Abstract
People socially connected with each other often share health risks, possibly due to shared environments and behaviors. In a retrospective cohort study, we examined whether incidence of diabetes was different for individuals with recently diagnosed partners compared to individuals similar on other characteristics but whose partners were never diagnosed with diabetes. We analyzed 2007–11 data from Kaiser Permanente Northern California (KNPC), an integrated health system with >3.5 million members. We estimated annual diabetes incidence controlling for demographic, socio-economic, behavioral, and health characteristics. Using propensity score matching and multivariate logistic regression, we compared odds of incident diabetes among co-residing partners ages 18–89 years of people who had been diagnosed with diabetes during the previous year (in robustness checks up to the previous three years) and people who had never been diagnosed but were similar on observed characteristics. Partners of newly-diagnosed people had annual diabetes incidence of 16.4/1,000, equivalent to10.8 times higher (95%CI: 9.2–12.6) than people whose spouses had never been diagnosed (1.5/1,000). Odds remained higher three years after a spouses’ diagnosis (45.4 vs. 11.7/1,000). Adjusting for other characteristics, odds of diabetes for those with a partner diagnosed in the previous year were 8.7 times higher CI: 7.4–10.2) than among those whose partner had never been diagnosed. Partners of persons with recently-diagnosed diabetes developed diabetes at much higher rates than partners of persons with similar characteristics who were never diagnosed with diabetes. Individuals with a recently diagnosed partner could be considered a high-risk population for screening and prevention.
Keywords: Family, clustering, longitudinal, social determinants
Introduction
Diabetes mellitus is a common, progressive condition, affecting over 29 million people in the United States.(Centers for Disease Control and Prevention, 2015). It is an especially costly chronic condition, identified as the leading cause of personal health spending. (Dieleman et al., 2016) Evidence from clinical trials has shown that lifestyle interventions and metformin can reduce diabetes incidence and that early recognition, lifestyle modification, self-management, and health checks can help reduce the morbidity burdens of diabetes.(Holman et al., 2008; Knowler et al., 2002; Lindstrom et al., 2006; Manley et al., 2000; Srikanth and Deedwania, 2005) However, awareness and perceived risk of diabetes are low in the U.S. – nearly 90% of people with pre-diabetes and approximately 25% of people with diabetes are unaware of their glycemic status.(Centers for Disease Control and Prevention, 2012, 2014)
To identify people with diabetes or at risk for diabetes and link them with preventive and health services, the American Diabetes Association and the U.S. Preventive Services Task Force developed guidelines recommending who should be offered glucose testing.(2015; Siu, 2015) These guidelines focus on established individual diabetes risk factors such as age, weight, and family history. However, social factors are emerging as important considerations in disease risk. For example, health-related characteristics, such as smoking and obesity tend to cluster within family, friendship, work, and neighborhood social networks.(Christakis and Fowler, 2007, 2008) Individuals who live together, even if they are not genetically related, such as spouses, may have related disease risks. One reason is that individuals who live together engage in many activities together and share health information, behavior patterns and habits such as diet, smoking and exercise, and environmental exposures. (Macken et al., 2000; Sonneville et al., 2012; Veinot et al., 2011; Wilson, 2002) Shared health risks may also be due to “assortative mating”, or the preference to partner with people with similar characteristics.(Mathews and Reus, 2001; Nakosteen et al., 2005; Wilson, 2002) Studies have shown that people whose spouse had a history of diabetes were also more likely to have diabetes. A clinic-based study in London found that the partners of people with diabetes were more than twice as likely to also have diabetes than partners of people without diabetes.(Khan et al., 2003) Other studies have reported concordance in spouses’ diabetes status in the United Kingdom, Sweden, China and Korea, and among Mexican adults in the U.S.(Hemminki et al., 2010; Hippisley-Cox et al., 2002; Jurj et al., 2006; Khan et al., 2003; Kim et al., 2006; Stimpson and Peek, 2005) Across studies, a spouse with a history of diabetes was associated with a 26% higher risk of also having diabetes.(Leong et al., 2014)
While previous studies have compared people who had spouses with diabetes and those who did not, they have not examined incident diabetes after a spouse’s diagnosis among people matched on risk factors. To better understand the role of a partner’s diagnosis independent of other shared risk factors, we matched people who had been diagnosed with diabetes in the previous year with people who were similar on other characteristics but who had never been diagnosed with diabetes; we then examined incidence of diabetes in their co-resident partners over the course of one and three years.
Materials and Methods
DATA SOURCE
Data were from the electronic health records of Kaiser Permanente Northern California (KPNC). KPNC is an integrated healthcare delivery system with a diverse population of about 3.5 million insured individuals who are broadly similar to the California population.(Gordon, 2012) We extracted demographic, health utilization, and laboratory data for all plan members for the period 2007 to 2011.
POPULATION
We selected all couples who were co-residing at the beginning and end of the study period and who were linked as “spouses” or “domestic partners” on a shared a health plan in the enrollment records. Address data from administrative records were used to confirm co-residence.
Couples were included if neither partner had ever been diagnosed with diabetes or if one partner was newly diagnosed in the previous year. Couples were excluded if either partner was outside the age range 18–89 years or did not have KPNC coverage throughout the year before and the year after the period of analysis, allowing for a one-month lapse. We included only couples enrolled in KPNC in that year and the previous year to ensure that we are not including as incident cases those who joined with prevalent diabetes. Specific exclusion criteria for KPNC members who had never been diagnosed with diabetes are listed and explained in the Supplementary Table.
VARIABLES
The main exposure was whether one partner had been diagnosed with diabetes during the previous year (for additional analysis, previous three years).
The outcome is whether a co-residing partner developed incident diabetes during the year following the diabetes diagnosis of the first partner or during the calendar year for the couples in which neither member had been diagnosed with diabetes. Diabetes incidence was based on the KPNC diabetes registry, a well-validated database of diabetes in KPNC. Following established protocol, KPNC members are considered to have an incident case of diabetes on the date they first met one of the following criteria: (1) at least two outpatient diabetes diagnoses; (2) at least one inpatient or emergency department diabetes diagnosis; or (3) using any diabetes medications, except metformin alone, in the previous two years.
We examined individually and included in multivariate models characteristics expected to be associated with diabetes: age, gender, race and ethnicity, BMI category, number of primary care visits in the previous year, and glucose screening in the previous year; and, from Census 2010 data, median household income and percentage of individuals aged 25 and older with at least a bachelor’s degree on the Census block of members’ residence.
ANALYSIS
One challenge to using descriptive or standard analytic methods to track incidence of diabetes within families is that some individuals or families may be more predisposed to diabetes for reasons other than the partner’s diabetes. Therefore, we used propensity score matching methods to compare incidence in individuals with spouses who were otherwise similar on observable characteristics but differed in diabetes diagnosis. This method replicates a counterfactual scenario, identifying individuals with similar predispositions who did and did not get a diabetes diagnosis and then observing incidence rates in their partners. It reduces selection bias and avoids imposing a linear relationship between exposure (one partner’s diagnosis) and outcome (the second partner’s diagnosis) by modeling the probability of exposure as a function of observed attributes that pre-date and may have shaped the probability of the first partner’s diagnosis.
We identified five couples with no diagnoses for each couple with one new diagnosis during the year. We selected 5 matched couples to maximize power without compromising the quality of the matches; greater than five matches result in less precision in the propensity score matching between the groups. We employed a nearest neighbor matching algorithm without replacement. The procedures first randomly sort the exposed and control cohorts and then step through each possible pairing, retaining the closest match; after a match has been identified at a given distance, a subsequent match must improve the distance to be selected. When there were ties, the control was selected by random selection. We calculated propensity scores - the probability of the first partner developing diabetes - using a logistic regression model with the first partner’s gender, race and ethnicity, and, in the previous year, age, BMI, neighborhood education and annual income, KPNC service area, and number of primary care visits. The mean difference in propensity scores for the completed matches was 0.0002 (SD 0.003).
Incidence was examined per 1,000 population for those whose partner had and had not developed diabetes. We calculated unadjusted odds ratios of developing diabetes, comparing partners of recently-diagnosed vs. never diagnosed persons; these were calculated for the entire population and by age, race, ethnicity, and gender.
Adjusted odds ratios of developing diabetes were then calculated, with models including whether the partner had developed diabetes, age, gender, race and ethnicity (Hispanic and Non-Hispanic White, Black, Asian), neighborhood education and income levels, BMI category, number of primary visits, and glucose screening in the previous year.
To provide context, we estimated the average annual incidence of diabetes at ages 18–79 years in the U.S. population and in the general KPNC population during the same period (2007–2011) and compare these with the patterns for partners of newly diagnosed people. Incidence among general KPNC members was calculated as the number who developed diabetes in that year divided by the number who did not have diabetes at the start of the year. The KPNC rate was a weighted average over the same five-year period. U.S. data were from the National Health Interview Survey as reported by the Centers for Disease Control and Prevention.(National Center for Chronic Disease Prevention and Health Promotion Division of Diabetes Translation, 2015).
Statistical analyses were performed with SAS software (version 9.3).
Results
Table 1 shows characteristics of partners of KPNC members who were diagnosed with diabetes in the previous year and of partners of KPNC members similar on other characteristics but never diagnosed with diabetes. The mean age of partners of newly diagnosed people was 54.3 years; 62.8% were women; 48.6% were White, with the second largest group being Asians, at 21.1% of the population. Among those for whom BMI data were available, 30.5% were obese, 28.4% were overweight, and 19.8% were normal weight. Partners of newly diagnosed members had had on average 2.5 primary care visits in the previous year and just over half had had glucose tested.
Table 1.
Baseline characteristics of co-residing partners of people with and without incident diabetes
| Characteristic | Partners of people with incident diabetes (N=30,155) |
Partners of people without incident diabetes (N=150,775) |
|---|---|---|
| Age | ||
| Mean, years | 54.3 ± 12.0 | 53.2 ± 13.4 |
| Categories, years - % | ||
| 18–44 | 21.3 | 27.3 |
| 45–64 | 59.5 | 52.8 |
| 65–79 | 17.0 | 16.4 |
| 80–89 | 2.2 | 3.5 |
| Gender (%) | ||
| Female | 62.8 | 65.4 |
| Male | 37.2 | 34.6 |
| Other or unknown | 0.01 | 0.01 |
| Race or ethnicity (%) | ||
| White | 48.6 | 53.2 |
| Black | 5.8 | 5.5 |
| Hispanic | 17.7 | 17.1 |
| Asian | 21.1 | 17.6 |
| Other or unknown | 6.7 | 6.6 |
| Census-track mean education (%) | ||
| <20% ≥ bachelor’s degree | 28.2 | 27.5 |
| 20%–34% ≥ bachelor’s degree | 30.5 | 30.2 |
| 35%–49% ≥ bachelor’s degree | 21.6 | 21.6 |
| ≥50% ≥ bachelor’s degree | 19.2 | 20.0 |
| Unknown | 0.6 | 0.8 |
| Census-track median annual household income (%) | ||
| <$60,000 | 28.4 | 27.9 |
| $60,000 – $79,999 | 26.0 | 25.6 |
| $80,000 – $99,999 | 20.5 | 20.2 |
| ≥$100,000 | 24.6 | 25.5 |
| Unknown | 0.6 | 0.8 |
| Body-mass index (%) | ||
| Normal (< 25 kg/m2) | 19.8 | 27.2 |
| Overweight (25–29 kg/m2) | 28.4 | 29.6 |
| Obese (≥ 30 kg/m2) | 30.5 | 21.8 |
| Unknown | 21.3 | 21.5 |
| No. of primary care visits | ||
| Mean | 2.5 ± 3.5 | 2.5 ± 3.3 |
| Categories - % | ||
| 0 | 22.9 | 23.1 |
| 1 | 22.9 | 23.6 |
| 2–3 | 30.7 | 30.6 |
| ≥4 | 23.5 | 22.8 |
| Any glucose testing in year before (%) | 50.3 | 47.3 |
Data Source: Kaiser Permanente Northern California Electronic Health Records
People who had been diagnosed with diabetes and people who had never been diagnosed were matched. Characteristics of their co-residing partners ages 18–89 years are shown here.
Chi-square or t-test
Compared with partners of newly diagnosed persons, those whose partner had never been diagnosed with diabetes were on average about one year older, were more often women, were more often White, and more often lived in neighborhoods with highest levels of education and income. The largest difference was in body weight, with over 7% more being normal weight and almost 9% fewer being obese compared with the spouses of people recently diagnosed with diabetes.
The diabetes incidence rates were 10.8 (95% CI: 9.2–12.6) times higher (16.4 vs. 1.5/1,000) among people whose partners had recently been diagnosed compared with people whose partners had not had a diabetes diagnosis (see Figure 1).
Figure 1. Average annual incidence of diabetes among people whose partner was and was not recently diagnosed with diabetes, 2007–2011.
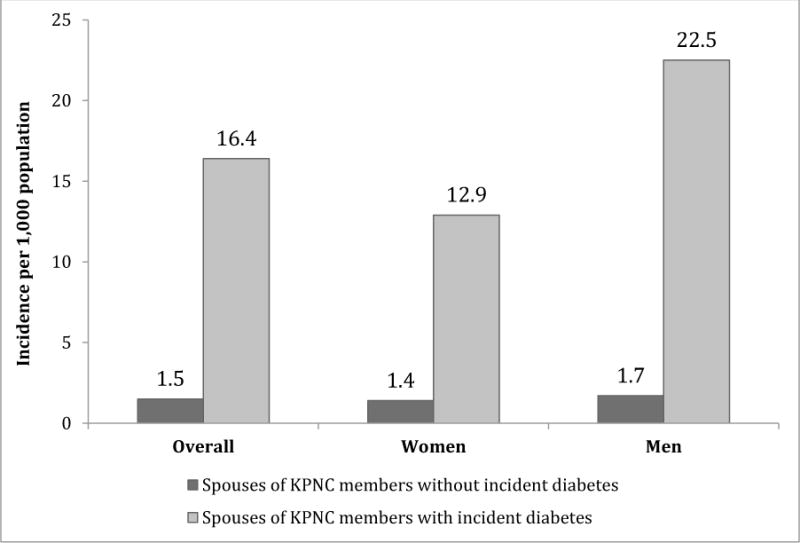
Data Source: Kaiser Permanente Northern California Electronic Health Records
Table 2 shows the unadjusted odds of diabetes incidence across characteristics for partners of otherwise similar people who had never been diagnosed and who had been diagnosed in the previous year. The odds ratio for those whose partner had been diagnosed compared with those whose partner had never been diagnosed was highest among partners who were aged 65–79 years (OR: 13.7, CI: 9.4–19.8), were male (OR: 13.1, CI: 10.3–16.7), were White (OR: 14.0, CI: 10.7–18.1), were in the highest-income (OR: 12.7; CI: 8.8–18.3) and highest-education (OR: 12.7; CI: 8.1–19.8) neighborhoods, and were normal weight (OR: 23.2; CI: 7.6–22.5). These patterns indicate that having a partner with diabetes elevates the risks among those who would otherwise be at relatively lower risk of diabetes, as many of these characteristics are associated with lower incidence. The odds were not higher among partners who had had glucose tests or multiple primary care visits, indicating that the elevated incidence is not the result of higher screening.
Table 2.
One-year incidence of diabetes and relative risk, by baseline characteristics
| Partners of people with incident diabetes: Incidence per 1,000 population | Partners of people without incident diabetes: Incidence per 1,000 population | Odds ratio | 95% CI | |
|---|---|---|---|---|
| Entire cohort | 16.4 | 1.5 | 10.8 | 9.2, 12.6 |
| Age | ||||
| 18–44 yr | 10.9 | 0.9 | 11.6 | 7.8, 17.2 |
| 45–64 yr | 17.2 | 1.8 | 9.8 | 8.0, 11.9 |
| 65–79 yr | 20.7 | 1.5 | 13.7 | 9.4, 19.8 |
| 80–89 yr | 15.3 | 2.6 | 5.8 | 2.6, 13.3 |
| Gender | ||||
| Female | 12.8 | 1.4 | 9.0 | 7.3, 11.1 |
| Male | 22.4 | 1.7 | 13.1 | 10.3, 16.7 |
| Race or ethnicity | ||||
| White | 13.6 | 1.0 | 14.0 | 10.7, 18.1 |
| Black | 22.1 | 2.9 | 7.8 | 4.7, 13.1 |
| Hispanic | 19.3 | 2.1 | 9.5 | 6.8, 13.3 |
| Asian | 18.7 | 2.2 | 8.8 | 6.4, 12.1 |
| Other or unknown | 16.8 | 2.0 | 8.6 | 4.9, 14.9 |
| Census-track mean education | ||||
| <20% ≥bachelor’s degree | 20.6 | 2.0 | 10.3 | 7.9, 13.3 |
| 20%–34% ≥ bachelor’s degree | 15.8 | 1.7 | 9.6 | 7.3, 12.7 |
| 35%–49% ≥ bachelor’s degree | 16.4 | 1.3 | 12.6 | 8.8, 18.0 |
| ≥50% ≥ bachelor’s degree | 11.2 | 0.9 | 12.7 | 8.1, 19.8 |
| Unknown | 11.2 | 1.8 | 6.3 | 0.9, 45.3 |
| Census-track median annual household income | ||||
| <$60,000 | 19.4 | 1.9 | 10.2 | 7.8, 13.4 |
| $60,000 – $79,999 | 16.2 | 1.8 | 9.2 | 6.9, 12.4 |
| $80,000 – $99,999 | 16.2 | 1.3 | 12.5 | 8.7, 18.1 |
| ≥$100,000 | 13.4 | 1.1 | 12.7 | 8.8, 18.3 |
| Unknown | 11.2 | 1.8 | 6.4 | 0.9, 45.4 |
| Body-mass index | ||||
| Normal (<25 kg/m2) | 6.4 | 0.5 | 13.1 | 7.6, 22.5 |
| Overweight (25–29 kg/m2) | 13.2 | 1.3 | 10.4 | 7.6, 14.4 |
| Obese (≥ 30 kg/m2) | 26.7 | 3.4 | 8.0 | 6.4, 10.0 |
| Unknown | 15.1 | 1.3 | 11.5 | 8.0, 16.5 |
| No. of primary care visits | ||||
| 0 | 14.5 | 1.2 | 12.5 | 8.7, 17.9 |
| 1 | 13.3 | 1.1 | 12.6 | 8.6, 18.4 |
| 2–3 | 16.5 | 1.4 | 11.9 | 8.9, 15.9 |
| 4+ | 21.1 | 2.6 | 8.3 | 6.4, 10.8 |
| Glucose testing in year before | ||||
| Any | 19.2 | 1.9 | 10.4 | 8.5, 12.8 |
| None | 13.5 | 1.2 | 11.0 | 8.6, 13.9 |
Data Source: Kaiser Permanente Northern California Electronic Health Records
Table 3 shows the odds of incident diabetes in models adjusting for partners’ diagnosis and other characteristics. The adjusted odds of diabetes for those with a newly diagnosed partner were 8.7 times higher (CI: 7.4–10.2) than for those whose partner had never been diagnosed. The odds of being diagnosed with diabetes were also higher among partners if they were older (highest at ages 80 to 89 years, OR: 3.2, CI: 2.0–5.1), male (OR: 1.4, CI: 1.2–1.6), non-White (highest for those of Asian descent, OR: 2.7, CI: 2.2–3.3), living in a poorer neighborhood (lowest for those in neighborhoods where the majority of residents have college degrees, OR: 0.6, CI: 0.5–0.7), or were heavier (highest for those with obesity, OR: 6.1, CI: 4.5–8.1). Relative risks of incident diabetes between partners of people with vs. without diabetes are also illustrated in Appendix Figure 1.
Table 3.
Adjusted odds ratios of incident diabetes
| Odds ratio | Odds ratio 95% CI | |
|---|---|---|
| Partner’s diabetes | ||
| Never diagnosed | 1.0 | |
| Diagnosed in last year | 8.7 | 7.4, 10.2 |
| Age | ||
| 18–44 yr | 1.0 | |
| 45–64 yr | 1.9 | 1.5, 2.3 |
| 65–79 yr | 2.3 | 1.8, 3.1 |
| 80–89 yr | 3.2 | 2.0, 5.1 |
| Gender | ||
| Female | 1.0 | |
| Male | 1.4 | 1.2, 1.6 |
| Race or ethnicity | ||
| White | 1.0 | |
| Black | 1.8 | 1.3, 2.3 |
| Hispanic | 1.6 | 1.3, 2.0 |
| Asian | 2.7 | 2.2, 3.3 |
| Other or unknown | 1.8 | 1.3, 2.4 |
| Census-track mean educationb | ||
| <20% ≥bachelor’s degree | 1.0 | |
| 20%–34% ≥ bachelor’s degree | 0.8 | 0.7, 1.0 |
| 35%–49% ≥ bachelor’s degree | 0.8 | 0.7, 1.0 |
| ≥50% ≥ bachelor’s degree | 0.6 | 0.5, 0.7 |
| Unknown | 0.7 | 0.2, 1.8 |
| Body-mass index | ||
| Normal (<25 kg/m2) | 1.0 | |
| Overweight (25–29 kg/m2) | 2.3 | 1.7, 3.1 |
| Obese (≥ 30 kg/m2) | 6.1 | 4.5, 8.1 |
| Unknown | 3.2 | 2.2, 4.7 |
| No. of primary care visits | ||
| 0 | 0.7 | 0.5, 1.0 |
| 1 | 0.6 | 0.5, 0.8 |
| 2–3 | 0.7 | 0.6, 0.9 |
| 4+ | 1.0 | |
| Glucose testing in year before | ||
| Any | 1.2 | 1.0, 1.5 |
| None | 1.0 |
Data Source: Kaiser Permanente Northern California Electronic Health Records
The census-track income and education variables were too closely correlated to both be included in this model.
To provide additional context, Figure 2 compares incidence of diabetes among partners of newly diagnosed KPNC members with the general KPNC membership and the U.S. population. The incidence of diabetes in the KPNC population was similar to that in the U.S. population overall, by gender (Panel 1), by age group (Panel 2), and by race and ethnicity (Panel 3). Incidence was almost double among partners of newly diagnosed people (16.4/1,000 vs. 8.3/1,000 in the general U.S. and KPNC populations); elevated risks persisted across gender, age groups, race, and ethnicity. Men whose partners were newly diagnosed experienced much higher incidence than did women whose partners had been recently diagnosed (22.4/1,000 vs. 12.8/1,000). While risks of diabetes diagnoses increased with age across all groups, the risks faced by people with newly diagnosed partners compared with the general population were highest for the youngest age group, those 18–44 years. People who identified as Black had the highest risk of diabetes in the general population, the highest risk if they had a newly diagnosed partner, and the highest increase in risk associated with having a diagnosed partner.
Figure 2. Average annual incidence of diabetes in the United States population, among KPNC members, and among partners of KPNC members recently diagnosed with diabetes, 2007–2011.
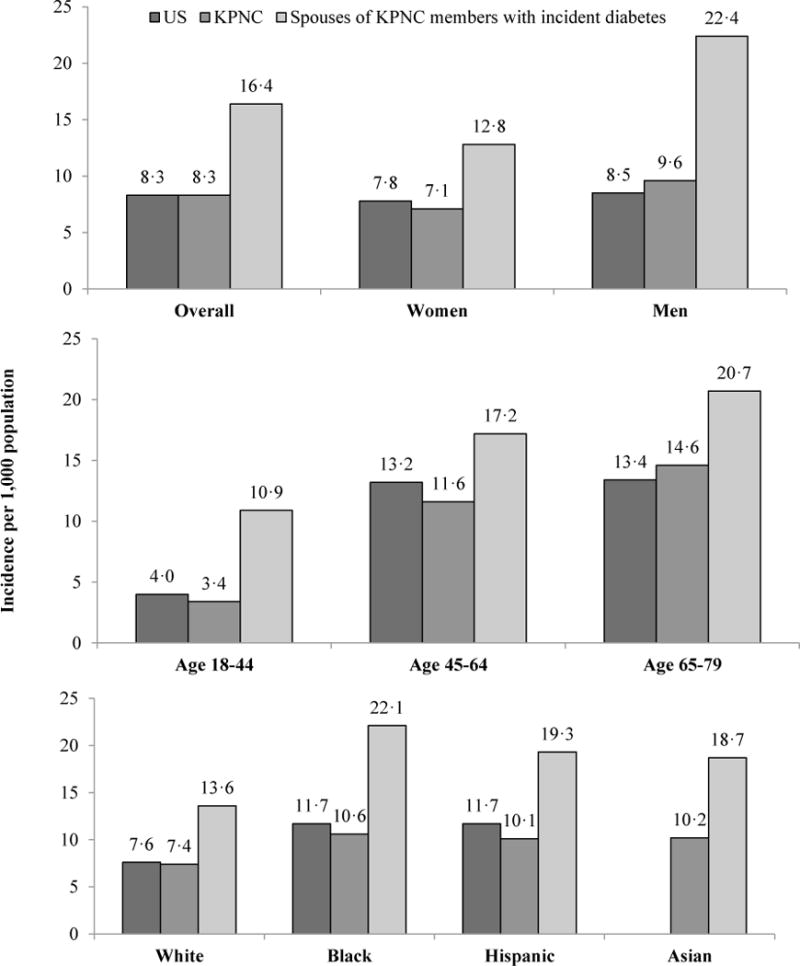
Data Source: Kaiser Permanente Northern California Electronic Health Records
Note: US figures are only for the population 18–79 years old, KPNC figures are for the population 18–89 years old.
Figure 3 illustrates cumulative incidence over 3-year of follow-up. After the incidence rates described at 1-year of follow-up, the risks of diabetes incidence in spouses of people recently diagnosed with diabetes continue to be higher than those of people similar on other characteristics but whose spouses were never diagnosed. Cumulative diabetes cases increased over time for everyone, as expected, but the cumulative incidence at 2 years of follow-up was 32.3/1,000 in spouses of recently diagnosed people, effectively double the cumulative incidence of the general Kaiser membership and 6 times higher than in people with never-diagnosed partners. At 3 years of follow-up, more than 45/1,000 people whose partners were diagnosed had also developed diabetes, compared to 24.1 among the general Kaiser membership and 11.7 among those with never-diagnosed partners.
Figure 3. Cumulative incidence of diabetes over 3 years among spouses of matched partners with and without incident diabetes and the general population, 2007–2011.
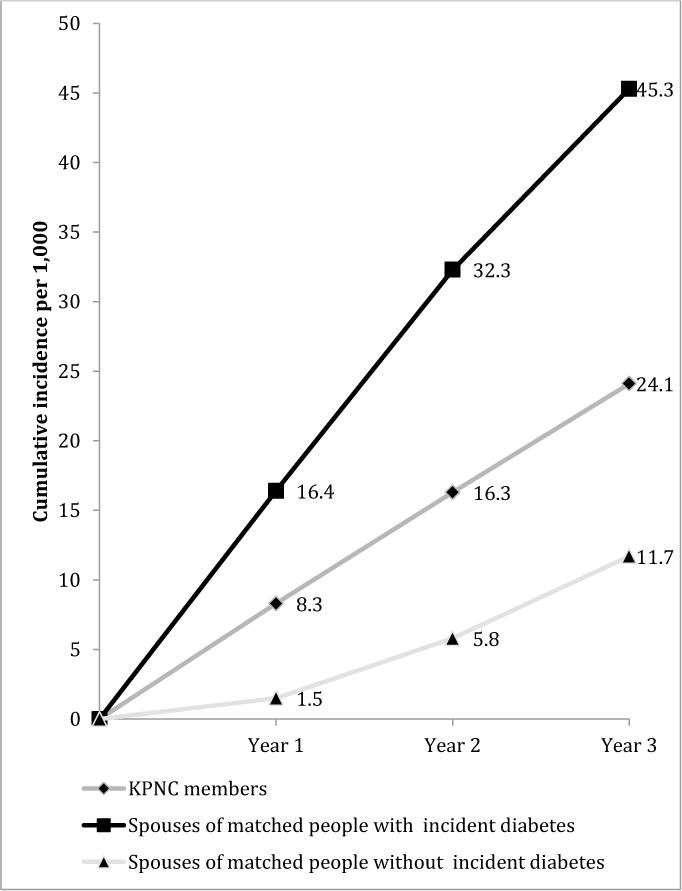
Data Source: Kaiser Permanente Northern California Electronic Health Records
Discussion
This study examined the risks of diabetes onset after a partner’s new diabetes diagnosis. When the risks of diabetes experienced by people with a recently diagnosed partner were compared with those experienced by people whose partners has never been diagnosed with diabetes but were similar in many other characteristics, the odds were almost nine times higher, even after adjusting for age, body weight, glucose testing, and other relevant characteristics. Incidence was much higher among partners of people recently diagnosed with diabetes across genders, age groups, races, and ethnicities.
The odds of diabetes in partners of newly diagnosed people were much higher when we examining among matched couples than when comparing in the general population, the typical point of comparison for previous studies. The patterns highlight that people with diabetes are different in many health, demographic, and economic characteristics from people without diabetes, and that when we thoroughly account for these differences, the risks associated with a partner’s diagnosis are even greater. They also highlights the very low risks of diabetes experienced by coupled individuals whose partners have never been diagnosed with diabetes. An important consideration is that, even after matching partner’s characteristics, the partners of people with newly diagnosed diabetes are much more often obese than the partners of people never diagnosed with diabetes.
Comparison with other risk factors emphasizes the potential clinical importance of considering spouses’ diabetes. With incidence of 16.4/1000 over 1 year, partners of newly diagnosed people are at very high risk. Annual incidence is 5–12% among people with pre-diabetes (Gerstein and et al., 2007). Obesity and overweight are associated with about 7 and 3 times the odds of diabetes, respectively, compared to normal weight individuals (2010) – substantially lower than to 8.6 higher odds among parters of diagnosed people.
The results highlight the clustering of diabetes within households for environmental or behavioral reasons. Individuals who live together engage in many activities together - eating, watching television, or being active; they share food, activity, safety and pollution environments.(Macken et al., 2000; Sonneville et al., 2012; Veinot et al., 2011; Wilson, 2002) Shared activities may be ones which one partner adopted from the other, which they developed together due to cohabiting, or which they both had individually before cohabiting.(Mathews and Reus, 2001; Nakosteen et al., 2005; Wilson, 2002) Risks of diabetes were higher among men with newly diagnosed partners than among women with newly diagnosed partners. This pattern could be linked to the greater role of women in deciding family meals and activities, but further research is needed to explore possible pathways.
Some of the elevated incidence rates of partners of people diagnosed with diabetes may be explained by increased screening among partners once one has been diagnosed. For example, the diagnosis of one member may prompt the other one to get screened also, where they may have otherwise gone undiagnosed until later. Unadjusted odds of diabetes diagnosis were higher among those who had been screened in the previous year than those who had not been screened, but the difference in risks of diagnosis among those whose partner had vs. had not already been diagnosed did not hinge on screening. Furthermore, adjusted for higher glucose testing and other characteristics, odds of developing diabetes were still 8.7 times higher for those with compared to those without a recent partner’s diabetes diagnosis. In addition, screening is recommended for everyone over age 45 years and, in this insured population, primary care use is high.
If partners are aware of their own elevated risks, they may be more inclined to seek screening, preventive care, or improve their own lifestyles. Learning about cancer, heart disease, or diabetes in a friend or family member has been shown to heighten concerns about developing the disease.(Montgomery et al., 2003) People with a family member or friend with diabetes have also reported greater concern about diabetes and its complications.(Mani et al., 2011) Perceived risk is a component of behavioral activation and contemplating behavior changes(Costello et al., 2012; Larsman et al., 2012; Lyna et al., 2002) and may encourage adoption of preventive behaviors such as physical activity, at least in the short-term.(Chang et al., 2011; Mani et al., 2011; Montgomery et al., 2003) Diabetes interventions engaging couples have reported that couples can achieve reductions in caloric intake and increases in physical activity together.(Gorin et al., 2008)
The study population was in Northern California, which is diverse but ethnically and socioeconomically different from other parts of the country. Still, the overall diabetes incidence in the KPNC system was very similar to that of the general U.S. population. The data are from a population with health insurance, and rate of detection of diabetes status may be lower among people without health insurance. Our study is about co-residing spouses and domestic partners who share a health plan with KPNC; relying on health insurance data, we do not know about the legal status of the individuals, nor about the closeness or duration of their relationship. Couples with separate health plans could not be identify in our data; this may entail that a higher proportion of dual-earner couples were excluded. We could not distinguish between type 1 and type 2 diabetes, but type 1 cases only account for five percent of all new adult cases nationally.(Adair and Prentice, 2004) Therefore, to the extent that a small proportion of our incident cases are type 1, and are unlikely to be linked to spousal connection, these are slightly biasing the observed relationship between spouses’ incident diabetes towards the null. While using matching techniques reduces some bias in analysis, it may not entirely eliminate bias due to unmeasured confounders.
This study builds on previous work by using electronic health records data from a large, integrated healthcare delivery system and using careful matching on a range of demographic and clinical variables not widely available in other datasets. By matching individuals on multiple characteristics and then examining incident cases over the course of a year in their co-residing partners we can better isolate the risks associated with a partner’s diagnoses.
Conclusion
The American Diabetes Association currently recommends that asymptomatic people be screened if they are 45 years or older or are any age with a BMI ≥25 kg/m2 and at least one diabetes risk factor, including having a first-degree relative with diabetes. Our findings suggest that having a partner with diabetes could be considered as a risk factor also, even more so after having matched people similar on other characteristics than previously documented in non-matched studies (Khan et al., 2003). Given that partners of people with diabetes have an elevated risk of developing diabetes themselves, they would benefit from receiving information about their own risk, preventive behavior changes, and screening options.
Living with a person with diabetes is an indicator of elevated diabetes risk, even among family members who are not genetically related. Understanding patterns of household influences on health behaviors may have implications for screening guidelines, for optimizing how providers give advice to newly-diagnosed persons, and for designing lifestyle or care delivery interventions that involve the family.
Supplementary Material
Highlights.
Partners of people newly-diagnosed with diabetes also had higher risks of diabetes
One-year incidence was 10.8 times higher than among those with similar partners
The partner’s odds remained higher three years after a spouses’ diagnosis
Diabetes clusters within households, even among people who are not related
Acknowledgments
Funding
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R21 DK098558-01 and P30 DK092924). The funders had no role in the study’s design, conduct, or reporting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Solveig A. Cunningham, Hubert Department of Global Health, Emory University
Sara R. Adams, Division of Research, Kaiser Permanente Northern California
Julie A. Schmittdiel, Division of Research, Kaiser Permanente Northern California
Mohammed K. Ali, Hubert Department of Global Health, Emory University
References
- 1.Diabetes Research and Clinical Practice. 2010;89:309–19. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Standards of Medical Care in Diabetes—2015: Summary of Revisions. Diabetes Care. 2015;38:S4. doi: 10.2337/dc15-S003. [DOI] [PubMed] [Google Scholar]
- 3.Adair LS, Prentice AM. A critical evaluation of the fetal origins hypothesis and its implications for developing countries. The Journal of nutrition. 2004;134:191–3. doi: 10.1093/jn/134.1.191. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Awareness of prediabetes—United States, 2005–2010. Morb Mortal Wkly Rep. 2012;62 [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention, editor. Centers for Disease Control and Prevention, 2014. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. U.S. Department of Health and Human Services; Atlanta, GA: [Google Scholar]
- 6.Centers for Disease Control and Prevention, 2015. Diabetes Report Card 2014. Centers for Disease Control and Prevention, US Dept of Health and Human Services; Atlanta, GA: [Google Scholar]
- 7.Chang M-h, Valdez R, Ned RM, Liu T, Yang Q, Yesupriya A, Dowling NF, Meigs JB, Bowen MS, et al. Influence of Familial Risk on Diabetes Risk–Reducing Behaviors Among U.S. Adults Without Diabetes. Diabetes Care. 2011;34:2393–99. doi: 10.2337/dc11-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–9. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 9.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358:2249–58. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello MJ, Logel C, Fong GT, Zanna MP, McDonald PW. Perceived Risk and Quitting Behaviors: Results From the ITC 4-Country Survey. American J Health Behav. 2012;36:681–92. doi: 10.5993/AJHB.36.5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, Hamavid H, Horst C, Johnson EK, et al. US Spending on Personal Health Care and Public Health, 1996–2013. JAMA. 2016;316:2627–46. doi: 10.1001/jama.2016.16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstein HC, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: A systematic overview and meta-analysis of prospective studies. Diabetes Research and Clinical Practice. 2007;78:305–12. doi: 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Gordon N. Internal Division of Research report. Kaiser Permanente Northern California Division of Research; Oakland, CA: 2012. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2009 California Health Interview Survey. http://www.dor.kaiser.org/external/chis_non_kp_2009/ [Google Scholar]
- 14.Gorin AA, Wing RR, Fava JL, Jakicic JM, Jeffery R, West DS, Brelje K, DiLillo VG. Weight loss treatment influences untreated spouses and the home environment: evidence of a ripple effect. Int J Obes. 2008;32:1678–84. doi: 10.1038/ijo.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemminki K, Li X, Sundquist K, Sundquist J. Familial risks for type 2 diabetes in Sweden. Diabetes Care. 2010;33:293–7. doi: 10.2337/dc09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hippisley-Cox J, Coupland C, Pringle M, Crown N, Hammersley V. Married couples’ risk of same disease: cross sectional study. BMJ. 2002;325:636. doi: 10.1136/bmj.325.7365.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 18.Jurj AL, Wen W, Li HL, Zheng W, Yang G, Xiang YB, Gao YT, Shu XO. Spousal correlations for lifestyle factors and selected diseases in Chinese couples. Annals of epidemiology. 2006;16:285–91. doi: 10.1016/j.annepidem.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 19.Khan A, Lasker SS, Chowdhury TA. Are spouses of patients with type 2 diabetes at increased risk of developing diabetes? Diabetes Care. 2003;26:710–2. doi: 10.2337/diacare.26.3.710. [DOI] [PubMed] [Google Scholar]
- 20.Kim HC, Kang DR, Choi KS, Nam CM, Thomas GN, Suh I. Spousal concordance of metabolic syndrome in 3141 Korean couples: a nationwide survey. Annals of epidemiology. 2006;16:292–8. doi: 10.1016/j.annepidem.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 21.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsman P, Eklöf M, Törner M. Adolescents’ risk perceptions in relation to risk behavior with long-term health consequences; antecedents and outcomes: A literature review. Saf Sci. 2012;50:1740–48. [Google Scholar]
- 23.Leong A, Rahme E, Dasgupta K. Spousal diabetes as a diabetes risk factor: a systematic review and meta-analysis. BMC medicine. 2014;12:12. doi: 10.1186/1741-7015-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, Hamalainen H, Harkonen P, Keinanen-Kiukaanniemi S, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–9. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 25.Lyna P, McBride C, Samsa G, Pollak KI. Exploring the association between perceived risks of smoking and benefits to quitting - Who does not see the link? Addict Behav. 2002;27:293–307. doi: 10.1016/s0306-4603(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 26.Macken LC, Yates B, Blancher S. Concordance of risk factors in female spouses of male patients with coronary heart disease. J Cardpulm Rehabil. 2000;20:361–8. doi: 10.1097/00008483-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Mani N, Caiola E, Fortuna RJ. The influence of social networks on patients’ attitudes toward type II diabetes. Journal of community health. 2011;36:728–32. doi: 10.1007/s10900-011-9366-6. [DOI] [PubMed] [Google Scholar]
- 28.Manley SE, Stratton IM, Cull CA, Frighi V, Eeley EA, Matthews DR, Holman RR, Turner RC, Neil HA. Effects of three months’ diet after diagnosis of Type 2 diabetes on plasma lipids and lipoproteins (UKPDS 45). UK Prospective Diabetes Study Group. Diabet Med. 2000;17:518–23. doi: 10.1046/j.1464-5491.2000.00320.x. [DOI] [PubMed] [Google Scholar]
- 29.Mathews CA, Reus VI. Assortative mating in the affective disorders: a systematic review and meta-analysis. Comprehensive psychiatry. 2001;42:257–62. doi: 10.1053/comp.2001.24575. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery GH, Erblich J, DiLorenzo T, Bovbjerg DH. Family and friends with disease: their impact on perceived risk. Preventive medicine. 2003;37:242–9. doi: 10.1016/s0091-7435(03)00120-8. [DOI] [PubMed] [Google Scholar]
- 31.Nakosteen R, Westerlund O, Zimmer M. Health-related disabilities and matching of spouses: Analysis of Swedish population data. J Popul Econ. 2005;18:491–507. [Google Scholar]
- 32.National Center for Chronic Disease Prevention and Health Promotion Division of Diabetes Translation, 2015. Diabetes Data and Statistics. Centers for Disease Control and Prevention; Atlanta, GA: http://www.cdc.gov/diabetes/data/ [Google Scholar]
- 33.Siu AL. Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: U.S. Preventive Services Task Force Recommendation StatementScreening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus. Annals of Internal Medicine. 2015;163:861–68. doi: 10.7326/M15-2345. [DOI] [PubMed] [Google Scholar]
- 34.Sonneville KR, Rifas-Shiman SL, Kleinman KP, Gortmaker SL, Gillman MW, Taveras EM. Associations of Obesogenic Behaviors in Mothers and Obese Children Participating in a Randomized Trial. Obesity (Silver Spring) 2012 doi: 10.1038/oby.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srikanth S, Deedwania P. Comprehensive risk reduction of cardiovascular risk factors in the diabetic patient: an integrated approach. Cardiology clinics. 2005;23:193–210. doi: 10.1016/j.ccl.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Stimpson JP, Peek MK. Concordance of chronic conditions in older Mexican American couples. Prev Chronic Dis. 2005;2:A07. [PMC free article] [PubMed] [Google Scholar]
- 37.Veinot TCE, Kim YM, Meadowbrooke CC. Health information behavior in families: Supportive or irritating? Proceedings of the American Society for Information Science and Technology. 2011;48:1–10. [Google Scholar]
- 38.Wilson SE. The health capital of families: an investigation of the inter-spousal correlation in health status. Social science & medicine. 2002;55:1157–72. doi: 10.1016/s0277-9536(01)00253-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


