Abstract
This study analyzed the distribution of endophytic fungi in 3 coastal environments with different climatic, geographical, and geological characteristics: the volcanic islands of Dokdo, the East Sea, and the West Sea of Korea. The isolated fungal endophytes were characterized and analyzed with respect to the characteristics of their host environments. For this purpose, we selected common native coastal halophyte communities from three regions. Molecular identification of the fungal endophytes showed clear differences among the sampling sites and halophyte host species. Isolates were also characterized by growth at specific salinities or pH gradients, with reference to previous geographical, geological, and climate studies. Unlike the East Sea or West Sea isolates, some Dokdo Islands isolates showed endurable traits with growth in high salinity, and many showed growth under extremely alkaline conditions. A smaller proportion of West Sea coast isolates tolerate compared to the East Sea or Dokdo Islands isolates. These results suggest that these unique fungal biota developed through a close interaction between the host halophyte and their environment, even within the same halophyte species. Therefore, this study proposes the application of specific fungal resources for restoring sand dunes and salt-damaged agricultural lands and industrialization of halophytic plants.
Keywords: Coastal fungi, Endophyte, Halophyte, Microbial diversity, Phylogenetic community structure
Endophytic microorganisms, such as the fungi inhabiting the inner tissues and/or organs of plants, are regarded as valuable ecological and agricultural resources [1]. Generally, endophytic fungal species have a positive impact on their host by enhancing plant growth or inducing systemic resistance [2,3]. Therefore, numerous recent studies have focused on positive fungus-plant interactions. Halophyte plant species can withstand a certain amount of salt in soil environment, which is the microecological zone surrounding the plant roots, and they can be used to restore salt-damaged environments, including agricultural lands, sand dunes, and mud flats. Thus, securing and characterizing such fungal species and revealing the phylogenetic relationships among these endophytic microorganisms are major strategies for utilizing these microbial resources [4,5].
Soil microbial communities can be modified by environmental factors. Thus, their functional and physiological features often reflect their environment [6]. Unique environments or interactions with halophytic hosts can affect the physiological or phylogenetic characteristics of the microbiome [7], and the structure and composition of fungal endophyte communities are influenced by many factors and complex interactions [8,9,10,11,12,13,14,15,16,17].
This study focused on the distribution and characteristics of endophytic fungal biota in relation to host plant species native to contrasting geographic and environment. To this end, endophytic fungi were isolated from 3 halophyte species, Sedum oryzifolium, Lysimachia mauritiana, and Aster spathulifolius, which are native to the coastal areas of Korea, including the sampling sites, the Dokdo Islands, West Sea, and East Sea. The fungi were then characterized both taxonomically, based on their nuclear ribosomal internal transcribed spacer (nrITS) sequences, and functionally, by analysis of their growth properties at different salinities and pH values. Depend on previous geographical studies [5,18,19] and the physiological properties of the host halophytes [4,20], we found differences between endophytic groups according to environmental conditions even if they shared the same halophyte host species [5,18,19]. Thus, comparative analysis and characterization of symbiotic fungi based on phenotype and host environment may facilitate environmental remediation by promoting the adaptation of halophyte species to harsh environments. To our knowledge, this is the first comparative study that reveals the fungal flora interacting with halophytic plants inhabiting coastal environments.
MATERIALS AND METHODS
Halophyte sampling and isolation of endophytic fungi
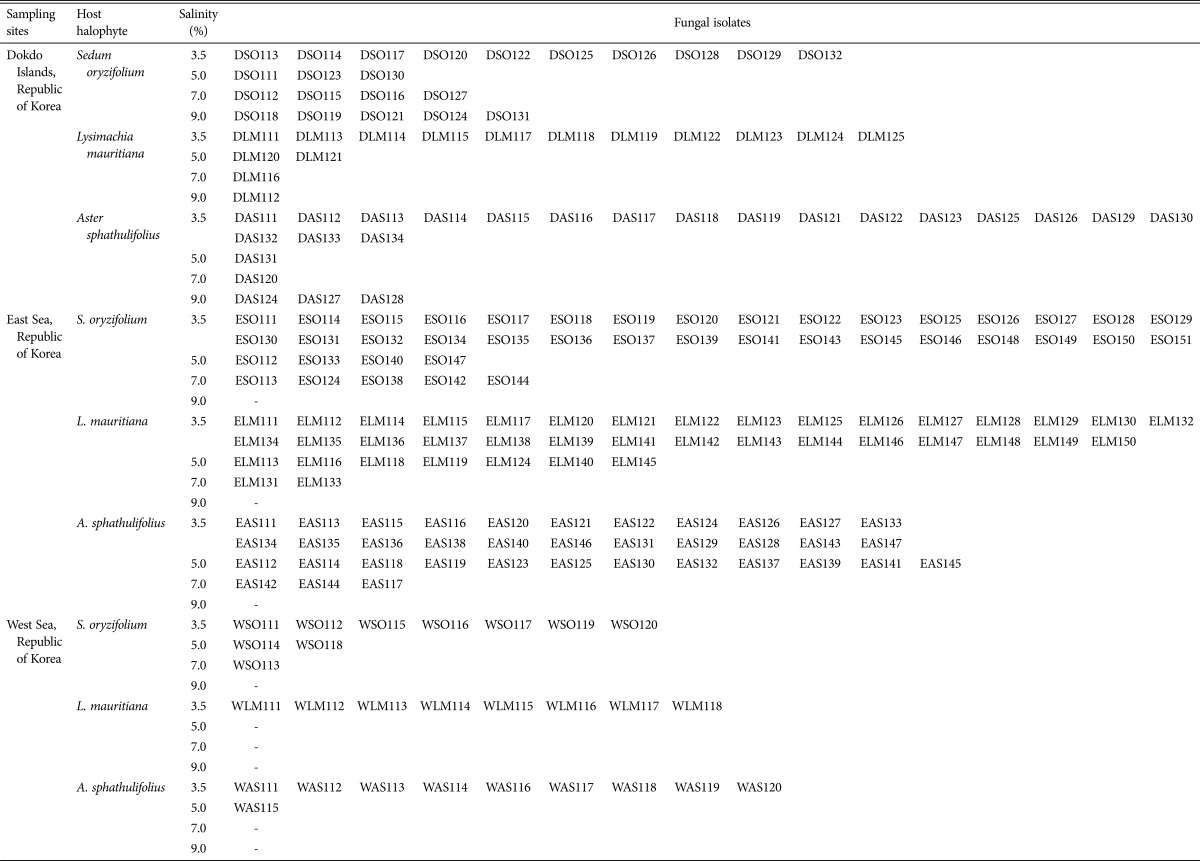
Three representative communities of halophytes (Sedum oryzifolium, Lysimachia mauritiana, and Aster spathulifolius) [20] native to the coasts of the Dokdo Islands, East Sea, and West Sea of Korea were sampled from the locations listed in Table 1. Fifty individuals per halophytic plant species per site were sampled (450 individuals in total). Plant specimens were collected in sterile bottles and stored at 4℃. The root was rinsed with sterile distilled water (SDW) and 0.1% Tween solution (Sigma-Aldrich, St. Louis, MO, USA) to eliminate suspended solids. Then, the root were treated with a 1.0% sodium hypochlorite solution for 5min to sterilize the root surface. Subsequently, the samples were submerged in 1% perchloric acid for 5 min and washed extensively with SDW twice. Residual water was eliminated with sterile gauze, and 50 pieces (3 cm in length) were cut from the roots of the plant. These samples were placed in Hagem minimal medium containing 80 ppm streptomycin (Sigma-Aldrich) to inhibit the growth of root bacteria or actinomycetes, and then incubated at 25℃ for 20 days. Endophytic fungi were isolated by subculturing in the same medium at the same temperature. Pure isolates were then subcultured on potato dextrose agar (PDA; Difco, Franklin Lakes, NJ, USA) and selected based on morphological (color, colony shape, etc.) differences [7].
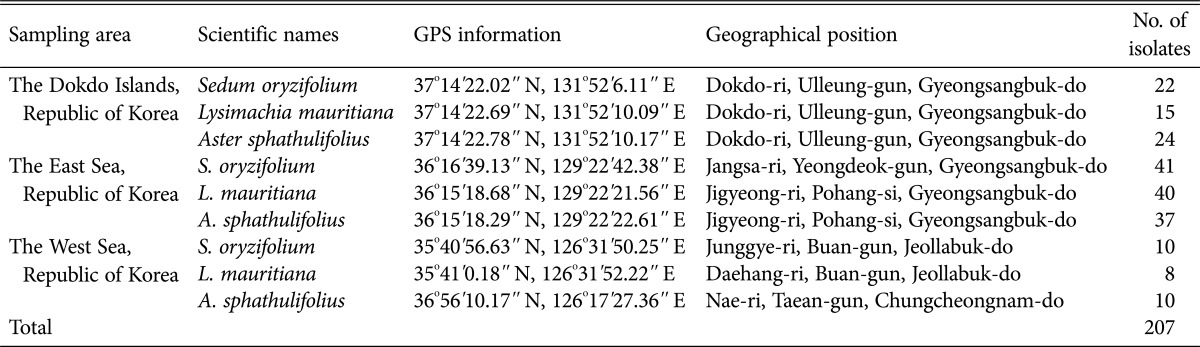
Table 1. Information of sampling sites and number of isolated endophytic fungal per each halophytic species.
Extraction of genomic DNA and PCR
Endophytic fungi from halophytic plants were inoculated into potato dextrose broth (PDB; Difco) medium and incubated at 25℃ with shaking at 120 rpm for 7 days. Filtered mycobionts were lyophilized for 2 days. Genomic DNA was extracted from lyophilized mycobionts using the DNeasy Plant Mini kit (Qiagen, Valencia, CA, USA). Primers (ITS1 and ITS4) targeting nrITS region were used for PCR amplification [21]. The PCR cycling conditions were as follows: predenaturation (94℃, 4min), followed by denaturation (94℃, 1 min), annealing (52–58℃, 1 min), and extension (72℃, 2 min) for 35 cycles, and a final extension step (72℃, 2min) [21]. PCR products were confirmed by electrophoresis on a 1.5% agarose gel stained with ethidium bromide. Bands were observed using a UV transilluminator, purified with the AccuPrep PCR & Gel Extraction kit (Bioneer, Daejeon, Korea), and sequenced using an ABI 3730XL DNA analyzer (Applied Biosystems, Carlsbad, CA, USA) [21].
Fungal identification and diversity
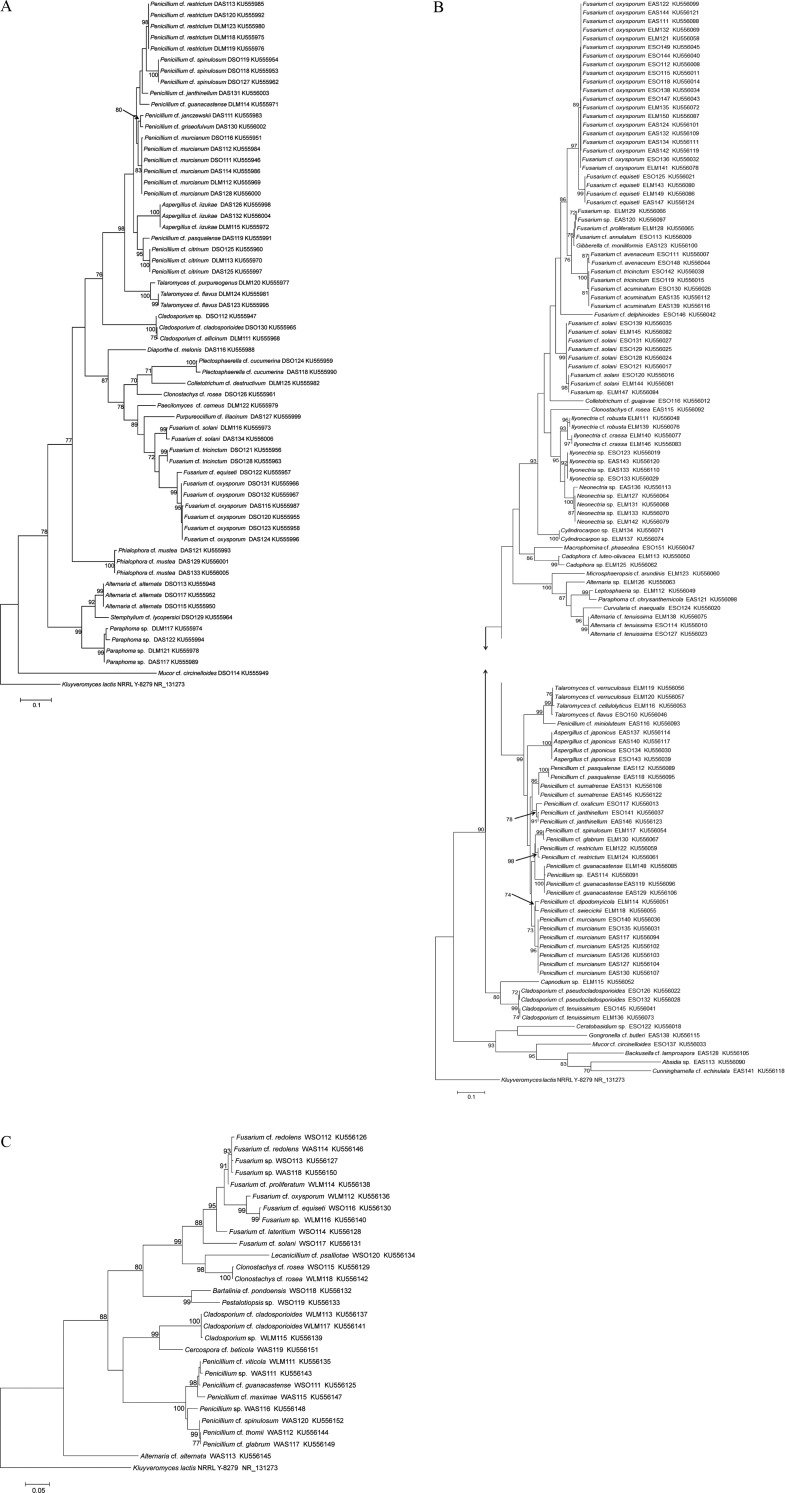
To confirm the identity of the isolates, a phylogenetic tree was constructed. Endophytic fungal nrITS sequences showed greater than 99% similarity with the sequences of other fungal species in the GenBank databases of the National Center for Biotechnology Information (NCBI). Phylogenetic relationships were analyzed by MEGA ver. 6.0 using alignments prepared with ClustalW [7]. Phylogenetic trees were inferred using the maximum-likelihood algorithm and Kimura 2-parameter distances. The stability of relationships was evaluated by bootstrap analysis with 1,000 replications [7,22]. All endophytic fungal isolate sequences (207) were deposited in NCBI GenBank (accession Nos. KU555946 to KU556152) (Supplemental Table 1). The diversity [6] of the isolates was determined by assessing species richness based on the Margalef (Dmg) [23] and Menhinick (Dmn) indices [24], and species diversity was measured by Shannon (H) [25] and Simpson (D) indices [25].
Physiological characterization in NaCl and pH gradients
Growth of fungal isolates at different NaCl concentrations and pH values was determined by weighing the dried fungal colonies following incubation in liquid media with different salt levels and pH values, respectively. The detailed experimental procedures are as follows. Fresh colonies of each isolate were cultivated on PDA medium and then used to inoculate PDB liquid medium containing different concentrations of NaCl (3.5%, 5.0%, 7.0%, and 9.0% [w/v]). The fungal strains were incubated for 7 days at 25℃ with shaking at 150 rpm and then harvested by filtration. The harvested fungal culture filtrates were lyophilized at −80℃, and then the dried biomass was measured. Growth at each pH value was determined in PDB medium adjusted to various pH values (pH 4.0, 5.5, 7.0, and 9.0) by the addition of hydrogen chloride (HCl) or sodium carbonate (Na2CO3) [26,27,28]. Incubation and measurement of each fungal isolate was done as described for the salinity tolerance test.
RESULTS AND DISCUSSION
Fungal identification
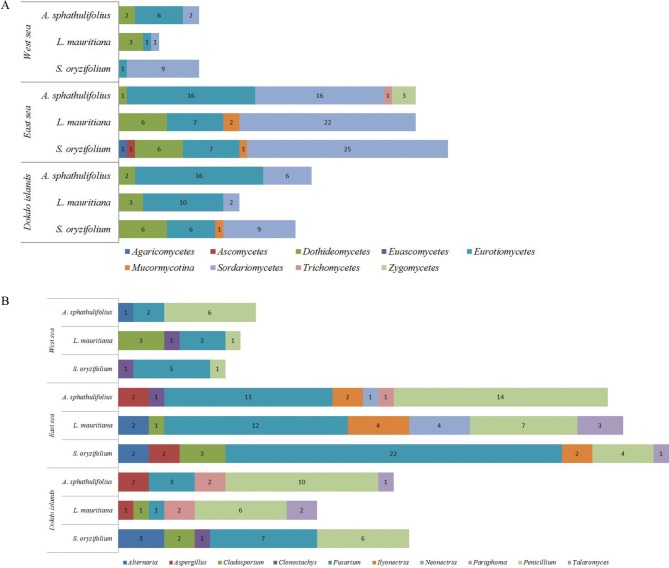
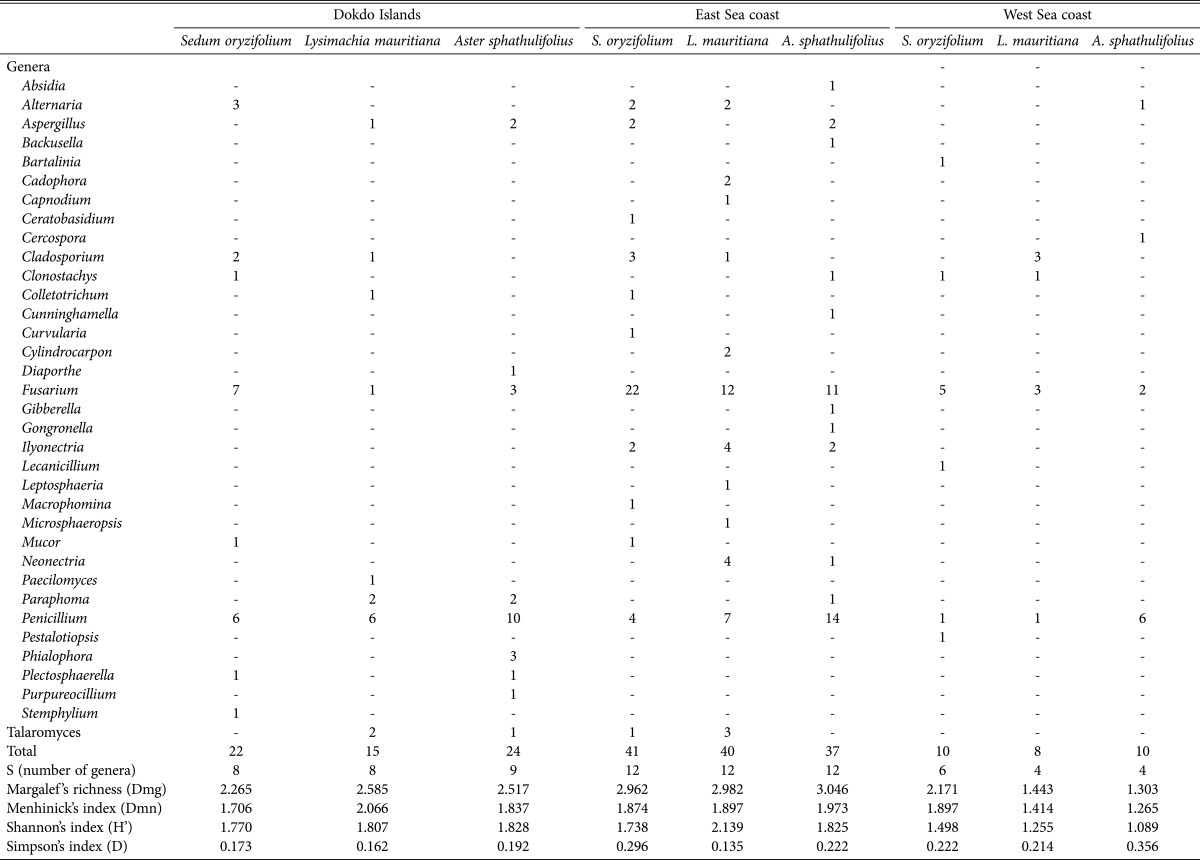
A total of 207 isolates were obtained from 3 species of halophytic plants native to the coasts of Dokdo (61 isolates) and the East Sea (118 isolates) and West Sea (28 isolates) (Table 1). The isolates from the East Sea coast were the most morphologically diverse. The distribution ratios (Fig. 1A and 1B) and phylogenetic relationships (Fig. 2A–2C), for each genus per halophytic host are presented (Supplementary Tables 1,2,3,4). The isolates were grouped into 3 phyla (Ascomycota, Zygomycota, and Basidiomycota), 8 classes (Agaricomycetes, Ascomycetes, Dothideomycetes, Euascomycetes, Mucoromycotina, Sordariomycetes, Trichomycetes, and Zygomycetes), and 36 genera based on comparison to nrITS sequences in databases by BLAST (Supplementary Tables 1,2,3,4). The Dokdo Islands and East Sea and West Sea coast isolates belonged to 16, 25, and 9 genera, respectively. Among 36 fungal genera, only Fusarium and Peniciliium species was commonly identified in all halophyte species from all geological regions. Interestingly, Ilyonectria species was commonly identified in all plant species in the East Sea coast. Depend on geological distribution, Penicillium was shown to dominant fungal species in the Dokdo Islands or East Sea coast, but Fusarium species showed dominance in the West Sea coast. Dominant fungal genera in specific halophyte species was varied by their native geological region: S. oryzifolium (Fusarium in all three coasts), L. mauritiana (Dokdo Islands: Penicillium, East Sea and West Sea coast: Fusarium), A. sphathulifolius (Dokdo Islands and the West Sea coasts: Penicillium, East Sea coast: Fusarium). As the final outcome, Fusarium and Penicillium are dominated in each halophyte species, and also identified as commonly distributed in all sites or all halophyte species. On the other hand, fungal species which showing low dominances in each of plant species or geological regions showed restricted distribution. Regarding the geological location of each sampling fields (segregated by at least 300 km), common fungal genera from ecologically segregated region were more likely to be closely associated with their host halophyte species. In this study, Fusarium or Penicillium might play a role as closer symbiosis to their host plants more than other fungal genera. Except these two taxa, other fungal genera might be under weak interdependency with their host plants, and variable by their geographic character.
Fig. 1. Endophytic distribution of isolates from halophytes native to each coastal region, based on class level (A), genus level (B) (described top 10 genera). A. spathulifolius, Aster spathulifolius; L. mauritiana, Lysimachia mauritiana; S. oryzifolium, Sedum oryzifolium.
Fig. 2. Phylogenetic trees of fungal endophytes from each coastal area. Trees were obtained using the maximum-likelihood algorithm with the Kimura 2-parameter. Kluyveromyces lactis NRRLY-827 NR131273 was used as the outgroup. The accession number is given in parentheses. Bootstrap values > 70% are shown alongside the branch considered. Trees of fungal isolates from the Dokdo Islands (A), the East Sea coast (B), the West Sea coast (C).
Species richness and diversity indices
The fungal diversity was analyzed (Table 2). According to the Margalef richness index, which describes the number of different species represented in an ecological community, East Sea coast showed higher value than that of the Dokdo Islands or the West Sea coast. Species richness in plant S. oryzifolium is as follows: East Sea coast (3.046) >Dokdo Islands (2.517) > West Sea coast (1.303), and this order is not different in other halophyte species. Unique, harse environment of the Dokdo Islands might limit the species richness in endophyte community, comparing the East Sea coast and the Dokdo Islands. Meanwhile, there was no meaningful pattern comparing the species richness between halophytic species native to same geological location. Regarding these results, fungal species richness was strongly affected by unique environment, rather than halophyte species. Nevertheless, commonly distributed fungal genera Penicillium or Fusarium in all sites or plant species indicates close interaction with their host halophyte with overcoming strong affection of geographical characteristic. Meanwhile, extraordinarily low dominance or restrictive distribution of fungal genera (except Penicillium or Fusarium) indicates environmental selective pressure to endophytic fungal community.
Table 2. Fungal diversity analysis from each of the halophytes native to each coast environment of Korea.
Characterization based on growth properties across a NaCl or pH gradient
To identify how each salt-damaged environment affected the endophytic fungal biota, growth was measured at various NaCl concentrations and pH values (Tables 3 and 4). In the case of the fungal isolates from Dokdo, about 15% of isolates grew optimally in 9.0% (w/v) NaCl, irrespective of halophyte species. In contrast, there was no isolates from the East Sea or West Sea which grew at 9% (w/v). Halophytes require salt for survival. Therefore, comparatively higher concentrations of Na+ might be absorbed by halophytes native to the Dokdo Islands than the concentrations absorbed by those on the East Sea or West Sea coasts [29]. This might be caused by the drastic seawater intrusion and accumulation of salt on the coast of the Dokdo Islands [18]. The West Sea samples presented a smaller percentage of isolates which showing endurance to such high salt gradient than the East Sea samples, presumably due to the lower seawater intrusion and following Na+ accumulation. We concluded that the fungal endophytes may have evolved such tolerable traits because of their symbiotic relationship with host halophytes that are adapted to high-salt coastal environments. Approximately 16% of the Dokdo isolates grew optimally in strong alkaline conditions (pH 9.0 In contrast, most isolates from the East Sea and West Sea grew well at pH 4.0–7.0), but failed to grow at 9% (w/v) except for ELM149. Moreover, isolates from L. mauritiana and A. spathulifolius, which are native to the West Sea, only grew at pH 4.0–7.0. Considering that the pH of seawater is approximately 8.3 [30], the large seawater intrusions or low soil buffering capacity of the Dokdo Islands might lead to Na+ accumulation in the halophytes' internal tissue [20,29,31]. Thus, the high proportion of alkalophilic fungal endophytes might have resulted from adaptation to this specialized plantenvironment interaction. Conversely, the neutral or alkaline pH of the West Sea may have arisen from low organic matter content and salt accumulation, rather than large seawater intrusions [18]. For this reason, halophytic Na+ absorption might not be as pronounced on the West Sea coast. Therefore, although the rhizosphere soil is alkaline, endophytic fungi from West Sea halophytes that cannot grow at alkaline pH values may adjust the environment inside the host plants, and thus show acidophilic traits. In contrast, the Dokdo Islands isolates grew at a broader pH range than isolates from the East Sea and West Sea coasts (Table 4). The Dokdo Islands are exposed to abrupt rainstorms, drying of volcanic soil by strong winds, sun reflected by water around the islands, and severe sea water intrusions. These complex climatic factors can lead to large variations in rhizosphere pH or salinity; adaptations to such conditions allow a wider distribution of cultivable isolates. The East Sea coast, mostly composed of sandy soil, showed limited glycophytic flora but domination of a halophytic community due to rapid seawater intrusion. Total organic material content is about 1.0%, and surface layer seawater affects coastal soil, which has a salinity of 3.3–3.4% [5,19]. Organic matter content is low due to the sandy soil and shows a pH range of 6.65–7.15 due to continuous wafting of seawater [5]. Water intrusion occurred more slowly than on the Dokdo Islands but rapid compared to West Sea coast [5,19]. In support of this observation, endophytic fungal biota from halophytic plants on the Dokdo Islands and East Sea coasts are affected by strong environmental characters (Fig. 1A and 1B).
Table 3. Growth character of each isolate at each concentration of NaCl.
Table 4. Growth character of each isolate at each pH gradient.
Numerous endophyte microbiomes have been studied with an aim to secure microbial resources for the Nagoya protocol. Endophytic microbiomes are essential resources for agriculture and environmental restoration. The results of this study indicates importance of physiologically appropriate selection of fungal biofertilizers to applicate under specific agricultural conditions. Endophytic fungal biota that have evolved or adapted independent of their unique coastal environment or halophyte host species are the fungal resources that are most needed for successful restoration of diverse and changing environments. A BLAST search revealed that several isolates show relatively high similarity to previously reported beneficial endophytic fungi. In particular, Aspergillus, Cadophora, Trichoderma, Penicillium, Curvularia, Clonostachys, Fusarium, and Lecanicillium are well known genera that promote plant growth and/or protect roots against biotic and abiotic stresses in agricultural lands [7,32,33,34,35,36,37,38]. Among the Dokdo Island isolates, most Penicillium isolates grew at alkaline pH (DLM113, DAS114, DAS128, and DAS130) or under high salt conditions (DSO118, DSO119, DLM112, and DAS128), in line with the results from the growth experiments at pH/saline gradients. These observations demonstrate the importance of applying appropriate fungal resources. Further studies are need to identify the relationships between host plants and the specific genera identified in this study in specific soil conditions.
In this study, diverse endophytic fungal isolates from the roots of halophytic plants on the coasts of the Dokdo Islands and the East Sea and West Sea of Korea were phylogenetically and physiologically characterized relative to their environment. These unique and specific microbial resources could be applied for environmental remediation purposes.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR201518201).
ELECTRONIC SUPPLEMENTARY MATERIAL
Supplementary data including four tables can be found with this article online at http://www.mycobiology.or.kr/src/sm/mb-45-150-s001.pdf.
Information of isolated endophytic fungi from hydrophytes
Distribution of enphytic fungi isolated from halophyte native to the Dokdo Islands
Distribution of enphytic fungi isolated from halophyte native to the East Sea of Korea
Distribution of enphytic fungi isolated from halophyte native to the West Sea of Korea
References
- 1.Bloemberg GV, Lugtenberg BJ. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol. 2001;4:343–350. doi: 10.1016/s1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 2.Gill SS, Gill R, Trivedi DK, Anjum NA, Sharma KK, Ansari MW, Ansari AA, Johri AK, Prasad R, Pereira E, et al. Piriformospora indica: potential and significance in plant stress tolerance. Front Microbiol. 2016;7:332. doi: 10.3389/fmicb.2016.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teixeira da, Tsavkelova EA, Zeng S, Ng TB, Parthibhan S, Dobranszki J, Cardoso JC, Rao MV. Symbiotic in vitro seed propagation of Dendrobium: fungal and bacterial partners and their influence on plant growth and development. Planta. 2015;242:1–22. doi: 10.1007/s00425-015-2301-9. [DOI] [PubMed] [Google Scholar]
- 4.Hameed A, Gulzar S, Aziz I, Hussain T, Gul B, Khan MA. Effects of salinity and ascorbic acid on growth, water status and antioxidant system in a perennial halophyte. AoB Plants. 2015;7:plv004. doi: 10.1093/aobpla/plv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HS, Cho JS, Lee JH, Lee JS. Soil environment of halophyte habitat in golaebul coastal sand-duens of east coast. Korean J Island Res. 2009;21:333–340. [Google Scholar]
- 6.Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You YH, Kwak TW, Kang SM, Lee MC, Kim JG. Aspergillus clavatus Y2H0002 as a new endophytic fungal strain producing gibberellins isolated from Nymphoides peltata in fresh water. Mycobiology. 2015;43:87–91. doi: 10.5941/MYCO.2015.43.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorbækmo MF, Carlsen T, Brysting A, Vralstad T, Høiland K, Ugland KI, Geml J, Schumacher T, Kauserud H. High diversity of root associated fungi in both alpine and arctic Dryas octopetala. BMC Plant Biol. 2010;10:244. doi: 10.1186/1471-2229-10-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haselwandter K. Mycorrhizal status of ericaceous plants in alpine and subalpine areas. New Phytol. 1979;83:427–431. [Google Scholar]
- 10.Haselwandter K, Read DJ. Fungal associations of roots of dominant and sub-dominant plants in high-alpine vegetation systems with special reference to mycorrhiza. Oecologia. 1980;45:57–62. doi: 10.1007/BF00346707. [DOI] [PubMed] [Google Scholar]
- 11.Johnson D, Ijdo M, Genney DR, Anderson IC, Alexander IJ. How do plants regulate the function, community structure, and diversity of mycorrhizal fungi? J Exp Bot. 2005;56:1751–1760. doi: 10.1093/jxb/eri192. [DOI] [PubMed] [Google Scholar]
- 12.Jumpponen A. Soil fungal community assembly in a primary successional glacier forefront ecosystem as inferred from rDNA sequence analyses. New Phytol. 2003;158:569–578. doi: 10.1046/j.1469-8137.2003.00767.x. [DOI] [PubMed] [Google Scholar]
- 13.Lekberg Y, Schnoor T, Kjøller R, Gibbons SM, Hansen LH, Al-Soud WA, Sørensen SJ, Rosendahl S. 454-sequencing reveals stochastic local reassembly and high disturbance tolerance within arbuscular mycorrhizal fungal communities. J Ecol. 2012;100:151–160. [Google Scholar]
- 14.Molina R, Massicotte H, Trappe JM. Specificity phenomena in mycorrhizal symbiosis: community-ecological consequences and practical implications. In: Allen MF, editor. Mycorrhizal functioning: an integrative plant-fungal process. New York: Chapman and Hall; 1992. pp. 357–423. [Google Scholar]
- 15.Natel P, Neumann P. Ecology of ectomycorrhizal-basidiomycete communities on a local vegetation gradient. Ecology. 1992;73:99–117. [Google Scholar]
- 16.Toljander JF, Eberhardt U, Toljander YK, Paul LR, Taylor AF. Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest. New Phytol. 2006;170:873–884. doi: 10.1111/j.1469-8137.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 17.Vare H, Vestberg M, Eurola S. Mycorrhiza and root-associated fungi in Spitsbergen. Mycorrhiza. 1992;1:93–104. [Google Scholar]
- 18.Ahn KD, Cha JS, Choo YS, Ghim SY, Hong SC, Hwang SI, Hwang UW, Jang YD, Jung SH, Kwon OS, et al. Natural heritage of Korea, Dokdo. Daejeon: Cultural Heritage Administration of Korea, Republic of Korea; [Google Scholar]
- 19.Jung YK, Kim W. Distributional characteristics of coastal mantle communities in Korean peninsula. Korean J Ecol. 2000;23:193–199. [Google Scholar]
- 20.Chapman VJ. Salt marshes and salt deserts of the world. In: Reimold RJ, Queen WH, editors. Ecology of halophytes. New York: Academic Press; 1974. pp. 3–19. [Google Scholar]
- 21.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sinsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 22.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margalef DR. Information theory in ecology. Gen Syst. 1958;3:36–71. [Google Scholar]
- 24.Whittaker RH. Evolution of species diversity in land communities. Evol Biol. 1972;10:1–67. [Google Scholar]
- 25.Lambshead PJ, Platt HM, Shaw KM. The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. J Nat Hist. 1983;17:859–874. [Google Scholar]
- 26.Kushner DJ. The halobacteriaceae. In: Woese CR, Wolfe R, editors. The bacteria. Vol. 8. New York: Academic Press; 2008. pp. 124–171. [Google Scholar]
- 27.Madigan MT, Martinko JM, Dunlap PV, Clark DP. Brock biology of microorganisms. 12th ed. Boston (MA): Addison-Wesley Press; 2008. [Google Scholar]
- 28.Krulwich TA, Guffanti AA. Physiology of acidophilic and alkalophilic bacteria. Adv Microb Physiol. 1983;24:173–214. doi: 10.1016/s0065-2911(08)60386-0. [DOI] [PubMed] [Google Scholar]
- 29.You YH, Park JM, Park JH, Kim JG. Specific rhizobacterial resources: characterization and comparative analysis from contrasting coastal environments of Korea. J Basic Microbiol. 2016;56:92–101. doi: 10.1002/jobm.201500195. [DOI] [PubMed] [Google Scholar]
- 30.Choi M, Han S. Remote sensing imageries for land cover and water quality dynamics on the west coast of Korea. Environ Monit Assess. 2013;185:9111–9124. doi: 10.1007/s10661-013-3240-1. [DOI] [PubMed] [Google Scholar]
- 31.Jjemba PK. Environmental microbiology: principles and applications. Enfield (NH): Science Publishers; 2004. [Google Scholar]
- 32.Gao L. Application of two-stage cultivation for exploring the nutritional requirements for sporulation of three biocontrol fungi. Biotechnol Res Int. 2015;2015:682839. doi: 10.1155/2015/682839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goettel MS, Koike M, Kim JJ, Aiuchi D, Shinya R, Brodeur J. Potential of Lecanicillium spp. for management of insects, nematodes and plant diseases. J Invertebr Pathol. 2008;98:256–261. doi: 10.1016/j.jip.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Mani VM, Soundari AP, Karthiyaini D, Preeth K. Bioprospecting endophytic fungi and their metabolites from medicinal tree Aegle marmelos in western ghats, India. Mycobiology. 2015;43:303–310. doi: 10.5941/MYCO.2015.43.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radhakrishnan R, Khan AL, Lee IJ. Endophytic fungal pretreatments of seeds alleviates salinity stress effects in soybean plants. J Microbiol. 2013;51:850–857. doi: 10.1007/s12275-013-3168-8. [DOI] [PubMed] [Google Scholar]
- 36.Razinger J, Lutz M, Schroers HJ, Urek G, Grunder J. Evaluation of insect associated and plant growth promoting fungi in the control of cabbage root flies. J Econ Entomol. 2014;107:1348–1354. doi: 10.1603/ec14004. [DOI] [PubMed] [Google Scholar]
- 37.Yamada A, Ogura T, Degawa Y, Ohmasa M. Isolation of Trichoderma matsutake and T. bakamatsutake cultures from field-collected ectomycorrhizas. Mycoscience. 2001;42:43–50. [Google Scholar]
- 38.You YH, Yoon H, Kang SM, Woo JR, Choo YS, Lee IJ, Shin JH, Kim JG. Cadophora malorum Cs-8-1 as a new fungal strain producing gibberellins isolated from Calystegia soldanella. J Basic Microbiol. 2013;53:630–634. doi: 10.1002/jobm.201200002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information of isolated endophytic fungi from hydrophytes
Distribution of enphytic fungi isolated from halophyte native to the Dokdo Islands
Distribution of enphytic fungi isolated from halophyte native to the East Sea of Korea
Distribution of enphytic fungi isolated from halophyte native to the West Sea of Korea