Abstract
Background
Octogenarians and nonagenarians with stage II/III rectal adenocarcinomas are underrepresented in randomized trials that have established standard of care therapy of preoperative chemoradiation followed by definitive resection (CRT+S). The purpose of our study was to evaluate the impact of therapies on overall survival (OS) in patients with stage II/III rectal cancers and determine predictors of therapy within the National Cancer Data Base (NCDB).
Methods
In the NCDB, patients ≥80 years old from 2004–2013 with clinical stage II/III rectal adenocarcinomas were queried. Kaplan-Meier, log-rank, logistic regression, Cox-proportional hazard regression, interaction-effect testing, and propensity-score matched analysis (PSM) were conducted.
Results
2,723 patients met criteria: 14.9% received no treatment, 29.7% had surgery alone, 5.0% received short-course radiation then surgery (RT+S), 45.4% received CRT+S, and 4.8% received surgery then chemoradiation (S+CRT). African-American race and residence in a lower-educated county were associated with not receiving treatment. Male sex, older age, worsening co-morbidities, and receiving no treatment or surgery alone were associated with worse OS. There was no statistical difference with OS between RT+S, S+CRT, and CRT+S. Interaction testing found CRT+S improved OS independent of age, co-morbidity status, sex, race, and tumor stage. On PSM, CRT+S was associated with improved OS compared to surgery alone.
Conclusions
A significant portion of octogenarians and nonagenarians with stage II/III rectal adenocarcinomas do not receive treatment. African-American race and living in a lower educated community were associated with not receiving therapy. Our series suggests that CRT+S appears to be a reasonable strategy in elderly patients who can tolerate therapy.
Keywords: Healthcare disparities, geriatric oncology, rectal cancer, chemotherapy and radiation therapy, total mesorectal excision
Introduction
Colorectal cancer is the second leading cause of death in the United States (US), with an estimated 40,000 cases of rectal cancer diagnosed in the United States annually1. The standard of care therapy for Stage II/III rectal cancers (cT3/4 and/or cN+) is neoadjuvant therapy, which in the US is generally concurrent chemoradiation followed by resection with TME technique (CRT+S) followed by adjuvant chemotherapy. These treatment paradigms were established via landmark randomized trials, including NSABP R-03 and the German Rectal Cancer Trial, which demonstrated improved disease and toxicity outcomes with preoperative chemoradiation2–4. Despite the literature justifying current treatment approaches, there is a paucity of data for the implementation in octogenarians and nonagenarians. These patients are routinely underrepresented on clinical trials and undertreated due to concerns of medical fragility or concerns about utility in patients who may die of competing risk factors5.
Given the rising number of oncologic patients ≥80 years old6, the infrequency with which a substantial population would be seen at any single institution, and the lack of information for the optimal treatment in these patients, we used the National Cancer Data Base (NCDB) to identify a large cohort of octogenarians and nonagenarians diagnosed with stage II and stage III rectal adenocarcinomas. Our primary goal was to determine the impact of treatment type on overall survival (OS) and to identify factors associated with the receipt of no treatment.
Methods
Patient Selection
The NCDB is a large, prospectively acquired database, capturing 70% of newly diagnosed malignancies in the US, all from Commission on Cancer (CoC) accredited facilities. The dataset includes detailed demographic, disease, and treatment characteristics in addition to survival outcomes.
The rectal cancer NCDB was queried for patients diagnosed from 2004 to 2013. To be included, patients had to be 80 years or older at diagnosis, have invasive adenocarcinoma histology, clinically be staged as II or III, and be without previous cancer diagnoses. Patients with metastatic disease at diagnosis or with incomplete treatment records were excluded (Supplementary Figure 1). Patients were classified by clinical stage based on the AJCC 7th edition7 into stage II or stage III. Treatment was classified as: no treatment, surgery alone (definitive low anterior resection or abdominoperineal resection), short-course radiation then surgery (less than 6 fractions and more than 4 Gy per fraction to be included), CRT+S, and definitive surgery followed by chemoradiation. The following patient characteristics were examined: age, sex, race, insurance type (government insurance, private insurance, or not insured), patient county of residence (urban, rural, or metro as defined by the US Census Bureau), distance from patient residence zip code to treatment facility, year of diagnosis, percentage of residents without a high school degree in patient’s census tract (<14, 14–19.9, 20–28.9, and ≥29% quartiles), median income of patient’s census tract (<30,000, 30,000–35,999, 36,000–45,999, and >46,000 dollars as determined by the American Community Survey), and co-morbidities as quantified by the Charlson-Deyo score8, 9. The following tumor characteristics were evaluated: size, grade (well, moderately, and poorly differentiated), lymphovascular space invasion (LVSI), and carcinoembryonic antigen level (CEA) (elevated, undetermined, and negative). The following treatment characteristics were evaluated: treatment facility type (community cancer center, comprehensive community cancer center, or academic program), treatment facility location within the United States (Northeast, South, Midwest, and West), extent of resection (R0 vs. R1), node positivity at time of surgery (pN0 or pN+), reason for no radiation (not part of treatment plan, not given due to patient risk factors, patient refusal, or unknown reason why not given), time from diagnosis to definitive treatment start (0–30 days, 31–90 days, and >90 days), and number of lymph nodes removed at surgery (none, <10, 10–14, >14). Chemotherapy type, number of cycles, and patient adherence cannot be reported using the NCDB. Age and tumor size were evaluated as continuous variables after testing revealed a linear effect on OS.
Statistical Methods
Statistical analysis was conducted using SAS Version 9.4 (Cary, NC) and SAS macros10. Descriptive statistics for each variable were determined. Variables were compared across treatment groups using Chi-Square and ANOVA tests where appropriate. Extent of resection, node positivity at surgery, and time from diagnosis to treatment start were compared across the four groups that received therapy only. Univariable and multivariable logistic regression models were fit to determine relevant predictors of receiving a no treatment and CRT+S, including all sociodemographic variables. The primary endpoint of OS was defined as months from diagnosis to death or last follow up. Univariable Cox proportional hazard models were fit for each variable. Multivariable Cox proportional hazard models were fit for the entire cohort, to include all relevant variables with a p-value of 0.1 or less after backwards elimination. Variables that directly correlated with no treatment (lymph node removal, extent of resection, etc.) were not included in the multivariable logistic regression or Cox models. Next, the effect of treatment type on OS stratified by different variables was estimated by testing the interaction effect between treatment type and each significant variable11. Interaction analyses was considered exploratory and was not powered for statistical testing. Lymphovascular invasion and CEA level were not included in the multivariable analysis due to the high number missing. Kaplan-Meier curves were generated for OS estimates for the entire cohort stratified by treatment group, with comparison of groups using log-rank tests with a P value of <0.05 considered statistically significant. Finally, propensity score matching was implemented to reduce treatment selection bias. A logistic regression model predicting for CRT versus surgery alone was carried out to estimate the propensity score of all covariates. Patients were then matched 1:1 based on propensity score using a greedy 5-1-digit match algorithm for sex, stage, age, Charlson-Deyo score, and facility location12. After matching, the balance of the two groups was evaluated by standardized differences with values <0.1 considered negligible13. The OS effect in the matched sample was estimated using a Cox model with a robust variance estimator14, 15. Do to the small number of patients treated with short-course preoperative radiation and postoperative chemoradiation, these patients were excluded from the propensity score-matched analysis. For each survival model, the proportional hazard assumption was assessed, and no statistically significant deviations were observed.
Results
Patient Characteristics
A total of 2,723 patients met study entry criteria (Supplementary Figure 1); 1,623 (59.6%) were Stage II and 1,100 (40.4%) were Stage III. The median age at diagnosis was 83.0 years old (80.0–91.0 years) with median follow-up time of 3.3 years (0.4–10.4 years). The median radiation dose was 25.0 Gy (22.5 Gy–30.0 Gy) in the short-course radiation then surgery cohort, 45.0 Gy (33.0 Gy–60.0 Gy) in the CRT+S cohort, and 45.0 Gy (39.0 Gy–62.0 Gy) in the postoperative chemoradiation cohort. Table 1 summarizes the study population by treatment group.
Table 1.
Summary of all patient, tumor, and treatment characteristics of the entire study population stratified by treatment received.
| Covariate | Statistics | Level | No Treatment (N=407) | Surgery Alone (N=807) | Short Course RT Then Surgery (N=137) | CRT Then Surgery (N=1233) | Surgery Then CRT (N=139) | P-Value |
|---|---|---|---|---|---|---|---|---|
| Patient Characteristics | ||||||||
| Age at Diagnosis | Median (Range) | 86 (80–90) | 84.9 (80–90) | 83.0 (80–90) | 82.8 (81–91) | 83.0 (80–91) | <0.01 | |
| Sex | N (Col %) | Male | 178 (43.7) | 349 (43.2) | 70 (51.1) | 655 (53.1) | 66 (47.5) | <0.01 |
| N (Col %) | Female | 229 (56.3) | 458 (56.8) | 67 (48.9) | 578 (46.9) | 73 (52.5) | ||
| Race | N (Col %) | White | 351 (87.5) | 732 (91.7) | 125 (92.6) | 1109 (90.8) | 126 (91.3) | 0.11 |
| N (Col %) | Black | 36 (9.0) | 40 (5.0) | 3 (2.2) | 67 (5.5) | 8 (5.8) | ||
| N (Col %) | Other | 14 (3.5) | 26 (3.3) | 7 (5.2) | 46 (3.8) | 4 (2.9) | ||
| Missing | 6 | 9 | 2 | 11 | 1 | |||
| Insurance Type | N (Col %) | Private Insurance + Medicare | 35 (8.7) | 77 (9.8) | 14 (10.3) | 126 (10.7) | 10 (7.4) | 0.79 |
| N (Col %) | Medicare | 366 (91.3) | 716 (90.2) | 122 (89.0) | 1093 (89.3) | 126 (92.6) | ||
| Missing | 6 | 14 | 1 | 14 | 3 | |||
| Distance from ZIP Code of Residence to Treatment Facility | N (Col %) | <10 miles | 253 (64.2) | 476 (59.9) | 82 (60.3) | 714 (58.5) | 79 (56.8) | 0.08 |
| N (Col %) | 10–50 miles | 116 (29.4) | 245 (30.8) | 35 (25.7) | 388 (31.8) | 52 (37.4) | ||
| N (Col %) | >50 miles | 25 (6.3) | 74 (9.3) | 19 (14.0) | 118 (9.7) | 8 (5.8) | ||
| Missing | 13 | 12 | 1 | 13 | 0 | |||
| Median Income of Census Tract of Residence | N (Col %) | < $30,000 | 56 (14.4) | 85 (10.8) | 10 (7.5) | 137 (11.4) | 15 (10.9) | 0.49 |
| N (Col %) | $30,000 – $35,999 | 76 (19.6) | 159 (20.3) | 32 (24.1) | 214 (17.9) | 24 (17.5) | ||
| N (Col %) | $36,000 – $45,999 | 116 (29.9) | 226 (28.8) | 43 (32.3) | 364 (30.4) | 41 (29.9) | ||
| N (Col %) | $46,000 + | 140 (36.1) | 314 (40.1) | 48 (36.1) | 483 (40.3) | 57 (41.6) | ||
| Missing | 19 | 23 | 4 | 35 | 2 | |||
| Percent of Census Tract of Residence without a High School Degree | N (Col %) | >=29% | 68 (17.5) | 116 (14.8) | 15 (11.3) | 165 (13.8) | 22 (16.1) | 0.19 |
| N (Col %) | 20–28.9% | 102 (26.3) | 171 (21.8) | 31 (23.3) | 263 (22.0) | 24 (17.5) | ||
| N (Col %) | 14–19.9% | 91 (23.5) | 199 (25.4) | 33 (24.8) | 319 (26.6) | 48 (35.0) | ||
| N (Col %) | < 14% | 127 (32.7) | 298 (38.0) | 54 (40.6) | 450 (37.6) | 43 (31.4) | ||
| Missing | 19 | 23 | 4 | 36 | 2 | |||
| County Type | N (Col %) | Metro | 308 (79.6) | 665 (85.3) | 101 (74.8) | 964 (80.3) | 104 (76.5) | 0.02 |
| N (Col %) | Urban | 74 (19.1) | 103 (13.2) | 30 (22.2) | 215 (17.9) | 28 (20.6) | ||
| N (Col %) | Rural | 10 (2.6) | 12 (1.5) | 4 (3.0) | 22 (1.8) | 4 (2.9) | ||
| Missing | 20 | 27 | 2 | 32 | 3 | |||
| Charlson-Deyo Score | N (Col %) | 0 | 282 (69.3) | 515 (63.8) | 97 (70.8) | 920 (74.6) | 98 (70.5) | <0.01 |
| N (Col %) | 1+ | 125 (30.7) | 292 (36.2) | 40 (29.2) | 313 (25.4) | 41 (29.5) | ||
| Year of Diagnosis | N (Col %) | 2004 – 2007 | 151 (37.1) | 268 (33.2) | 45 (32.8) | 248 (20.1) | 36 (25.9) | <0.01 |
| N (Col %) | 2008 – 2010 | 111 (27.3) | 286 (35.4) | 45 (32.8) | 468 (38.0) | 56 (40.3) | ||
| N (Col %) | 2011 – 2013 | 145 (35/6) | 253 (31.4) | 47 (34.3) | 517 (41.9) | 47 (33.8) | ||
| Tumor Characteristics | ||||||||
| Stage | N (Col %) | Stage II | 256 (62.9) | 541 (67.0) | 80 (58.4) | 675 (54.7) | 71 (51.1) | <0.01 |
| N (Col %) | Stage III | 151 (37.1) | 266 (33.0) | 57 (41.6) | 558 (42.3) | 68 (48.9) | ||
| Size of Tumor (cm) | Median (Range) | 5.0 (0.5–15.0) | 4.5 (3.0–14.0) | 4.6 (0.8–9.0) | 4.3 (0.9–9.1) | 5.3 (1.1–9.5) | <0.01 | |
| Tumor Grade | N (Col %) | Well Differentiated | 20 (7.1) | 58 (7.3) | 12 (10.0) | 92 (8.8) | 9 (7.0) | 0.32 |
| N (Col %) | Moderately Differentiated | 217 (76.7) | 601 (75.8) | 82 (68.3) | 811 (77.4) | 97 (75.2) | ||
| N (Col %) | Poorly Differentiated | 46 (16.3) | 134 (16.9) | 26 (21.7) | 145 (13.8) | 23 (17.8) | ||
| Missing | 124 | 14 | 17 | 185 | 10 | |||
| Lymphovascular Invasion | N (Col %) | Not present | 22 (91.7) | 242 (73.3) | 39 (72.2) | 463 (86.9) | 32 (68.1) | <0.01 |
| N (Col %) | Present | 2 (8.3) | 88 (26.7) | 15 (27.8) | 70 (13.1) | 15 (31.9) | ||
| Missing | 383 | 477 | 83 | 700 | 92 | |||
| Carcinoembryonic Antigen (CEA) | N (Col %) | Elevated | 125 (57.1) | 212 (47.0) | 43 (53.1) | 357 (45.6) | 44 (48.4) | 0.10 |
| N (Col %) | Undetermined | 3 (1.4) | 3 (0.7) | 0 (0.0) | 5 (0.6) | 0 (0) | ||
| N (Col %) | Normal | 91 (41.6) | 236 (52.3) | 38 (46.9) | 421 (53.8) | 47 (51.6) | ||
| Missing | 188 | 356 | 56 | 450 | 48 | |||
| Treatment Characteristics | ||||||||
| Extent of Resection | N (Col %) | R0 | N/A | 701 (89.0) | 119 (90.2) | 1141 (94.8) | 109 (85.8) | <0.01 |
| N (Col %) | R1 | 87 (11.0) | 13 (9.8) | 63 (5.2) | 18 (14.2) | |||
| N (Col %) | No surgery | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Missing | 19 | 5 | 29 | 12 | ||||
| Regional Lymph Nodes Examined | N (Col %) | 0 | 388 (98.5) | 66 (8.2) | 16 (11.7) | 162 (13.3) | 28 (20.3) | <0.01 |
| N (Col %) | <10 | 3 (0.8) | 137 (17.0) | 29 (21.2) | 339 (27.9) | 15 (10.9) | ||
| N (Col %) | 10 – 14 | 0 (0) | 177 (22.0) | 37 (27.0) | 322 (26.5) | 24 (17.4) | ||
| N (Col %) | >14 | 0 (0) | 424 (52.7) | 55 (40.1) | 393 (32.3) | 71 (51.4) | ||
| Missing | 16 | 3 | 0 | 17 | 1 | |||
| Regional Lymph Nodes Positive at Surgery | N (Col %) | pN0 | N/A | 426 (57.6) | 77 (63.6) | 787 (74.7) | 36 (32.7) | <0.01 |
| N (Col %) | pN+ | 313 (42.4) | 44 (36.4) | 266 (25.3) | 74 (67.3) | |||
| Missing | 68 | 16 | 180 | 27 | ||||
| Reason for No Radiation (RT) | N (Col %) | RT Given | 0 (0) | 0 (0) | 137 (100.0) | 1233 (100.0) | 139 (100.0) | N/A |
| N (Col %) | RT Not Part of Planned First Course Treatment | 232 (57.0) | 603 (74.7) | 0 (0) | 0 (0) | 0 (0) | ||
| N (Col %) | RT Contraindicated Due to Patient Risk Factors | 51 (12.5) | 79 (9.8) | 0 (0) | 0 (0) | 0 (0) | ||
| N (Col %) | RT Recommended but Refused | 124 (30.5) | 125 (15.5) | 0 (0) | 0 (0) | 0 (0) | ||
| Time from Diagnosis to Treatment Start | N (Col %) | 0–30 days | N/A | 499 (63.8) | 66 (48.5) | 523 (44.9) | 95 (69.9) | <0.01 |
| N (Col %) | 31–90 days | 245 (31.3) | 66 (48.5) | 609 (52.3) | 39 (28.7) | |||
| N (Col %) | >90 days | 38 (4.9) | 4 (2.9) | 33 (2.8) | 2 (1.5) | |||
| Missing | 25 | 1 | 68 | 3 | ||||
| Facility Type | N (Col %) | Community Cancer Center | 89 (21.9) | 159 (19.7) | 24 (17.5) | 226 (18.3) | 22 (15.8) | 0.62 |
| N (Col %) | Comprehensive Community Cancer Program | 212 (52.1) | 423 (52.4) | 73 (53.3) | 637 (51.7) | 82 (59.0) | ||
| N (Col %) | Academic Center | 106 (26.0) | 225 (27.9) | 40 (29.2) | 370 (30.0) | 35 (25.2) | ||
| Facility Location | N (Col %) | Northeast | 96 (23.6) | 199 (24.7) | 32 (23.4) | 289 (23.4) | 29 (20.9) | <0.01 |
| N (Col %) | South | 112 (27.5) | 246 (30.5) | 26 (19.0) | 325 (26.4) | 41 (29.5) | ||
| N (Col %) | Midwest | 125 (30.7) | 201 (24.9) | 51 (37.2) | 421 (34.1) | 43 (30.9) | ||
| N (Col %) | West | 74 (18.2) | 161 (20.0) | 28 (20.4) | 198 (16.1) | 26 (18.7) | ||
The parametric p-value is calculated by ANOVA for numerical covariates and chi-square test for categorical variates.
RT = Radiation Therapy
CRT = Concurrent chemotherapy and radiation
N/A = Not applicable
For variables comparing treatment results, the reported p-value is a log-rank comparison of the four groups that received therapy (the no treatment group was not included).
Bold indicates statistical significance.
Predictors of Receiving No Treatment
In total, 407 patients (14.9%) did not receive any treatment. On univariable analysis, age per one year increase, female sex (vs. male sex), black race (vs. white race), living in a lower-income census tract of residence (<$30,000) vs. higher-income census tract of residence (S46,000+), and living in a census tract with ≥20% of the population without a high-school degree (vs. <20% of the population in a census tract with a high-school degree) were associated with receiving no treatment (all P <0.05, Table 2). On multivariable analysis, age per one year increase (OR=1.21, 95%CI: 1.17–1.25), black race vs. white race (OR=1.89, 95%CI: 1.22–2.92), and living in a lower educated census tract with ≥29% without a high-school degree vs. <14% without a high-school degree (OR=1.38, 95%CI: 1.05–1.97) or between 20–28.9% without a high school degree vs. <14% without a high-school degree (OR=1.53, 95%CI: 1.12–2.09), were associated with receipt of no therapy (Table 2).
Table 2.
Univariable and multivariable association of patient, tumor, and treatment characteristics with receiving no therapy amongst all patients.
| Univariable Analysis | Multivariable Analysis* | ||||
|---|---|---|---|---|---|
| Covariate | Level | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value |
| Age at Diagnosis (per 1 year increase) | 1.20 (1.16–1.24) | <0.01 | 1.21 (1.17–1.25) | <0.01 | |
| Stage | III | 0.85 (0.68–1.06) | 0.14 | -- | -- |
| II | - | - | -- | -- | |
| Sex | Male | 0.80 (0.65–0.99) | 0.04 | -- | -- |
| Female | - | - | -- | -- | |
| Race | Other | 1.01 (0.56–1.79) | 0.99 | 1.05 (0.56–1.99) | 0.93 |
| Black | 1.82 (1.23–2.69) | <0.01 | 1.89 (1.22–2.92) | <0.01 | |
| White | - | - | - | - | |
| Insurance Type | Private & Medicare | 0.84 (0.58–1.21) | 0.35 | -- | -- |
| Medicare | - | - | -- | -- | |
| Facility Type | Community Cancer Program | 1.31 (0.96–1.78) | 0.09 | -- | -- |
| Comprehensive Community Cancer Program | 1.11 (0.86–1.42) | 0.43 | -- | -- | |
| Academic Program | - | - | -- | -- | |
| Facility Location | Northeast | 0.97 (0.70–1.35) | 0.87 | -- | -- |
| South | 0.98 (0.71–1.34) | 0.88 | -- | -- | |
| Midwest | 0.97 (0.71–1.33) | 0.87 | -- | -- | |
| West | - | - | -- | -- | |
| Median Income of Census Tract of Residence | < $30,000 | 1.47 (1.05–2.07) | 0.03 | ||
| $30,000 – $35,999 | 1.14 (0.84–1.54) | 0.39 | |||
| $36,000 – $45,999 | 1.11 (0.85–1.44) | 0.44 | |||
| $46,000 + | - | - | |||
| Percent of Census Tract of Residence Without a High School Degree | >=29% | 1.43 (1.04–1.97) | 0.03 | 1.38 (1.05–1.97) | 0.04 |
| 20–28.9% | 1.39 (1.05–1.84) | 0.02 | 1.53 (1.12–2.09) | <0.01 | |
| 14–19.9% | 1.02 (0.76–1.36) | 0.91 | 1.07 (0.79–1.46) | 0.65 | |
| < 14% | - | - | |||
| County Type | Metro | 0.80 (0.40–1.60) | 0.44 | 0.65 (0.29–1.42) | 0.28 |
| Urban | 0.93 (0.45–1.92) | 0.76 | 0.93 (0.42–2.05) | 0.86 | |
| Rural | - | - | - | - | |
| Charlson-Deyo Score | 1+ | 1.05 (0.84–1.32) | 0.66 | -- | -- |
| 0 | - | - | -- | -- | |
| Year of Diagnosis | 2011 – 2013 | 0.66 (0.51–0.85) | <0.01 | 0.77 (0.58–1.04) | 0.07 |
| 2008 – 2010 | 0.52 (0.39–0.67) | <0.01 | 0.75 (0.53–1.01) | 0.06 | |
| 2004 – 2007 | - | - | - | - | |
| Distance from County of ZIP code to Treatment Facility | <10 miles | 1.65 (1.07–2.54) | 0.02 | 2.13 (1.26–3.61) | <0.01 |
| 10–50 miles | 1.42 (0.90–2.24) | 0.14 | 1.77 (1.04–2.87) | 0.03 | |
| >50 miles | - | - | - | - | |
Bold indicates statistical significance.
Backward selection with an alpha level of removal of 0.1 was used, leading to the removal of sex, Charlson-Deyo Score, Insurance Type, and Stage.
Predictors of Receiving Chemoradiotherapy then Surgery
A total of 1,233 patients (45.4%) received CRT+S. On univariable analysis, age per one year increase, male sex (vs. female sex), treatment facility in the Midwest (vs. West), Charlson-Deyo score of 0 (vs. 1+), diagnosis between 2008–2010 or 2011–2013 (vs.2004–2007), and stage III tumors (vs. stage II) were associated with receipt of CRT+S (all P <0.05; Table 3). On multivariable analysis, male sex vs. female sex (OR=1.31, 95%CI: 1.11–1.55), treatment at a facility in the Midwest vs. West (OR=1.60, 95%CI: 1.26–2.05) or in the Northeast vs. West (OR=1.34, 95%CI: 1.03–1.73), treatment between 2008–2010 vs. 2004–2007 (OR=1.90, 95%CI: 1.54–2.35) or 2011–2013 vs. 2004–2007 (OR=2.05, 95%CI: 1.67–2.53), and stage III tumors vs. stage II (OR=1.33, 95%CI: 1.13–1.58) were associated with receipt of CRT+S. Charlson-Deyo comorbidity score of 1+ vs. 0 (OR=0.62, 95%CI: 0.52–0.75) and age per one year increase (OR=0.81, 95%CI: 0.78–0.83) were associated with not receiving CRT+S (Table 3).
Table 3.
Univariable and multivariable association of patient, tumor, and treatment characteristics with receiving chemoradiotherapy followed by surgery.
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Covariate | Level | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value |
| Age at Diagnosis (per 1 year increase) | 0.80 (0.78–0.83) | <0.01 | 0.80 (0.78–0.83) | <0.01 | |
| Stage | III | 1.45 (1.24–1.69) | <0.01 | 1.33 (1.13–1.58) | <0.01 |
| II | - | - | - | - | |
| Sex | Male | 1.41 (1.21–1.64) | <0.01 | 1.31 (1.11–1.55) | <0.01 |
| Female | - | - | |||
| Race | Other | 1.09 (0.72–1.63) | 0.71 | -- | -- |
| Black | 0.93 (0.67–1.29) | 0.67 | -- | -- | |
| White | - | - | -- | -- | |
| Insurance Type | Private & Medicare | 1.15 (0.89–1.47) | 0.31 | -- | -- |
| Medicare | - | - | -- | -- | |
| Facility Type | Community Cancer Program | 0.84 (0.68–1.06) | 0.14 | -- | -- |
| Comprehensive Community Cancer Program | 0.88 (0.74–1.05) | 0.19 | -- | -- | |
| Academic Program | - | - | -- | -- | |
| Facility Location | Northeast | 1.18 (0.93–1.50) | 0.17 | 1.34 (1.03–1.73) | 0.03 |
| South | 1.12 (0.89–1.41) | 0.37 | 1.21 (0.94–1.55) | 0.14 | |
| Midwest | 1.46 (1.17–1.83) | <0.01 | 1.60 (1.26–2.05) | <0.01 | |
| West | - | - | |||
| Median Income of Census Tract of Residence | < $30,000 | 0.96 (0.74–1.25) | 0.78 | -- | -- |
| $30,000 – $35,999 | 0.85 (0.69–1.05) | 0.14 | -- | -- | |
| $36,000 – $45,999 | 0.99 (0.82–1.19) | 0.94 | -- | -- | |
| $46,000 + | - | - | -- | -- | |
| Percent of Census Tract of Residence Without a High School Degree | >=29% | 0.87 (0.69–1.11) | 0.26 | -- | -- |
| 20–28.9% | 0.93 (0.76–1.14) | 0.50 | -- | -- | |
| 14–19.9% | 1.00 (0.82–1.21) | 0.95 | -- | -- | |
| < 14% | - | - | -- | -- | |
| County Type | Metro | 1.36 (0.80–2.33) | 0.25 | -- | -- |
| Urban | 1.45 (0.83–2.54) | 0.19 | -- | -- | |
| Rural | - | - | -- | -- | |
| Charlson-Deyo Score | 1+ | 0.68 (0.57–0.80) | <0.01 | 0.62 (0.52–0.75) | <0.01 |
| 0 | - | - | - | - | |
| Year of Diagnosis | 2011 – 2013 | 2.12 (1.74–2.58) | <0.01 | 2.05 (1.67–2.53) | <0.01 |
| 2008 – 2010 | 1.89 (1.55–2.31) | <0.01 | 1.90 (1.54–2.35) | <0.01 | |
| 2004 – 2007 | - | - | - | - | |
| Distance from ZIP Code of Residence to Treatment Facility | <10 miles | 0.86 (0.65–1.12) | 0.28 | -- | -- |
| 10–50 miles | 0.92 (0.69–1.23) | 0.62 | -- | -- | |
| >50 miles | - | - | -- | -- | |
Bold indicates statistical significance.
Backward selection with an alpha level of removal of 0.1 was used which removed the following variables from the model; county type, insurance type, and race.
Overall Survival
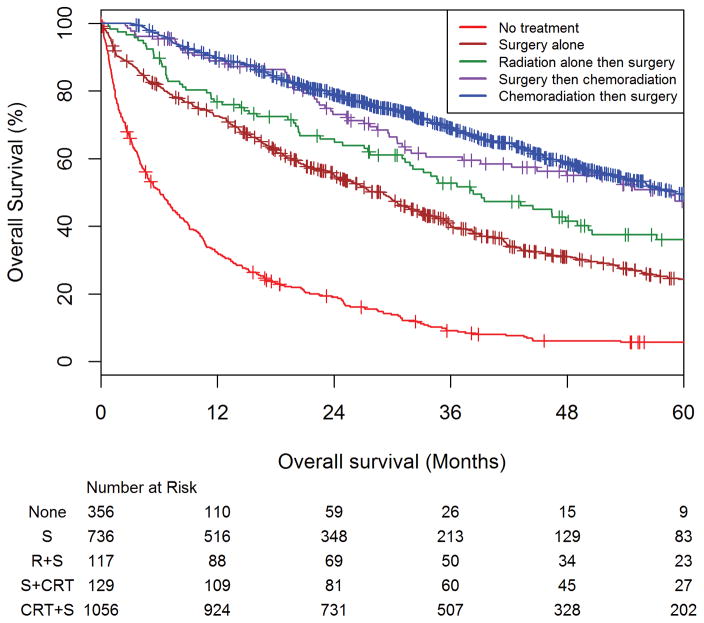
Among all patients, the 3 and 5-year overall survival estimated rates for patients receiving CRT+S were 69.0% (95% CI 65.9–71.9%) and 49.6% (95% CI 45.7–53.3%) respectively. The 3 and 5-year overall survival estimated rates for patients receiving surgery then chemoradiation were 60.5% (95%CI: 50.8–68.9%) and 47.4% (36.9%–57.2%) respectively. The 3 and 5-year survival estimated rates for patients receiving short-course radiation followed by surgery were 52.7% (95%CI: 42.8–61.7%) and 36.0% (95%CI: 26.3–45.7%) respectively. The 3 and 5-year overall survival estimated rates for patients receiving definitive surgery alone were 40.3% (95% CI: 36.5–44.1%) and 24.3% (95%CI: 20.6–28.1%) respectively. The 3 and 5-year overall survival estimated rates for patients not receiving therapy of were 9.1% (95% CI 6.3–12.6%) and 5.7% (95% CI 3.5–8.8%) respectively (Figure 1).
Figure 1. Overall survival amongst elderly rectal adenocarcinoma patients stratified by treatment received: chemoradiation then surgery, surgery the chemoradiation, short-course radiation then surgery, surgery alone, and no treatment.
Kaplan-Meier curve showing the overall survival (OS) estimates of the entire cohort of patients as stratified by treatment group, with the number at risk below the x-axis. Among all patients, the 3 and 5-year overall survival estimated rates for patients receiving CRT+S were 69.0% (95% CI 65.9–71.9%) and 49.6% (95% CI 45.7–53.3%) respectively. The 3 and 5-year overall survival estimated rates for patients receiving surgery then chemoradiation were 60.5% (95%CI: 50.8–68.9%) and 47.4% (36.9%–57.2%) respectively. The 3 and 5-year survival estimated rates for patients receiving short-course radiation followed by surgery were 52.7% (95%CI: 42.8–61.7%) and 36.0% (95%CI: 26.3–45.7%) respectively. The 3 and 5-year overall survival estimated rates for patients receiving definitive surgery alone were 40.3% (95% CI: 36.5–44.1%) and 24.3% (95%CI: 20.6–28.1%) respectively. The 3 and 5-year overall survival estimated rates for patients not receiving therapy of were 9.1% (95% CI 6.3–12.6%) and 5.7% (95% CI 3.5–8.8%) respectively.
On univariable analysis, age per one year increase, Charlson-Deyo score of 1+ (vs. 0), LVSI presence (vs. no LVSI), elevated CEA (vs. negative CEA), no treatment, surgery alone, short-course radiation then surgery (as compared to CRT+S), no lymph nodes removed (vs. >14), R1 resections (vs. R0 resections), and treatment at a facility located in the South (vs. West) were associated with worse OS (all P <0.05; Table 4). On multivariable analysis, not receiving treatment (HR=5.66, 95%CI: 4.84–6.66) or receiving surgery alone (HR=1.98, 95%CI: 1.73–2.27) was associated with worse OS compared to CRT+S. No statistical OS difference was observed between patients receiving short-course radiation followed by resection (HR=1.51, 95%CI: 0.97–1.88) or surgery followed by chemoradiation (HR=1.17, 95%CI: 0.90–1.52) compared to CRT+S. Additionally, male sex vs. female sex (HR=1.12, 95%CI: 1.01–1.25), age per one year increase (HR=1.07, 95%CI: 1.05–1.09), Charlson-Deyo score of 1+ vs. 0 (HR=1.35, 95%CI: 1.20–1.51), and stage III tumors vs. stage II (HR=1.28, 95%CI: 1.14–1.42) were associated with worse OS (Table 4). On exploratory interaction testing, the influence of treatment received on OS was not dependent on age, race, sex, Charlson-Deyo score, stage, indicating a benefit to CRT+S across these variables (Supplementary Table 1).
Table 4.
Univariable and multivariable association of patient, tumor, and treatment characteristics of the entire cohort with overall survival.
| Univariable Analysis | Multivariable Analysis* | ||||
|---|---|---|---|---|---|
| Covariate | Level | Hazard Ratio (95% CI) | P -value | Hazard Ratio (95% CI) | P -value |
| Patient Characteristics | |||||
| Age at Diagnosis (per 1 year increase) | 1.12 (1.10–1.14) | <0.01 | 1.07 (1.05–1.09) | <0.01 | |
| Sex | Male | 1.01 (0.91–1.12) | 0.92 | 1.12 (1.01–1.25) | 0.04 |
| Female | - | - | - | - | |
| Race | Other | 0.79 (0.58–1.08) | 0.13 | -- | -- |
| Black | 1.11 (0.88–1.39) | 0.42 | -- | -- | |
| White | - | - | -- | -- | |
| Insurance Type | Private & Medicare | 0.98 (0.82–1.17) | 0.84 | -- | -- |
| Medicare | - | - | -- | -- | |
| Distance from ZIP Code of Residence to Treatment Facility | <10 miles | 1.25 (1.02–1.53) | 0.03 | 1.21 (0.98–1.48) | 0.07 |
| 10–50 miles | 1.07 (0.87–1.33) | 0.53 | 1.09 (0.88–1.36) | 0.43 | |
| >50 miles | - | - | - | - | |
| Median Income of Census Tract of Residence | < $30,000 | 1.18 (0.98–1.40) | 0.07 | -- | -- |
| $30,000 – $35,999 | 1.05 (0.90–1.22) | 0.53 | -- | -- | |
| $36,000 – $45,999 | 1.13 (1.00–1.29) | 0.06 | -- | -- | |
| $46,000 + | - | - | -- | -- | |
| Percent of Census Tract of Residence without a High School Degree | >=29% | 1.01 (0.85–1.19) | 0.92 | -- | -- |
| 20–28.9% | 1.07 (0.93–1.23) | 0.37 | -- | -- | |
| 14–19.9% | 1.03 (0.89–1.18) | 0.71 | -- | -- | |
| < 14% | - | - | -- | -- | |
| County Type | Metro | 1.07 (0.73–1.56) | 0.74 | -- | -- |
| Urban | 1.05 (0.70–1.56) | 0.82 | -- | -- | |
| Rural | - | - | -- | -- | |
| Charlson-Deyo Score | 1+ | 1.38 (1.24–1.55) | <0.01 | 1.35 (1.20–1.51) | <0.01 |
| 0 | - | - | - | - | |
| Year of Diagnosis | 2011 – 2013 | 0.76 (0.65–0.88) | <0.01 | -- | -- |
| 2008 – 2010 | 0.80 (0.71–0.91) | <0.01 | -- | -- | |
| 2004 – 2007 | - | - | -- | -- | |
| Tumor Characteristics | |||||
| Stage | III | 1.09 (0.98–1.21) | 0.09 | 1.28 (1.14–1.42) | <0.01 |
| II | - | - | - | - | |
| Size of Tumor (cm) | 1.02 (1.01–1.03) | 0.04 | N/A | ||
| Tumor Grade | Well Differentiated | 0.86 (0.68–1.10) | 0.23 | N/A | |
| Moderately Differentiated | 0.81 (0.70–0.94) | <0.01 | |||
| Poorly Differentiated | - | - | |||
| Lymphovascular Invasion | Present | 1.95 (1.51–2.53) | <0.01 | N/A | |
| Not present | - | - | |||
| Carcinoembryonic Antigen (CEA) | Elevated | 1.42 (1.24–1.64) | <0.01 | N/A | |
| Undetermined | 1.77 (0.79–3.96) | 0.17 | |||
| Negative | - | - | |||
| Treatment Characteristics | |||||
| Treatment Type | No Treatment | 6.52 (5.62–7.55) | <0.01 | 5.66 (4.84–6.63) | <0.01 |
| Surgery Alone | 2.22 (1.95–2.52) | <0.01 | 1.98 (1.73–2.27) | <0.01 | |
| Short-Course RT Then Surgery | 1.58 (1.23–2.02) | <0.01 | 1.51 (0.97–1.88) | 0.09 | |
| S+CRT | 1.18 (0.90–1.53) | 0.46 | 1.17 (0.90–1.52) | 0.25 | |
| CRT+S | - | - | - | - | |
| Extent of Resection | No Surgery | 4.98 (4.37–5.68) | <0.01 | N/A | |
| R1 | 2.15 (1.79–2.60) | <0.01 | |||
| R0 | - | - | |||
| Regional Lymph Nodes Examined | 0 | 1.93 (1.69–2.21) | <0.01 | N/A | |
| <10 | 0.92 (0.79–1.07) | 0.27 | |||
| 10 – 14 | 0.87 (0.75–1.02) | 0.09 | |||
| >14 | - | - | |||
| Regional Lymph Nodes Positive at Surgery | pN0 | 0.61 (0.64–0.70) | <0.01 | N/A | |
| pN+ | - | - | |||
| Time from Diagnosis to Treatment Start | 0–30 days | 1.22 (0.88–1.69) | 0.25 | N/A | |
| 31–90 days | 0.88 (0.63–1.23) | 0.44 | |||
| >90 days | - | - | |||
| Facility Type | Community Cancer Program | 1.10 (0.94–1.28) | 0.24 | -- | -- |
| Comprehensive Community Cancer Program | 1.09 (0.97–1.24) | 0.15 | -- | -- | |
| Academic Program | - | - | -- | -- | |
| Facility Location | Northeast | 1.13 (0.96–1.33) | 0.11 | 1.17 (0.98–1.39) | 0.08 |
| South | 1.23 (1.05–1.44) | <0.01 | 1.28 (1.08–1.50) | <0.01 | |
| Midwest | 1.02 (0.87–1.19) | 0.85 | 1.18 (1.00–1.40) | 0.06 | |
| West | - | - | - | - | |
RT = Radiation Therapy
CRT+S = Definitive concurrent chemoradiation followed by definitive resection
S+CRT = Definitive surgery followed by concurrent chemoradiation
N/A = Not applicable
Bold indicates statistical significance
Note that variable directly related to receiving some treatment (as indicated by N/A) could not be included in the multivariable model due to their high-collinearity with receipt of no therapy. Lymphovascular invasion and CEA level could not be incorporated into the multivariable model due to the high number missing this data. Race and year of diagnosis were not included in the multivariable model after exclusion of variables with an alpha value of 0.1 or greater.
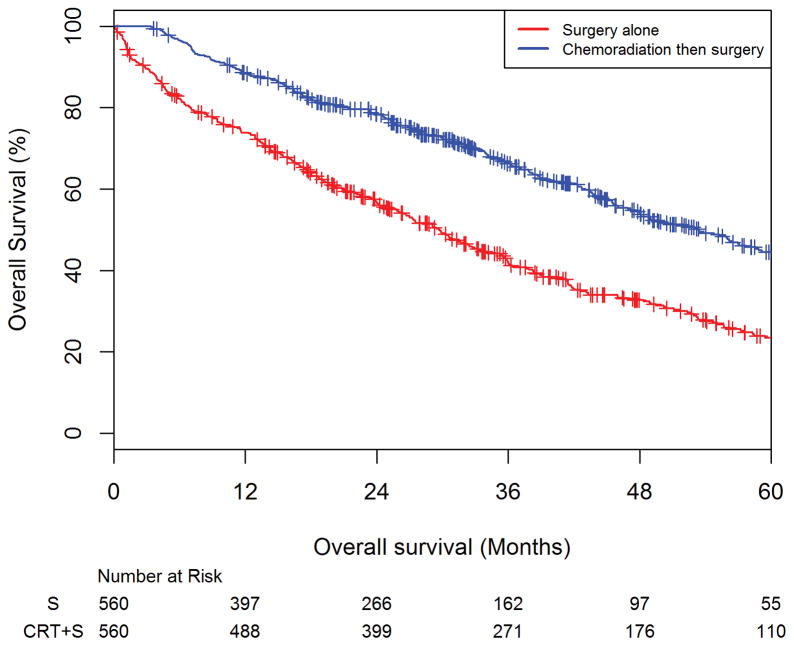
After propensity-score matching, there were 560 patients in the surgery alone cohort and 560 patients in the CRT+S cohort. Receiving surgery alone was associated with worse OS compared with CRT+S (HR=1.94, 95%CI: 1.65–2.27) in this model. Among the matched-cohort, the 3 and 5-year overall survival estimated rates for patients receiving surgery alone were 42.1% (95%CI: 37.6–46.4%) and 23.5% (95%CI: 19.2%–28.1%) respectively. The 3 and 5-year overall survival estimated rates for patients receiving chemoradiation followed by surgery were 66.2% (95%CI: 61.9–70.2%) and 44.5% (95%CI: 39.4%–49.5%) respectively (Figure 2).
Figure 2. Overall survival amongst elderly rectal adenocarcinoma patients on propensity-score matched analysis comparing surgery alone versus chemoradiation followed by surgery.
Kaplan-Meier curve showing the overall survival (OS) estimates of the propensity-score matched cohort of patients as stratified by surgery alone versus chemoradiation followed by surgery, with the number at risk below the x-axis. The 3 and 5-year overall survival estimated rates for patients receiving surgery alone were 42.1% (95%CI: 37.6–46.4%) and 23.5% (95%CI: 19.2%–28.1%) respectively. The 3 and 5-year overall survival estimated rates for patients receiving chemoradiation followed by surgery were 66.2% (95%CI: 61.9–70.2%) and 44.5% (95%CI: 39.4%–49.5%) respectively.
Discussion
Our series is the largest in the literature examining treatment patterns and survival outcomes of octogenarians and nonagenarians with stage II and stage III rectal adenocarcinomas. We found that, despite an older population, deviation from guideline care was associated with worse OS. These results persisted on multivariable and exploratory interaction variable testing, suggesting that the benefit to receiving therapy is independent of age, race, sex, and co-morbidity status as measured by the Charlson-Deyo score. Additionally, we saw an OS benefit for patients treated with CRT+S compared to surgery alone on propensity-score matched analysis. Our series also confirmed known poor prognostic factors in rectal cancer including incomplete resection, higher stage, male sex, advancing age, positive lymph nodes at time of surgery, and higher co-morbidities3, 16–18. Alarmingly, our series found that black race and living in a lower-educated census tract were found to be associated with not receiving any therapy. Conversely, we found that male sex, treatment in the Midwest or Northeast, and lower comorbidity status were associated with receiving CRT+S.
Patients ≥80 years old are not well represented on the randomized trials that have defined CRT+S as the standard of care for stage II/III rectal cancer. In the landmark German Phase III rectal cancer trial, 823 patients with stage II/III rectal cancer were randomized to preoperative chemoradiation followed by TME and adjuvant 5FU chemotherapy versus the same schedule of chemoradiation used postoperatively2, 3. At 10 years, there was improvement in local control in the preoperative arm (92.9%) versus the postoperative arm (89.9%) without improvement in disease-free survival. These results shifted the historical paradigm of postoperative chemoradiation19, 20 to a preoperative approach. However, the oldest person on this trial was 77 years old, leading to concerns about its applicability in octogenarians and nonagenarians. In a similarly designed trial in the United States population, 267 patients with cT3, cT4, or cN+ rectal cancers were randomized to preoperative versus postoperative chemoradiation4. Unlike the German trial there was no observed difference in locoregional control (5-year rates of 10.7% in both arms) but there was a 5-year disease-free survival benefit to preoperative versus postoperative therapy (64.7% vs. 53.4% respectively). In this trial, patients were stratified at age 60 years old, but there is no information about the range of patient age nor about the percentage of patients aged 80 years or older. In our current series, we demonstrate that receipt of CRT+S was associated with improved OS on multivariable, exploratory interaction testing, and propensity-score matched analysis, suggesting that preoperative CRT be explored in octogenarians and nonagenarians with stage II/III rectal cancers, and not be omitted simply given the patient’s age.
Due to their underrepresentation on landmark trials, there have been retrospective studies looking at the utility of treatment in elderly patients. In a study of 642 patients ≥75 years old with resectable rectal cancer in the Dutch Cancer Registry comparing short-course radiation followed by surgery (RT+S) versus surgery alone, local recurrence was less common in the radiation group compared to surgery alone (2% vs. 6% respectively)21. However, increased toxicity was seen in the RT+S group, and there was a significant increase in perioperative deaths for patients with “severe comorbidities” in the RT+S cohort. The authors concluded that omitting preoperative radiation may be suitable in elderly patients with additional risks. Conversely, a recent study from the Swedish Cancer Registry of 2,300 patients with resectable rectal cancer, where 70.3% had preoperative radiation, found that in patients with higher comorbidity (Charlson-Deyo score ≥1) there was an OS improvement with RT+S (HR=0.65) versus surgery alone17. The study included 433 patients ≥80 years old, and subset analysis showed a benefit in local control but not OS with RT+S. The authors concluded that omitting preoperative radiation in patients with comorbidities should be cautioned. In our series, short-course radiation followed by surgery was infrequently used (137 patients, 5.0% of entire group) and on multivariable analysis was found to trend towards lower OS compared with CRT+S (HR=1.52, 95%CI: 0.98–1.96). In an analysis of the Surveillance, Epidemiology, and End Results Database (SEER) of 21,390 localized rectal cancer patients with median age of 68 (interquartile range of 55 to 77) was undertaken to examine the impact of age on therapy received and cancer outcomes22. The authors found that for every 5 years past 70 years old, there was a 37% relative risk increase in cancer-related mortality as well as a 46% relative risk decrease in receiving appropriate cancer-directed surgery. The authors concluded that elderly patients have worse disease outcomes, but are also more likely to not receive standard therapy. Taken together, these findings are congruent to our series as rising age and rising comorbidity status were associated with worse OS amongst all stages, but an OS benefit was still seen with the delivery of CRT+S on multiple statistical methodologies. Taken together, there may be a benefit to CRT+S in this elderly cohort, independent of age, race, sex, and comorbidities. Additionally, we found that on multivariable analysis that adjuvant chemoradiation had no OS difference (HR=1.10, 95%CI: 0.83–1.46) when compared to preoperative chemoradiation. This suggests that either approach can be justified, even in this elderly cohort, with the caveat that only 4.8% of our series had surgery followed by chemoradiation. We saw a significant number of patients ≥80 years old receiving no therapy (14.9% in the entire cohort), higher than reported in the SEER study.
Sociodemographic factors have been known to influence outcomes of patients with rectal cancer. Previous studies have shown that African-American race is associated with higher mortality compared to Whites23, 24, with some suggestion that this is related to unequal access to screening25. These disparate outcomes are likely related to socioeconomic status (SES), which may serve as a proxy for income, education, and access to care26. Our series confirms many of these findings using a database that captures 70% of newly diagnosed malignancies in the US. Additionally, our series is the first to recapitulate these SES factors leading to lack of care specifically in an elderly cohort of rectal cancers. As no patients in our series lacked insurance, as would be expected in an elderly population where Medicare is available, these disparities in treatment received are even more alarming.
Our series has several strengths and limitations. The strengths include our sample size; the largest assessment of stage II and stage III rectal cancer in octogenarians and nonagenarians ever reported. We are the first to summarize practice patterns of this population in the United States as well as SES and demographic factors associated with receiving different therapies. The limitations of our study are namely the lack of uncaptured variables and outcomes in the NCDB, like other cancer registries and retrospective data analyses. We performed stratified analysis, multivariable, interaction variable testing, and propensity score matched analysis to attempt to account for these confounding variables, and there continued to be a persistent OS benefit to receiving CRT+S. The HR generated comparing patients receiving surgery alone to those receiving CRT+S was similar in the multivariable model (HR=1.98, 95%CI: 1.73–2.27) as with the propensity-score matched model (HR=1.94, 95%CI: 1.65–2.27), suggesting that the adjustments for cofounders in the multivariable analysis may be adequate. However, despite our statistical methodology, there are likely cofounders that cannot be evaluated. We cannot accurately report on the effect of margin status, lymph node-yield at surgery, and other treatment related factors on OS as these variables were direct functions of treatment type. We cannot comment on the impact of adjuvant chemotherapy on OS due to NCDB coding limitations. Additionally, we cannot comment on the tumor control rates or cancer-specific survival as this information is not reported in the NCDB, and it is possible the OS benefit to CRT+S is due to competing risks of death, as the patients that received CRT+S were more likely to be younger and have lower medical comorbidities. Most prospective studies examining preoperative therapies for stage II/III rectal cancers in the TME surgical era have found a local control benefit to therapy but no survival differences2, 4, 27, further implying the overall survival differences seen in our series may be patient selection driven, despite our efforts to reduce bias. We also cannot comment on if all definitive resections used TME technique as this is not delineated in the NCDB, but our series is purposefully limited to the TME-era to address this. We also cannot comment on the effect of short-course preoperative radiation or postoperative chemoradiation in a propensity-score matched analysis, both utilized treatment approaches, as there was a limited number of these patients in this series. In the multivariable model, use of short-course radiation or adjuvant chemoradiation appeared to statistically not be associated with worse OS compared with CRT+S. Treatment toxicity is extremely relevant in any population, specifically an elderly cohort, and we cannot comment on that in this series. CRT is associated with fibrosis, fecal incontinence, and myelosuppression28–30. Conflicting evidence exists regarding treatment toxicity in elderly patients, with one series showing that CRT+S is comparably tolerated and adhered to as younger patients31 while another series reported worse severe toxicity and more permanent stomas compared to younger patients32.
Conclusion
In this analysis of the NCDB, we found that 14.9% of octogenarians and nonagenarians diagnosed with stage II and III rectal cancer do not receive cancer-directed curative therapy. Patients who received standard of care therapy of preoperative chemoradiation followed by definitive resection had an OS benefit compared to those who received surgery alone or no therapy. We found that older age, African-American race, and living in a lower-educated census tract among other variables were associated with not receiving therapy, despite all patients in our series having health insurance. Overall this series suggests that adherence to the standard of care established through trials with younger patients, should be a consideration in decision making about the risks and benefits of treatment for patients ≥80 years old with stage II and III rectal adenocarcinoma. Efforts to limit health care disparities should be further investigated.
Supplementary Material
Supplementary Figure 1: The Consolidated Standards of Reporting Trials (CONSORT) diagram of patients 80 years and older with stage II/III rectal adenocarcinoma in the National Cancer Data Base.
Acknowledgments
Financial/Grant Support: This work was supported by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and National Cancer Institute at the National Institute of Health (Grant Number P30CA138292). This funding supported the statisticians who helped with study design, performed the analysis and helped with data interpretation. This funding did not have any role in the writing of the manuscript or the decision to submit it for publication. As the corresponding author, I have had full access to the data in the study and hold final responsibility for the decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
Conflicts of Interest/Disclosures: None
Author contributions: RJC (all aspects), JMS (data collection, statistical analysis), EC (data collection, statistical analysis), RJ (data collection), JJ (planning, review), KP (planning, review), DGT (planning, review), MCR (planning, review), CES (planning, review), TWG (planning, data collection, editing, review), MWD (planning, review), JCL (all aspects)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 6.Kent EE, Malinoff R, Rozjabek HM, et al. Revisiting the Surveillance Epidemiology and End Results Cancer Registry and Medicare Health Outcomes Survey (SEER-MHOS) Linked Data Resource for Patient-Reported Outcomes Research in Older Adults with Cancer. J Am Geriatr Soc. 2016;64:186–192. doi: 10.1111/jgs.13888. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB American Joint Committee on Cancer. AJCC cancer staging manual. 7. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Nickleach DLY, Shrewsberry A, et al. SAS® Macros to Conduct Common Biostatistical Analyses and Generate Reports. SESUG 2013: The Proceeding of the SouthEast SAS User Group. 2013 [Google Scholar]
- 11.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: Regression modeling. Bone Marrow Transplant. 2001;28:1001–1011. doi: 10.1038/sj.bmt.1703271. [DOI] [PubMed] [Google Scholar]
- 12.LSP. Reducing bias in a propensity score matched pair sample using greedy matching techniques. SAS SUGI. 2001;26:214–226. [Google Scholar]
- 13.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson P. On robustness and model flexibility in survival analysis: transformed hazard models and average effects. Biometrics. 2007;63:69–77. doi: 10.1111/j.1541-0420.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 15.Lin DY, LW The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074–1078. [Google Scholar]
- 16.Saraste D, Gunnarsson U, Janson M. Local excision in early rectal cancer-outcome worse than expected: a population based study. Eur J Surg Oncol. 2013;39:634–639. doi: 10.1016/j.ejso.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Elliot AH, Martling A, Glimelius B, Johansson H, Nilsson PJ. Impact of pre-treatment patient-related selection parameters on outcome in rectal cancer. Eur J Surg Oncol. 2016 doi: 10.1016/j.ejso.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Siegel R, Burock S, Wernecke KD, et al. Preoperative short-course radiotherapy versus combined radiochemotherapy in locally advanced rectal cancer: a multi-centre prospectively randomised study of the Berlin Cancer Society. BMC Cancer. 2009;9:50. doi: 10.1186/1471-2407-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 20.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 21.Maas HA, Lemmens VE, Nijhuis PH, de Hingh IH, Koning CC, Janssen-Heijnen ML. Benefits and drawbacks of short-course preoperative radiotherapy in rectal cancer patients aged 75 years and older. Eur J Surg Oncol. 2013;39:1087–1093. doi: 10.1016/j.ejso.2013.07.094. [DOI] [PubMed] [Google Scholar]
- 22.Chang GJ, Skibber JM, Feig BW, Rodriguez-Bigas M. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg. 2007;246:215–221. doi: 10.1097/SLA.0b013e318070838f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30:401–405. doi: 10.1200/JCO.2011.37.5527. [DOI] [PubMed] [Google Scholar]
- 24.Tawk R, Abner A, Ashford A, Brown CP. Differences in Colorectal Cancer Outcomes by Race and Insurance. Int J Environ Res Public Health. 2015;13:ijerph13010048. doi: 10.3390/ijerph13010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkins T, Gillies RA, Harbuck S, Garren J, Looney SW, Schade RR. Racial disparities and barriers to colorectal cancer screening in rural areas. J Am Board Fam Med. 2012;25:308–317. doi: 10.3122/jabfm.2012.03.100307. [DOI] [PubMed] [Google Scholar]
- 26.Lee W, Nelson R, Mailey B, Duldulao MP, Garcia-Aguilar J, Kim J. Socioeconomic factors impact colon cancer outcomes in diverse patient populations. J Gastrointest Surg. 2012;16:692–704. doi: 10.1007/s11605-011-1809-y. [DOI] [PubMed] [Google Scholar]
- 27.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 28.Minsky BD, Conti JA, Huang Y, Knopf K. Relationship of acute gastrointestinal toxicity and the volume of irradiated small bowel in patients receiving combined modality therapy for rectal cancer. J Clin Oncol. 1995;13:1409–1416. doi: 10.1200/JCO.1995.13.6.1409. [DOI] [PubMed] [Google Scholar]
- 29.Temple LK, Wong WD, Minsky B. The impact of radiation on functional outcomes in patients with rectal cancer and sphincter preservation. Semin Radiat Oncol. 2003;13:469–477. doi: 10.1016/S1053-4296(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 30.Prabhu RS, Cassidy RJ, Landry JC. Radiation therapy and neutropenia. Curr Probl Cancer. 2015;39:292–296. doi: 10.1016/j.currproblcancer.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Guimas V, Boustani J, Schipman B, et al. Preoperative Chemoradiotherapy for Rectal Cancer in Patients Aged 75 Years and Older: Acute Toxicity, Compliance with Treatment, and Early Results. Drugs Aging. 2016;33:419–425. doi: 10.1007/s40266-016-0367-0. [DOI] [PubMed] [Google Scholar]
- 32.Francois E, Azria D, Gourgou-Bourgade S, et al. Results in the elderly with locally advanced rectal cancer from the ACCOR12/PRODIGE 2 phase III trial: tolerance and efficacy. Radiother Oncol. 2014;110:144–149. doi: 10.1016/j.radonc.2013.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: The Consolidated Standards of Reporting Trials (CONSORT) diagram of patients 80 years and older with stage II/III rectal adenocarcinoma in the National Cancer Data Base.