Abstract
Galantamine, a drug used to treat Alzheimer’s disease, protects guinea pigs against the acute toxicity and lethality of organophosphorus (OP) compounds, including soman. Here, we tested the hypothesis that a single exposure of guinea pigs to 1xLD50 soman triggers cognitive impairments that can be counteracted by galantamine. Thus, animals were injected intramuscularly with saline (0.5 ml/kg) or galantamine (8 mg/kg) and 30 min later injected subcutaneously with soman (26.3 µg/kg) or saline. Cognitive performance was analyzed in the Morris water maze (MWM) four days or three months after the soman challenge. Fifty percent of the saline-injected animals that were challenged with soman survived with mild-to-moderate signs of acute toxicity that subsided within a few hours. These animals showed no learning impairment and no memory retention deficit, when training in the MWM started four days post-soman challenge. In contrast, animals presented significant learning impairment when testing started three months post-challenge. Though the magnitude of the impairment correlated with the severity of the acute toxicity, animals that presented no or only mild signs of toxicity were also learning impaired. All guinea pigs that were treated with galantamine survived the soman challenge with no signs of acute toxicity and learned the MWM task as control animals, regardless of when testing began. Galantamine also prevented memory extinction in both saline-and soman-challenged animals. In conclusion, learning impairment develops months after a single exposure to 1xLD50 soman, and galantamine prevents both the acute toxicity and the delayed cognitive deficits triggered by this OP poison.
Keywords: Cholinesterease, galantamine, guinea pig, Morris water maze, organophosphorus, soman
Introduction
Organophosphorus (OP) compounds, including pesticides and the nerve agents soman, sarin and VX, are among the most toxic man-made chemicals. Although different OPs interact with specific targets in the peripheral and central nervous sytems (Albuquerque et al., 1985), their acute toxicity is characterized by overstimulation followed by desensitization of cholinergic muscarinic and nicotinic receptors that results in part from the irreversible inhibition of acetylcholinesterase (AChE) – the enzyme that hydrolyzes acetylcholine (Newmark, 2007).
Some of the nerve agents have been used with catastrophic results in wars and terrorist attacks (Coupland and Leins, 2005; Romano and King, 2001). The 1995 terrorist attack with sarin in the Tokyo subway is the largest documented exposure of a civilian population to a nerve gas. Approximately 95% of the victims who were admitted to hospitals and diagnosed as moderately or severely intoxicated were treated intravenously with atropine to block the muscarinic receptors and pralidoxime to reactivate OP-inhibited AChE; diazepam was used as needed to control the convulsions (Okumura et al., 1996). Extended follow-up studies of six months to ten years reported an increased incidence of post-traumatic stress disorder (Ohtani et al., 2004) and chronic memory decline (Hood, 2001; Nishiwaki et al., 2001) among victims of the sarin attack, suggesting that the approved treatments of OP poisoning did not effectively prevent the delayed development of neurological disorders.
Studies from multiple laboratories have successfully identified neurobehavioral deficits in rats, guinea pigs, and non-human primates following a single exposure to nerve agents. For instance, following low-level inhalation exposure to sarin, rats showed significant impairment in spatial discrimination in the Y-Maze (Kassa et al., 2002). In that study the levels of sarin were sufficiently low to trigger no or only mild signs of cholinergic hyperstimulation. Other studies reported that rats and mice that developed severe signs of acute toxicity, including convulsions, following a single subcutaneous (sc) exposure to 1-1.2xLD50 soman presented acute and delayed cognitive impairments in the Morris water maze (MWM) (Filliat et al., 1999, 2007; Raveh et al., 2002). Of particular interest is a report that in asymptomatic, soman-challenged mice cognitive deficits could be detected at three months, but not one month following the challenge (Filliat et al., 2007).
Reports that the intensity and duration of convulsions in rodents exposed to soman correlate with the magnitude of neuropathology scores and behavioral deficits (McDonough and Shih, 1997; Raveh et al., 2002) suggested that early management of soman-induced convulsions would be sufficient to reduce the neuropathology and the accompanying cognitive impairments. However, neurodegeneration and the resulting cognitive deficits observed in soman-intoxicated rodents can be significantly reduced by therapeutic interventions that, although unable to control the seizures, effectively decrease glutamate excitotoxicity (Filliat et al., 1999). Identifying an antidote capable of counteracting the delayed neurotoxic effects of an exposure to nerve agents is crucial for management of a population exposed to these agents either during military operations or in the event of a terrorist attack.
It is well accepted that guinea pigs are the best non-primate model for predicting the effectiveness of antidotal therapies for OP poisoning in humans (Maxwell et al., 1987). We have demonstrated that galantamine, a drug approved for treatment of Alzheimer’s disease (Corey-Bloom, 2003), effectively counteracts the lethality and the acute toxicity of OPs in guinea pigs (Albuquerque et al., 2006). Functional analyses of synaptic transmission and plasticity, histological evaluation of neuronal viability, and magnetic resonance imaging (MRI) analysis of the structural integrity of the brains of nerve agent-challenged guinea pigs subjected to different treatments provided additional evidence that galantamine is an effective and safe antidote against the acute toxicity induced by OPs (Alexandrova et al., 2010; Alkondon et al. 2009; Gullapalli et al., 2010).
The present study was designed to test the hypothesis that a single exposure of guinea pigs to soman triggers cognitive deficits that can be prevented by galantamine. To test this hypothesis, guinea pigs were tested in the MWM at four days or three months after their challenge with 1xLD50 soman and/or treatment with galantamine. Earlier studies have reported that AChE activity is quickly inhibited in guinea pigs injected with 1xLD50 soman (Lintern et al., 1998; Shih et al., 2005). Recovery of the enzyme activity is very slow and seven days following the soman challenge AChE activity remains significantly inhibited in some brain regions of guinea pigs (Lintern et al., 1998). Thus, studying the guinea pigs four days and three months after the initial soman challenge is essential to delineate the contribution of AChE inhibition to behavioral deficits induced by the nerve agent. The MWM, which was originally developed to assess spatial learning in rats (Morris, 1984), has proven to be a valuable task to assess spatial behavior, learning, and memory processes in guinea pigs (Byrnes et al., 2004; de Groot et al., 2001; Dringenberg et al., 2001; Filliat et al., 2002; Lewejohann et al., 2010). The results presented here strongly support the hypothesis and demonstrate that galantamine is a highly effective medical countermeasure to prevent the delayed learning impairment that develops as a result of an acute exposure to soman.
Material and methods
Animal care and treatments
Female Hartley guinea pigs [Crl(HA)Br; Charles River Laboratories, Wilmington, MA] were housed in groups of four in stainless steel cages (60 × 60 × 25 cm) in a climate controlled animal-care facility (21 ± 0.5°C; 12-h light/dark cycle). Animals were 30–33 days old on arrival, and were acclimated for at least 48 h before any treatment. Food and water was available ad libitum. All investigators complied with the regulations and standards of the Animal Welfare Act and adhered to the principles of the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Guinea pigs received an intramuscular (im) injection of saline (0.9% NaCl) or galantamine (8 mg/kg) in one hind limb 30 min before they were injected subcutaneously with 1xLD50 soman (26.3 µg/kg) or saline between the shoulder blades. This dose of galantamine prevents the acute toxicity of 1xLD50 soman and is well tolerated by guinea pigs (Aracava et al., 2009; Gullapalli et al., 2010). Disposable tuberculin syringes with 26-gauge needles were used. Sterile saline was used to dissolve galantamine and to dilute the stock solution of soman (1.8–1.9 mg/ml). Injection volumes did not exceed 0.5 ml/kg.
Guinea pigs were monitored every 15 min during the first 2 h after the treatments, hourly during the next 6 h, and daily subsequently. Gross behavior and body weight were recorded daily. Acute reactions to treatments were scored according to a modified Racine scale (Aracava et al., 2009) as described in Results. The experimenter who performed the behavioral tests and analyzed the data was blind to the severity of the acute toxicity. Animals were euthanized according to the protocol approved by the Institutional Animal Care and Use Committee (IACUC) as soon as they developed life-threatening signs of intoxication, including gasping and unremitting motor convulsions.
Chemicals
Stock solution of soman (1.88–1.9 mg/ml) was obtained from the US Army Edgewood Chemical Biological Center via an agreement with the United States Army Medical Research Institute of Chemical Defense. Soman was stored, handled, and disposed according to the regulations set forth by the Institute. Galantamine.HBr was generously provided by Dr. Alfred Maelicke (Galantos Pharma, Mainz, Germany). The systematic names of the chemicals used are: (i) soman – methylphosphonofluoridic acid 1,2,2-trimethylpropyl ester, and (ii) galantamine – (4aS,6R,8aS)-5,6,9,10,11,12-hexahydro-3-methoxy-11-methyl-4aH-[1]benzofuro[3a,3,2-ef] [2] benzazepin-6-ol.
Apparatus
The water maze consisted of a large grey galvanized metal circular tank (180-cm diameter; 60-cm tall) filled with tap water to a depth of 38 cm. Water was made opaque with non-toxic black tempera paint. The pool was located in a room with distinct three-dimensional distal cues that aided orientation. It was divided virtually into four quadrants (N, S, E, W). A black circular (20-cm diameter) escape platform was submerged (hidden) in the center of one of the quadrants. The platform consisted of two levels, which were designed to provide comfortable escape from water for guinea pigs, which are known to have difficulties with climbing on the platform (Filliat et al., 2002). The outer level, a 3.75-cm diameter strip, was located 2 cm below the center of the platform. Both platform levels had circular groves 4-mm deep. The central platform level was submerged 3 cm below the water surface.
A video camera was mounted on the ceiling right above the center of the pool to capture images of the swimming animals. The video input of the camera was relayed to an online computer system running the video-tracking Any-Maze software (Stoelting Co, Wood Dale, IL). A curtain separated the pool from the computer system.
Behavioral testing
Training in the MWM started either four days or three months after the treatments (Figure 1A). In tests that started four days after treatments, training consisted of four consecutive trials per day during five days. In tests that started three months after the treatments, training lasted six days, primarily due to the slower learning of the adult animals compared to the younger, pre-pubertal guinea pigs. All tests were conducted between 11 AM and 4 PM.
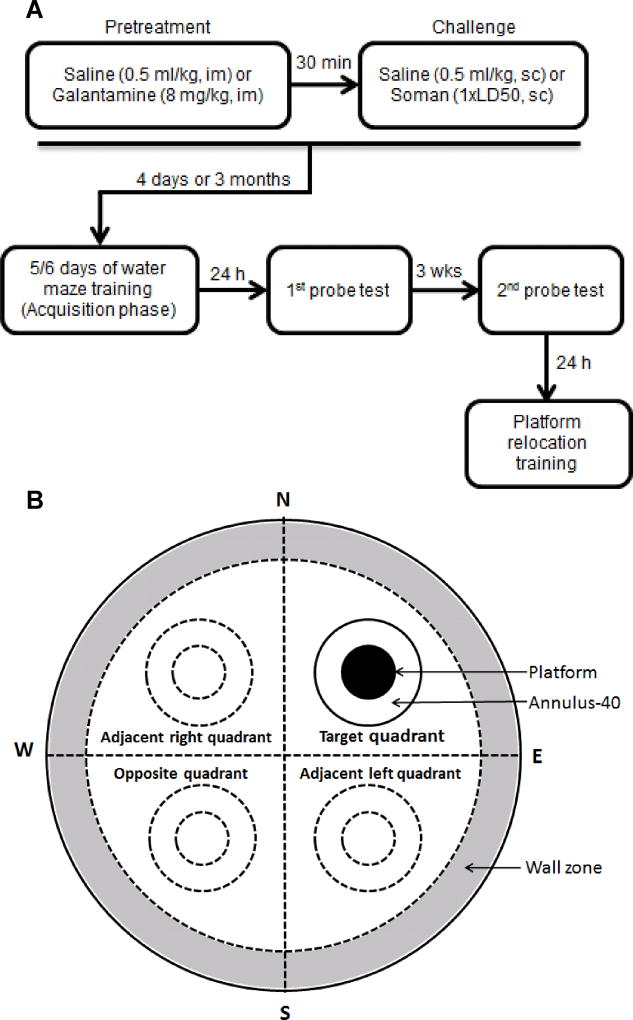
Figure 1. Flow chart of the treatments and behavioral testing the guinea pigs were subjected to and schematic representation of the zones in the water maze.
A. Flow chart showing the times the animals were injected with saline, soman, and/or galantamine and the times at which they were subjected to the different tests in the MWM. B. Diagram shows that, for purposes of analyses, the water maze was virtually divided into four quadrants and a 15-cm wide zone along the wall of the maze. The inner doted circles in the centers of non-target quadrants represent areas of hypothetical platforms and the outer doted circles represent the annulus-40. The filled circle in the target (training) quadrant represents the target platform and the outer circle represents the annulus-40 in the target quadrant.
On each training day, guinea pigs were transported to the drying room next to the testing room and allowed to acclimate for at least 2 h prior to testing. The hidden escape platform remained in the same position during the acquisition phase of the task.
As shown in a previous study (Wilber, 1959), water temperatures in the ranges of 28–40°C and 28–35°C maximize the swimming performance of guinea pigs weighing approximately 400 g and 1000 g, respectively. In addition, mean body temperature of guinea pigs has been reported to decrease less than 0.5°C following 5-min forced swimming in water maintained at 30 ± 1°C (Wicke et al., 2007). In the present study, water temperature on the first day of training was 30 ± 0.25°C, as in Filliat et al. (2002), and was decreased 1°C each subsequent day. On the fifth training day and during the probe and the platform relocation tests, the water temperature was 26 ± 0.25°C. This experimental design provided the animals comfort of swimming as well as motivation for platform searching, while preventing excessive loss of body heat.
On the first training day, prior to the first trial, each animal was placed onto the submerged platform for 15 s, after which time they were placed into a cage adjacent to the maze for 10–15 s prior to starting the first training trial. On each training day, animals received four consecutive swimming trials interspaced by placing them into the waiting cage for 10–15 s. Each trial was started by placing an animal into the water facing the wall, with the start locations varying pseudorandomly (N, S, E, or W). The animals were allowed a maximum of 90 s to locate and mount the hidden escape platform. After mounting the platform, the animal was allowed to remain on it for 15 s before beginning of the next trial. Animals that were unsuccessful in locating the platform within 90 s were guided there by the experimenter. After finishing the block of four trials, guinea pigs were placed in a cage with paper bedding under a ceramic infrared heating lamp until completely dry. When completely dried, they were transported back to their homeroom.
The acquisition phase was followed by two probe trials to test memory retention (Figure 1A). The first trial was performed 24 h and the second, three weeks after the training. During the probe trial, the platform was removed and animals were allowed to freely explore the pool for 90 s. One hour after the first probe test, the platform was reinstalled in the same position as that used during the acquisition phase and the animals received one additional training trial. They were then dried as described above and transported back to their homeroom for three weeks, after which time the second probe test was performed.
Twenty four h following the second probe test, animals were trained for three consecutive days to find a different platform position per day (Figure 1A). The 4-trial/day training procedure was the same as that described for the acquisition phase and started each day by placing the animals on the platform for 15 s before the first trial. On the first day, the platform was positioned in the center of the quadrant opposite to that of the platform during the acquisition phase. On the second day, the platform was placed in the center of the quadrant adjacent to both previous locations. Finally, on the third day, the platform was placed in the center of the quadrant opposite to that where the platform was in the second day.
Behavioral data acquisition and analysis
Data was collected and analyzed using the Any-Maze software. The software was set to turn off tracking at either 90 s after the beginning of each trial or when the animal stopped on the platform for at least 2 s. The minimum period of time on the platform was used because guinea pigs very often crossed the platform without stopping or immediately escaped from it. If they stopped on the platform for at least 2 s, they rarely escaped it.
Dependent measures of performance during the acquisition phase and the training to relocated platforms included escape latency (time it took for guinea pigs to escape onto the hidden platform) and distance traveled. During the probe tests, number of crossings of the target platform was compared to crossing of areas equal to the platform area that were virtually positioned in the center of non-training quadrants. These areas are designated as virtual platform positions. The same type of comparison was performed for an extended platform area referred to as annulus-40, i.e. the 40-cm diameter area around the center of the quadrant (Markowska et al., 1993). Such comparison allows excluding the possibility that number of platform crossings relates to the pattern of swimming rather than to the preference of the training site. A schematic representation of the different areas of the water maze is presented in Figure 1B.
The effects of galantamine and soman on the performance of the animals were examined using a two-way repeated measures analysis of variance (ANOVA) with pretreatment (galantamine or saline) and challenge (soman or saline) as between-animal factors, and training day, quadrant position, platform position, or annulus-40 position as a repeated measure factor. Whenever interactions between the repeated measure factor and pretreatment or challenge were significant, one-way ANOVA followed by Fisher’s LSD post-hoc test was performed to evaluate differences between experimental groups. One-way ANOVA followed by Dunnett’s post-hoc test was used to compare preference to training vs. non-training quadrant, platform position, or annulus-40 position. Statistical analyses were done using the SPSS software (SPSS Inc., Chicago, IL).
Fluoro Jade-B staining of guinea pig brain slices
Guinea pigs were injected with saline (0.5 ml/kg) or galantamine (8 mg/kg) intramuscularly. Thirty minutes later guinea pigs were subcutaneously injected with saline or 1xLD50 soman. Two days after the injections, animals were anesthetized with ketamine (75 mg/kg, ip) and perfused with 300 ml of 0.1 M phosphate-buffered 10% formalin via the ascending aorta, while clamping the descending aorta. Their brains were removed post-fixed overnight in 10% formalin, cryoprotected in 30% sucrose, and frozen in −75°C isopentane. Frozen brains were cut in 20-µm thick slices, which were post-fixed with freshly prepared 4% paraformaldehyde in phosphate-buffered saline, dehydrated in 70% and 100% alcohol and stored at −80°C until further processing. On the day of the staining, slides were thawed to −20°C, then to 4°C, and finally allowed to thaw further in ice-cold 100% ethanol for 5 min. This was followed by three washes in 70% ethanol, 50% ethanol, and water, each for 1 min. The slides were subsequently washed in PBS and water for 3 min each. The slides were immersed in 0.06% potassium permanganate solution for 15 min on a rotating platform, rinsed in water for 1 min, and finally transferred to the Fluoro Jade-B staining solution for 30 min, gently shaking in the dark. The staining solution was prepared from a 0.01% stock solution of Fluoro Jade-B (Chemicon, Temecula, CA) that was made by dissolving 10 mg of the dye powder in 100 ml of distilled water. To make up 100 ml of staining solution, 4 ml of the stock solution was added to 96 ml of 0.1% acetic acid vehicle. This results in a final dye concentration of 0.0004%. The stock solution, when stored in the refrigerator, was stable for 3 months, whereas the staining solution was prepared within 15 min of use and was not reused.
Slides were rinsed with water, dried on a slide warmer (50°C), immersed in xylene, and cover slipped with Cytoseal® mounting media (Richard Allan Scientific, Kalamazoo, MI). Slides were imaged with a Nikon Eclipse 80i upright microscope equipped with a Ds-FiZ camera controlled by NIS-Elements BR 3.0 SP4 software (Nikon Instruments Inc., Melville, NY). The fluorescence intensity was visualized using a fluorescein isothiocyanate filter set (Excitation: 465 nm; Emission: 520 nm). All imaging was done at room temperature and files saved as jpeg format with each field subjected to ‘auto-white balance’ before capturing, with no other manipulation.
Results
Acute signs of intoxication presented by guinea pigs challenged with 1xLD50 soman
The modified Racine scale described in Aracava et al. (2009) was used to define qualitatively the severity of the acute signs of intoxication presented by guinea pigs that were challenged with 1xLD50 soman (26.5 µg/kg, sc). Animals in stage 0–1, 2–3, and 4–5 (see Table 1) were considered mildly, moderately, and severely intoxicated, respectively. In animals that reached stages 0–3, signs of acute intoxication were not life-threatening and subsided within a few hours after the soman challenge. In the present study, animals were euthanized according to the IACUC-approved protocol as soon as they reached stages 4–5 because, in more than 80% of these animals, signs of acute toxicity quickly become life-threatening (Alexandrova et al., 2010). Twenty six out of the 50 soman-injected guinea pigs presented signs of acute toxicity that did not advance beyond stage 3 and were tested behaviorally at four days or three months after the injection of the nerve agent.
Table 1.
Qualitative classification of toxic signs of intoxication presented by guinea pigs challenged with lxLD50 soman
| Score | Signs of toxicity | Classification of toxicity |
|---|---|---|
| 0 | No abnormal gross behavior | Mild |
| 1 | Facial twitches, pawing at whiskers and mouth, chewing | |
| 2 | Head tremor and/or nodding, short periods of immobility | Moderate |
| 3 | Forelimb clonus | |
| 4 | Rearing with no loss of balance, strong grinding, gnashing or bruxism | Severe |
| 5 | Rearing with loss of balance, frank convulsions |
The severity of the soman-induced acute toxicity was classified as described in Aracava et al. (2009).
Guinea pigs that were treated with galantamine (8 mg/kg, im) and 30 min later injected with saline (0.5 ml/kg, sc) or 1xLD50 soman survived with no apparent signs of acute toxicity. As in other studies (Alexandrova et al., 2010; Aracava et al., 2009; Gullapalli et al., 2010), daily weight gain and gross behavior of galantamine-treated animals were comparable to those of control animals (data not shown).
Acquisition of the MWM task four days after the injections of guinea pigs with galantamine and/or soman
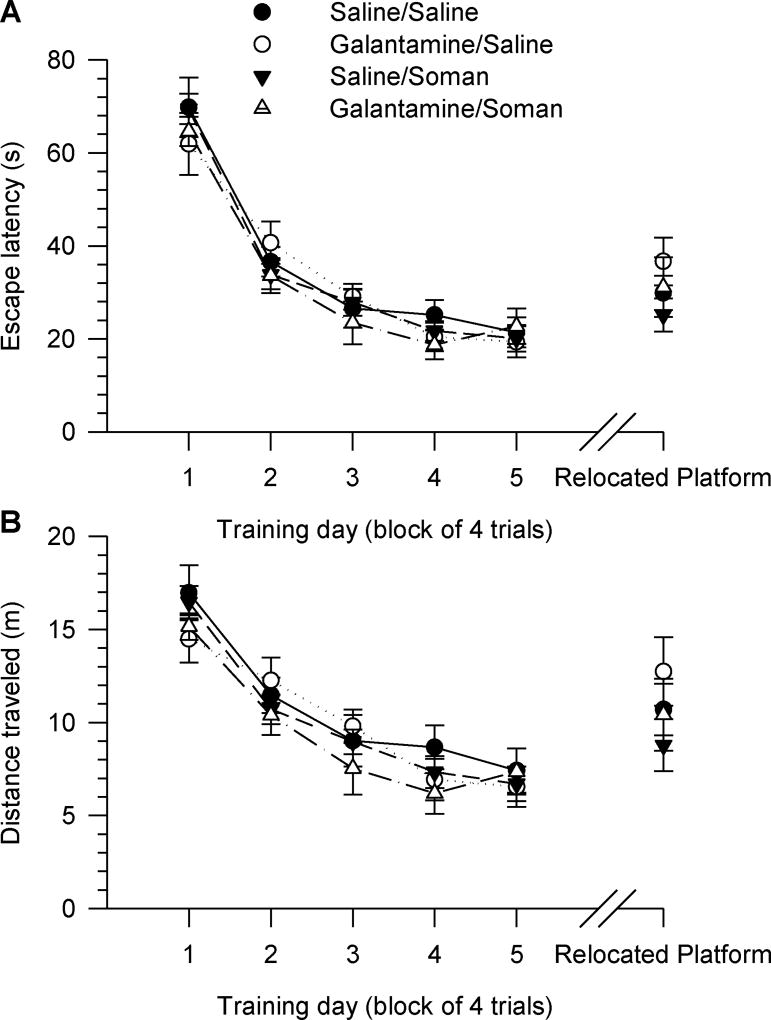
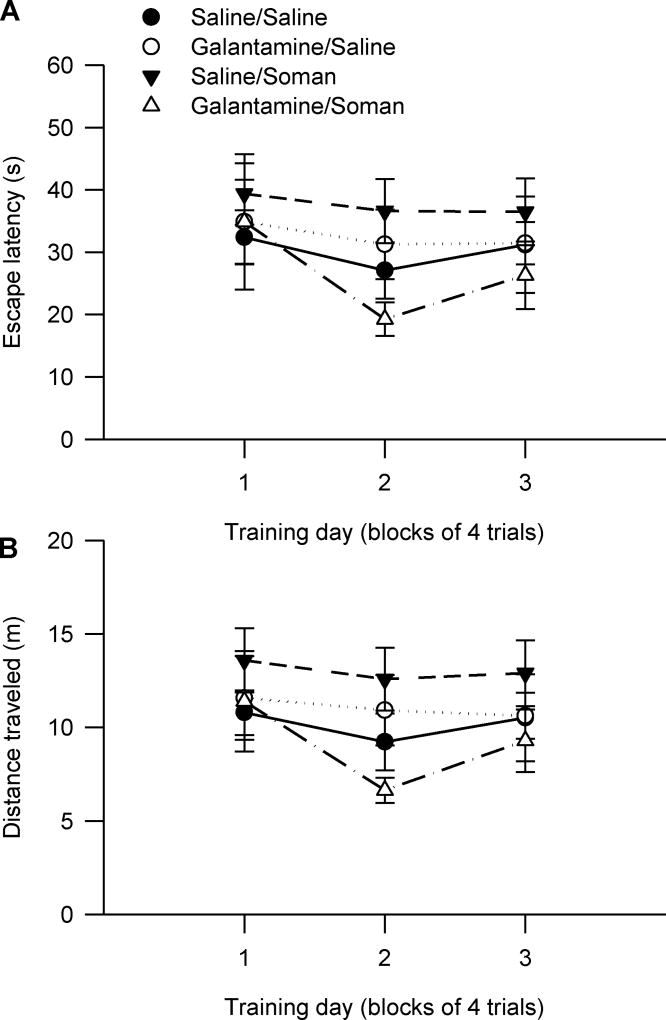
All guinea pigs learned to find the hidden platform and escape onto it during the acquisition phase of the MWM task when training began four days after their treatments (Figure 2A). Escape latency decreased with the training days, and all animals reached a near-asymptotic level of performance, with latencies of approximately 20–24 s between days three and five of training (Figure 2A). Two-way repeated measures ANOVA with pre-treatment (galantamine or saline) and challenge (soman or saline) as between animal factors and training day as the repeated measure showed significant main effect of the training day on both escape latency [F(4,164) = 122.81, p < 0.001] and distance travelled [F(4,164) = 55.41, p < 0.001] (Figure 2A, 2B). Pre-treatment and challenge had no significant main effect on escape latency or distance travelled. In addition, there were no significant interactions between these two factors and the training day (Figure 2A, 2B).
Figure 2. Acquisition of the MWM task starting four days after injection of guinea pigs with saline, soman and/or galantamine.
Experimental groups consisted of animals that were injected with: saline (0.5 ml/kg, im)/saline (0.5 ml/kg, sc), galantamine (8 mg/kg, im)/saline (0.5 ml/kg, sc), saline (0.5 ml/kg, im)/soman (1xLD50, sc), or galantamine (8 mg/kg, im)/soman (1xLD50, sc). Inter-injection intervals were 30 min. Four days after the injections, animals received five days (four trials/day) of reference memory training, followed three weeks later by training to find the hidden platform positioned in the quadrant opposite to the training quadrant. Graphs of escape latency (A) and distance traveled (B) per training day revealed no significant difference among the test groups during the acquisition phase and the training to new platform position. Data points and error bars represent mean and SEM, respectively, of results obtained from 10–15 animals/group.
Swimming speed was not affected by the treatments. The mean swimming speed of all animals increased between days one and two of training, and remained constant thereafter (see Table 2).
Table 2.
Swimming speed of prepubertal and young adult guinea pigs that had been subjected to various treatments four days or three months before training in the MWM.
| Saline/Saline | Saline/Soman | Galantamine/Saline | Galantamine/Soman | |
|---|---|---|---|---|
| Training day | Daily swimming speed (m/s) (Start of training: 4 days after injections) | |||
| 1 | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.24 ± 0.01 | 0.24 ± 0.01 |
| 2 | 0.32 ± 0.01 | 0.33 ± 0.05 | 0.31 ± 0.01 | 0.31 ± 0.01 |
| 3 | 0.34 ± 0.01 | 0.34 ± 0.01 | 0.34 ± 0.01 | 0.33 ± 0.01 |
| 4 | 0.34 ± 0.01 | 0.33 ± 0.01 | 0.34 ± 0.01 | 0.32 ± 0.01 |
| 5 | 0.34 ± 0.01 | 0.33 ± 0.01 | 0.34 ± 0.01 | 0.32 ± 0.01 |
| Training day | Daily swimming speed (m/s) (Start of training: 3 months after injections) | |||
| 1 | 0.24 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 |
| 2 | 0.27 ± 0.01 | 0.24 ± 0.05 | 0.26 ± 0.02 | 0.26 ± 0.02 |
| 3 | 0.32 ± 0.03 | 0.27 ± 0.02 | 0.29 ± 0.03 | 0.29 ± 0.03 |
| 4 | 0.33 ± 0.01 | 0.29 ± 0.03 | 0.32 ± 0.02 | 0.32 ± 0.03 |
| 5 | 0.33 ± 0.02 | 0.31 ± 0.03 | 0.34 ± 0.04 | 0.32 ± 0.02 |
| 6 | 0.35 ± 0.01 | 0.31 ± 0.03 | 0.35 ± 0.02 | 0.33 ± 0.02 |
Prepubertal guinea pigs received injections of: (i) saline (0.5 ml/kg, im) followed 30 min later by saline (0.5 ml/kg, sc); (ii) saline (0.5 ml/kg, im) followed 30 min later by soman (26.3 µg/kg, sc); (iii) galantamine (8 mg/kg, im) followed 30 min later by saline (0.5 ml/kg, sc); or (iv) galantamine (8 mg/kg, im) followed 30 min later by soman (26.3 µg/kg, sc). Data are presented as mean ± SEM of results obtained from 7–11 animals/group.
Memory retention of animals that had been challenged with soman and/or treated with galantamine four days before the acquisition phase of the MWM task
Spatial memory retention was assessed during two probe tests. In these tests, the escape platform was removed from the pool and the animals were allowed to swim freely for 90 s, as described in Materials and methods.
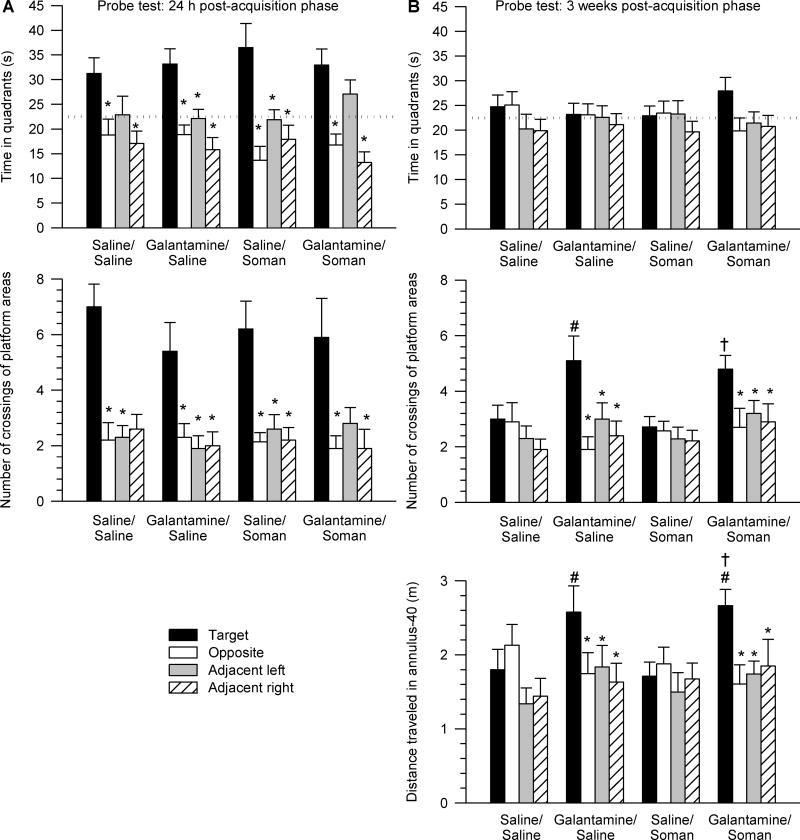
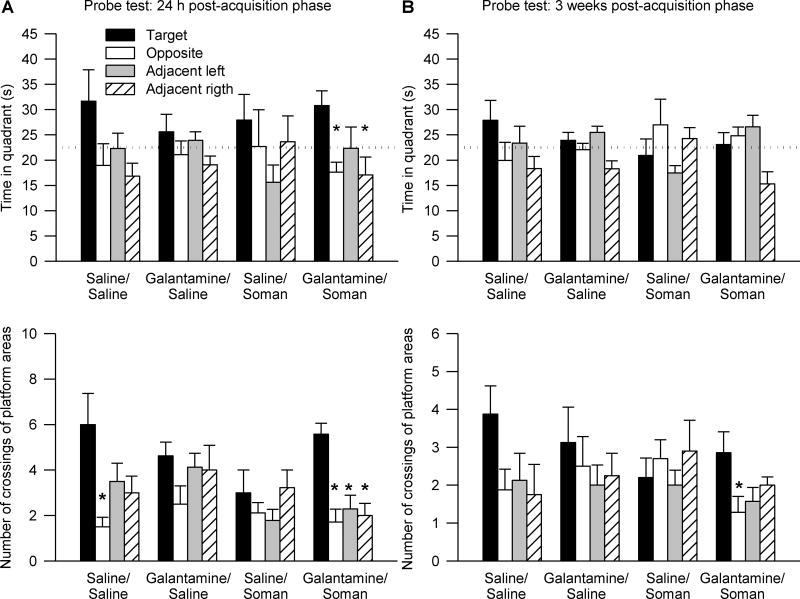
The first probe test was performed 24 h after the last training session. One-way ANOVA followed by Dunnett’s post-hoc test revealed that, despite their treatment (saline or galantamine) and challenge (soman or saline), all animals expressed bias toward the target quadrant, i.e. the quadrant where the training platform was positioned during the acquisition phase. Similar to saline/saline-injected animals, all tested animals spent more time (Figure 3A) and swum longer distances (data not shown) in the target quadrant than in the other quadrants. Two-way repeated measures ANOVA with pre-treatment (galantamine or saline) and challenge (soman or saline) as between animal factors and quadrant position as the repeated measure revealed a significant main effect of the quadrant position [F(3,123) = 22.01, p < 0.001] on time spent in the quadrants. There were no significant main effects of pre-treatment and/or challenge and no significant interactions of the factors.
Figure 3. Peformance of guinea pigs in probe tests performed one day and three weeks after completion of the acquisition phase of the MWM training started four days following the injections of saline, soman, and/or galantamine.
Results are from the same animals as those that completed the MWM training shown in Figure 2. A. Data are from the probe test performed 24 h after completion of the acquisition phase. Top graph shows the time animals spent in each of the four quadrants of the pool. Bottom graph shows number of crossings of the platform areas virtually positioned in the center of each quadrant of the pool. Within each experimental group, animals showed bias to the target quadrant and to the platform area in the center of the training quadrant (* p < 0.05 according to one-way ANOVA followed by Dunnett post-hoc test). B. Data are from the probe test performed three weeks after completion of the acquisition phase. Top graph depicts the time spent in each quadrant of the pool. Middle graph shows the number of crossings of the platform areas virtually positioned in the center of each quadrant. Bottom graph shows the distance travelled in the pre-defined 40-cm annulus surrounding the center of each quadrant. Within each experimental group, animals showed no bias to the target quadrant. However, animals pre-treated with galantamine showed preference to crossing the platform and the annulus-40 area vitually positioned in the center of the training quadrant (* p < 0.05 according to one-way ANOVA followed by Dunnett post-hoc test). In addition, galantamine-pretreated animals showed significantly better performance than saline-pretreated expressed as a higher number of the target platform crossings and larger distance traveled in the target annulus-40 zone (# p < 0.05 vs. saline/saline, † p < 0.05 vs. saline/soman according to one-way ANOVA followed by Fisher’s LSD post-hoc test). In A and B, the training (target) area was used as the reference against which the other three areas were named as shown in Figure 1. Graph and error bars represent mean and SEM, respectively, of results obtained from 10–15 animals. The horizontal dotted lines in the top graphs represent the chance level (22.5 s).
To further examine accuracy of performance during the first probe test, number of crossings of the target platform area positioned in the center of the training quadrant was compared with crossing of equivalent areas virtually positioned in the center of non-training quadrants (virtual platform positions). One-way ANOVA followed by Dunnett’s post-hoc test showed that despite of their treatment and/or challenge, all animals showed bias toward crossing the target platform area in the center of the training quadrant (Figure 3A). Two-way repeated measures ANOVA revealed significant main effect of the platform position [F(3,96) = 23.9, p < 0.001], but no significant main effect of pre-treatment and challenge, in addition to no significant interactions of the factors.
The second probe test was performed three weeks after the acquisition of the MWM task. In this test, none of the experimental groups showed spatial bias toward the training quadrant (Figure 3B). However, one-way ANOVA followed by Dunnett’s post-hoc test indicated that all galantamine-pretreated animals showed spatial bias expressed as crossing of the target platform position (Figure 3B). Two-way repeated measures ANOVA revealed a significant main effect of pre-treatment (galantamine or saline) [F(1,41) = 6.7, p < 0.05] and platform position [F(3,123) = 8.2, p < 0.001], in addition to a significant pre-treatment × platform position interaction [F(3,123) = 4.44, p < 0.05]. One-way ANOVA followed by Fisher’s post-hoc test showed that guinea pigs injected with galantamine prior to the challenge with saline or soman crossed the target platform position significantly more times than saline/saline-injected (control) and saline/soman-injected animals (Figure 3B).
To further assess the accuracy of the performance of the animals in the second probe test, the distance traveled in a 40-cm-diameter zone around the center of the quadrants (annulus-40; Markowska at al., 1993) was analyzed. One-way ANOVA followed by Dunnett’s post-hoc test confirmed that all galantamine-pretreated animals showed spatial bias toward the area that included the target platform position (Figure 3B). Two-way repeated measures ANOVA revealed significant main effect of the annulus-40 position [F(3,123) = 4.47, p < 0.01] and significant pre-treatment × annulus-40 position interaction [F (3,123) = 3.87, p < 0.05]. One-way ANOVA followed by Fisher’s post-hoc comparison indicated that guinea pigs treated with galantamine and subsequently injected with saline or soman travelled significantly longer distances in the target annulus-40 area than did control and saline/soman-injected animals (Figure 3B). Therefore, the second probe test demonstrated that galantamine given alone or as a pre-treatment to the soman challenge has long-lasting effects on memory retention.
After the second probe test, the guinea pigs were required to learn to find the platform positioned in the quadrant opposite to the training quadrant, as described in Materials and Methods. During this training to the new platform position, there was no significant main effect of pre-treatment and challenge on the escape latency [F (3,43) = 1.08, p = 0.39] or distance traveled [F (3,43) = 1.03, p = 0.39] (Figure 1). There were also no significant interactions between the factors.
Acquisition of the MWM task three months after a single challenge with 1xLD50 soman and/or pre-treatment with galantamine
A second set of MWM-naïve animals was trained in the MWM three months after they were injected with saline or galantamine and subsequently challenged with soman or saline. At this time, guinea pigs were slightly over four months old. None of the animals showed deficiency in their swimming ability. On the third day of training, all 4-month old animals reached swimming speeds that were comparable to those of younger guinea pigs on the second training day (Table 2). However, 4-month old animals did not climb onto the platform as easily as the prepubertal (~40-day-old) animals. The larger size of the 4-month old compared to the prepubertal animals (700–900 g vs. 400–450 g) may have contributed to the problem with climbing and to the slower task acquisition of the older animals.
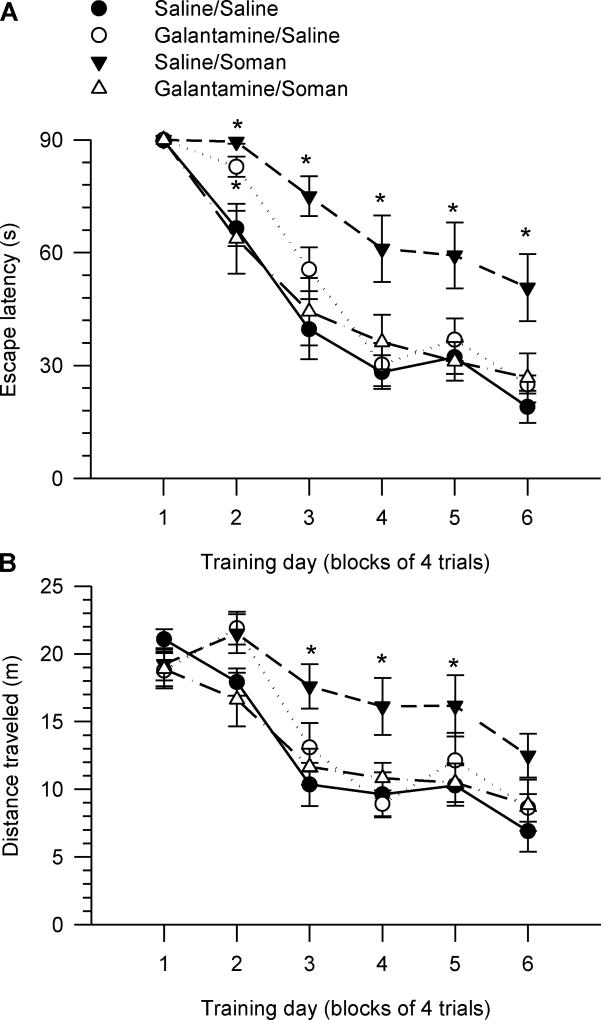
As with the younger animals, escape latency decreased with the training days. All 4-month old guinea pigs reached a near-asymptotic level of performance between days four and six of training, with mean escape latencies in the range of 30–35 s (for animals that had received three months earlier injections of saline/saline, galantamine/saline, or galantamine/soman) and 60–65 s (for groups that had been injected three months earlier with saline/soman) (Figure 4A).
Figure 4. Acquisition of the MWM task starting three months after injection of guinea pigs with saline, soman and/or galantamine.
Female prepubertal guinea pigs were injected intramuscularly with saline (0.5 ml/kg) or galantamine (8 mg/kg) 30 min prior to the sc injection of 1xLD50 soman (26 µg/kg) or saline (0.5 ml/kg). Three months later, animals received six days (four trials/day) of reference memory training. Graphs of escape latency (A) and distance traveled (B) per training day revealed that saline/soman-injected animals showed significant learning impairment. Galantamine/saline-injected animals also presented a delay in their ability to learn to find the hidden platform; on the second day of training, their escape latency was still longer than that of control (saline/saline-injected) animals. Data points and error bars represent mean and SEM, respectively, of results obtained from 7–11 animals/group. * p < 0.05 vs. saline/saline according to one-way ANOVA followed by Bonfferoni test.
Two-way repeated measures ANOVA with pre-treatment (galantamine or saline) and challenge (soman or saline) as between animal factors and training day as the repeated measure showed significant main effects of training day [F(5,150) = 91.94, p < 0.001] and challenge F(1,30) = 5.96, p < 0.001] on escape latency (Figure 4A). The analysis also revealed the following significant interactions: pre-treatment × challenge [F(1,30) = 12.83, p < 0.01], challenge × training day [F(5,150) = 2.52, p < 0.05], and pre-treatment × challenge × training day [F(5,150) = 2.79, p < 0.05]. There was a significant main effect of training day [F(5,150) = 118.47, p < 0.001] and a significant pre-treatment × challenge interaction [F(1,30) = 2.79, p < 0.05] on traveled distance. One-way ANOVA comparison of individual means for each day of training followed by Fisher’s LSD test revealed that animals that had been injected three months earlier with saline followed by soman did not learn the task as effectively as control animals (Figures 4A, 4B). Two of the eleven saline/soman-injected guinea pigs were unable to learn the task during the 6-day acquisition phase; based on our experience with such OP-exposed animals, it can take them more than two weeks to learn the task.
The learning curve of galantamine/soman-injected animals was similar to that of control (saline/saline-injected animals); escape latency and distance traveled for galantamine/soman-injected animals were not significantly different from control (Figures 4A, 4B). The learning curve of galantamine/saline-injected guinea pigs was also comparable to control (Figure 4A, 4B). Exception is made to the second day of the training, when animals that had been injected with galantamine/saline were locating the platform more slowly than control animals (Figure 4A).
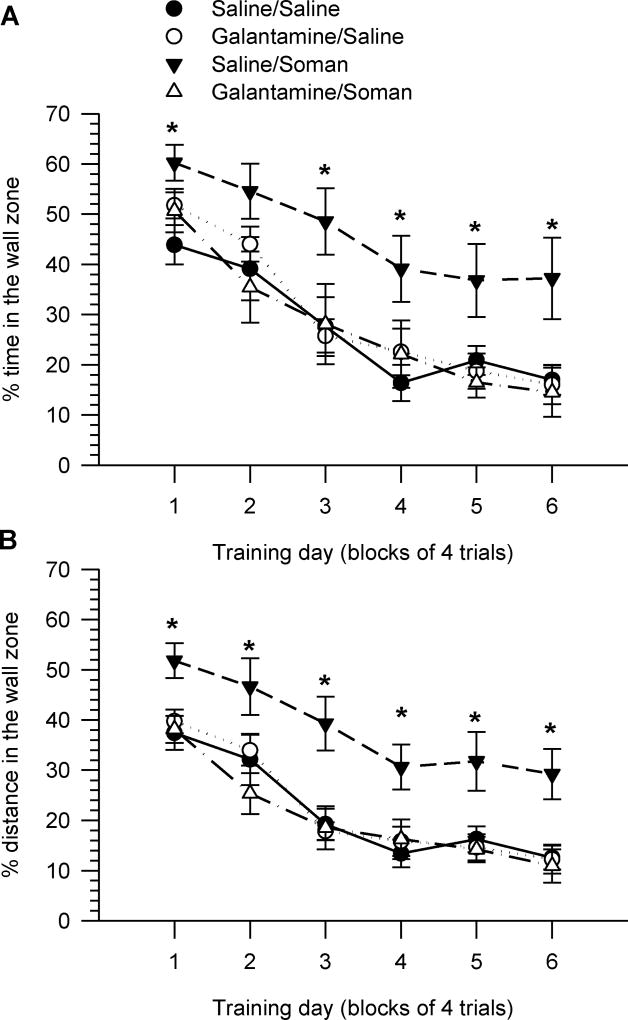
To reveal the nature of the learning impairment induced by soman and the learning delay of animals that had been treated with galantamine, we analyzed time and distance travelled in the wall zone. Swimming close to the wall is a natural defensive behavior referred to as thigmotaxis (Simon et al, 1994; Treit and Fundytus, 1988). To estimate thigmotactic behavior, for each trial, time spent in the wall zone was expressed as a percentage of the escape latency, and distance in the wall zone was expressed as a percentage of the total distance traveled.
Two-way repeated measures ANOVA revealed that the thigmotactic behavior of all animals decreased over the course of training, reaching near-asymptotic levels between training days four and six (Figures 5A, 5B). There were significant main effects of training day on both time [F(5,150) = 83.5, p < 0.001] and distance [F(5,150) = 51.22, p < 0.001] swum in the wall zone. There were also significant main effects of pre-treatment [F(1,30) = 6.57, p < 0.05] and challenge [F(1,30) = 4.75, p < 0.05], in addition to a significant pre-treatment × challenge interaction [F(1,30) = 7.43, p < 0.05] on the distance the animals swam in the wall zone (Figure 5B). Although there were no significant main effects of pre-treatment and challenge on the time spent on the wall zone ([F(1,30) = 2.61, p = 0.117] and [F(1,30) = 3.4, p = 0.075], respectively), there was a significant pre-treatment × challenge interaction [F(1,30) = 4.46, p < 0.05]. Time and distance traveled in wall zone were significantly longer for animals that had been injected with saline/soman three months earlier than for control animals (p < 0.05 for most training days; Figure 5A, 5B). Thigmotactic behavior of animals that had been injected with galantamine/saline or galantamine/soman was not significantly different from that of controls (Figure 5A, 5B).
Figure 5. Thigmotactic behavior during the acquisition phase of the MWM initiated three months after injection of guinea pigs with saline, soman and/or galantamine.
Results presented here are from the same animals as those in Figure 4. Thigmotactic behavior was defined by the time the animals spent swimming close to the wall (A) and the distance they swum close to the wall zone (B). Data points and error bars represent mean and SEM, respectively, of results obtained from 7–11 animals per treatment group. * p < 0.05 according to one-way ANOVA followed by Fisher’s LSD post-hoc test.
Probe tests and training to relocated platforms for animals that had been challenged with soman and/or treated with galantamine three months before the acquisition phase of the MWM task
The first probe test was performed 24 h after the last training of the acquisition phase performed three months after the injections. Two saline/soman-challenged guinea pigs that showed no progress in platform searching and stayed swimming close to the wall during the 6-day acquisition phase were excluded from the probe tests.
Two-way repeated measures ANOVA with pre-treatment and challenge as between animal factors and quadrant position as the repeated measure revealed no significant main effect of pre-treatment and challenge and no significant interaction between the factors on time in the quadrants. There was, however, a significant main effect of the quadrant position [F(3,84) = 3.48, p < 0.05] on time spent in the quadrants. Only animals that had been pre-treated with galantamine and challenged with soman spent more time in the training quadrant (p < 0.05, Dunnett’s post-hoc test) (Figure 6A).
Figure 6. Peformance of guinea pigs in probe tests performed one day and three weeks after completion of the acquisition phase of the MWM training initiated three months following the injections of saline, soman, and/or galantamine.
Results are from the same animals as those that completed the MWM training shown in Figure 4. A and B show data from the probe test performed 24 h and three weeks, respectively, after completion of the acquisition phase. Top graphs show the time animals spent in the each of the four quadrants of the pool. Bottom graphs shown the number of crossings of the platform areas virtually positioned in the center of each quadrant of the pool. The training (target) quadrant was used as the reference against which the other three quadrants were named. In A, galantamine/soman-injected animals showed bias to the target quadrant and to the platform area in the center of the training quadrant (* p < 0.05 according to one-way ANOVA followed by Dunnett post-hoc test). In B, galantamine/soman-injected animals showed preference to crossing the target platform area in comparison with the platform area virtually positioned in the opposite quadrant (* p < 0.05 according to one-way ANOVA followed by Dunnett post-hoc test). Graph and error bars represent mean and SEM, respectively, of results obtained from 7–9 animals. The horizontal dotted lines in the top graphs represent the chance level (22.5 s).
When platform position was used as the repeated measure, ANOVA revealed no significant main effect of pre-treatment and challenge on number of crossings of platform areas virtually positioned in the center of the quadrants. There was, however, a significant main effect of the platform position [F(3,84) = 11.01, p < 0.001] and a significant pre-treatment × challenge × platform position interaction [F(3,84) = 3.68, p < 0.05]. Control animals showed significant bias toward the training platform area only when compared to the virtual platform area in the quadrant opposite to the training quadrant (p < 0.05, Dunnett’s post-hoc test) (Figure 6A). Soman-challenged guinea pigs that had been pre-treated with galantamine showed preference to the training platform area vs. any of the virtual platform areas (p < 0.05, Dunnett’s post-hoc test) (Figure 6A).
During the second probe trial, performed three weeks after the first, there was no significant main effect of quadrant position, challenge or pre-treatment and no significant interactions among these factors (Figure 6B). However, analysis of number of crossings of platform areas virtually positioned in the center of the quadrants revealed significant main effect of position of the areas [F(3,84) = 2.8, p < 0.05]. Again, spatial bias was present only in the group of animals that had been treated with galantamine before the soman challenge. These animals showed preference to crossing the platform area in the center of the training quadrant vs. the platform area in the center of the opposite quadrant (p < 0.05, Dunnett’s post-hoc test) (Figure 6B).
A series of trainings to three different platform positions was completed after the second probe trial to further evaluate spatial navigation of animals. The two soman-challenged animals that did not learn how to escape onto the platform during the 6-day acquisition phase of the task were excluded from the training to relocated platforms. Two-way repeated measures ANOVA of escape latency (Figure 7A) and distance traveled (Figure 7B) with pre-treatment and challenge as between animal factors, and reversal training day as the repeated measure revealed no significant main effect and no significant interaction of the factors. These results suggest that the learning impairment observed during the acquisition phase of the MWM task performed three months after the exposure to soman was more likely due to procedural learning impairment, rather than deficits in spatial navigation.
Figure 7. Performance of guinea pigs to find relocated platforms following the acquisition phase of the MWM training initiated three months after injections of saline, soman and/or galantamine.
Results are from the same animals that completed the second probe test after the MWM training initiated three months after the injections. Each day animals were trained to find the platform in a different position as described in Materials and Methods. They received four trials per day. There were no significant differences among the experimental groups. Data points and error bars represent mean and SEM of results obtained from 7–9 animals/group.
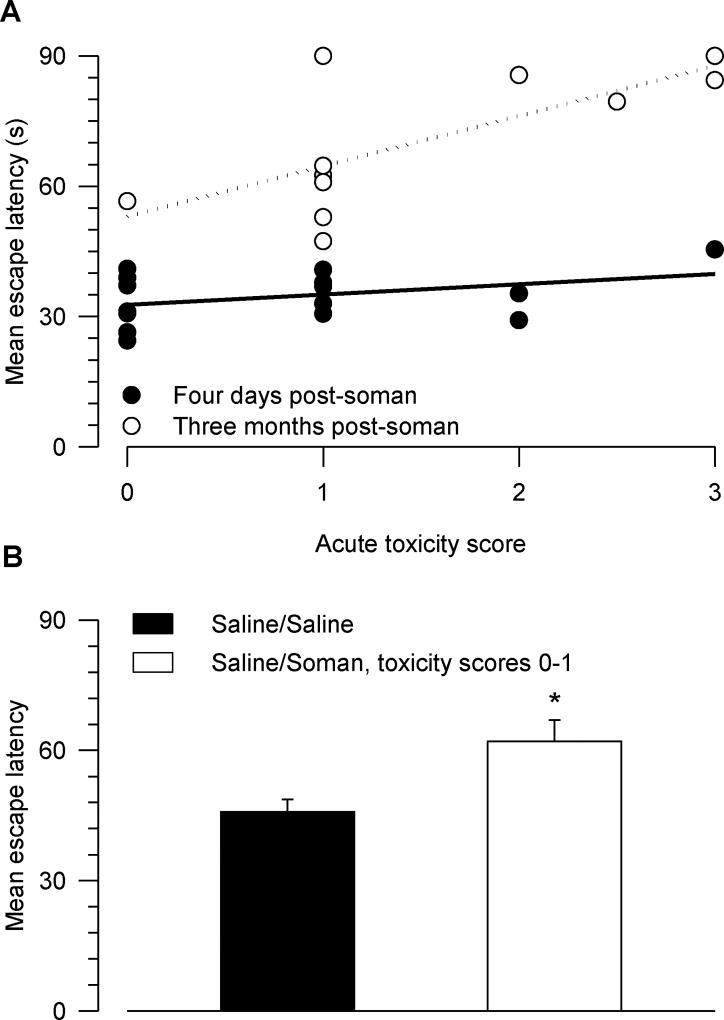
Correlation between the severity of the acute toxicity induced by soman and the magnitude of the learning impairment detected three months after the soman injection
The qualitative scores of acute toxicity presented by the animals soon after their exposure to 1xLD50 soman were plotted against the mean escape latency during the acquisition phase of the MWM task (Figure 8A). The mean escape latency was taken as a learning index; the longer the latency was, the more learning impaired the animals were. A Pearson correlation analysis revealed no significant correlation between the severity of the acute toxicity and the learning index of guinea pigs tested four days after their exposure to soman (r2 = 0.14, p = 0.16). In contrast, there was a positive correlation between the severity of the acute signs of intoxication and the learning index of guinea pigs tested three months post-soman challenge (r2 = 0.51, p = 0.014). Thus, the more severe the signs of acute intoxication were, the larger the magnitude of the learning impairment was. However, when compared to control animals, guinea pigs that had been considered mildly intoxicated (scores 0–1) still showed some degree of learning impairment three months after the soman challenge (Figure 8B).
Figure 8. Relationship between scores of acute toxicity and mean escape latency.
A. Plot of mean escape latency vs. score of acute toxicity. The escape latency across five or six days of training was averaged for each animal. Lines through the data points are the linear regressions. The Pearson correlation coefficients (r2) of the linear regressions of data from animals tested four days and three months after their injection with saline/soman were 0.14 and 0.51, respectively. The correlation between the severity of the acute toxicity and the learning impairment the animals presented three months after their injection with saline/soman was significant (F = 9.35, p = 0.014). The higher the toxicity score was, the longer the mean escape latency was. B. When tested three months after their treatments, mean escape latency of saline/saline-injected animals was significantly shorter than that of saline/soman-injected animals that showed either no or only mild signs of acute toxicity (scores 0–1). * p < 0.05 according to one-way ANOVA. Graph and error bars are mean and SEM of results obtained from 8–11 animals.
Fluoro Jade-B staining of the brains of saline- or galantamine-pretreated guinea pigs that are subsequently exposed to soman
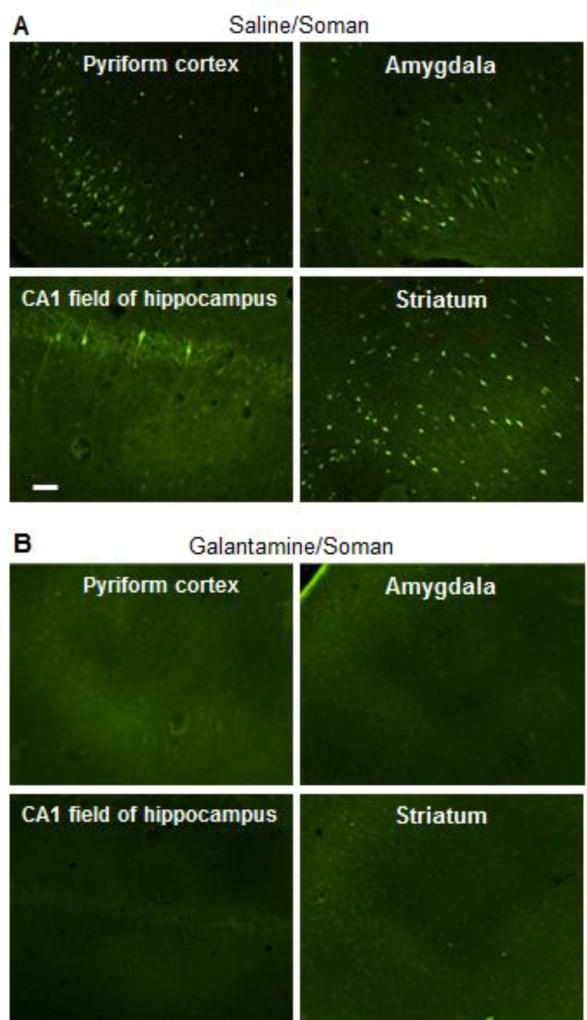
Guinea pigs were injected with saline (0.5 ml/kg, im) or galantamine (8 mg/kg, im) and thirty min later with soman (1xLD50, sc). The brains of saline/soman-injected guinea pigs classified as mildly to moderately intoxicated and of all galantamine/soman-injected guinea pigs were processed 48 h later for Fluoro Jade-B staining as described in Materials and methods. Fluoro-Jade B is known as a high-affinity fluorescent marker for localization of neurodegeneration during acute neuronal distress (Schmued and Hopkins, 2000). As shown in Figure 9A, large numbers of Fluoro Jade-B-positive cells were detected in the pyriform cortex, amygdala, and striatum of a guinea pig that scored 2 in the modified Racine scale following the injection of 1xLD50 soman. Only few Fluoro Jade-B-positive neurons were seen in the CA1 field of the hippocampus (Figure 9A) and no labeled neuron was visualized in the CA3 field of the hippocampus or in the dendate gyrus of this animal. As shown in Figure 9B, pre-treatment with galantamine prevented the soman-induced neurodegeneration. Fluoro Jade-B-positive neurons were not seen in the brains of galantamine/soman-injected animals.
Figure 9. Fluro Jade-B staining of different regions of the brains of guinea pigs treated with galantamine or saline and subsequently challenged with 1xLD50 soman.
Guinea pigs were injected with saline (0.5 ml/kg, im) or galantamine (8 mg/kg, im) and thirty min later with soman (1xLD50, sc). Saline/soman-injected guinea pigs classified as mildly to moderately intoxicated and all galantamine/soman-injected guinea pigs were euthanized 48 h after the treatments. Their brains were processed for Fluoro Jade-B staining. Photomicrographs are representative of the pyriform cortex, amygadala, CA1 field of the hippocampus, and striatum of a saline/soman-injected guinea pig that scored 2 in the modified Racine scale (A) and of a galantamine/soman-injected animal (B). No Fluoro Jade-B-positive cells were seen in the brains of galantamine/soman-injected guinea pigs. Results are representative of each treatment group, which had four animals. Calibration bar: 50 µm.
Discussion
The present study demonstrates that guinea pigs considered mildly to moderately intoxicated when challenged with 1xLD50 soman present learning impairment in the MWM task three months after the challenge, despite the fact that neurodegeneration is detectable as early as 48 h following the challenge.. Evidence is also provided that pre-treatment with a clinically relevant dose of galantamine effectively counteracts the soman-induced neurodegeneration and cognitive deficits. Mechanisms that contribute to the deleterious effects of soman and the effectiveness of galantamine are discussed herein.
Delayed cognitive impairment induced by a single exposure to soman may be accounted for by deficits in procedural learning
While four days after presenting mild-to-moderate signs of acute toxicity when injected with 1xLD50 soman guinea pigs learned the MWM task as well as did control animals, three months later soman-injected animals were learning impaired when compared to controls. These findings are in agreement with previous reports that immediate cognitive impairments are evident only in rodents that experience severe signs of acute toxicity, particularly prolonged convulsions, when exposed to 1-1.2xLD50 soman (Filliat et al., 1999, 2007).
There are two learning components in the classical version of the MWM that uses a hidden platform and extra-maze distal cues (reviewed in Izquierdo et al., 2006). During the procedural learning of the task, animals learn to swim in order to escape onto the hidden platform. During the spatial learning, animals learn to use the extra-maze cues to guide themselves toward the platform. Procedural and spatial learning are heavily dependent on the functional integrity of the striatum (McDonald and White, 1994; Packard and McGaugh 1992) and the hippocampus (Morris et al., 1986), respectively, and are modulated by the amygdala (Packard et al., 1994) and thalamic nuclei (Cain et al., 2006). Anterior thalamic nuclei contain a large population of cells that control head direction (Taube, 1995), which appears to be important for acquisition of efficient strategies to navigate in novel environments. In fact, lesions that damage thalamic nuclei of water maze-naïve rats increase thigmotactic behavior and impair learning of the classical version of the MWM task, while having no significant effect on a purely procedural version of the task (Cain et al., 2006).
The findings that, three months after their exposure to soman, guinea pigs performed as well as control animals in the probe test and in learning to find the platform relocated to different quadrants suggest that their learning impairment during the acquisition phase was due to deficits in procedural, rather than spatial learning. The limited neurodegeneration seen in the hippocampi of guinea pigs that presented mild-to-moderate signs of intoxication when challenged with 1xLD50 soman is in line with the finding that hippocampal-dependent spatial learning is not impaired by the nerve agent.
A recent magnetic resonance imaging study reported time-dependent increases in T2-weighted signal intensities in the thalamus of soman (1xLD50)-challenged guinea pigs that showed signs of acute toxicity ranging from 0 to 3 in the modified Racine scale (Gullapalli et al., 2010). The increase in T2-weighted signal intensity, which is suggestive of extracellular edema (Gröhn et al., 1998), reached significance by seven days after the soman challenge. Thus, it is tempting to speculate that progressive damage to the thalamus contributed to the learning impairment observed months after animals showed mild-to-moderate signs of acute toxicity when exposed to soman. The involvement of the striatum and other brain regions on the soman-induced learning impairment cannot be ruled out, however, particularly in light of the large numbers of Fluoro Jade-B-positive neurons detected in the striatum, amygdala, and other cerebral cortical regions of guinea pigs 48 h after their challenge with 1xLD50 soman. It is likely that the early neurodegeneration induced by the nerve agent leads to time-dependent aberrant remodeling of the neuronal circuitries that, in turn, contributes to the development of cognitive deficits.
Thigmotaxis is known to inhibit acquisition of spatial relationships of contextual cues, and, thereby, impair performance in the MWM (Acheson et al., 2011; Kallai et al., 2005). Although typically considered a measure of anxiety (Simon et al, 1994; Treit and Fundytus, 1988), thigmotaxis in the MWM can be seen in the absence of anxiety-related behavior in the open field. Specifically, striatal lesions trigger thigmotactic behavior in the MWM, without inducing anxiety-related behavior in the open field (Devan et al., 1999). Thus, it remains to be determined whether thigmotactic behavior of soman-exposed animals in the MWM, which can contribute to the learning impairment presented by the animals, is a result of the recently reported anxiety-inducing effect of the nerve agent (Mamczarz et al., 2010).
The severity of acute toxicity induced by soman correlated positively with the magnitude of the delayed learning impairment. This finding is in agreement with other reports that rodents exhibiting severe signs of acute toxicity after a single exposure to soman have impaired cognitive performance in the MWM (Filliat et al., 1999). However, in the current study, guinea pigs that exhibited very mild or no signs of acute toxicity still presented learning deficits three months after the initial challenge with 1xLD50 soman. Another study examined the MWM performance of male guinea pigs at seven days and three months after they had been injected once a day for ten days with a non-convulsant dose of soman (0.4xLD50) (Johnson et al., 2008). In that study, soman-exposed animals showed neither immediate nor delayed learning impairment in the MWM. However, the animals that were tested three months after the exposure to soman were not naïve to the MWM; they had already been tested at seven days post-exposure. Assuming that the delayed learning deficit induced by soman is the result of impaired procedural learning, it would have been detected only if the animals were naïve to the task at the time of testing.
Brain AChE activity is rapidly inhibited following an exposure of guinea pigs to 1xLD50 soman (Lintern et al., 1998). Shih et al. (1995) reported that AChE activity in red blood cells is inhibited by approximately 80% and 90–95% at 5 min and 10 min, respectively, after the sc injection of 1xLD50 soman in guinea pigs. Recovery of AChE activity in the brain is time dependent and region specific, with activity returning to control levels in many regions by seven days after the exposure (Lintern et al., 1998). Therefore, it is unlikely that at three months after a single challenge with 1xLD50 soman AChE remains inhibited to any significant extent to contribute to the delayed effect of soman on learning.
The long time span between the exposure to soman and the development of learning deficits suggests that these deficits may be the result of changes in expression of genes that control synaptic plasticity and/or neuronal viability in specific areas of the brain. Such changes could be indirectly triggered by: (i) excessive activation followed by desensitization of nicotinic and muscarinic receptors during the time AChE activity is inhibited by soman, (ii) activation or inactivation of second messenger mechanisms secondary to direct interactions of soman with specific neurotransmitter receptors (Lau et al., 1988; Silveira et al., 1990), and/or (iii) direct activation by soman of intracellular pathways that control gene expression (Osterreicher et al., 2007).
Effectiveness of galantamine as a medical countermeasure to prevent soman-induced learning impairment
Galantamine has emerged as a safe and effective medical countermeasure against acute intoxication with soman and other OP compounds (Albuquerque et al., 2006). When administered to guinea pigs 30 min before their challenge with 1xLD50 soman, galantamine (6–10 mg/kg, im) prevents the lethality and acute toxicity of the nerve agent. No additional supportive therapy is necessary (Aracava et al., 2009; Pereira et al., 2010). A recent MRI study also demonstrated the effectiveness of galantamine in preventing or limiting the brain damage induced by soman over time (Gullapalli et al., 2010). Of clinical relevance, doses of galantamine needed to prevent the acute toxicity and the brain damage induced by OP compounds are compatible with human use (Albuquerque et al., 2006).
The results presented here demonstrate for the first time that learning impairment and thigmotactic behavior developed months after an acute exposure to 1xLD50 soman are effectively prevented by galantamine. Other studies have reported the beneficial effects of galantamine on acquisition of the MWM task in learning-impaired rodents (Sweeney et al., 1988, 1990; Woodruff-Pak and Santos, 2000; Woodruff-Pak et al., 2001).
When testing started four days after treatment of the guinea pigs with galantamine alone, acquisition of the MWM task was not different from that of control animals. These results are in agreement with reports that in normal mice galantamine does not alter acquisition of the MWM task, even when applied before every training session (Van Dam et al., 2005). However, galantamine-treated guinea pigs were slightly impaired on the second day of the training that started three months after treatment. The cause underlying this delayed learning, which was not detected in soman-challenged guinea pigs that had been pre-treated with galantamine and could not be accounted for by increased thigmotactic behavior, remains to be determined.
Galantamine had a long-lasting effect on memory retention. In the first probe test performed 24 h following the acquisition phase of the training that started four days after treatment, galantamine-treated animals showed the same bias as control animals to the training quadrant or the training platform area. During the second probe test, performed three weeks after the first one, control animals showed no significant bias to the training quadrant or the training platform area. However, regardless of whether they had been injected with saline or 1xLD50 soman, galantamine-pretreated guinea pigs continued to show preference to the training platform area. Improved memory retention was also detected in the older guinea pigs that had been pre-treated with galantamine and subsequently challenged with soman. A similar finding has been reported in a study of transgenic mice overexpressing the human amyloid precursor protein (Vam Dam and De Deyn, 2006). In that study, mice received a prolonged treatment with galantamine and were tested in the MWM three weeks after withdrawal of the drug. While galantamine had no significant effect on acquisition, it improved memory retention in the probe test performed four days after the last training session (Vam Dam and De Deyn, 2006). A recent study revealed that galantamine, via activation of ERK1/2 through the allosteric potentiation of nAChRs, ameliorates methamphetamine-induced memory impairment in rats (Noda et al., 2010). It is, therefore, tempting to speculate that allosteric potentiation of nAChRs contributes to the memory-enhancing effect of galantamine in guinea pigs.
Potential molecular mechanisms underlying the effectiveness of galantamine to prevent soman-induced learning impairment
Although there was a positive correlation between the severity of the acute intoxication and the magnitude of the learning impairment presented three months after the initial exposure to soman, animals that showed no or only mild signs of toxicity still developed learning deficits. Thus, the ability of galantamine to curtail the acute toxicity of soman is not the sole mechanism that prevents the delayed learning impairment. A number of concurrent mechanisms may contribute to the effectiveness of galantamine to counter soman-induced learning deficits.
Reversible inhibition of brain AChE by galantamine may protect a significant pool of the enzyme from irreversible inhibition by soman (Albuquerque et al., 2006). Such action can prevent prolonged overactivation followed by desensitization of cholinergic receptors, which are known to be coupled to intracellular mechanisms that regulate cognitive functioning (reviewed in Albuquerque et al., 2009). Second, galantamine has neuroprotective actions that curtail soman-induced neurodegeneration (Gullapalli et al., 2010; see also Figure 9B). Third, as a nicotinic allosteric potentiating ligand, galantamine can increase the activity of nAChR-associated signaling mechanisms (Albuquerque et al., 2009) to prevent changes in gene expression that are likely to contribute to the delayed effects of soman on cognitive functions.
In conclusion, the results presented herein support the hypothesis that learning deficits develop months after mild-to-moderate acute intoxication with soman and are effectively counteracted by pre-treatment with a clinically relevant dose of galantamine.
Acknowledgments
This work was funded by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke [Grant UO1NS059344].
The authors would like to thank Dr. Yasco Aracava for her help with treating and caring for the animals. The authors are also indebted to Ms. Mabel Zelle for her technical assistance.
Abbreviations
- AChE
Acetylcholinesterase
- ACh
acetylcholine
- ANOVA
analysis of variance
- IACUC
Institutional Animal Care and Use Committee
- MRI
magnetic resonance imaging
- MWM
Morris water maze
- OP
organophosphorus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
The use of galantamine as an antidote against OP poisoning is protected under the International Patent Application PCT/US05/33789 filed on September 23, 2005.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as reflecting the view of the National Institutes of Neurological Disorders and Stroke, the Department of the Army, the Department of Defense, or the federal government.
References
- Acheson SK, Moore NL, Kuhn CM, Wilson WA, Swartzwelder HS. The synthetic cannabinoid WIN 55212-2 differentially modulates thigmotaxis but not spatial learning in adolescent and adult animals. Neurosci Lett. 2011;487:411–4. doi: 10.1016/j.neulet.2010.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Deshpande SS, Kawabuchi M, Aracava Y, Idriss M, Rickett DL, Boyne AF. Multiple actions of anticholinesterase agents on chemosensitive synapses: molecular basis for prophylaxis and treatment of organophosphate poisoning. Fundam Appl Toxicol. 1985;5:S182–203. doi: 10.1016/0272-0590(85)90129-0. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Aracava Y, Fawcett WP, Oliveira M, Randall WR, Hamilton TA, Kan RK, Romano JA, Jr, Adler M. Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc Natl Acad Sci USA. 2006;103:13220–5. doi: 10.1073/pnas.0605370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrova EA, Aracava Y, Pereira EFR, Albuquerque EX. Pretreatment of Guinea pigs with galantamine prevents immediate and delayed effects of soman on inhibitory synaptic transmission in the hippocampus. J Pharmacol Exp Ther. 2010;334:1051–8. doi: 10.1124/jpet.110.167700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Aracava Y, Pereira EFR, Albuquerque EX. A single in vivo application of cholinesterase inhibitors has neuron type-specific effects on nicotinic receptor activity in guinea pig hippocampus. J Pharmacol Exp Ther. 2009;328:69–82. doi: 10.1124/jpet.108.146068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracava Y, Pereira EFR, Akkerman M, Adler M, Albuquerque EX. Effectiveness of donepezil, rivastigmine, and ±huperzine A in counteracting the acute toxicity of organophosphorus nerve agents: comparison with galantamine. J Pharmacol Exp Ther. 2009;331:1014–24. doi: 10.1124/jpet.109.160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes ML, Richardson DP, Brien JF, Reynolds JN, Dringenberg HC. Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult guinea pig following brain growth spurt--prenatal ethanol exposure. Neurotoxicol Teratol. 2004;26:543–51. doi: 10.1016/j.ntt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Cain DP, Boon F, Corcoran ME. Thalamic and hippocampal mechanisms in spatial navigation: a dissociation between brain mechanisms for learning how versus learning where to navigate. Behav Brain Res. 2006;170:241–56. doi: 10.1016/j.bbr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J. Galantamine: a review of its use in Alzheimer's disease and vascular dementia. Int J Clin Pract. 2003;57:219–23. [PubMed] [Google Scholar]
- Coupland R, Leins KR. Science and prohibited weapons. Science. 2005;308:1841. doi: 10.1126/science.1115436. [DOI] [PubMed] [Google Scholar]
- de Groot DM, Bierman EP, Bruijnzeel PL, Carpentier P, Kulig BM, Lallement G, Melchers BP, Philippens IH, van Huygevoort AH. Beneficial effects of TCP on soman intoxication in guinea pigs: seizures, brain damage and learning behaviour. J Appl Toxicol. 2001;21:S57–65. doi: 10.1002/jat.812. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Richardson DP, Brien JF, Reynolds JN. Spatial learning in the guinea pig: cued versus non-cued learning, sex differences, and comparison with rats. Behav Brain Res. 2001;124:97–101. doi: 10.1016/s0166-4328(01)00188-7. [DOI] [PubMed] [Google Scholar]
- Filliat P, Baubichon D, Burckhart MF, Pernot-Marino I, Foquin A, Masqueliez C, Perrichon C, Carpentier P, Lallement G. Memory impairment after soman intoxication in rat: correlation with central neuropathologyImprovement with anticholinergic and antiglutamatergic therapeutics. Neurotoxicology. 1999;20:535–49. [PubMed] [Google Scholar]
- Filliat P, Coubard S, Pierard C, Liscia P, Beracochea D, Four E, Baubichon D, Masqueliez C, Lallement G, Collombet JM. Long-term behavioral consequences of soman poisoning in mice. Neurotoxicology. 2007;28:508–19. doi: 10.1016/j.neuro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Filliat P, Foquin A, Lallement G. Effects of chronic administration of huperzine A on memory in guinea pigs. Drug Chem Toxicol. 2002;25:9–24. doi: 10.1081/dct-100108469. [DOI] [PubMed] [Google Scholar]
- Gröhn OH, Lukkarinen JA, Oja JM, van Zijl PC, Ulatowski JA, Traystman RJ, Kauppinen RA. Noninvasive detection of cerebral hypoperfusion, reversible ischemia from reductions in the magnetic resonance imaging relaxation time, T2. J. Cereb Blood Flow Metab. 1998;18:911–20. doi: 10.1097/00004647-199808000-00012. [DOI] [PubMed] [Google Scholar]
- Gullapalli RP, Aracava Y, Zhuo J, Helal Neto E, Wang J, Makris G, Merchenthaler I, Pereira EFR, Albuquerque EX. Magnetic resonance imaging reveals that galantamine prevents structural brain damage induced by an acute exposure of guinea pigs to soman. Neurotoxicology. 2010;31:67–76. doi: 10.1016/j.neuro.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Hood E. The Tokyo attacks in retrospect: sarin leads to memory loss. Environ Health Perspect. 2001;109:A542. doi: 10.1289/ehp.109-a542a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. 7. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington DC: 1996. [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Da Silva WC, Medina JH, Cammarota M. The connection between the hippocampal and the striatal memory systems of the brain: a review of recent findings. Neurotox Res. 2006;10:113–21. doi: 10.1007/BF03033240. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Daugherty KS, Gallagher SJ, Moran AV, DeFord SM. Glutamate receptor pathology is present in the hippocampus following repeated sub-lethal soman exposure in the absence of spatial memory deficits. Neurotoxicology. 2008;29:73–80. doi: 10.1016/j.neuro.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kallai J, Makany T, Karadi K, Jacobs WJ. Spatial orientation strategies in Morris-type virtual water task for humans. Behav Brain Res. 2005;159:187–96. doi: 10.1016/j.bbr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kassa J, Krejcová G, Vachek J. The impairment of spatial memory following low-level sarin inhalation exposure and antidotal treatment in rats. Acta Medica (Hradec Kralove) 2002;45:149–53. [PubMed] [Google Scholar]
- Lau WM, Freeman SE, Szilagyi M. Binding of some organophosphorus compounds at adenosine receptors in guinea pig brain membranes. Neurosci Lett. 1988;94:125–30. doi: 10.1016/0304-3940(88)90282-0. [DOI] [PubMed] [Google Scholar]
- Lewejohann L, Pickel T, Sachser N, Kaiser S. Wild genius - domestic fool? Spatial learning abilities of wild and domestic guinea pigs. Front Zool. 2010;7:9. doi: 10.1186/1742-9994-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintern MC, Wetherell JR, Smith ME. Differential recovery of acetylcholinesterase in guinea pig muscle and brain regions after soman treatment. Hum Exp Toxicol. 1998;17:157–62. doi: 10.1177/096032719801700306. [DOI] [PubMed] [Google Scholar]
- Mamczarz J, Pereira EFR, Aracava Y, Adler M, Albuquerque EX. An acute exposure to a sub-lethal dose of soman triggers anxiety-related behavior in guinea pigs: interactions with acute restraint. Neurotoxicology. 2010;31:77–84. doi: 10.1016/j.neuro.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Long JM, Johnson CT, Olton DS. Variable-interval probe test as a tool for repeated measurements of spatial memory in the water maze. Behav Neurosci. 1993;107:627–32. doi: 10.1037//0735-7044.107.4.627. [DOI] [PubMed] [Google Scholar]
- Maxwell DM, Brecht KM, O'Neill BL. The effect of carboxylesterase inhibition on interspecies differences in soman toxicity. Toxicol Lett. 1987;39:35–42. doi: 10.1016/0378-4274(87)90254-2. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–70. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–79. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–76. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Newmark J. Nerve agents. Neurologist. 2007;13:20–32. doi: 10.1097/01.nrl.0000252923.04894.53. [DOI] [PubMed] [Google Scholar]
- Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Minami M, Omae K. Sarin Health Effects Study Group Effects of sarin on the nervous system in rescue team staff members and police officers 3 years after the Tokyo subway sarin attack. Environ Health Perspect. 2001;109:1169–73. doi: 10.1289/ehp.011091169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Mouri A, Ando Y, Yukari Wakia1, Yamada S-N, Yoshimi A, Yamada K, Ozaki N, Wang D, Nabeshim T. Galantamine ameliorates the impairment of recognition memory in mice repeatedly treated with methamphetamine: involvement of allosteric potentiation of nicotinic acetylcholine receptors and dopaminergic-ERK1/2 systems. Intern J Neuropsychopharmacol. 2010;13:1343–54. doi: 10.1017/S1461145710000222. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Iwanami A, Kasai K, Yamasue H, Kato T, Sasaki T, Kato N. Post-traumatic stress disorder symptoms in victims of Tokyo subway attack: a 5-year follow-up study. Psychiatry Clin Neurosci. 2004;58:624–9. doi: 10.1111/j.1440-1819.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- Okumura T, Takasu N, Ishimatsu S, Miyanoki S, Mitsuhashi A, Kumada K, Tanaka K, Hinohara S. Report on 640 victims of the Tokyo subway sarin attack. Ann Emerg Med. 1996;28:129–35. doi: 10.1016/s0196-0644(96)70052-5. [DOI] [PubMed] [Google Scholar]
- Osterreicher J, Pejchal J, Kassa J. Alteration of mitogen-activated protein kinase pathway after soman poisoning. Drug Chem Toxicol. 2007;30:283–91. doi: 10.1080/01480540701380190. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci USA. 1994;91:8477–81. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci. 1992;106:439–46. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Pereira EFR, Aracava Y, Alkondon M, Akkerman M, Merchenthaler I, Albuquerque EX. Molecular and cellular actions of galantamine: clinical implications for treatment of organophosphorus poisoning. J Mol Neurosci. 2010;40:196–203. doi: 10.1007/s12031-009-9234-3. [DOI] [PubMed] [Google Scholar]
- Raveh L, Weissman BA, Cohen G, Alkalay D, Rabinovitz I, Sonego H, Brandeis R. Caramiphen and scopolamine prevent soman-induced brain damage and cognitive dysfunction. Neurotoxicology. 2002;23:7–17. doi: 10.1016/s0161-813x(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Romano JA, Jr, King JM. Psychological casualties resulting from chemical and biological weapons. Mil Med. 2001;166:21–2. [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Shih TM, Kan RK, McDonough JH. In vivo cholinesterase inhibitory specificity of organophosphorus nerve agents. Chem Biol Interact. 2005;157–158:293–303. doi: 10.1016/j.cbi.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Silveira CL, Eldefrawi AT, Eldefrawi ME. Putative M2 muscarinic receptors of rat heart have high affinity for organophosphorus anticholinesterases. Toxicol Appl Pharmacol. 1990;103:474–81. doi: 10.1016/0041-008x(90)90320-t. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Sweeney JE, Höhmann CF, Moran TH, Coyle JT. A long-acting cholinesterase inhibitor reverses spatial memory deficits in mice. Pharmacol Biochem Behav. 1988;31:141–7. doi: 10.1016/0091-3057(88)90325-5. [DOI] [PubMed] [Google Scholar]
- Sweeney JE, Bachman ES, Coyle JT. Effects of different doses of galanthamine, a long-acting acetylcholinesterase inhibitor, on memory in mice. Psychopharmacology (Berl.) 1990;102:191–200. doi: 10.1007/BF02245921. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–62. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Van Dam D, Abramowski D, Staufenbiel M, De Deyn PP. Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23 model. Psychopharmacology (Berl) 2005;180:177–90. doi: 10.1007/s00213-004-2132-z. [DOI] [PubMed] [Google Scholar]
- Van Dam D, De Deyn PP. Cognitive evaluation of disease-modifying efficacy of galantamine and memantine in the APP23 model. Eur. Neuropsychopharmacol. 2006;16:59–69. doi: 10.1016/j.euroneuro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wicke KM, Rex A, Jongen-Relo A, Groth I, Gross G. The guinea pig forced swim test as a new behavioral despair model to characterize potential antidepressants. Psychopharmacology (Berl) 2007;195:95–102. doi: 10.1007/s00213-007-0874-0. [DOI] [PubMed] [Google Scholar]
- Wilber CG. Some factors which are correlated with swimming capacity in guinea pigs. J Appl Physiol. 1959;14:199–203. doi: 10.1152/jappl.1959.14.2.199. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Santos IS. Nicotinic modulation in an animal model of a form of associative learning impaired in Alzheimer's disease. Behav Brain Res. 2000;113:11–9. doi: 10.1016/s0166-4328(00)00196-0. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Vogel RW, 3rd, Wenk GL. Galantamine: effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning. Proc Natl Acad Sci USA. 2001;98:2089–94. doi: 10.1073/pnas.031584398. [DOI] [PMC free article] [PubMed] [Google Scholar]