CPF) on butyrylcholinesterase (BuChE) and acetylcholinesterase (AChE) in different blood compartments and brain regions. Measurements of BuChE in plasma (A), hippocampus (C), and thalamus (E) and of AChE in red blood cells (RBCs, B), hippocampus (D), and thalamus (F) are from offspring (n = 5 males and 4 females) on the day they were born to pregnant guinea pigs that had been injected with peanut oil (PO, n = 2) and from offspring (n = 7 males and 4 females) on the day they were born to pregnant guinea pigs that had been injected with CPF (n = 3). Post-hoc analysis using the Tukey-Kramer test revealed plasma BuChE activity was significantly lower in plasma and brain tissue from offspring prenatally exposed to CPF than from those exposed to PO (***, p < 0.001). Likewise, AChE activity was significantly lower in RBCs from CPF-exposed than from PO-exposed offspring (**, p < 0.01).
The random effect ANOVA revealed a significant main effect of maternal exposure to CPF on RBC AChE in offspring [F(1,3) = 20.16; p = 0.02]. There was no significant main effect of sex [F(1,13) = 0.07; p = 0.798] and no prenatal exposure × sex interaction [F (1,13) = 1.66; p = 0.220] on RBC AChE. Maternal CPF exposure reduced by approximately 75% the activity of AChE in RBCs of both male and female offspring (Fig. 2B). This degree of RBC AChE inhibition was comparable to that seen in RBCs from the CPF-exposed dams (mean ± SE: 81.6 ± 3.50%, n = 3). In contrast, the random effect ANOVA revealed that AChE activity in the different brain regions analyzed, including the hippocampus and thalamus, was not significantly different between CPF- and peanut oil-exposed offspring (Fig. 2D, F).
3.4. Open field and novel object exploration
There was a significant main effect of testing day on the total distance the animals traveled in the open field, with the locomotor activity decreasing significantly between the first and second days of testing [F(1,198) = 137.81, p < 0.0001] (Fig. 3A). There was no significant main effect of sex or exposure and no significant interaction between these two factors on the total distance or on the inter-session decline of the total distance traveled by the animals in the open fields (Fig. 3A).
Fig. 3.
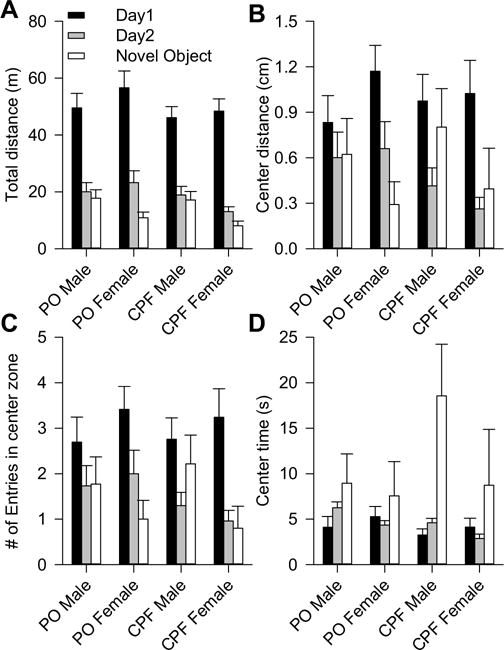
Locomotor activity, habituation, and novel object exploration of prepubertal offspring prenatally exposed to chlorpyrifos (CPF) or peanut oil (PO). Graphs show total distance (A) as well as distance (B), number of entries (C), and time (D) in the center zone recorded during testing animals in 10-min sections in two consecutive days in open fields followed by 10-min exploration of a novel object in the center of the same fields. Graph and error bars are mean and SEM, respectively, of results from the same groups of animals as in Fig. 1B.
There was a significant main effect of testing day on the distance traveled [F(1,198) = 22.059, p < 0.0001] and number of entries in the center zone [F (1,198) = 23.35, p < 0.0001], with exploration of the center of the field decreasing significantly between the first and second day of testing (Fig. 3B and C). These results are in agreement with the notion that the animals habituated when successively exposed to the open fields. However, prenatal exposure and sex had no significant main effect on distance, number of entries, and time in the center zone (Fig. 3B–D).
Behavior of the animals in the presence of a novel object placed on the center of the open field was compared to that recorded during the preceding open field session. A three-way random effect ANOVA using test (i.e., novel object and 2nd day of open field), sex, and exposure as factors revealed a significant main effect of test [F (1,198) = 7.44, p = 0.0069] and sex [F(1,198) = 4.22, p = 0.0412], no significant main effect of exposure, and no significant interaction between sex and test on total distance (Fig. 3A).
Distance and number of entries in the center zone during the novel object session were comparable to those measured in the preceding open field test without the object (Fig. 3B, C). However, there was a significant main effect of test on time in the center zone [F(1,198) = 6.84, p = 0.0096], with the presence of the novel object triggering a significant increase of time the animals spent in the center of the fields (Fig. 3C). Sex and prenatal exposure had no significant effect on the parameters measured during the novel object exploration test.
Exploration of the center of open fields is normally taken as a measure of anxiety-related behavior in rodents (Prut and Belzung, 2003). Because even control animals rarely entered and explored the center of the open fields (note in Fig. 3B that distance in the center of the fields was < 2% of the total distance traveled in each test day), results should not be interpreted as an indication of lack of effect of CPF on anxiety-related behavior.
3.5. Morris water maze
3.5.1. Reference memory training
During the first two days of the reference memory training in the MWM, seven animals were retired due to their inability to swim (2 peanut oil-exposed females, 4 CPF-exposed males, and 1 CPF-exposed female). Over the course of 5 days of training in the MWM animals progressively learned the task as suggested by a significant main effect of training day on the escape latency [F (4,503) = 158.71, p < 0.0001] and distance [F(4,503) = 64.31, p < 0.0001] (Fig. 4A, B). There was also a significant main effect of sex on both escape latency [F(1,503) = 12.53, p = 0.0004] and distance [F(1,503) = 15.77, p < 0.0001], with males outperforming females. Finally, there was a significant main effect of prenatal exposure on escape latency [F(1,27) = 9.36, p = 0.0050] and distance traveled to reach the hidden platform [F(1,27) = 6.48, p = 0.0169].
Fig. 4.
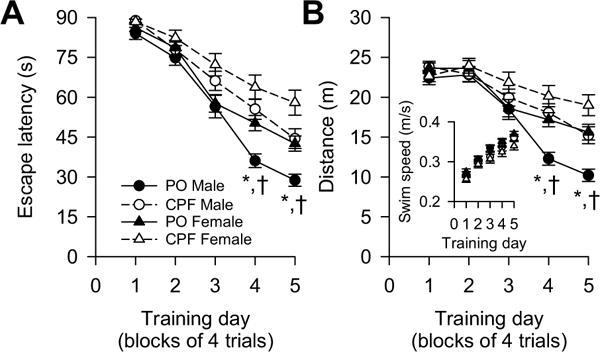
Effect of prenatal exposure to chlorpyrifos (CPF) on the acquisition phase of Morris water maze task. Graphs show (A) average escape latency and (B) average distance traveled by CPF- and peanut oil (PO)-exposed offspring per training day. Inset in B shows swim speed of male and female offspring prenatally exposed to CPF or PO. Data points and error bars represent mean and SEM, respectively, of results obtained from same groups of animals as in Fig. 1C. According to Tukey-Kramer post-hoc test for pairwise comparisons: *, p < 0.05 PO males vs. CPF males; †, p < 0.05 PO males vs. PO females.
There was a significant training day × sex interaction [escape latency: F(4,503) = 3.59, p = 0.0067; distance: F(4,503) = 4.40, p = 0.0017] and analysis of simple main effects of each factor in this interaction revealed that the performance of male and female guinea pigs became significantly different on the 4th and 5th days of training [escape latency on 4th day: F(1,503) = 10.66, p = 0.0012; escape latency on 5th day: F(1,503) = 16.86, p < 0.0001; distance on 4th day: F(1,503) = 12.36, p = 0.0005; distance on 5th day: F (1,503) = 21.93, p < 0.0001].
The training day × prenatal exposure interaction was also significant [escape latency: F(4, 503) = 4.90, p = 0.0007; distance: F (4,503) = 4.88, p = 0.0007]. Analysis of the simple main effect of each factor in this interaction revealed that prenatal exposure to CPF significantly increased both the escape latencies and distances measured on the 3rd, 4th, and 5th training days [escape latency: 3rd day: F(1,503) = 9.97, p = 0.0049; 4th day: F(1,503) = 15.27, p = 0.0001; 5th day: F(1,503) = 13.55, p = 0.0003. Distance: 3rd day: F(1,503) = 3.93, p = 0.048; 4th day: F(1,503) = 12.85, p = 0.0004; 5th day: F(1,503) = 12.02, p = 0.0006].
The prenatal exposure × sex interaction was significant when distance was the dependent variable [F(1,503) = 4.58, p = 0.0329]. Examination of this interaction revealed that the effect of prenatal CPF exposure on distance was stronger among male than female offspring Male: [F(1,503) = 10.72, p = 0.0011; Female: F (1,503) = 1.78, p = 0.1827]. There was no significant prenatal exposure × sex interaction when escape latency was the dependent variable [F(1,503) = 2.90, p = 0.0895], because the prenatal exposure to CPF had a significant main effect on the escape latency of both males and females as revealed by the analysis of simple main effects [Males: F(1,503) = 2.90, p = 0.0004; Females: F(1,503) = 4.09, p = 0.0438]. A previous study also reported that prenatal exposure of female guinea pigs to the same CPF dose regimen used here significantly increased escape latency in the MWM without affecting their swimming speed (Mullins et al., 2015).
Post-hoc pairwise comparisons confirmed that the performance of control male guinea pigs was significantly better than that of their female counterparts and that of CPF-exposed male offspring on the 4th and 5th training days (Fig. 4A, B). Because the effect of CPF was weak among female offspring, post-hoc pairwise comparisons revealed no significant differences between control and CPF-exposed female offspring on each day of training (Fig. 4A, B).
The poor performance of control female offspring and CPF-exposed offspring in the MWM could not be attributed to impaired locomotor activity. There was no significant main effect of training day, sex, or prenatal exposure on the swim speed of the animals (Fig. 4B inset) or the distance they traveled in the open fields (see Fig. 3). In addition, it is unlikely that visual acuity deficits contributed to the poor MWM performance of those animals, because there are reports that the locomotor activity of visually impaired guinea pigs is significantly higher than that of control guinea pigs (Ishikawa et al., 1991; O’Hara and Dyer, 1974).
Thigmotaxis refers in the MWM to a tendency of the animals to swim close to the wall around the perimeter of the pool (Dalm et al., 2000). It is a non-spatial strategy rodents typically use in the first day of training in the MWM before they learn that they cannot escape the water if they stay close to the wall (Dalm et al., 2000). In fact, on the first training day, the percent of time and distance traveled in the wall zone was similar among all experimental groups (Fig. 5A, B). These parameters progressively declined with the training days as revealed by a significant main effect of training day on both % time and % distance traveled in the wall zone [% Time: F (4,503) = 133.95, p < 0.0001; % distance: F(4,503) = 144.79, p < 0.0001]. Sex also had a significant main effect on these parameters, with females spending longer time and swimming longer distances in the wall zone than males [% time: F(1, 503) = 23.65, p < 0.0001; % distance: F(1,503) = 25.14, p <0.0001]. Post-hoc analysis confirmed that this sex dimorphism was confined to the control group; on the 4th and 5th training days the thigmotactic behavior of female offspring prenatally exposed to peanut oil was significantly more pronounced than that of their male counterparts (Fig. 5A, B).
Fig. 5.
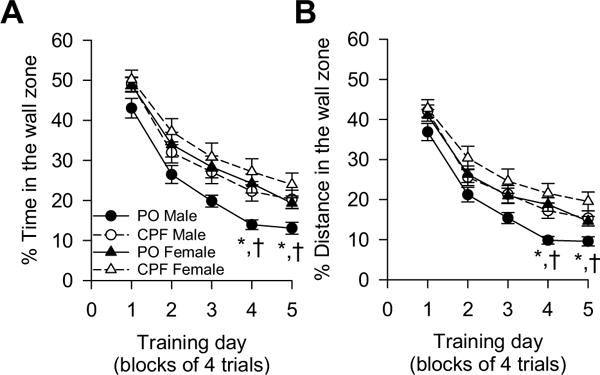
Effect of prenatal exposure to chlorpyrifos (CPF) on thigmotactic behavior during the acquisition phase of the Morris water maze. Thigmotactic behavior was defined by the % of time (A) and % of distance (B) that animals swam within a 20 cm-wide zone around the tank wall. Data points and error bars represent mean and SEM, respectively, of results obtained from the same animals as in Fig. 1C. According to the Tukey-Kramer post-hoc test for parwise comparisons: *, p < 0.05 PO males vs. CPF males; †, p < 0.05 PO males vs. PO females.
Prenatal exposure to CPF had no significant main effect on the % of time and % of distance in the wall zone (Fig. 5A, B). However, the sex × prenatal exposure interaction was significant for both dependent variables [% time: F(1,503) = 12.03, p = 0.0006; % distance: F(1,503) = 13.03, p = 0.0003]. Analysis of the simple main effect of each factor in the interactions indicated that prenatal exposure to CPF significantly increased thigmotactic behavior only among male offspring [% time: F(1,503) = 7.98, p = 0.0049; % distance: F(1,503) = 8.80, p = 0.0032]. Post-hoc analysis confirmed that on the 4th and 5th training days the thigmotactic behavior of male offspring prenatally exposed to CPF was significantly more pronounced than that of control male offspring (Fig. 5A, B). The thigmotactic behavior of female offspring prenatally exposed to peanut oil and CPF did not differ significantly (Fig. 5A, B).
3.5.2. Memory retention
Analysis of time spent in the water maze quadrants during the first probe test revealed a significant main effect of quadrant [F (3,397) = 2.89, p = 0.0352] and a significant quadrant × prenatal exposure × sex interaction [F(3,397) = 3.10, p = 0.0267]. Analysis of the simple main effect of each factor in this interaction revealed that only male offspring that had been prenatally exposed to peanut oil spent more time in one of the quadrants [peanut oil males: F(3,397) = 4.35, p = 0.0049; CPF males: F(3,397) = 2.37, p = 0.0701; peanut oil females: F(3,397) = 0.81, p = 0.4860; CPF females: F(3,397) = 0.60, p = 0.6171]. The quadrant × preanatal exposure × sex interaction approached significance when distance swum in the quadrants was the dependent variable [F (3,397) = 2.20, p = 0.087]. Based on a within-subject comparison using a one-way random effect ANOVA followed by the Dunnett’s post-hoc test, only male offspring prenatally exposed to peanut oil spent significantly more time and swam longer distances in the quadrant where the platform used to be located than in the other quadrants (Fig. 6A, B).
Fig. 6.
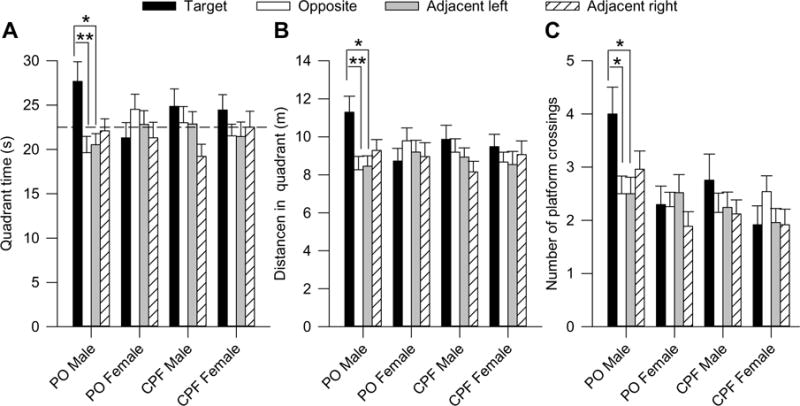
Performance of prepubertal guinea pigs in a probe test performed 72 h after completion of the acquisition phase of the Morris water maze training. (A) Time animals spent in each of the four quadrants of the pool. (B) Distance traveled in each quadrant of the pool. (C) Based on a within-subject comparison, a one-way random effect ANOVA followed by the Dunnett’s post-hoc test revealed that only male offspring prenatally exposed to peanut oil (PO) showed significant preference towards the target quadrant and the platform area in the center of the target quadrant (*, p < 0.05; **, p < 0.01). Graph and error bars represent mean and SEM, respectively, of results obtained from the same animals as in Fig. 1C.
To assess search accuracy during the probe tests, number of crossings of the platform area in the training quadrant was compared to number of crossings of equivalent areas positioned in the center of the non-training quadrants. The three-way random effect ANOVA revealed a significant main effect of sex [F (1,397) = 5.36, p = 0.0211] and a significant sex × quadrant interaction [F(3,397) = 3.15, p = 0.0249]. The one-way random effect ANOVA followed by the Dunnett’s post-hoc test indicated that only male offspring that had been prenatally exposed to peanut oil crossed the platform position in the training quadrant more often than the same area in the other quadrants (Fig. 6C).
Analysis of the total distance traveled during the first probe test showed that locomotor performance was not significantly affected by sex or prenatal exposure to CPF (Table 2). According to the three-way random effect ANOVA, prenatal exposure to CPF and sex had no significant main effect on the% of time or distance the animals swam in the wall zone during the first probe test. However, there was a significant prenatal exposure × sex interaction for the% of time [F(1,79) = 4.02, p = 0.0484]. Analysis of the simple main effect of each factor in this interaction indicated that offspring males prenatally exposed to peanut oil spent less time and swam shorter distances in the wall zone than did the animals in the other groups [Prenatal exposure: F(1,79) = 7.01, p = 0.0098 for % time and F(1,79) = 5.61, p = 0.0203 for % distance. Sex: F(1,79) = 6.25, p = 0.0145 for % time and F(1,79) = 5.84, p = 0.018 for % distance]. Post-hoc multiple comparisons revealed that the thigmotactic behavior of male offspring prenatally exposed to peanut oil was less pronounced than that of their female counterparts (Table 2). They also indicated that thigmotaxis was less pronounced among male offspring prenatally exposed to peanut oil than those exposed to CPF (Table 2).
Table 2.
Overall swimming performance and thigmotactic behavior of male and female offspring prenatally exposed to peanut oil or chlorpyrifos (CPF).
| Peanut Oil
|
CPF
|
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Total distance (m) | 37.30 ± 0.49 | 36.67 ± 0.66 | 36.15 ± 0.67 | 35.8 ± 0.68 |
| % Time in wall zone | 23.3% ± 2.8*,† | 33.5% ± 2.2 | 35.1% ± 3.3 | 35.7% ± 3.5 |
| % Distance in wall zone | 21.3% ± 2.8* | 29.6% ± 2.0 | 31.4% ± 3.1 | 32.6% ± 3.2 |
Thigmotactic behavior was defined as the percentage of time and the percentage of distance animals swam in a 20 cm-wide zone from the wall of the pool. Results are presented as mean ± SEM of measures obtained during the first probe test from the same animals as in Fig. 4. According to the random effect ANOVA model followed by Tukey-Krammer post-hoc test for pairwise comparisons:
p < 0.05 (peanut oil-exposed males compared to peanut oil-exposed females);
p < 0.05 (peanut oil-exposed males compared to CPF-exposed males).
During the second probe test, motor function assessed as total distance traveled was not significantly different among the test groups (data not shown). In addition, analysis of the time and distance in the quadrants and of number of platform crossings revealed that during this test none of the animals showed significant preference toward the target quadrant (data not shown).
3.5.3. Platform relocations
Due to the workflow, offspring from one dam that had been exposed to peanut oil and from three dams that had been exposed to CPF could not be tested. The hierarchical distribution of number of offspring and dams per treatment is included in the legend of Fig. 7. During the first platform relocation test, there was a significant main effect of trial on escape latency [F(3,340) = 4.49, p = 0.0042] and distance traveled to reach the platform [F (3,340) = 6.51, p = 0.0003]. Sex had no significant main effect on escape latency or distance. However, there was a near-significant main effect of prenatal exposure on distance [F(1,24) = 5.66, p = 0.056] and a significant main effect of prenatal exposure to CPF on escape latency [F(1,24) = 6.04, p = 0.0216] (Fig. 7A inset). There was also a significant trial × prenatal exposure interaction [escape latency: F(3,340) = 2.94, p = 0.0334; distance: F (3,340) = 2.91, p = 0.0348]. Examination of the simple main effect of each factor in this interaction revealed a significant effect of exposure on the 4th trial [escape latency: F(1,340) = 13.47, p = 0.0003; distance: F(1,340) = 12.87, p = 0.004]. Post-hoc analysis indicated that on the 4th trial male and female offspring prenatally exposed to CPF swam longer distances and took longer to find the hidden platform than did male and female offspring prenatally exposed to peanut oil (Fig. 7A, B).
Fig. 7.
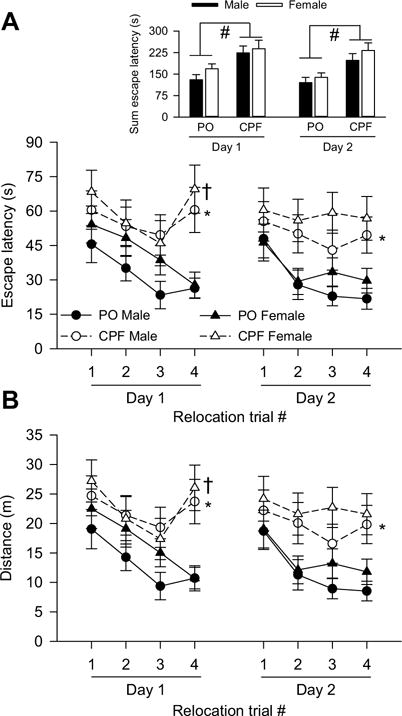
Effect of prenatal exposure to chlorpyrifos (CPF) on performance of guinea pigs trained in two platform relocation tests. Graphs of escape latency (A) and distance (B) measured during the 1st and 2nd relocation tests. Data points and error bars represent mean and SEM, respectively, of results originated from 23 male offspring (1–4/litter) and 24 female offspring (1–4/litter) of 12 PO-exposed sows and from 26 male offspring (1–4/litter) and 22 female offspring (1–4/litter) of 10 CPF-exposed sows. According to the Tukey-Kramer post-hoc test for multi-group comparisons: *, p < 0.05 PO males vs. CPF males; † p = 0.05 PO females vs. CPF females. Inset in A depicts the overall significant main effect of prenatal exposure to CPF on escape latency during the 1st and 2nd relocation tests (#, p < 0.05).
During the second relocation test, trial also had a significant main effect on escape latency [F(3,336) = 3.81, p = 0.0104] and distance [F(3,336) = 4.73, p = 0.0030]. There was no significant main effect of sex. However, prenatal exposure to CPF had a near-significant main effect on the distance traveled to reach the hidden platform [F(1,24) = 4.13, p = 0.0533] and a significant main effect on the escape latency [F(1,24) = 4.90, p = 0.0366] (Fig. 7A inset). There was no significant sex × prenatal exposure interaction. Post-hoc analysis of the data revealed that escape latency and distance to reach the hidden platform during the 4th trial were longer among male offspring exposed to CPF than among those exposed peanut oil (Fig. 7A, B).
To determine whether the poor performance of CPF-exposed animals during the relocation tests was due to increased thigmotaxis, propensity for swimming in the wall zone was analyzed. During the first relocation test, there was a significant main effect of trial on % of time [F(1,340) = 14.40, p < 0.0001] and % of distance in the wall zone [F(1,340) = 13.17, p < 0.0001], with both parameters decreasing significantly with training. Prenatal exposure to CPF had a nearly significant main effect on the% of distance swum in the wall zone [F(25,340) = 4.23, p = 0.0508]. There was no significant main effect of sex on distance or time swum in the wall zone. However, sex significantly modified the effect of prenatal exposure to CPF on time and distance swum in the wall zone as indicated by significant sex × prenatal exposure interactions [% of time: F(3,340) = 8.93, p = 0.0030; % of distance: F(3,340) = 9.00, p = 0.0029]. Analysis of the simple main effect of each factor in the interactions revealed a significant main effect of prenatal exposure to CPF on thigmotactic behavior of males [% of time: F(1,340) = 8.34 p = 0.0278; % of distance: F(1,340) = 10.51, p = 0.0013].
Post-hoc data analysis indicated that male offspring prenatally exposed to CPF swam significantly longer time and distance in the wall zone than their control counterparts during the 1st trial (Fig. 8A, B), while significant cognitive impairment was not detected in this trial (see Fig. 7A, B). During the 4th trial, when CPF-exposed males and females showed deterioration of cognitive performance (see Fig. 7A, B), only CPF-exposed males swam significantly longer distance in the wall zone (Fig. 8B). Therefore, deterioration of cognitive performance of CPF-exposed female offspring cannot be explained by increased swimming in the wall zone.
Fig. 8.
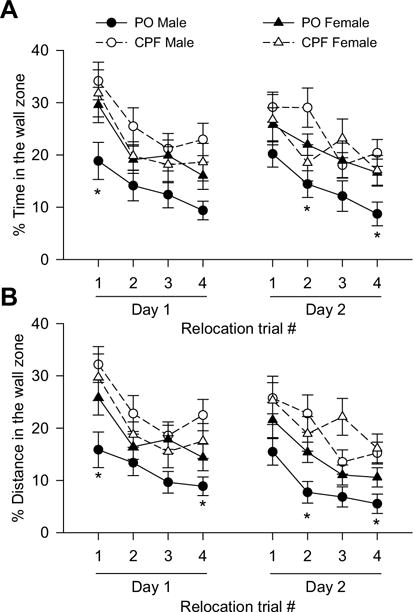
Effect of prenatal exposure to chlorpyrifos (CPF) on thigmotactic behavior of guinea pigs during the platform relocation tests. Thigmotactic behavior was defined by the % of time (A) and % of distance (B) that animals swam within a 20 cm-wide zone around the tank wall. Data points and error bars represent mean and SEM, respectively, of results obtained from the same animals as in Fig. 7 with exception of 1 CPF-exposed male offspring, which was not tested. According to the Tukey-Kramer post-hoc test for multi-group comparisons: *, p < 0.05 PO males vs. CPF males.
During the second relocation test, there was a significant main effect of trial on % of time and % of distance swum in wall zone, with both parameters decreasing significantly with training [% of time in wall zone: F(3,336) = 9.68, p < 0.0001; % of distance: F (3,336) = 15.20, p < 0.0001]. Prenatal exposure to CPF had a significant main effect on the% of distance swum in the wall zone [F(24,336) = 4.92, p = 0.0363]. Although sex had no significant main effect on time or distance swum in the wall zone, there was a significant sex × prenatal exposure interaction [% of time: F (3,336) = 11.37, p = 0.0008; % of distance: F(3,336) = 6.84, p = 0.0093]. Examination of the main simple effect of each factor in the interactions showed that prenatal exposure to CPF significantly increased swimming in the wall zone among males [% of time: F(1,336) = 9.36, p = 0.0024; % of distance: F(1,336) = 7.67, p = 0.0059]. Post-hoc analysis of the data indicated that during the 2nd and 4th trials, % of time and% of distance in the wall zone were significantly longer among male offspring prenatally exposed to CPF than among those exposed to peanut oil (Fig. 8B).
4. Discussion
The present study is the first to provide evidence of a direct relationship between prenatal exposure to CPF and cognitive deficits in guinea pigs, which, like humans, are considered a precocial species. As shown here, prenatal exposure of guinea pigs to sub-acute doses of CPF had no significant effect on locomotor activity or habituation, a form of non-associative memory. However, it significantly impaired spatial learning, with the effect being more pronounced among male than female offspring.
4.1. Effect of prenatal exposure of guinea pigs to CPF on locomotor activity and non-associative learning
In this study, guinea pigs were injected subcutaneously between approximate GD 53 and 63 with CPF (25 mg/kg/day) or its vehicle (peanut oil). Two lines of evidence supported the notion that the fetuses were exposed in utero to CPF and/or its oxon metabolite, which is known to block AChE and BuChE. First, on the day of birth, plasma BuChE and RBC AChE activities were approximately 75% and 85%, respectively, lower in offspring of CPF-exposed than of peanut oil-exposed (control) dams. Second, BuChE activity in various brain regions was also reduced by nearly 80–90% among offspring of CPF-exposed dams.
The finding that AChE activities measured in different brain regions, including the hippocampus, were not significantly different between CPF-exposed and control offspring may be accounted for by CPF and/or CPF-oxon binding with high affinity to other molecular targets (reviewed in Terry, 2012). It could also help to explain why neither locomotor activity nor locomotor habituation differed significantly between offspring prenatally exposed to CPF and those exposed to vehicle. Locomotor activity and habituation are controlled in part by cholinergic activity in the hippocampus (Leussis and Bolivar, 2006). Specifically, when microinfused in the hippocampus muscarinic agonists or AChE inhibitors increase while muscarinic antagonists decrease loco-motor habituation and activity in open fields (Izquierdo et al., 1992; Zheng et al., 1983). Thus, it is likely that the prenatal exposure to CPF did not result in significant changes in hippocampal cholinergic activity.
Developmental exposure of mice and rats to CPF doses that elicit no signs of acute toxicity can increase, decrease, or have no effect on their locomotor activity, measured as distance traveled in open field or figure-8 apparatuses or in a social recognition task; the outcome depends upon the time and level of exposure, time of testing, and type of test (Carr et al., 2001; Dam et al., 2000; Levin et al., 2001, 2002; Ricceri et al., 2003; Venerosi et al., 2006). Of particular interest, no significant changes in locomotor activity have been observed in Sprague-Dawley rats or CD-1 mice exposed to CPF at PND11-14 or 1-21 (Carr et al., 2001; Dam et al., 2000; Levin et al., 2001; Venerosi et al., 2006). These ages span a period of brain growth spurt in mice and rats comparable to that experienced by fetal guinea pigs at end of gestation (Dobbing and Sands, 1970).
4.2. Spatial learning in the MWM is sexually dimorphic among control guinea pigs
Sexual dimorphism of spatial learning has been described in different animal species, with males outperforming females in most instances (Berger-Sweeney et al., 1995; Bimonte et al., 2000). However, the present study is the first to demonstrate that pre-pubertal male guinea pigs perform significantly better than their female counterparts during the acquisition phase of the non-cued version of the MWM. Since learning in the MWM is a hippocampus-dependent task (Chersi and Burgess, 2015), it is likely that the sexual dimorphism of the hippocampal morphology of guinea pigs contributes to the sex differences observed here in the MWM (Bartesaghi et al., 2003). Previous studies may have missed the sexual dimorphism of the learning performance of prepubertal guinea pigs in the MWM (Byrnes et al., 2004; Richardson et al., 2002), because of the tank size, size and architecture of the platform, duration of the training trials, and water temperature, all of which are factors that influence the performance of rodents in this task (Vorhees and Williams, 2006).
4.3. Learning and memory deficits in guinea pigs prenatally exposed to CPF
The sexual dimorphism observed during the acquisition phase of the MWM task in control guinea pigs was absent in guinea pigs prenatally exposed to CPF, because the performance of CPF-exposed males was more severely impaired than that of CPF-exposed females. Association of prenatal CPF exposure with cognitive deficits in children is also generally stronger among boys than girls (Horton et al., 2012; Marks et al., 2010; Rauh et al., 2012). However, in preclinical studies, whether cognitive impairments are more pronounced in males or females depends on the time at which animals are exposed to CPF. In general, cognitive deficits are more apparent among female than male rats exposed to CPF during the late days of gestation (Levin et al., 2002; Haviland et al., 2010). The different outcomes observed in rats and guinea pigs exposed in utero during late gestation to CPF can be accounted for by fact that the brain growth spurt of rats is a postnatal event whereas that of guinea pigs is a prenatal event (Dobbing and Sands, 1970, 1979). This notion is supported by reports that cognitive deficits resulting from continued CPF exposure of rats between late gestation and early postnatal ages are more pronounced among males than females (Aldridge et al., 2005; Johnson et al., 2009; Levin et al., 2001).
In the classical version of the MWM, animals first learn that they have to swim in order to escape onto a hidden platform from which they can be rescued (Izquierdo et al., 2006). As this procedural learning proceeds, animals learn to use the extra-maze cues to triangulate the position of the hidden platform and guide themselves towards it (Izquierdo et al., 2006). The finding that learning differences between CPF-exposed and control offspring became significant towards the last days of training in the MWM suggests that the prenatal exposure to CPF had a more detrimental effect on spatial than on procedural learning. This notion is supported by the finding that CPF-exposed offspring also presented significant deficits in the platform relocation tests during which the animals had to learn to find the hidden platform in different locations using the same contextual information as that used during the acquisition phase.
The platform relocation tests performed here differed from the more traditional delayed match-to-sample task used to evaluate episodic-like memory, because here the animals were placed on the platform 15 s prior to the first trial each day. To accurately evaluate episodic-like memory, animals would have been allowed to search randomly for the platform in the first trial and tested in a second trial for their ability to process and retain the spatial information needed to find the platform in the new position (Vorhees and Williams, 2006). Nevertheless, the results obtained from the relocation tests suggest that episodic-like memory may also be impaired in guinea pigs as it is in children prenatally exposed to CPF (Horton et al., 2012; Rauh et al., 2012).
Spatial learning deficits presented by male, but not female offspring prenatally exposed to CPF could be explained at least in part by increased thigmotaxis, which has been shown to inhibit acquisition of spatial relationships of contextual cues (Acheson et al., 2011). Increased thigmotactic behavior can be a result of increased anxiety (Treit and Fundytus, 1988) and/or damage to brain regions such as the thalamus and the dorsal medial striatum, which are known to play a central role in the regulation of spatial search strategies (Cain and Boon, 2003). Of interest, a recent resonance magnetic imaging study of female guinea pigs prenatally exposed to CPF revealed that diffusion measures indicative of reduced white matter integrity within the striatum and amygdala correlated with their poor spatial learning performance in the MWM (Mullins et al., 2015).
Results from the probe tests confirmed the spatial cognitive impairment resulting from prenatal CPF exposure of male guinea pigs and the sex differences in the cognitive behavior of control guinea pigs in the MWM. However, they were inconclusive regarding the potential detrimental effects of prenatal exposure to CPF specifically on memory retention. During the first test, performed 72 h after the completion of training, only control male offspring presented signs of memory retention; they preferentially swam in the area where the platform used to be located. The lack of preference toward the target quadrant seen among CPF-exposed male offspring could be interpreted as evidence of significant memory deficits. Alternatively, it could have resulted from the fact that these animals did not reach the same near-asymptotic level of learning at the end of the acquisition phase as their control counterparts.
4.4. Critical features of the guinea pig model
The acute toxicity of OP pesticides results primarily, though not exclusively, from their ability to block AChE in the peripheral and central nervous systems. Since RBC AChE is very sensitive to inhibition by most OP compounds, RBC AChE inhibition is commonly used as a surrogate endpoint for health risk assessment of OP intoxication (Strelitz et al., 2014).
On the day of birth, although neither CPF-exposed dams nor their offspring presented clinical signs of OP intoxication, they had approximately 75% lower RBC AChE activity than their control counterparts. In contrast, brain AChE activity was not significantly different between control and CPF-exposed offspring. Following repeated exposure to OP compounds, the degree of inhibition of RBC AChE does not reflect the degree of inhibition of tissue AChE, because recovery of AChE activity between exposures will be due to combined reactivation of the inhibited enzyme and synthesis of new enzyme (Mason, 2000). While the half-life of AChE in brain ranges from 2 to 3 days (Wenthold et al., 1974), synthesis of new RBC AChE is only due to new RBC production and the half-life of RBCs is approximately 120 days (D’Alessandro et al., 2010).
In the U.S., California and Washington have instituted programs in which RBC AChE activity can be longitudinally monitored in workers handling OP pesticides (Ames et al., 1989; Hofmann et al., 2010). At any time workers enrolled in these programs present RBC AChE activity at or below 70% of their baselines, they are removed from OP pesticide handling until their RBC AChE activity is within 20% of their personal baseline. However, this is not common practice within the U.S. or worldwide and evidence exists that RBC AChE activity can be reduced by as much as 40–80% from baseline in workers who otherwise present no overt clinical sign or symptom of OP intoxication (Ames et al., 1989; Farahat et al., 2011; Singleton et al., 2015). Thus, based on measurements of RBC AChE, the CPF dose regimen the guinea pigs were subjected to in the present study translates to levels relevant to occupational exposure of people who handle OP pesticides throughout the world. It remains to be determined how the prenatal CPF levels achieved in this study compare to those experienced by children of mothers exposed to this pesticide during pregnancy.
In conclusion, the results presented here demonstrate that, similar to children, prepubertal guinea pigs exposed in utero to the pesticide CPF develop sexually dimorphic cognitive deficits. These results are far reaching because: (i) the CPF dose regimen administered to the pregnant animals induced levels of RBC AChE inhibition that can be relevant to humans occupationally exposed to this pesticide, and (ii) the pattern of brain development and the placenta structure of humans are closer to those of guinea pigs than of other rodents commonly used in preclinical studies. Thus, the guinea pig emerges as a valuable model to advance our understanding of the toxicokinetics and the cellular and molecular mechanisms underlying the developmental neurotoxicity of OP pesticides. Such studies can ultimately lead to the identification of potential medical countermeasures to treat and/or prevent the neurotoxic effects of these pesticides in the developing brain.
Acknowledgments
The authors are indebted to Ms. Mabel A. Zelle for her technical assistance and for her assistance editing the manuscript. Part of this research was presented as posters during the 2012 meeting of the Society for Neuroscience (Gavrushenko L, Pescrille JD, Pereira EFR, Albuquerque EX, Mamczarz J. Effect of prenatal exposure to chlorpyrifos on cognitive performance of guinea pigs. Program No. 840.02. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012. Online). This work was funded by the National Institutes of Health through the National Institute of Environmental Health Sciences [Grant ES019282].
Footnotes
Conflict of interest
None.
References
- Acheson SK, Moore NL, Kuhn CM, Wilson WA, Swartzwelder HS. The synthetic cannabinoid WIN 55212-2 differentially modulates thigmotaxis but not spatial learning in adolescent and adult animals. Neurosci Lett. 2011;487:411–414. doi: 10.1016/j.neulet.2010.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames RG, Brown SK, Mengle DC, Kahn E, Stratton JW, Jackson RJ. Cholinesterase activity depression among California agricultural pesticide applicators. Am J Ind Med. 1989;15:143–150. doi: 10.1002/ajim.4700150203. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Guidi S, Severi S, Contestabile A, Ciani E. Sex differences in the hippocampal dentate gyrus of the guinea pig before puberty. Neuroscience. 2003;121:327–339. doi: 10.1016/s0306-4522(03)00434-2. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Arnold A, Gabeau D, Mills J. Sex differences in learning and memory in mice: effects of sequence of testing and cholinergic blockade. Behav Neurosci. 1995;109:859–873. doi: 10.1037//0735-7044.109.5.859. [DOI] [PubMed] [Google Scholar]
- Bimonte H, Hyde L, Hoplight B, Denenberg V. In two species, females exhibit superior working memory and inferior reference memory on the water version of the spatial radial-arm maze. Physiol Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Richardson DP, Brien JF, Reynolds JN, Dringenberg HC. Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult guinea pig following brain growth spurt-prenatal ethanol exposure. Neurotox Teratol. 2004;26:543–551. doi: 10.1016/j.ntt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Cain DP, Boon F. Detailed behavioral analysis reveals both task strategies and spatial memory impairments in rats given bilateral middle cerebral artery stroke. Brain Res. 2003;972:64–74. doi: 10.1016/s0006-8993(03)02486-7. [DOI] [PubMed] [Google Scholar]
- Carr RL, Chambers HW, Guarisco JA, Richardson JR, Tang J, Chambers JE. Effects of repeated oral postnatal exposure to chlorpyrifos on open-field behavior in juvenile rats. Toxicol Sci. 2001;59:260–267. doi: 10.1093/toxsci/59.2.260. [DOI] [PubMed] [Google Scholar]
- Carter AM. Animal models of human placentation–a review. Placenta. 2007;28(Suppl A):S41–47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Cattani M, Cena K, Edwards J, Pisaniello D. Potential dermal and inhalation exposure to chlorpyrifos in Australian pesticide workers. Ann Occup Hyg. 2001;45:299–308. [PubMed] [Google Scholar]
- Chersi F, Burgess N. The cognitive architecture of spatial navigation: hippocampal and striatal contributions. Neuron. 2015;88:64–77. doi: 10.1016/j.neuron.2015.09.021. [DOI] [PubMed] [Google Scholar]
- D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8:82–88. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalm S, Grootendorst J, de Kloet ER, Oitzl MS. Quantification of swim patterns in the Morris water maze. Behav Res Methods Instrum Comput. 2000;32:134–139. doi: 10.3758/bf03200795. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res Dev Brain Res. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- de Jong LP, van Dijk C, Berhitoe D, Benschop HP. Hydrolysis and binding of a toxic stereoisomer of soman in plasma and tissue homogenates from rat, guinea pig and marmoset, and in human plasma. Biochem Pharmacol. 1993;46:1413–1419. doi: 10.1016/0006-2952(93)90106-7. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, Lasarev MR, Rohlman DS, Anger WK, Lein PJ, Olson JR. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Perspect. 2011;119:801–806. doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett WP, Aracava Y, Adler M, Pereira EF, Albuquerque EX. Acute toxicity of organophosphorus compounds in guinea pigs is sex- and age-dependent and cannot be solely accounted for by acetylcholinesterase inhibition. J Pharmacol Exp Ther. 2009;328:516–524. doi: 10.1124/jpet.108.146639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Farahat FM, Galvin K, Fenske EK, Olson JR. Contributions of inhalation and dermal exposure to chlorpyrifos dose in Egyptian cotton field workers. Int J Occup Environ Health. 2012;18:198–209. doi: 10.1179/1077352512Z.00000000030. [DOI] [PubMed] [Google Scholar]
- Grand View Research, Chlorpyrifos market analysis, market size, application analysis, regional outlook, competitive strategies, and forecasts, 2015–2022. http://www.grandviewresearch.com/industry-analysis/chlorpyrifos-market.
- Haviland JA, Butz DE, Porter WP. Long-term sex selective hormonal and behavior alterations in mice exposed to low doses of chlorpyrifos in utero. Reprod Toxicol. 2010;29:74–79. doi: 10.1016/j.reprotox.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Hofmann JN, Keifer MC, Checkoway H, De Roos AJ, Farin FM, Fenske RA, Richter RJ, van Belle G, Furlong CE. Biomarkers of sensitivity and exposure in Washington state pesticide handlers. Adv Exp Med Biol. 2010;660:19–27. doi: 10.1007/978-1-60761-350-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Kahn LG, Perera F, Barr DB, Rauh V. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory. Neurotoxicol Teratol. 2012;34:534–541. doi: 10.1016/j.ntt.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals Institute of Laboratory Animal Resources, Commission on Life Sciences. 7th. National Research Council; Washington, DC: 1996. [Google Scholar]
- Ishikawa K, Yamada M, Masaki M, Togowa K. One-eye and locomotor compensation in guinea pigs. Laryngoscope. 1991;101:1213–1215. doi: 10.1288/00005537-199111000-00009. [DOI] [PubMed] [Google Scholar]
- Israel B. Widely used pesticide seems to harm boys’ brains more than girls’. Environm Hlth News August, 20 2012. 2012 http://www.environmentalhealthnews.org/ehs/news/2012/boys-and-chlorpyrifos.
- Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MB, Medina JH. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol. 1992;58:16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Da Silva WC, Medina JH, Cammarota M. The connection between the hippocampal and the striatal memory systems of the brain: a review of recent findings. Neurotox Res. 2006;10:113–121. doi: 10.1007/BF03033240. [DOI] [PubMed] [Google Scholar]
- Johnson FO, Chambers JE, Nail CA, Givaruangsawat S, Carr RL. Developmental chlorpyrifos and methyl parathion exposure alters radial-arm maze performance in juvenile and adult rats. Toxicol Sci. 2009;109:132–142. doi: 10.1093/toxsci/kfp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Mamczarz J, Kulkarni GS, Pereira EFR, Albuquerque EX. Galantamine counteracts development of learning impairment in guinea pigs exposed to the organophosphorus poison soman: clinical significance. Neurotoxicology. 2011;32:785–798. doi: 10.1016/j.neuro.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HJ. The recovery of plasma cholinesterase and erythrocyte acetylcholinesterase activity in workers after over-exposure to dichlorvos. Occup Med (Lond) 2000;50:343–347. doi: 10.1093/occmed/50.5.343. [DOI] [PubMed] [Google Scholar]
- McCollister SB, Kociba RJ, Humiston CG, McCollister DD. Studies on the acute and long-term oral toxicity of chlorpyrifos (0,0-diethyl-0(3,5,6-trichloro-2-pyridyl) phosphorothioate) Food Cosmet Toxicol. 1974;12:45–61. doi: 10.1016/0015-6264(74)90321-6. [DOI] [PubMed] [Google Scholar]
- Mullins RJ, Xu S, Pereira EFR, Pescrille JD, Todd SW, Mamczarz J, Albuquerque EX, Gullapalli RP. Prenatal exposure of guinea pigs to the organophosphorus pesticide chlorpyrifos disrupts the structural and functional integrity of the brain. Neurotoxicology. 2015;48:9–20. doi: 10.1016/j.neuro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara MP, Dyer RS. Locomotor exploratory activity in blind and normal guinea pigs. Physiol Behav. 1974;13:701–702. doi: 10.1016/0031-9384(74)90244-3. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, Bernert T, Garfinkel R, Tu YH, Diaz D, Dietrich J, Whyatt RM. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl Acad Sci USA. 2012;109:7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, Markina N, Valanzano A, Fortuna S, Cometa MF, Meneguz A, Calamandrei G. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicol Appl Pharmacol. 2003;191:189–201. doi: 10.1016/s0041-008x(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Richardson DP, Byrnes ML, Brien JF, Reynolds JN, Dringenberg HC. Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig. Eur J Neurosci. 2002;16:1593–1598. doi: 10.1046/j.1460-9568.2002.02214.x. [DOI] [PubMed] [Google Scholar]
- Rosas LG, Eskenazi B. Pesticides and child neurodevelopment. Curr Opin Pediatr. 2008;20:191–197. doi: 10.1097/MOP.0b013e3282f60a7d. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH. Neurochemical mechanisms in soman-induced seizures. J Appl Toxicol. 1997;17:255–264. doi: 10.1002/(sici)1099-1263(199707)17:4<255::aid-jat441>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Singleton ST, Lein PJ, Dadson OA, McGarrigle BP, Farahat FM, Farahat T, Bonner MR, Fenske RA, Galvin K, Lasarev MR, Anger WK, Rohlman DS, Olson JR. Longitudinal assessment of occupational exposures to the organophosphorous insecticides chlorpyrifos and profenofos in Egyptian cotton field workers. Int J Hyg Environ Health. 2015;218:203–211. doi: 10.1016/j.ijheh.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slob AK, Goy RW, van der Werff ten Bosch JJ. Sex differences in growth of guinea-pigs and their modification by neonatal gonadectomy and prenatally administered androgen. J Endocrinol. 1973;58:11–19. doi: 10.1677/joe.0.0580011. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol Appl Pharmacol. 2008;233:211–219. doi: 10.1016/j.taap.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout DM, 2nd, Bradham KD, Egeghy PP, Jones PA, Croghan CW, Ashley PA, Pinzer E, Friedman W, Brinkman MC, Nishioka MG, Cox DC. American Healthy Homes Survey: a national study of residential pesticides measured from floor wipes. Environ Sci Technol. 2009;43:4294–4300. doi: 10.1021/es8030243. [DOI] [PubMed] [Google Scholar]
- Strelitz J, Engel LS, Keifer MC. Blood acetylcholinesterase and butyrylcholinesterase as biomarkers of cholinesterase depression among pesticide handlers. Occup Environ Med. 2014;71:842–847. doi: 10.1136/oemed-2014-102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV., Jr Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther. 2012;134:355–365. doi: 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Calamandrei G, Ricceri L. A social recognition test for female mice reveals behavioral effects of developmental chlorpyrifos exposure. Neurotoxicol Teratol. 2006;28:466–771. doi: 10.1016/j.ntt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PE, Leatherdale ST, Dubin JA. Advantages of mixed effects models over traditional ANOVA models in developmental studies: a worked example in a mouse model of fetal alcohol syndrome. Dev Psychobiol. 2007;49:664–674. doi: 10.1002/dev.20245. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Mahler HR, Moore WJ. The half-life of acetylcholinesterase in mature rat brain. J Neurochem. 1974;22:941–943. doi: 10.1111/j.1471-4159.1974.tb04319.x. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, Tang D, Kinney PL, Perera FP. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber CG. Some factors which are correlated with swimming capacity in guinea pigs. J Appl Physiol. 1959;14:199–203. doi: 10.1152/jappl.1959.14.2.199. [DOI] [PubMed] [Google Scholar]
- Zheng S, Berman HA, Geyer MA. Behavior during hippocampal microinfusions: anticholinesterase-induced locomotor activation. Behav Brain Res. 1983;9:295–304. doi: 10.1016/0166-4328(83)90134-1. [DOI] [PubMed] [Google Scholar]


