Abstract
Organophosphorus (OP) insecticides are pest-control agents heavily used worldwide. Unfortunately, they are also well known for the toxic effects that they can trigger in humans. Clinical manifestations of an acute exposure of humans to OP insecticides include a well-defined cholinergic crisis that develops as a result of the irreversible inhibition of acetylcholinesterase (AChE), the enzyme that hydrolyzes the neurotransmitter acetylcholine (ACh). Prolonged exposures to levels of OP insecticides that are insufficient to trigger signs of acute intoxication, which are hereafter referred to as subacute exposures, have also been associated with neurological deficits. In particular, epidemiological studies have reported statistically significant correlations between prenatal subacute exposures to OP insecticides, including chlorpyrifos, and neurological deficits that range from cognitive impairments to tremors in childhood. The primary objectives of this article are: (i) to address the short- and long-term neurological issues that have been associated with acute and subacute exposures of humans to OP insecticides, especially early in life (ii) to discuss the translational relevance of animal models of developmental exposure to OP insecticides, and (iii) to review mechanisms that are likely to contribute to the developmental neurotoxicity of OP insecticides. Most of the discussion will be focused on chlorpyrifos, the top-selling OP insecticide in the United States and throughout the world. These points are critical for the identification and development of safe and effective interventions to counter and/or prevent the neurotoxic effects of these chemicals in the developing brain.
Keywords: acetylcholinesterase, brain, chlorpyrifos, development, learning, memory
For over half a century, organophosphorus (OP) insecticides have been among the most heavily and ubiquitously used insecticides throughout the world, with chlorpyrifos (CPF) leading the market for many years. Unfortunately, similar to other xenobiotics, these insecticides are toxic to humans, and their widespread usage has become a major global public health concern. Yet, given their effectiveness against insects, ease of application, and low cost, their use is predicted to grow worldwide through 2022 (Grand View Research, 2014).
The fatalities and poor health outcomes resulting from exposures of humans of all ages to high doses of OP insecticides are well documented and result primarily from the common action of these chemicals and/or their metabolites as irreversible inhibitors of acetylcholinesterase (AChE), the enzyme that hydrolyzes the neurotransmitter acetylcholine (Dharmani and Jaga 2005). Cases of accidental and intentional acute OP poisoning occur throughout the world, including the United States (US) (Jaga and Dharmani 2003). However, they are particularly insidious in developing countries, where OP insecticides are readily available, poorly regulated, and account for hundreds of thousands of deaths every year (Buckley et al. 2004; Gunnell et al. 2007).
There are also concerns regarding the health effects of long-term exposures of humans throughout the world to levels of OP insecticides that are insufficient to trigger overt signs of acute intoxication (Jaga and Dharmani 2003; Bouvier et al. 2005). Different research groups have reported that these subacute OP exposures are associated with neurological deficits in adults (reviewed in Jamal et al. 2002; Levin and Rodnitzky 1976; Ross et al. 2013) and, as it will be further discussed in the next section, in children (reviewed in Eaton et al. 2008; Engel et al. 2011; Reiss et al. 2015; Rosas and Eskenazi 2008). In fact, out of concern of the potential dangers posed by subacute exposures of developing children to CPF, in 2000 the Environmental Protection Agency (EPA) restricted the household use of this OP insecticide in the US (Lemus and Abdelghani 2000). As of 2006, however, CPF could still be detected in 78% of homes surveyed in the US (Stout et al. 2009). In addition, agricultural exposures in the United States were not addressed by the EPA restriction, and biological markers of CPF exposure, including the CPF metabolite 3,5,6-trichloro-2-pyridinol (TCPY) in urine and the parent compound (CPF) in blood serum, have been detected in samples from American agricultural workers and their families (Fenske et al. 2002; Eskenazi et al. 2004, 2007; Huen et al. 2012). Thus, chronic subacute exposures to CPF remain a serious public health concern, particularly for children, in the United States and throughout the world.
Epidemiological assessment of the developmental neurotoxicity of CPF
Exposure to CPF is a major issue during pregnancy because, similar to other hydrophobic compounds, this insecticide readily crosses the placenta (Abdel-Rahman et al. 2002) and, as such, has the potential to induce untoward effects in the developing organism.
Longitudinal epidemiological studies carried out in a multiethnic inner-city population of children traced statistically significant correlations between prenatal subacute exposures that resulted in CPF concentrations > 6.17 pg/g in cord blood collected at birth and: (i) reduced weight and length at birth (Perera et al. 2003), (ii) impaired cognition and motor function, attention deficit hyperactive disorder, and developmental problems at the age of 3 (Rauh et al. 2006), (iii) deficits in working memory and reduced full-scale intelligence quotient at the age of 7 (Rauh et al. 2011), and (iv) childhood tremors at the age of 11 (Rauh et al. 2015). Some authors have argued that levels of CPF in umbilical cord blood are too low to cause biologically meaningful AChE inhibition (critical reviews of the subject can be found in Eaton et al. 2008; Reiss et al. 2015). While this is true, mechanistic studies that will be discussed later in this review have provided evidence that CPF can interact with and change the activity of non-AChE targets. Therefore, the neurodevelopmental toxicity of CPF may develop in the absence of significant AChE inhibition. In addition, one cannot rule out the possibility that in utero levels of CPF are substantially higher than those measured in umbilical cord because the half-life of CPF has been estimated to be approximately 27 h (Timchalk et al. 2002). Umbilical cord levels of CPF provide a snapshot of the degree of exposure rather than an accurate assessment of the total prenatal OP burden experienced by the developing organism in utero.
In studies that used urine or blood level of TCPY as a biomarker of CPF exposure, either no associations or only weak associations were traced between prenatal CPF exposure and fetal growth indices (Eskenazi et al. 2004; Whyatt et al. 2004) or cognitive deficits in children (Fortenberry et al. 2014). Although the presence of TCPY in blood and/or urine reflects exposure to CPF, levels of the metabolite do not necessarily correlate with the internal CPF dose, that is, the amount of CPF absorbed by an organism. Specifically, CPF can be hydrolyzed to TCPY in the environment (Morgan et al. 2005; Lu et al. 2006). As such, measured levels of TCPY in body fluids or tissue represent not only the amount of TCPY generated by the metabolism of CPF in vivo but also the amount of TCPY absorbed together with CPF. In cases in which the amount of TCPY absorbed from external sources overwhelms the amount of TCPY produced by the in vivo breakdown of CPF, blood or urine levels of TCPY do not accurately correlate with levels of CPF absorbed and, consequently, do not correlate with the magnitude of the biological effects of CPF. This emphasizes the limitations of some biomarkers of exposure in health risk assessments (Ryan et al. 2007).
Prenatal exposures that resulted in CPF concentrations ≥ 4.39 pg/g in cord blood collected at birth have also been associated with significant structural abnormalities in the brain of 7–9-year-old children when compared with age-matched children who were either not exposed to CPF during pregnancy or experienced prenatal exposures that resulted in cord blood CPF levels < 4.39 pg/g. These abnormalities included a significant enlargement of the mesial surface of the superior frontal gyrus bilaterally in addition to frontal and parietal cortical thinning (Rauh et al. 2012). In addition, the statistically significant positive correlations normally traced between the full-scale intelligence quotient (FSIQ) and the surface area of the superior temporal, inferior frontal, inferior precentral, and inferior postcentral gyri bilaterally, and the precuneus of the left hemisphere were either absent or reversed among children, particularly boys, who experienced prenatal exposures producing cord levels of CPF ≥ 4.39 ng/g (Rauh et al. 2012). This finding lent support to the hypothesis that disruption of the structural integrity of the brain can be an important determinant of the cognitive deficits associated with prenatal exposure to CPF.
The limitations of the epidemiological studies reviewed above, including assessment of biomarkers of exposure only at birth rather than throughout pregnancy, lack of an ideal biomarker of exposure or effect, and the potential influence of other risk factors on the measured neurological outcomes, have been extensively discussed in critical reviews published earlier (Eaton et al. 2008; Reiss et al. 2015). However, some findings strongly indicate that the association between cord blood levels of CPF and neurodevelopmental outcomes cannot be ignored. First, following the government-mandated ban of residential CPF use in 2001, decreased use of CPF was accompanied by significant decreases in CPF levels in both breathing air samples and cord blood. Specifically, maternal personal air samples collected over 48 h during the third trimester of pregnancies in 1999 and 2002 dropped from 17.2 to 4.8 ng/m3 (Whyatt et al. 2005). Likewise, CPF levels in umbilical cord blood sampled from deliveries in 1999 and 2002 dropped from 6.9 to 1.3 pg/g (Whyatt et al. 2005). In parallel, the significant inverse correlations between CPF levels in umbilical cord plasma and birth weight and length observed in a cohort of children born before the mandated ban were not detected in a cohort of children born after the ban (Whyatt et al. 2005). The monotonic relationships traced between cord blood levels of CPF and neurological or morphological outcomes in these and other studies support the notion that a biological gradient exists between the level of CPF exposure and the biological response (Whyatt et al. 2005; Rauh et al. 2006, 2012). Second, infants born after the ban had significantly better Mental Development Index and Psychomotor Development Index scores than those born before the ban (Rauh et al. 2006).
Developmental toxicity also has been observed in children born to mothers who are exposed during gestation to levels of OP insecticides that cause marked inhibition of serum cholinesterase. Specifically, Farahat et al. (2016) compared the birth outcomes of pregnant women who lived in close proximity to or worked in agricultural fields in the Menoufia governorate in Egypt to those of pregnant women living in the governorate capital. The activity of serum cholinesterase measured in blood collected at 20–22 weeks of pregnancy from women in the rural cohort was approximately 73% lower than that measured in blood collected from women in the urban cohort. Gestational age at delivery was shorter in the rural cohort compared to the urban cohort. In addition, birth weight was lower and head circumference was smaller in the rural than in the urban cohort. In the rural cohort, there was a statistically significant correlation between serum cholinesterase activity and reduced gestational age at delivery, low birth weight, and small head circumference. Additional studies with larger samples, analyses of both serum cholinesterase and red blood cell (RBC) AChE activities at multiple time points during pregnancy, and clinical follow up of children’s neurodevelopment will help to establish the health risks for pregnant women at risk of exposure to cholinesterase-inhibiting levels of OP insecticides. This is especially critical for agricultural communities in the developing world, where use of these insecticides is not well regulated.
Given the inherent limitations of epidemiological studies, controlled preclinical studies using translationally relevant animal models can be extremely useful to trace cause-consequence relationships between exposures to specific toxicants and health outcomes. They can also play a critical role in the identification of mechanisms that contribute to the pathological conditions triggered by a given toxicant, and lay the groundwork for the discovery of potential interventions to mitigate the health issues resulting from an exposure to that toxicant.
Preclinical assessment of the developmental neurotoxicity of CPF in rats and mice
Studies of different rodent models have provided evidence supporting the notion that subacute exposures of the developing mammalian brain to CPF result in significant neurobehavioral alterations, including cognitive deficits and locomotor impairments.
Developmental subacute exposure of mice and rats to CPF results in an increase, a decrease, or no change in locomotor activity. The outcome is dependent upon the CPF dose, the age of the animals when they are tested, the time of exposure, and the animal species, among other factors (see Table 1). For instance, Dam et al. (2000) reported that male rats exposed on postnatal day (PND) 1–4 to CPF [1 mg/kg/day, subcutaneous (s.c.)] and tested in open fields on PND21 and PND30 presented lower locomotor activity than sex- and age-matched rats exposed to vehicle. Rats injected with CPF (1 mg/kg/day, s.c.) on PND1–4 presented no change in locomotor activity when tested in the figure 8 apparatus on PND35 (Levin et al. 2001). Finally, an increase in locomotor activity was observed when rats that had been neonatally exposed to the same CPF dose regimen were tested in the elevated plus maze on PND52–53 (Aldridge et al. 2005). These results suggest that developmental subacute exposure to CPF triggers an age-dependent alteration in the locomotor activity of rats, with hypolocomotion in pre-adolescent ages giving way to hyperlocomotion in young adulthood. In fact, there is evidence in the literature that behavioral deficits induced by another OP insecticide – parathion – wax and wane with the age of rats (Levin et al. 2010). However, one cannot rule out that the different types of test apparatuses also influenced the outcomes of the studies.
Table 1.
Locomotor phenotypes observed following developmental exposure of mice and rats to CPF
| CPF dose regimen | Vehicle | Species/strain | Time of exposure | Testing age | Type of test | Sex tested | Locomotor activitya | Rate of habituation | Sex affected | Brain AChE inhibition 24 h after last dose (%)b | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 or 5 mg/kg/day; s.c. | DMSO | SD rats | GD17–20 | PND35 | Figure-8 | M/F | ↔ | ↑ | NA | 1 mg/kg: NS 5 mg/kg: 45%c |
1 |
| 3 or 6 mg/kg/day; p.o. | Peanut Oil | CD-1 mice | GD15–18 | PND120 | Social recognition | F | ↔ | NR | NA | 3 mg/kg: NS 6 mg/kg: 40% |
2 |
| 3 or 6 mg/kg/day; p.o. | Peanut Oil | CD-1 mice | GD15–18 | PND60 | Open field | M | ↑ (6 mg/kg) | NR | M | 3 mg/kg: NS 6 mg/kg: 40% |
3 |
| 1 or 3 mg/kg/day; s.c. | Peanut Oil | CD-1 mice | PND1–4 | PND25 | Open field | M/F | ↔ | NR | NA | NS | 4 |
| 1 mg/kg/day; s.c. | DMSO | SD rats | PND1–4 | PND35 | Figure-8 | M/F | ↔ | NR | NA | NR | 5 |
| 1 mg/kg/day; s.c. | DMSO | SD rats | PND1–4 | PND21 & 30 | Open field | M/F | ↓ | NR | M | 20–25%d | 6 |
| 1 mg/kg/day; s.c. | DMSO | SD rats | PND1–4 | PND35 | Figure-8 | M/F | ↑ | ↔ | M | 20–25%d | 7e |
| 1 mg/kg/day; s.c. | DMSO | SD rats | PND1–4 | PND52–53 | Plus Maze | M/F | ↑ | NR | M | 20–25%d | 8 |
| 5 mg/kg/day; s.c. | DMSO | SD rats | PND11–14 | PND21 & 30 | Open field | M/F | ↔ | NR | NA | 65%d | 6 |
| 5 mg/kg/day; s.c. | DMSO | SD rats | PND11–14 | PND35 | Figure-8 | M/F | ↔ | ↓ | M/F | NR | 5 |
| 1 or 3 mg/kg/day; s.c. | Peanut Oil | CD-1 mice | PND11–14 | PND25 | Open field | M/F | ↑ | NR | M/F | NS | 4 |
| 1 or 3 mg/kg/day; s.c. | Peanut Oil | CD-1 mice | PND11–14 | PND60 | Open field | M | ↑ (3 mg/kg) | NR | M | NS | 3 |
| 1 or 3 mg/kg/day; s.c. | Peanut Oil | CD-1 mice | PND11–14 | PND120 | Social recognition | F | ↔ | NR | NA | NS | 2 |
| 3 mg/kg/48 h; p.o. | Corn oil | SD rats | PND1–21 | PND10–30 | Open field | M/F | ↔ | NR | NA | NRf | 9g |
AChE, acetylcholinesterase; CPF, chlorpyrifos; GD, gestation day; NA, not applicable; NR, not reported; NS, not significant; SD, Sprague–Dawley; s.c., subcutaneous; p.o., per os (oral gavage); PND, postnatal day; M, male; F, female.
References:
Locomotor activity refers to the total distance traveled or the number of photobeam breaks in specific apparatuses.
Data available for earlier time points are discussed in the paper.
Degree of brain AChE inhibition is reported in Qiao et al., 2002c and Song et al., 1997d.
In this study, dams received one s.c. injection of vehicle per day between GD17 and 19.
AChE activity in different brain regions was inhibited by approximately 30% on PND20 and 25% on PND25.
In this study, male and female rats born from dams exposed to higher doses of CPF (escalating from 3 mg/kg on PND1 to either 6 mg/kg or 12 mg/kg on PND21) presented reduced locomotor activity on PND 25 and PND30.
In 2014, Levin and collaborators reported that 35-day-old male rats that were exposed to CPF (1 mg/kg/day, s.c.) on PND1–4 exhibited higher locomotor activity than their control counterparts when tested in the figure 8 apparatus. The CPF dose regimen, the rat strain, the age at which the animals were tested, and the testing apparatus in this study were the same as those in the 2001 study, in which the authors reported that the locomotor activity of 35-day-old rats had not been impacted by the neonatal CPF exposure. The apparent discrepancy could be reconciled by the fact that offspring in the 2014 study were born to dams that had been injected with saline once a day between gestation days (GD) 17 and 19. Results obtained from CPF-exposed offspring of rats injected with saline during pregnancy were compared to those obtained from offspring of dams that had also been injected with saline. Therefore, potential effects of the prenatal injections were controlled for. However, potential interactions between the neonatal injections of CPF and the stress imparted by the prenatal injections of saline were not. Aldridge et al. (2005) reported that neonatal rats exposed to CPF present hyperlocomotion in young adulthood. Thus, it is possible that the stress induced by the prenatal saline injections could have precipitated the early development of hyperlocomotion in the CPF-exposed rats.
The alterations observed in the locomotor activity of mice and rats subjected to different developmental CPF exposures do not correlate well with the degree of brain AChE inhibition measured at 24 h after the last dose (see Table 1). Because AChE activity in the developing brain can quickly recover between repeated exposures to CPF, in part because of the rapid turnover of the enzyme, measurement of enzyme inhibition 24 h after the last dose underestimates the actual degree of inhibition induced by CPF (Lassiter et al. 1998). Thus, in their study, Dam et al. (2000) measured AChE activity in different brain regions 2 and 4 h after rats were exposed to CPF (1 mg/kg/day, s.c.) on PND1. Brain AChE was inhibited by approximately 60% among males and 20% among females. This higher degree of brain AChE inhibition 2–4 h after the administration of the first dose of CPF in males than females could have affected the brain development of males more pronouncedly such that only males presented reduced locomotor activity when tested at 21 or 30 days of age in open fields (Dam et al. 2000). However, it remains unclear whether AChE inhibition is in fact the main driving mechanism of the developmental neurotoxicity of CPF, because female but not male rats presented righting reflex deficits and impaired negative geotaxis when tested at PND5–8 (Dam et al. 2000).
Preclinical studies have also reported that developmental subacute exposure of rats and mice to CPF, in different vehicles and through different routes of administration, results in spatial learning and memory deficits that are sexually dimorphic (Table 2). Whether the impairments are more pronounced in males or females depends on the time at which the animals are exposed to CPF. In general, subacute exposure of rats and mice to CPF exclusively during the prenatal period results in cognitive deficits that are more pronounced among females than males (Levin et al. 2002; Haviland et al. 2010). In contrast, cognitive deficits resulting from neonatal (with or without prenatal) subacute exposure of rats to CPF are more pronounced among males than females (Levin et al. 2001; Aldridge et al. 2005; Johnson et al. 2009; Gómez-Giménez et al. 2017).
Table 2.
Cognitive phenotypes observed following developmental exposure of mice and rats to CPF
| CPF dose regimen | Vehicle | Species/strain | Time of exposure | Testing age | Type of test | Sex tested | Spatial learning | Memory | Sex affected | Brain AChE inhibition 24 h after last dose (%) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 or 5 mg/kg/day; s.c. | DMSO | ND4 mice | GD17–20 | PND60 | Foraging maze | M/F | ↓ | ↓ | F | NR | 1 |
| 1 or 5 mg/kg/day; s.c. | DMSO | SD rat | GD17–20 | Young adulthood | 16-arm radial maze | M/F | ↓ | ↓ | F | NR | 2 |
| 1 mg/kg/day; s.c. | DMSO | SD rats | PND1–4 | PND64 | 16-arm radial maze | M/F | ↓ | ↓ | Ma | 20–25%b | 3 |
| 0.1, 0.3, or 1 mg/kg/day; oral diet | Corn Oil and sweet jelly | Wistar rats | GD7 through lactation (PND21) | PND60–90 | MWM 8-arm radial maze | M/F | ↓ | ↓ | Ma | NR | 4 |
| 1, 4, or 6 mg/kg/dayc; p.o. | Corn Oil | SD rats | PND1–21 | PND36–60 | 12-arm radial maze | M/F | ↓ | ↓ | M | NRd | 5 |
| 1 mg/kg/day; s.c. | DMSO | SD rats | PND1–4 | Young adulthood | 16-arm radial maze | M/F | ↓ | ↓ | M | 20–25%a | 6 |
| 0.3, 1, or 5 mg/kg/day; p.o. | Corn Oil | SD rats | GD6 through lactation (PND10) | PND22–24 PND 61–90 | T-maze delayed spatial alternation | M/F | NA | ↔e | NA | NRf | 7 |
| 1 or 5 mg/kg/day, s.c. | DMSO | ICR mice | GD13–17 | PND45–60 | T-maze delayed spatial alternation | M/F | NA | ↓g | M | NR | 8 |
AChE, acetylcholinesterase; CPF, chlorpyrifos; GD, gestation day; NA, not applicable; NR, not reported; NS, not significant; SD, Sprague–Dawley; s.c., subcutaneous; p.o., per os (oral gavage); PND, postnatal day; MWM, Morris water maze; M, male; F, female.
References: Haviland et al. (2010); Levin et al. (2002); Aldridge et al. (2005); Gómez-Giménez et al. (2017); Johnson et al. (2009); Levin et al. (2001); Maurissen et al. (2000); Chen et al. (2012).
Performance of female rats exposed to the highest CPF dose was significantly better than that of control females in the radial mazes.
Degree of brain AChE inhibition is reported in Qiao et al., 2002.
The medium and high doses were escalated to starting from the lower dose of 1 or 1.5 mg/kg, respectively, and doubling it on PND6 and again on PND14.
On PND20, hippocampal AChE was inhibited by 14%, 50%, and 53% among rats that had been exposed to the low, medium, and high CPF dose, respectively.
Outcome measured: % correct first choice.
On GD20, maternal brain AChE activity was inhibited by approximately 18% and 90% in the groups that had been exposed to the medium and the high CPF dose, respectively.
Outcomes measured: % correct first choices; mean number of win-shift errors; mean number of lose-shift errors. Outcome affected: mean number of lose-shift errors.
In one study, rat pups born from dams continuously exposed to CPF (0.3–5 mg/kg/day, p.o. gavage) during gestation and lactation presented no learning or memory deficits (Maurissen et al. 2000). One cannot rule out that those results could have been confounded by the rapid and robust effects that gavage has on stress-related responses (Balcombe et al. 2004) and the long-lasting effects of maternal stress on cognitive functions of offspring (Richetto and Riva 2014). However, even more importantly, the battery of behavioral tests used by Maurissen et al. may not have been sufficiently sensitive to detect the cognitive domain(s) affected by CPF. For instance, the authors concluded that gestational followed by lactational exposure of rats to CPF had no effect on spatial memory, because it did not reduce the percentage of correct choices pre-adolescent and adult rats made in a spatial delayed alternation task in a T-maze. This is a classic spatial working memory task in which rats are required to remember the spatial location of a reward, in this study a food pellet, within a short delay (Tsutsui et al. 2016). Using the same task, Chen et al. (2012) also reported that the percentage of correct choices made by adult mice prenatally exposed to CPF (1 or 5 mg/kg/day, s.c.; GD13–17) was not significantly different from that made by control mice. However, Chen et al. introduced a correction procedure in to the task. If the animals made an error choice, they were given a chance to shift their selection in consecutive trials during which the same arm was kept baited until the animals made a correct choice. A win-shift error was counted every time the animals did not shift their choice after they had selected a correct arm in the previous trial. On the other hand, a lose-shift error was counted if the animals repeated an incorrect choice made in the previous trial. While the inability to use win-shift failures is associated with working memory deficits, the inability to use lose-shift strategies is suggestive of perseverative behavior or executive function deficits (Zhang et al. 2013). The prenatal CPF exposure had no effect on the percentage of win-shift errors. However, it did increase the percentage of lose-shift errors made by the mice in the task (Chen et al. 2012). Since these results suggest that cognitive deficits induced by prenatal exposure are function-specific, the negative results reported by Maurissen et al. have to be interpreted with a great deal of caution.
Translational relevance of preclinical models of the developmental neurotoxicity of OP insecticides
The advancement of research in the field of developmental OP neurotoxicity rests on the use of models carefully selected for: (i) elucidation of endpoints of toxicity and their dose–response relationships, (ii) identification of the toxicodynamics of the different OP insecticides, and (iii) characterization of mechanisms of action underlying the developmental neurotoxicity of these insecticides. Since all models are approximations, selecting an appropriate model for research is indeed a challenging task and must be driven by the test hypothesis and scientific rationale, while considering humane and ethical endpoints.
Undoubtedly, research conducted in rats and mice in the past decades has critically increased our understanding of the sensitivity of the developing mammalian brain to the neurotoxic effects of CPF and other OP insecticides. These animal models present a number of advantages to toxicological research, including: (i) wide availability, (ii) manageable handling, maintenance, and breeding, (iii) large litter sizes, (iv) validated neurobehavior assays, and (v) well-established genomic sequences that facilitate genetic manipulations. However, as discussed in the following paragraphs, there are limitations associated with these models that can be overcome by the use of additional models.
Rats and mice are notoriously unsuitable to high-throughput large-scale screening of large numbers of toxicants. Animal models such as the zebrafish are generally used for this purpose. In fact, methods are being developed and validated for the use of zebrafish in the characterization of the persisting neurobehavioral impairments caused by developmental exposure to CPF and other OP insecticides (Eddins et al. 2010; Richendrfer and Creton 2015).
There is also a shift in the temporal brain development of rats and mice compared to humans (Dobbing and Sands 1970, 1973). While at birth humans display advanced neural and perceptual development, rats and mice are comparatively immature (Dobbing and Sands 1979). Based on brain growth spurt and development of the GABAergic system in the brain, rat postnatal days (PNDs) 2–7 equate to the human fetal age during the third trimester of pregnancy (Clancy et al. 2007 and refs. therein). Consequently, in utero exposure of rats and mice to toxicants does not target the same developmental stages of the brain as those targeted by in utero exposure of humans. This has been carefully taken into account in the assessment of the developmental neurotoxicity of CPF and other toxicants in mice and rats. To target the period of brain development that corresponds to the last trimester of human pregnancy, researchers expose rats during the first postnatal week to the toxicants of interest. As such, potential effects that interactions between the toxicants and the placenta would have on the developing fetus in the last trimester of human pregnancy are missed.
The structure of the human placenta is also quite distinct from that of the rat and mouse placenta. While in humans the placenta is hemomonochorial, that is, it has a single layer of trophoblasts, in rats and mice the placenta is hemotrichorial, that is, it has a triple trophoblastic layer, making it difficult to compare placental transfer of chemicals in humans to that in rats and mice (Carter 2007).
Finally, while rats and mice have high levels of circulating carboxylesterases, which metabolically inactivate OP compounds, humans have low levels of these enzymes (de Jong et al. 1993). Since age-related differences in sensitivity of rats to CPF have been correlated with age-dependent expression of these enzymes (Benke and Murphy 1975; Moser et al. 1998), the age- and species-dependent expression of carboxylesterases need to be taken into account in physiologically based pharmacokinetic and pharmacodynamics models developed to convert biologically active doses of CPF from rats and mice of various ages to humans.
It is in this context that the guinea pig emerges as a potentially useful animal model to address specific questions related to the developmental neurotoxicity of OP insecticides. First, brain growth spurt in humans and guinea pigs is predominantly a prenatal event (Dobbing and Sands 1970, 1973). Second, once developed, the overall brain structure of guinea pigs is remarkably similar to that of humans, particularly in limbic regions that are known to play a critical role in cognitive processing, including the hippocampus, and in the Circle of Willis, an arterial polygon that sits at the base of the brain and supplies blood to the brain and surrounding structures (Librizzi et al. 1999). Third, the sensitivity of guinea pigs to OP compounds is also more similar to that of non-human primates and humans than to that of rats and mice (see Pereira et al. 2014 and references therein), in part because levels of circulating carboxylesterases are markedly lower in guinea pigs than in rats or mice. Finally, the guinea pig has a hemomonochorial placenta with a fetal/maternal transport barrier very similar to that of the human placenta (Mess 2007). Therefore, the guinea pig can be a valuable model for elucidating the involvement of the placenta in regulating the neurotoxicity of OP insecticides in the developing organism. They can also be suitable to aid in the translation of biologically active doses of CPF to humans and in the identification of medical interventions that used during pregnancy can effectively prevent the neurotoxic effects of the insecticide during the prenatal brain growth spurt.
Developmental neurotoxicity of CPF in guinea pigs
In recent years, our laboratory has assessed the developmental toxicity of CPF in guinea pigs (Mullins et al. 2015; Mamczarz et al. 2016). In those studies, guinea pigs were exposed in utero to CPF (25 mg/kg/day formulated in peanut oil, 10 days, starting on approximate GD53–55) during the period of brain growth spurt and rapid brain myelination (Dobbing and Sands 1970).
The CPF dose regimen used in the guinea pig study captured important features associated with occupational OP exposures of humans. First, the daily s.c. injections of the guinea pigs were intended to recapitulate the repetitive nature of the human occupational exposures (Farahat et al. 2011). Second, the s.c. route was used to approximate the slow sustained release of CPF from the dermal route, a prevalent route of occupational human exposures (Cattani et al. 2001; Fenske et al. 2012). Third, the dose of CPF injected in the pregnant guinea pigs was selected to model a scenario in which occupational human exposure to levels that produce no overt signs of acute toxicity may be presumed safe.
Although CPF-exposed guinea pig dams presented no clinical signs of acute OP intoxication, they had approximately 75% lower AChE activity in RBC than control animals at the time of delivery. As mentioned earlier, there are several reports that RBC AChE activity can be reduced by as much as 40–80% from baseline in workers who otherwise present no overt sign of OP intoxication (Ames et al. 1989; Lakew and Mekonnen 1998; Ohayo-Mitoko et al. 1999; Farahat et al. 2011; Singleton et al. 2015). Thus, the CPF dose regimen administered to the pregnant guinea pigs translates into levels that can be experienced by workers who handle OP insecticides throughout the world.
As reported in Mamczarz et al. (2016), on the day they were born, guinea pigs prenatally exposed to CPF (25 mg/kg/day, s.c., 10 days starting on ~ GD52) had significantly lower RBC AChE and plasma butyrylcholinesterase activities than their control counterparts. This finding demonstrated that CPF crossed the placenta and reached the fetuses. Brain butyrylcholinesterase was also markedly inhibited in different brain regions of CPF-exposed pups, indicating that CPF crossed the fetal blood–brain barrier as well. However, AChE activities in different brain regions of CPF-exposed pups were not significantly different from those measured in control offspring (Mamczarz et al. 2016).
The degree of RBC AChE inhibition following continued exposure to OP compounds is a result of cumulative inhibition of the enzyme in the RBCs. As such, it does not necessarily reflect the degree of AChE inhibition in tissues, where recovery of AChE activity between exposures is a result of both reactivation of the inhibited enzyme and synthesis of new enzyme (Mason 2000). Since RBCs do not have a nucleus and, therefore, do not synthesize proteins, RBC AChE activity is only recovered when new RBCs come into circulation, and the half-life of RBCs is approximately 120 days (D’Alessandro et al. 2010). In the brain, on the other hand, the half-life of AChE is approximately 2–3 days (Wenthold et al. 1974). However, the possibility cannot be ruled out that AChE is not significantly inhibited in the brain of the CPF-exposed offspring, because CPF and/or CPF-oxon can interact with high affinity with molecular targets other than AChE in the brain (Terry 2012).
Cholinergic activity in the hippocampus controls locomotor activity and habituation (Izquierdo et al. 1992; Leussis and Bolivar 2006). Thus, the finding that neither locomotor activity nor locomotor habituation was affected by the prenatal CPF exposure could be explained, at least in part, by the finding that AChE was not significantly inhibited in the brain of the guinea pigs (Mamczarz et al. 2016). Sprague–Dawley rats tested at different ages after being exposed to CPF (5 mg/kg/day, s.c.) between PND 11 and 14 or (3 mg/kg/48 h, p.o.) between PND1 and 21 also presented no changes in locomotor activity (Dam et al. 2000; Carr et al. 2001; Levin et al. 2001). It is important to note that the ages at which the rat pups were exposed to CPF in these studies covered a period of brain growth spurt comparable to that experienced by fetal guinea pigs during the last third portion of gestation (Dobbing and Sands 1970). In addition, the CPF dose regimens administered to the rats in those studies resulted in variable degrees of total cholinesterase inhibition in the brain that did not exceed 35% 1 day before and 20% 1 day after the last CPF dose (Dam et al. 2000; Carr et al. 2001).
To assess the impact of prenatal exposure to CPF on cognitive functions, the guinea pigs were subjected to the classical non-cued version of the Morris water maze. Starting on approximate PND38, guinea pigs were trained to escape onto a hidden platform during five consecutive trial days. Among control animals, time to escape onto the hidden platform (hereafter referred to as escape latency) and distance to reach the platform significantly decreased with increased number of training days, with control male outperforming control female guinea pigs (Mamczarz et al. 2016). Both male and female guinea pigs prenatally exposed to CPF presented learning deficits in this task; they swam longer distances and times to escape onto the hidden platform (Fig. 1). However, CPF-exposed male guinea pigs were more severely affected than their female counterpart, such that the task was no longer sexually dimorphic among guinea pigs prenatally exposed to the insecticide (Mamczarz et al. 2016).
Fig. 1.
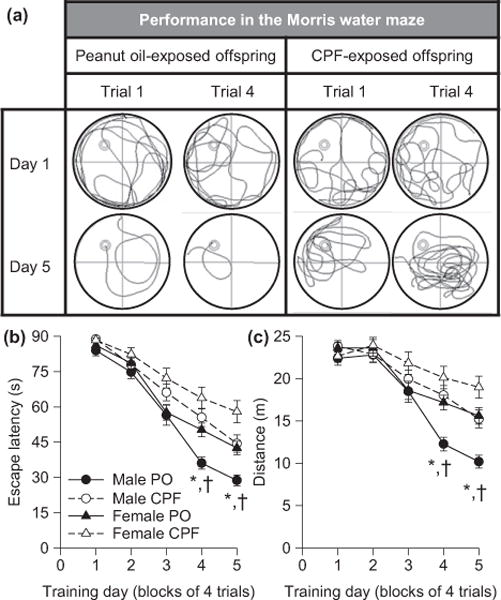
Learning deficits presented by guinea pigs prenatally exposed to chlorpyrifos (CPF). Learning performance of offspring born to pregnant guinea pigs that had been injected with peanut oil (PO) or with CPF (25 mg/kg/day for 10 days starting on approximate GD53–55) was examined in the Morris water maze, as described in Mamczarz et al. (2016). (a) Swim paths of a vehicle (peanut oil)-exposed and a CPF-exposed male offspring on the 1st and 4th trials of the first and last days of training to find the hidden platform in the Morris water maze. The swim path of the male guinea pig prenatally exposed to peanut oil became shorter with training as the animal learned to use the contextual cues to find the platform. In contrast, the swim path of the male prenatally exposed to CPF did not improve substantially with training. (b and c) Graphs show mean escape latency (b) and distance (c) traveled by CPF- and peanut oil (PO)-exposed offspring per training day. Results are presented as mean ± standard error of the mean. A random effect ANOVA model revealed that: (i) among control animals, learning performance was sex dimorphic, with performance being better among control males than control females, and (ii) prenatal exposure to CPF impaired learning of male and female offspring, with the effect being more pronounced among males. According to Tukey– Kramer post hoc test for pairwise comparisons: *p < 0.05 PO males versus CPF males; †p < 0.05 PO males versus PO females. Details of the analysis are provided in the article by Mamczarz et al. (2016), from which this figure was adapted.
After completion of the behavioral tests, the structural integrity of the brain of female offspring that had been prenatally exposed to CPF or peanut oil was analyzed by means of in vivo magnetic resonance imaging methods that included T2-weighted images and diffusion kurtosis imaging, as described in Mullins et al. (2015). That study revealed that prenatal exposure of female guinea pigs to CPF resulted in a significant reduction of brain volume, specifically in the frontal brain regions that included the striatum. It also provided evidence that, compared to control age- and sex-matched offspring, female offspring prenatally exposed to CPF presented in the striatum, amygdala, and corpus callosum: (i) decreased fractional anisotropy, and (ii) increased mean and radial diffusivity (Fig. 2). These results led to the hypothesis that prenatal exposure of female guinea pigs to CPF disrupts the axonal integrity and/or results in demyelination within the striatum, amygdala, and corpus callosum. In support of this hypothesis was the finding that the intensity of Luxol Fast Blue staining, which has been used to clarify the role of myelination in various disease states (Deshmukh et al. 2013), was significantly reduced in the lateral amygdala following the prenatal CPF exposure (Mullins et al. 2015).
Fig. 2.
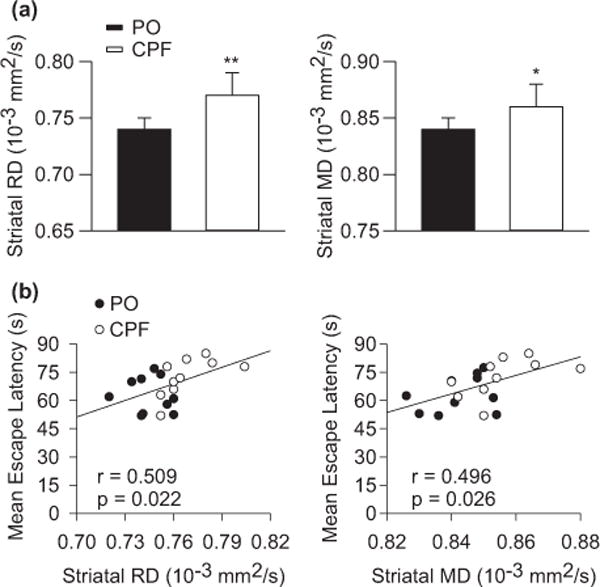
Diffusion kurtosis imaging (DKI) parameters (mean diffusivity, MD and radial diffusivity, RD) obtained from the striatum of female guinea pigs prenatally exposed to chlorpyrifos (CPF) or peanut oil and correlation of these parameters with the learning performance of the animals. (a) Mean striatal RD and MD measured from female guinea pigs born to dams exposed to peanut oil or CPF (25 mg/kg/day for 10 days starting on approximate gestation day 53–55). Results are presented as mean and standard error of the mean. *p < 0.05; **p < 0.01. (b) Scatterplots of the correlation between striatal DKI measures and mean escape latency measures of CPF- and peanut oil-exposed offspring. Filled circles are data from CPF-exposed offspring, whereas open circles are data from peanut-oil exposed offspring. Details on the statistical analysis are provided in the article by Mullins et al. (2015), from which this figure was adapted.
Diffusion kurtosis imaging measures obtained from the striatum, amygdala, and corpus callosum were significantly correlated with the performance of the guinea pigs in the Morris water maze (Mullins et al. 2015; see also Fig. 2). These correlations do not establish a cause-consequence relationship between the structural brain damage and the behavioral impairment presented by the guinea pigs exposed prenatally to CPF. However, they align well with reports that lesions in the striatum, amygdala, and corpus callosum result in cognitive deficits in laboratory animals and humans (Block et al. 1993; Sauerwein and Lassonde 1994; Galliot et al. 2010; Chida et al. 2011).
The findings that guinea pigs subjected to prenatal subacute exposure to CPF presented spatial learning deficits that were sexually dimorphic and correlated with disruption of the structural integrity of different brain regions are in line with reports that: (i) correlations between prenatal CPF exposure and cognitive deficits in children are generally stronger among boys than girls (Marks et al. 2010; Horton et al. 2012; Rauh et al. 2012), and (ii) normal correlations between FSIQ and the surface area of different brain regions were either absent or reversed among children, particularly boys, who experienced prenatal exposures producing cord blood levels of CPF ≥ 4.39 ng/g (Rauh et al. 2012). However, it is important to note that the CPF dose regimen administered to the pregnant guinea pigs caused marked inhibition of RBC AChE, whereas RBC AChE is not markedly inhibited following environmental exposures of humans to CPF. A dose–response relationship analysis is necessary to determine the dose dependence of the developmental neurotoxicity of CPF in the guinea pig model and establish the relevance of the model for human environmental exposures.
Perspectives on potential mechanisms underlying the developmental neurotoxicity of OP insecticides: emphasis on CPF
As mentioned earlier, irreversible inhibition of AChE in the peripheral and central nervous systems contributes to the cholinergic syndrome induced by an acute exposure to OP insecticides (reviewed in Pereira et al. 2014). However, as discussed here, several lines of evidence suggest that additional compound-specific mechanisms of action contribute not only to the acute toxicity of high doses of these insecticides but also to the neurotoxic effects that develop following continued low-level exposures, particularly in the developing brain.
Based on the notion that AChE is the primary molecular target accounting for the acute toxicity of OP insecticides, the LD50 of these insecticides should directly correlate with their IC50 to inhibit the enzyme and/or with the rate of reactivation of the inhibited enzyme. However, that is not always the case (e.g., Santhoshkumar et al. 1996). In addition, if AChE inhibition were the sole mechanism underlying the acute toxicity of OP compounds, mice with a null mutation in the gene that encodes AChE would be resistant to the toxicity of these chemicals. Instead, AChE−/− mice are more sensitive than wild-type mice to the acute toxicity of OP compounds, including CPF-oxon (Lockridge et al. 2005). In addition, while treatment of wild-type mice with the muscarinic antagonist atropine counters the acute toxicity of OP compounds, treatment of AChE−/− mice does not (Duysen et al. 2001).
Based on the assumption that AChE inhibition is a common mechanism underlying the developmental neurotoxicity of OP insecticides, one could also predict that exposure of a developing organism to any OP insecticide would trigger exactly the same effect with a biological gradient proportional to the degree of the inhibition of the catalytic enzyme activity. This is, however, not the case. Exposure of developing organisms to different OP insecticides triggers different effects. For instance, a statistically significant up-regulation of the serotonin (5HT) receptor subtypes 5HT1 and/or 5HT2 is observed in different brain regions of adult rats exposed neonatally to CPF (1 mg/kg/day, s.c.; PND1–4), with the effects being more pronounced among males than females (Aldridge et al. 2004). In contrast, a statistically significant down-regulation of 5HT1 receptors is noted in the brain of male (but not female) adult rats exposed neonatally to doses of the OP insecticide diazinon (0.5 mg/kg/day, s.c.; PND1–4) (Slotkin et al. 2008) that cause similar degree of AChE inhibition as that induced by CPF.
Numerous studies have demonstrated that CPF can directly interact with and change the activity of serine hydrolases, including carboxylesterases, muscarinic receptors, cannabinoid receptors, and such structural proteins as tubulin (reviewed in Jett and Lein 2006; Terry 2012). The paragraphs that follow discuss how some of these molecular interactions and hitherto unexplored mechanisms may contribute to the developmental neurotoxicity of CPF.
Some studies have proposed that disruption of the structural and functional integrity of the brain following exposure to low levels of CPF may be a result of disruption of axonal transport and outgrowth mediated by tubulin and related structural proteins (Howard et al. 2005; Prendergast et al. 2007; Grigoryan et al. 2008; Yang et al. 2008; Middlemore-Risher et al. 2011). This proposal was built upon the initial in vitro demonstration that CPF oxon, among other OP compounds, binds covalently to tubulin and disrupts tubulin polymerization, with 5–10 μM CPF-oxon decreasing and 25 μM CPF-oxon increasing microtubule length and density (Prendergast et al. 2007; Grigoryan et al. 2008, 2009). Subsequent studies demonstrated that organophosphorylated tubulin and disrupted microtubule structures could be detected in the brain of adult female mice treated with doses of CPF that did not cause significant AChE inhibition (3 mg/kg/day, 14 days, s.c.) (Jiang et al. 2010). Direct covalent binding of CPF to kinesin has also been proposed to explain the concentration-dependent inhibition by CPF (IC50 ≈ 9 μM) and CPF-oxon (IC50 ≈ 2 μM) of kinesin-dependent microtubule motility observed in vitro (Gearhart et al. 2007). One can hypothesize that disruption of axonal transport and outgrowth resulting from direct interactions of CPF and/or CPF-oxon with structural proteins in the developing brain can generate abnormal patterns of neuronal connectivity and, thereby, contribute to the neurobehavioral alterations reported to be associated with developmental exposure to CPF.
The work of Yang et al. (2008) demonstrated that, at concentrations that did not inhibit the catalytic activity of AChE, CPF (1 nM) and CPF oxon (1 pM) inhibited axonal outgrowth in primary cultures of rat and mice DRG. The inhibitory effect of CPF or CPF oxon on axonal outgrowth was: (i) smaller in magnitude in primary DRG cultures from AChE+/− mice than in primary DRG from wild-type mice, (ii) absent in primary cultures of dorsal root ganglia of AChE−/− mice, and (iii) restored when primary DRG cultures from AChE−/− mice were transfected with the full-length wild-type AChE. Taken together these findings reveal that the ability of CPF and CPF oxon to inhibit axonal outgrowth: (i) is independent of their ability to block the catalytic activity of AChE, and (ii) cannot be solely explained by the direct interactions of CPF and/or CPF-oxon with structural proteins. They also suggest that CPF/CPF-oxon-induced inhibition of the morphogenic activity of AChE may contribute to the neurotoxicity of concentrations of the insecticide that are insufficient to inhibit the catalytic activity of the enzyme.
Previous studies demonstrated that, with IC50s of approximately 22 nM and 14 nM, CPF-oxon displaces binding of the m2 muscarinic receptor ligand [3H]-cismethyldioxolane from rat striatal membranes and of the cannabinoid receptor CB1 ligand [3H]CP559940 from mouse brain membranes, respectively (Huff et al. 1994; Quistad et al. 2002). While these binding studies suggested that CPF-oxon can directly interact with m2 and CB1 receptors, they did not elucidate whether the interaction leads to receptor activation or inhibition. In 2001, however, Olivier et al. (2001) reported that CPF-oxon blocked forskolin-induced cAMP production in cerebral cortical slices, and it did so more potently in slices from 7-day-old rats than from adult rats. Since the effect was only partially blocked by the non-selective muscarinic agonist atropine, CPF-oxon-induced suppression of cAMP signaling in the cerebral cortex may be mediated, at least in part, by the ability of CPF-oxon to interact with and activate m2 muscarinic receptors and CB1 receptors. During fetal life, CB1 receptor signaling regulates neural progenitor cell differentiation and guides axonal migration and synaptogenesis (reviewed in Fride et al. 2009). Thus, it remains to be determined whether CB1 is a molecular target that contributes to the developmental neurotoxicity of CPF.
In vivo studies have reported that changes in downstream signaling involving neurotrophins could contribute to the developmental neurotoxicity of CPF. A significant reduction in the expression of neurotrophins in the superfamily of fibroblast growth factor has been observed in the brainstem and forebrain of 5-day-old rats exposed to CPF (1 mg/kg/day, s.c.) between PND1 and PND4 (Slotkin et al. 2007). A transient reduction in the levels of nerve growth factor has also been noted in the forebrain of rat pups gavaged with CPF (1.5–3 mg/kg/day) between PND1 and PND6 (Betancourt and Carr 2004). Although the molecular mechanisms underlying these effects remain to be elucidated, CPF-induced down-regulation of fibroblast growth factor and nerve growth factor expression in the developing brain can certainly contribute to suppression of neurite outgrowth, cell differentiation, and neuronal repair, all of which are largely regulated by these neurotrophins (Rydel and Greene 1987; Limke et al. 2003; Bernd 2008).
Additional mechanisms that have been proposed to contribute to the developmental neurotoxicity of CPF and have been discussed in the literature include: (i) exacerbated oxidative stress (Crumpton et al. 2000; Jett and Navoa 2000; Slotkin and Seidler 2010), (ii) imbalanced intracellular Ca2+ homeostasis (Giordano et al. 2007), (iii) increased signaling mediated by inflammatory mediators, such as interleukins and cytokines (Tian et al. 2015), and (iv) increased activity/expression of protein kinases, including protein kinase C and mitogen-activated kinases (Slotkin and Seidler 2009; Zhang et al. 2015).
In more recent years, attention has been directed to epigenetic mechanisms, which play critical roles in the development of the nervous systems, as potential determinants of the etiology of neurological disorders resulting from exposure of the developing brain to toxicants such as heavy metals and OP insecticides (Senut et al. 2012; Kim et al. 2016). Among the epigenetic modifications most studied to date are DNA methylation, histone modifications, and non-coding RNAs (reviewed in Bannister and Kouzarides 2011; Esteller 2011; Roidl and Hacker 2014).
Exposure of pregnant mice to CPF-methyl (4, 20, 100 mg/kg/day, p.o.; GD7–12), an OP insecticide chemically related to but less acutely toxic than CPF, has been shown to cause a dose-dependent hypomethylation of the H19 gene in different organs of the fetuses on GD13 (Shin et al. 2015). Demethylation of the imprinting control region of this gene has been associated with intrauterine and postnatal growth retardation (Murphy et al. 2012). If exposure of developing fetuses to toxicologically relevant doses of CPF also leads to hypomethylation of the H19 gene, this mechanism could prove to be an important determinant of the developmental toxicity of this insecticide.
A recent study also demonstrated that in vitro exposure of proliferating and differentiating human neuronal progenitor cells to CPF induced a concentration-dependent hypermethylation of histone H3 on the lysine (K) 4 residue (H3K4) that became statistically significant at 57 μM (Kim et al. 2016). H3K4 methylation controls the expression of a number of pluripotency-associated genes during the differentiation of embryonic stem cells into neural stem cells and neurons (Roidl and Hacker 2014). Thus, it remains to be determined whether CPF-induced increased methylation of H3K4 is also observed following exposure of developing fetuses to CPF and, if so, whether it contributes to the developmental neurotoxicity of the insecticide.
No study has assessed whether the expression of non-coding RNAs is affected by exposure of the developing brain to CPF. However, an increased expression of the non-coding micro RNAs miRNA-132 and miRNA-212 has recently been observed in the hippocampi of young adult rats exposed for 21 days to doses of CPF (10 mg/kg/day, s.c.) that caused significant inhibition of brain AChE activity but were not sufficient to trigger overt signs of acute toxicity (Lee et al. 2016). These miRNAs play an important role in synaptogenesis, as indicated by the findings that: (i) miRNA-132 over-expression in forebrain neurons increases synaptic density and (ii) loss of miRNA-132/212 suppresses spine formation and reduces dendritic length and branching in newborn hippocampal neurons (Hansen et al. 2010; Magill et al. 2010). Therefore, if CPF were to induce up-regulation of these miRNAs in the developing brain as well, this mechanism could contribute to improper neuronal wiring that would culminate in neurological deficits later in life.
Another question that remains unexplored relates to whether potential effects of CPF in the placenta create an environment that is detrimental to the healthy development of the fetuses. In the placenta, a proper balance between a pro- and an anti-inflammatory environment, which is maintained in part by placental muscarinic and nicotinic receptors (Satyanarayana 1986; Paparini et al. 2015), is essential to nurture fetal growth. There is evidence that at high micromolar concentrations CPF and CPF-oxon disturb the redox balance in and induce apoptosis of human placental JEG-3 cells (Saulsbury et al. 2008; Chiapella et al. 2013). However, no study has assessed whether toxicologically relevant concentrations/doses of CPF impair the ability of the placenta to support normal fetal development and, if so, by what mechanism.
Conclusions
The epidemiological studies reviewed herein have reported statistically significant correlations between prenatal exposures to CPF and postnatal neurological complications, particularly cognitive deficits that are also associated with disruption of the structural integrity of the brain. Based on scientific evidence provided by these and other studies, the US EPA has given serious consideration to a potential ban of all uses of CPF in the United States. A major limitation of epidemiological studies, however, lies on the fact that they are generally not suitable to establish cause-consequence relationships between exposures and health outcomes. It is in this context that preclinical studies become extremely relevant. Various preclinical research groups throughout the world have consistently demonstrated that CPF is a developmental neurotoxicant. The developmental CPF neurotoxicity, which is well supported by studies using different animal models, routes of exposure, vehicles, and testing methods, is generally characterized by cognitive deficits and disruption of the structural integrity of the brain. Nevertheless, there is still controversy as to whether the effects observed in animal models can be extrapolated to humans exposed to low levels of CPF.
Researchers have argued that the doses of CPF reported to induce developmental neurotoxicity in animals are orders of magnitude greater than incidental environmental exposures in humans (Juberg 2012; Reiss et al. 2015). However, based on a fundamental principle of pharmacology and toxicology, the concentration of a xenobiotic at its site of action and the affinity of the xenobiotic for the molecular target(s) that mediate its effects are the primary drivers of the biological effect of that chemical. Although the concentration of a xenobiotic at its site of action correlates with the dose, this relationship is dependent upon the xenobiotics, pharmacokinetics, which is species specific. Many studies that attempted to translate rodent to human levels of CPF exposure did so on the basis of the pharmacokinetics of CPF in these species (reviewed in Eaton et al. 2008). Others have developed physiologically based pharmacokinetic and pharmacodynamic models that take into account the pharmacokinetics of CPF and use the ability of CPF to block AChE in the different species as a measure of the pharmacodynamics of CPF (e.g., Poet et al. 2017). Unfortunately, as discussed in the previous section, it is unclear to what extent, if any, AChE inhibition contributes to the developmental neurotoxicity of CPF. In addition, as discussed in the previous section, there is evidence that CPF interacts directly with and changes the activity of non-AChE targets. The challenge is to determine the contribution of these targets to the developmental neurotoxicity of CPF. Therefore, the use of AChE inhibition as the pharmacodynamic parameter in physiologically based pharmacokinetic and pharmacodynamic models is likely to result in inaccurate translation of animal to human doses of CPF (or vice-versa). Until the mechanism(s) underlying the developmental neurotoxicity of CPF is(are) identified, a comparison of the CPF effects (rather than doses) across different species seems to be more appropriate. Such comparison may lead to the identification of a biomarker of effect that, together with a biomarker of exposure, will more accurately guide the human health risk assessment for CPF.
Undoubtedly, addressing the questions that remain unanswered regarding the developmental neurotoxicity of CPF is critically needed to provide the basis for the creation and enforcement of programs to better monitor and control the agricultural, industrial, and domestic use and handling of CPF throughout the world.
Acknowledgments
This work was supported by the NIH grant ES019282.
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- BChE
butyrylcholinesterase
- CPF
chlorpyrifos
- DKI
diffusion kurtosis imaging
- DMSO
dimethyl sulfoxide
- EPA
Environmental Protection Agency
- FA
fractional anisotropy
- FGF
fibroblast growth factor
- GD
gestation day
- H3K4
lysine 4 of histone 3
- MD
mean diffusivity
- miRNA
micro RNA
- MRI
magnetic resonance imaging
- NGF
nerve growth factor
- OP
organophosphorus
- p.o
per os
- PND
postnatal day
- RBC
red blood cell
- RD
radial diffusivity
- s.c
subcutaneous
- TCPY
3,5,6-trichloro-2-pyridinol
Footnotes
Conflict of interest disclosure
The authors have no conflict of interest to declare.
References
- Abdel-Rahman A, Blumenthal G, Abou-Donia S, Ali F, Abdel-Monem A, Abou-Donia M. Pharmacokinetic profile and placental transfer of a single intravenous injection of [14C] chlorpyrifos in pregnant rats. Arch Toxicol. 2002;76:452–459. doi: 10.1007/s00204-002-0366-2. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames RG, Brown SK, Mengle DC, Kahn E, Stratton JW, Jackson RJ. Cholinesterase activity depression among California agricultural pesticide applicators. Am J Ind Med. 1989;15:143–150. doi: 10.1002/ajim.4700150203. [DOI] [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke GM, Murphy SD. The influence of age on the toxicity and metabolism of methyl parathion and parathion in male and female rats. Toxicol Appl Pharmacol. 1975;31:254–269. doi: 10.1016/0041-008x(75)90161-1. [DOI] [PubMed] [Google Scholar]
- Bernd P. The role of neurotrophins during early development. Gene Expr. 2008;14:241–250. doi: 10.3727/105221608786883799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt AM, Carr RL. The effect of chlorpyrifos and chlorpyrifos-oxon on brain cholinesterase, muscarinic receptor binding, and neurotrophin levels in rats following early postnatal exposure. Toxicol Sci. 2004;77:63–71. doi: 10.1093/toxsci/kfh003. [DOI] [PubMed] [Google Scholar]
- Block F, Kunkel M, Schwarz M. Quinolinic acid lesion of the striatum induces impairment in spatial learning and motor performance in rats. Neurosci Lett. 1993;149:126–128. doi: 10.1016/0304-3940(93)90752-7. [DOI] [PubMed] [Google Scholar]
- Bouvier G, Seta N, Vigouroux-Villard A, Blanchard O, Momas I. Insecticide urinary metabolites in nonoccupationally exposed populations. J Toxicol Environ Health. 2005;8:485–512. doi: 10.1080/10937400591007284. [DOI] [PubMed] [Google Scholar]
- Buckley NA, Roberts D, Eddleston M. Overcoming apathy in research on organophosphate poisoning. BMJ. 2004;329:1231–1233. doi: 10.1136/bmj.329.7476.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Chambers HW, Guarisco JA, Richardson JR, Tang J, Chambers JE. Effects of repeated oral postnatal exposure to chlorpyrifos on open-field behavior in juvenile rats. Toxicol Sci. 2001;59:260–267. doi: 10.1093/toxsci/59.2.260. [DOI] [PubMed] [Google Scholar]
- Carter AM. Animal models of human placentation–a review. Placenta. 2007;28(Suppl. A):S41–S47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Cattani M, Cena K, Edwards J, Pisaniello D. Potential dermal and inhalation exposure to chlorpyrifos in Australian pesticide workers. Ann Occup Hyg. 2001;45:299–308. [PubMed] [Google Scholar]
- Chen XP, Chen WZ, Wang FS, Liu JX. Selective cognitive impairments are related to selective hippocampus and prefrontal cortex deficits after prenatal chlorpyrifos exposure. Brain Res. 2012;1474:19–28. doi: 10.1016/j.brainres.2012.07.036. [DOI] [PubMed] [Google Scholar]
- Chiapella G, Flores-Martín J, Ridano ME, Reyna L, Magnarelli de Potas G, Panzetta-Dutari GM, Genti-Raimondi S. The organophosphate chlorpyrifos disturbs redox balance and triggers antioxidant defense mechanisms in JEG-3 cells. Placenta. 2013;34:792–798. doi: 10.1016/j.placenta.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Chida Y, Kokubo Y, Sato S, Kuge A, Takemura S, Kondo R, Kayama T. The alterations of oligodendrocyte, myelin in corpus callosum, and cognitive dysfunction following chronic cerebral ischemia in rats. Brain Res. 2011;1414:22–31. doi: 10.1016/j.brainres.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJS. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Brain Res Dev Brain Res. 2000;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8:82–88. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res Dev Brain Res. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Deshmukh VA, Tardif V, Lyssiotis CA, et al. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502:327–332. doi: 10.1038/nature12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmani C, Jaga K. Epidemiology of acute organophosphate poisoning in hospital emergency room patients. Rev Environ Health. 2005;20:215–232. doi: 10.1515/reveh.2005.20.3.215. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Li B, Xie W, Schopfer LM, Anderson RS, Broomfield CA, Lockridge O. Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J Pharmacol Exp Ther. 2001;299:528–535. [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38(Suppl. 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, Furlong CE, Holland NT. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112:1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, et al. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Perspect. 2011;119:801–806. doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat T, Shaheen HM, Sanad Z, Farag NA. Organophosphate pesticide exposure during pregnancy and adverse perinatal outcome. J Womens Health Care. 2016;5:336. [Google Scholar]
- Fenske RA, Lu C, Barr D, Needham L. Children’s exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environ Health Perspect. 2002;110:549–553. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Farahat FM, Galvin K, Fenske EK, Olson JR. Contributions of inhalation and dermal exposure to chlorpyrifos dose in Egyptian cotton field workers. Int J Occup Environ Health. 2012;18:198–209. doi: 10.1179/1077352512Z.00000000030. [DOI] [PubMed] [Google Scholar]
- Fortenberry GZ, Meeker JD, Sánchez BN, et al. Urinary 3,5,6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: distribution, temporal variability, and relationship with child attention and hyperactivity. Int J Hyg Environ Health. 2014;217:405–412. doi: 10.1016/j.ijheh.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E, Gobshtis N, Dahan H, Weller A, Giuffrida A, Ben-Shabat S. The endocannabinoid system during development: emphasis on perinatal events and delayed effects. Vitam Horm. 2009;81:139–158. doi: 10.1016/S0083-6729(09)81006-6. [DOI] [PubMed] [Google Scholar]
- Galliot E, Levaillant M, Beard E, Millot J-L, Pourié G. Enhancement of spatial learning by predator odor in mice: involvement of amygdala and hippocampus. Neurobiol Learn Mem. 2010;93:196–202. doi: 10.1016/j.nlm.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol. 2007;218:20–29. doi: 10.1016/j.taap.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Giordano G, Afsharinejad Z, Guizzetti M, Vitalone A, Kavanagh TJ, Costa LG. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol Appl Pharmacol. 2007;219:181–189. doi: 10.1016/j.taap.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Gómez-Giménez B, Llansola M, Hernández-Rabaza V, Cabrera-Pastor A, Malaguarnera M, Agusti A, Felipo V. Sex-dependent effects of developmental exposure to different pesticides on spatial learning. The role of induced neuroinflammation in the hippocampus. Food Chem Toxicol. 2017;99:135–148. doi: 10.1016/j.fct.2016.11.028. [DOI] [PubMed] [Google Scholar]
- Grand View Research. Chlorpyrifos market analysis, market size, application analysis, regional outlook, competitive strategies, and forecasts, 2015 to 2022. 2014 http://www.grandviewresearch.com/industry-analysis/chlorpyrifos-market [accessed on February 16, 2016]
- Grigoryan H, Schopfer LM, Thompson CM, Terry AV, Masson P, Lockridge O. Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: a potential mechanism of long term toxicity by organophosphorus agents. Chem Biol Interact. 2008;175:180–186. doi: 10.1016/j.cbi.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan H, Schopfer LM, Peeples ES, Duysen EG, Grigoryan M, Thompson CM, Lockridge O. Mass spectrometry identifies multiple organophosphorylated sites on tubulin. Toxicol Appl Pharmacol. 2009;240:149–158. doi: 10.1016/j.taap.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell D, Eddleston M, Phillips MR, Konradsen F. The global distribution of fatal pesticide self-poisoning: systematic review. BMC Public Health. 2007;7:357. doi: 10.1186/1471-2458-7-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KF, Sakamoto K, Wayman GA, Impey S, Obrietan K. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS ONE. 2010;5:e15497. doi: 10.1371/journal.pone.0015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviland JA, Butz DE, Porter WP. Long-term sex selective hormonal and behavior alterations in mice exposed to low doses of chlorpyrifos in utero. Reprod Toxicol. 2010;29:74–79. doi: 10.1016/j.reprotox.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Horton MK, Kahn LG, Perera F, Barr DB, Rauh V. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicol Teratol. 2012;34:534–541. doi: 10.1016/j.ntt.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. 2005;207:112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Huen K, Bradman A, Harley K, Yousefi P, Boyd Barr D, Eskenazi B, Holland N. Organophosphate pesticide levels in blood and urine of women and newborns living in an agricultural community. Environ Res. 2012;117:8–16. doi: 10.1016/j.envres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff RA, Corcoran JJ, Anderson JK, Abou-Donia MB. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J Pharmacol Exp Ther. 1994;269:329–335. [PubMed] [Google Scholar]
- Izquierdo I, da Cunha C, Rosat R, Jerusalinsky D, Ferreira MB, Medina JH. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol. 1992;58:16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Jaga K, Dharmani C. Sources of exposure to and public health implications of organophosphate pesticides. Rev Panam Salud Publica. 2003;14:171–185. doi: 10.1590/s1020-49892003000800004. [DOI] [PubMed] [Google Scholar]
- Jamal GA, Hansen S, Julu PO. Low level exposures to organophosphorus esters may cause neurotoxicity. Toxicology. 2002;181–182:23–33. doi: 10.1016/s0300-483x(02)00447-x. [DOI] [PubMed] [Google Scholar]
- Jett DA, Lein PJ. Noncholinesterase mechanisms of central and peripheral neurotoxicity: Muscarinic receptors and other targets. In: Gupta RC, editor. Toxicology of Organophosphate & Carbamate Compounds. Elsevier Inc; Oxford, UK: 2006. pp. 233–245. [Google Scholar]
- Jett DA, Navoa RV. In vitro and in vivo effects of chlorpyrifos on glutathione peroxidase and catalase in developing rat brain. Neurotoxicology. 2000;21:141–145. [PubMed] [Google Scholar]
- Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol Sci. 2010;115:183–193. doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FO, Chambers JE, Nail CA, Givaruangsawat S, Carr RL. Developmental chlorpyrifos and methyl parathion exposure alters radial-arm maze performance in juvenile and adult rats. Toxicol Sci. 2009;109:132–142. doi: 10.1093/toxsci/kfp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong LP, van Dijk C, Berhitoe D, Benschop HP. Hydrolysis and binding of a toxic stereoisomer of soman in plasma and tissue homogenates from rat, guinea pig and marmoset, and in human plasma. Biochem Pharmacol. 1993;46:1413–1419. doi: 10.1016/0006-2952(93)90106-7. [DOI] [PubMed] [Google Scholar]
- Juberg DR. Differentiating experimental animal doses from human exposures to chlorpyrifos. Proc Natl Acad Sci USA. 2012;109:E2195. doi: 10.1073/pnas.1208081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Wegner SH, Van Ness KP, Park JJ, Pacheco SE, Workman T, Hong S, Griffith W, Faustman EM. Differential epigenetic effects of chlorpyrifos and arsenic in proliferating and differentiating human neural progenitor cells. Reprod Toxicol. 2016;65:212–223. doi: 10.1016/j.reprotox.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakew K, Mekonnen Y. The health status of northern Omo State Farm workers exposed to chlorpyrifos and profenofos. Ethiop Med J. 1998;36:175–184. [PubMed] [Google Scholar]
- Lassiter TL, Padilla S, Mortensen SR, Chanda SM, Moser VC, Barone S., Jr Gestational exposure to chlorpyrifos: apparent protection of the fetus? Toxicol Appl Pharmacol. 1998;152:56–65. doi: 10.1006/taap.1998.8514. [DOI] [PubMed] [Google Scholar]
- Lee YS, Lewis JA, Ippolito DL, Hussainzada N, Lein PJ, Jackson DA, Stallings JD. Repeated exposure to neurotoxic levels of chlorpyrifos alters hippocampal expression of neurotrophins and neuropeptides. Toxicology. 2016;340:53–62. doi: 10.1016/j.tox.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus R, Abdelghani A. Chlorpyrifos: an unwelcome pesticide in our homes. Rev Environ Health. 2000;15:421–433. doi: 10.1515/reveh.2000.15.4.421. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Levin HS, Rodnitzky RL. Behavioral effects of organophosphate in man. Clin Toxicol. 1976;9:391–403. doi: 10.3109/15563657608988138. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Timofeeva OA, Yang L, Petro A, Ryde IT, Wrench N, Seidler FJ, Slotkin TA. Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behav Brain Res. 2010;208:319–327. doi: 10.1016/j.bbr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Cauley M, Johnson JE, Cooper EM, Stapleton HM, Ferguson PL, Seidler FJ, Slotkin TA. Prenatal dexamethasone augments the neurobehavioral teratology of chlorpyrifos: significance for maternal stress and preterm labor. Neurotoxicol Teratol. 2014;41:35–42. doi: 10.1016/j.ntt.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librizzi L, Biella G, Cimino C, De Curtis M. Arterial supply of limbic structures in the guinea pig. J Comp Neurol. 1999;411:674–682. doi: 10.1002/(sici)1096-9861(19990906)411:4<674::aid-cne11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Limke TL, Cai J, Miura T, Rao MS, Mattson MP. Distinguishing features of progenitor cells in the late embryonic and adult hippocampus. Dev Neurosci. 2003;25:257–272. doi: 10.1159/000072273. [DOI] [PubMed] [Google Scholar]
- Lockridge O, Duysen EG, Voelker T, Thompson CM, Schopfer LM. Life without acetylcholinesterase: the implications of cholinesterase inhibitor toxicity in AChE-knockout mice. Environ Toxicol Pharmacol. 2005;19:463–469. doi: 10.1016/j.etap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu L, Newman J, Faber B, Gan J. Degradation of pesticides in nursery recycling pond waters. J Agric Food Chem. 2006;54:2658–2663. doi: 10.1021/jf060067r. [DOI] [PubMed] [Google Scholar]
- Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci USA. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamczarz J, Pescrille JD, Gavrushenko L, Burke RD, Fawcett WP, DeTolla LJ, Chen H, Jr, Pereira EFR, Albuquerque EX. Spatial learning impairment in prepubertal guinea pigs prenatally exposed to the organophosphorus pesticide chlorpyrifos: Toxicological implications. Neurotoxicology. 2016;56:17–28. doi: 10.1016/j.neuro.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HJ. The recovery of plasma cholinesterase and erythrocyte acetylcholinesterase activity in workers after overexposure to dichlorvos. Occup Med (Lond) 2000;50:343–347. doi: 10.1093/occmed/50.5.343. [DOI] [PubMed] [Google Scholar]
- Maurissen JP, Hoberman AM, Garman RH, Hanley TR., Jr Lack of selective developmental neurotoxicity in rat pups from dams treated by gavage with chlorpyrifos. Toxicol Sci. 2000;57:250–263. doi: 10.1093/toxsci/57.2.250. [DOI] [PubMed] [Google Scholar]
- Mess A. The Guinea pig placenta: model of placental growth dynamics. Placenta. 2007;28:812–815. doi: 10.1016/j.placenta.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Adam BL, Lambert NA, Terry AV., Jr Effects of chlorpyrifos and chlorpyrifos-oxon on the dynamics and movement of mitochondria in rat cortical neurons. J Pharmacol Exp Ther. 2011;339:341–349. doi: 10.1124/jpet.111.184762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, Wilson NK, Lyu CW. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Expo Anal Environ Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- Moser VC, Chanda SM, Mortensen SR, Padilla S. Age-and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol Sci. 1998;46:211–222. doi: 10.1006/toxs.1998.2526. [DOI] [PubMed] [Google Scholar]
- Mullins RJ, Xu S, Pereira EFR, Pescrille JD, Todd SW, Mamczarz J, Albuquerque EX, Gullapalli RP. Prenatal exposure of guinea pigs to the organophosphorus pesticide chlorpyrifos disrupts the structural and functional integrity of the brain. Neurotoxicology. 2015;48:9–20. doi: 10.1016/j.neuro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R, Ibáñez L, Hattersley A, Tost J. IGF2/H19 hypomethylation in a patient with very low birthweight, preocious pubarche and insulin resistance. BMC Med Genet. 2012;13:42. doi: 10.1186/1471-2350-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayo-Mitoko GJ, Kromhout H, Karumba PN, Boleij JS. Identification of determinants of pesticide exposure among Kenyan agricultural workers using empirical modelling. Ann Occup Hyg. 1999;43:519–525. [PubMed] [Google Scholar]
- Olivier K, Jr, Liu J, Pope C. Inhibition of forskolin-stimulated cAMP formation in vitro by paraoxon and chlorpyrifos oxon in cortical slices from neonatal, juvenile, and adult rats. J Biochem Mol Toxicol. 2001;15:263–269. doi: 10.1002/jbt.10002. [DOI] [PubMed] [Google Scholar]
- Paparini D, Gori S, Grasso E, Scordo W, Calo G, Pérez Leirós C, Ramhorst R, Salamone G. Acetylcholine contributes to control the physiological inflammatory response during the peri-implantation period. Acta Physiol (Oxf) 2015;214:237–247. doi: 10.1111/apha.12494. [DOI] [PubMed] [Google Scholar]
- Pereira EFR, Aracava Y, DeTolla LJ, Beecham EJ, Jr, Basinger GW, Jr, Wakayama EJ, Albuquerque EX. Animal models that best reproduce the clinical manifestations of human intoxication with organophosphorus compounds. J Pharmacol Exp Ther. 2014;350:313–321. doi: 10.1124/jpet.114.214932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poet TS, Timchalk C, Bartels MJ, Smith JN, McDougal R, Juberg DR, Price PS. Use of a probabilistic PBPK/PD model to calculate data derived extrapolation factors for chlorpyrifos. Regul Toxicol Pharmacol. 2017;86:59–73. doi: 10.1016/j.yrtph.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV., Jr Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience. 2007;146:330–339. doi: 10.1016/j.neuroscience.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: what is the vulnerable period? Environ Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Nomura DK, Sparks SE, Segall Y, Casida JE. Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides. Toxicol Lett. 2002;135:89–93. doi: 10.1016/s0378-4274(02)00251-5. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119:1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl Acad Sci USA. 2012;109:7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garcia WE, Whyatt RM, Horton MK, Barr DB, Louis ED. Prenatal exposure to the organophosphate pesticide chlorpyrifos and childhood tremor. Neurotoxicology. 2015;51:80–86. doi: 10.1016/j.neuro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss R, Chang ET, Richardson RJ, Goodman M. A review of epidemiologic studies of low-level exposures to organophosphorus insecticides in non-occupational populations. Crit Rev Toxicol. 2015;45:531–641. doi: 10.3109/10408444.2015.1043976. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Markina N, Valanzano A, Fortuna S, Cometa MF, Meneguz A, Calamandrei G. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicol Appl Pharmacol. 2003;191:189–201. doi: 10.1016/s0041-008x(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, Calamandrei G. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol Sci. 2006;93:105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Richendrfer H, Creton R. Chlorpyrifos and malathion have opposite effects on behaviors and brain size that are not correlated to changes in AChE activity. Neurotoxicology. 2015;49:50–58. doi: 10.1016/j.neuro.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetto J, Riva MA. Prenatal maternal factors in the development of cognitive impairments in the offspring. J Reprod Immunol. 2014;104–105:20–25. doi: 10.1016/j.jri.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Roidl D, Hacker C. Histone methylation during neural development. Cell Tissue Res. 2014;356:539–552. doi: 10.1007/s00441-014-1842-8. [DOI] [PubMed] [Google Scholar]
- Rosas LG, Eskenazi B. Pesticides and child neurodevelopment. Curr Opin Pediatr. 2008;20:191–197. doi: 10.1097/MOP.0b013e3282f60a7d. [DOI] [PubMed] [Google Scholar]
- Ross SM, McManus IC, Harrison V, Mason O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol. 2013;43:21–44. doi: 10.3109/10408444.2012.738645. [DOI] [PubMed] [Google Scholar]
- Ryan PB, Burke TA, Cohen Hubal EA, Cura JJ, McKone TE. Using biomarkers to inform cumulative risk assessment. Environ Health Perspect. 2007;115:833–840. doi: 10.1289/ehp.9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydel RE, Greene LA. Acidic and basic fibroblast growth factors promote stable neurite outgrowth and neuronal differentiation in cultures of PC12 cells. J Neurosci. 1987;7:3639–3653. doi: 10.1523/JNEUROSCI.07-11-03639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhoshkumar P, Karanth S, Shivanandappa T. Neurotoxicity and pattern of acetylcholinesterase inhibition in the brain regions of rat by bromophos and ethylbromophos. Fundam Appl Toxicol. 1996;32:23–30. doi: 10.1006/faat.1996.0103. [DOI] [PubMed] [Google Scholar]
- Satyanarayana M. A correlative review of acetylcholine synthesis in relation to histopathology of the human syncytiotrophoblast. Acta Obstet Gynecol Scand. 1986;65:567–572. doi: 10.3109/00016348609158388. [DOI] [PubMed] [Google Scholar]
- Sauerwein HC, Lassonde M. Cognitive and sensori-motor functioning in the absence of the corpus callosum: neuropsychological studies in callosal agenesis and callosotomized patients. Behav Brain Res. 1994;64:229–240. doi: 10.1016/0166-4328(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Saulsbury MD, Heyliger SO, Wang K, Round D. Characterization of chlorpyrifos-induced apoptosis in placental cells. Toxicology. 2008;244:98–110. doi: 10.1016/j.tox.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senut MC, Cingolani P, Sen A, Kruger A, Shaik A, Hirsch H, Suhr ST, Ruden D. Epigenetics of early-life lead exposure and effects on brain development. Epigenomics. 2012;4:665–674. doi: 10.2217/epi.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HS, Seo JH, Jeong SH, Park SW, Park YI, Son SW, Kang HG, Kim JS. Effect on the H19 gene methylation of sperm and organs of offspring after chlorpyrifos-methyl exposure during organogenesis period. Environ Toxicol. 2015;30:1355–1363. doi: 10.1002/tox.21923. [DOI] [PubMed] [Google Scholar]
- Singleton ST, Lein PJ, Dadson OA, et al. Longitudinal assessment of occupational exposures to the organophosphorous insecticides chlorpyrifos and profenofos in Egyptian cotton field workers. Int J Hyg Environ Health. 2015;218:203–211. doi: 10.1016/j.ijheh.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Protein kinase C is a target for diverse developmental neurotoxicants: transcriptional responses to chlorpyrifos, diazinon, dieldrin and divalent nickel in PC12 cells. Brain Res. 2009;1263:23–32. doi: 10.1016/j.brainres.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Oxidative stress from diverse developmental neurotoxicants: antioxidants protect against lipid peroxidation without preventing cell loss. Neurotoxicol Teratol. 2010;32:124–131. doi: 10.1016/j.ntt.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environ Health Perspect. 2007;115:909–916. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull. 2008;75:640–647. doi: 10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Stout DM, 2nd, Bradham KD, Egeghy PP, et al. American healthy homes survey: a national study of residential pesticides measured from floor wipes. Environ Sci Technol. 2009;43:4294–4300. doi: 10.1021/es8030243. [DOI] [PubMed] [Google Scholar]
- Terry AV., Jr Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther. 2012;134:355–365. doi: 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Dai H, Deng Y, et al. The effect of HMGB1 on subtoxic chlorpyrifos exposure-induced neuroinflammation in amygdala of neonatal rats. Toxicology. 2015;338:95–103. doi: 10.1016/j.tox.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Nolan RJ, Mendrala AL, Dittenber DA, Brzak KA, Mattsson JL. A Physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol Sci. 2002;66:34–53. doi: 10.1093/toxsci/66.1.34. [DOI] [PubMed] [Google Scholar]
- Tsutsui KI, Oyama K, Nakamura S, Iijima T. Comparative overview of visuospatial working memory in monkeys and rats. Front Syst Neurosci. 2016;10:99. doi: 10.3389/fnsys.2016.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Calamandrei G, Ricceri L. A social recognition test for female mice reveals behavioral effects of developmental chlorpyrifos exposure. Neurotoxicol Teratol. 2006;28:466–471. doi: 10.1016/j.ntt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Mahler HR, Moore WJ. The half-life of acetylcholinesterase in mature rat brain. J Neurochem. 1974;22:941–943. doi: 10.1111/j.1471-4159.1974.tb04319.x. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, Garfinkel R, Andrews H, Hoepner L, Barr DB. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol Appl Pharmacol. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol. 2008;228:32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Liu SS, Yi F, Zhuo M, Li BM. Delay-dependent impairment of spatial working memory with inhibition of NR2B-containing NMDA receptors in hippocampal CA1 region of rats. Mol Brain. 2013;6:13. doi: 10.1186/1756-6606-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dai H, Deng Y, Tian J, Zhang C, Hu Z, Bing G, Zhao L. Neonatal chlorpyrifos exposure induces loss of dopaminergic neurons in young adult rats. Toxicology. 2015;336:17–25. doi: 10.1016/j.tox.2015.07.014. [DOI] [PubMed] [Google Scholar]


