Abstract
Diabetes mellitus is a chronic disorder which affects millions of population worldwide. Global estimates published in 2010 reported the world diabetic prevalence as 6.4%, affecting 285 million adults. Foot ulceration and wound infection are major forms of disabilities arising from diabetic diseases. This study was aimed to develop a natural antimicrobial finishing on medical grade textile that meets American Association of Textiles Chemists and Colorists (AATCC) standard. The textile samples were finished with the ethanolic extract of Penicillium amestolkiae elv609, an endophytic fungus isolated from Orthosiphon stamineus Benth (common name: cat's whiskers). Endophyte is defined as microorganism that reside in the living plant tissue, without causing apparent disease symptom to the host. The antimicrobial efficacy of the ethanolic extract of P. minioluteum was tested on clinical pathogens isolated from diabetic wound. The extract exhibited significant inhibitory activity against 4 bacteria and 1 yeast with the minimal inhibitory concentration ranged from 6.25 to 12.5 mg/mL. The results indicate different susceptibility levels of the test microorganism to the ethanolic extract. However, the killing activity of the extract was concentration-dependent. The finished medical textile showed excellent antimicrobial efficacy on AATCC test assays. All the microbial cultures treated with the textile sample displayed a growth reduction of 99.9% on Hoheinstein Challenge Test. The wash durability of the finished textile was found good even after 50 washes with commercial detergent. Besides, the gas chromatography mass spectrometry analysis showed that 6-octadecenoic acid and diethyl phthalate were the main bioactive constituents of the extract. In conclusion, the developed medical textile showed good antimicrobial efficacy on laboratory tests. This work can be extended to in vivo trials for developing healthcare textile products for antimicrobial applications.
Keywords: Antimicrobial textiles, Diabetic ulcer, Penicillium amestolkiae
The National Health and Morbidity Survey revealed that 17.5% of Malaysian adult citizens are estimated to be affected by diabetes in 2015 [1]. About 24% of diabetes patients were amputated due to microbial infection [2]. The issue identified with diabetic ulcer is the expensive medical treatment cost. Diabetic foot complications resulted in increased hospital bed occupancy and further increase the healthcare cost and resources. Besides, these sorts of wound need more in-depth research to comprehend the right and proficient approach to understand the right and efficient way to increase the chances for the wound to heal. Foot ulceration and wound infection are major forms of disabilities arising from diabetic diseases. The most common pathogens isolated from infected wound are Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, and Entrobacter spp. [3]. The severity of the wound infection can be influenced by the type and density of microorganisms, the host immune response, and the type of tissue involved [4]. The large number of pathogenic species in the diabetic wound fluid indicate the slow healing process.
Antimicrobial textiles are widely used as wound dressing materials. They provide a physical barrier to cover and protect the wound from microorganisms that can cause wound infection. According to Alavi et al. [5], silver is the most commonly used antimicrobial dressings in antimicrobial textile. However, silver dressing causes tissue toxicity and inhibits fibroblast growth. In addition, synthetic based antimicrobial textiles also cause skin rashes, eco-toxicity and induction of drug resistance [6]. Hence, new antimicrobial finishing is necessary due to the limitations of the current options available. However, only limited studies were conducted by using fungal extracts as the antimicrobial finishing.
By definition, endophytes are microorganisms that reside in living plant tissues for all or part of their life cycle [7]. They are known for the production of bioactive metabolites, particularly antimicrobials, antioxidants, and anti-inflammatory compounds [8,9]. However, thus far, no study was conducted to evaluate the application potential of these compounds in medical textile to combat microbial infection. Thus, in this study, we aim to develop a natural antimicrobial finishing on medical grade textile with extract obtained from Penicillium amestolkiae elv609, an endophytic fungus residing in medicinal herb, Orthosiphon stamineus Benth. P. amestolkiae are known for enzyme production, especially cellulases, xylosidase and hemicellulases [10,11]. They also known as the producer of monascus-like red pigments. Up to date, no report is available on antimicrobial activity of P. amestolkiae.
MATERIALS AND METHODS
Isolation of endophytic fungi
The endophytic fungus P. amestolkiae elv609 was previously isolated by Tong et al. [12]. The fungus culture was deposited at Upstream Bioprocess Laboratory, Universiti Kuala Lumpur.
Cultivation and extraction
Yeast extract sucrose broth (yeast extract, 20 g/L; sucrose, 40 g/L; magnesium sulfate 0.5 g/L) with pH 5.8 ± 0.2 was used to cultivate the fungal isolate [12]. Two agar plugs with 2 cm in diameter were inoculated into the medium. The cultures were cultivated at 30℃ with 120 rpm rotational speed in a shake flask system. After 2 wk of cultivation, the fermented broth and fungal biomass were separated by using Whatman No.1 filter paper. Then, the granular fungal biomass was soaked in ethanol for overnight at ratio of 1 : 20 (w/v). The extract was concentrated under reduced pressure by using rotator evaporator at 60℃ to obtain the crude extract paste. The extract was kept at 4℃ in dark until further use.
Test microorganisms
The test bacteria used in this study include 4 gram-positive bacteria (Bacillus cereus, Bacillus coagulans, Streptococcus sp., and Staphylococcus aureus), 4 gram-negative bacteria (Escherichia coli, Proteus mirabilis, Yersinia sp., and Pseudomonas aeruginosa), and 2 yeasts (Candida albicans and Candida utilis). All the test microorganisms were isolated from wound of diabetic patient in Hospital Seberang Jaya, Penang. The test microorganisms were sub-cultured on nutrient agar prior to use for every 2 wk in order to maintain its viability. The microbial inoculum was prepared by transferring a loopful of microbial colonies into a universal bottle of sterile distilled water. The turbidity of the suspension was adjusted so that it matches with 0.5 Mc Farland standards which contain approximately 108 colony-forming unit (CFU)/mL.
Disc diffusion assay
The antimicrobial efficacy of the extract was evaluated according to standards created by the Clinical Laboratory Standards Institute (CLSI) with modifications [12]. The assay was performed by transferring the inoculums to the surface of Mueller Hinton agar (Merck, Darmstadt, Germany) using cotton swap. Sterile paper disc impregnated with 20 µL of 50 mg/mL extract was placed on the inoculated medium. Then, 20 µL of 100 µg/mL chloramphenicol was used as positive control and 20 µL of 20% Tween 80 was used as negative control. The experiment was done in triplicate in separate occasions. All plates were incubated at 37℃ for 24 hr. After the incubation period, the diameters of inhibition zone surrounding discs were measured [13].
Broth microdilution assay
The minimal inhibitory concentration (MIC) was determined by using broth microdilution assay in sterile 96-well microtiter plate. A serial two-fold dilution of the extract was carried out with double strength Mueller-Hinton broth. One hundred microliters of inoculums and extract were then added into each wells for a final volume of 200 µL. The final concentration of the extract was ranged from 0.78 to 100 mg/mL. Chloramphenicol was used as reference antibiotic. Then, the plate was incubated at 37℃ for 24 hr. After incubation period, 40 µL of 0.2 mg/mL p-iodonitrotetrazolium violet salt (Sigma, St. Louis, MO, USA) dissolved in 99.5% ethanol and was added into each well. The plate was incubated for 30 min at 37℃. The color changed from yellow to purple indicates the microbial growth in the well. The lowest extract concentration that produced a significant growth inhibition effect on test microorganism was identified as the MIC [14]. To check the viability of the test microorganisms, the mixture in every well was streaked on nutrient agar. The plates were incubated at 37℃ for 24 hr. The minimal lethality concentration (MLC) was recorded as the lowest extract concentration to kill the test microorganisms.
Antimicrobial finishing of cotton textile
Plain, 100% cotton woven textile, made up of Ne60 combed yarn with 110 ends/cm and 80 picks per inch was chosen for antimicrobial finishing [15]. Firstly, the textile was mordated with acetic acid at a liquor ratio of 1 : 40 (w/v) and boiled for 3 hr at 100℃. Then, followed by finishing of extract at 55℃ for 45 min with continuous stirring. Lastly, textile was soaked in 10% citric acid at 50℃ for 5min with continuous stirring in order to fix antimicrobial finishing. The coated textile was dried at 50℃ for 24 hr. A solvent control was prepared by replacing the extract with methanol.
Hoheinstein Challenge Test (AATCC-100)
The textile samples were cut to the size of 2 × 2 cm, 50 µL microbial inoculum was inoculated into 100 mL of nutrient broth followed by the transfer of textile sample. The cultures were incubated at 37℃ for 24 hr with a rotational speed of 120 rpm. After the incubation period, the culture was suitably diluted and plated on nutrient agar plate. The colony counts were obtained after 24 hr of incubation at 37℃. The antimicrobial efficiency of the sample was determined by comparing the percentage reduction of bacteria relative to the solvent control.
Wash durability test (AATCC-147)
The wash durability of the developed textile was evaluated after different wash cycles according to Sarkar et al. [16]. The samples were washed using 1% standard detergent. The antimicrobial activity was assessed after 30 and 50 washes according to protocol described in previous section (AATCC-100).
Gas chromatography mass spectroscopy analysis
The analysis was performed by using gas chromatography instrument (Hewlett-Packard 6890N, Palo Alto, CA, USA) with mass spectrometer (Hewlett-Packard 5973 inert mass selective mass detector) with mass spectrometry (Hewlett-Packard 5973 inert mass selective detector). The column HP-MS (30.0 m × 0.25 mm; Agilent Technologies, Santa Clara, CA, USA) was used for this analysis. The instrument was calibrated using absolute methanol (blank sample). The oven temperature was fixed at 70 to 285℃ at 30℃/min and a hold for 2 min. Helium was used as carrier gas with a flow rate of 1.2 mL/min. The injector temperature was 280℃, injection volume of 1 µL with a split ratio of 1 : 5. The mass spectra were taken at 70 eV with a mass scan range of 35–650 amu. The identification of compounds was based on the comparison of their mass spectra with National Institute of Standards and Technology (NIST 02) Library.
RESULTS AND DISCUSSION
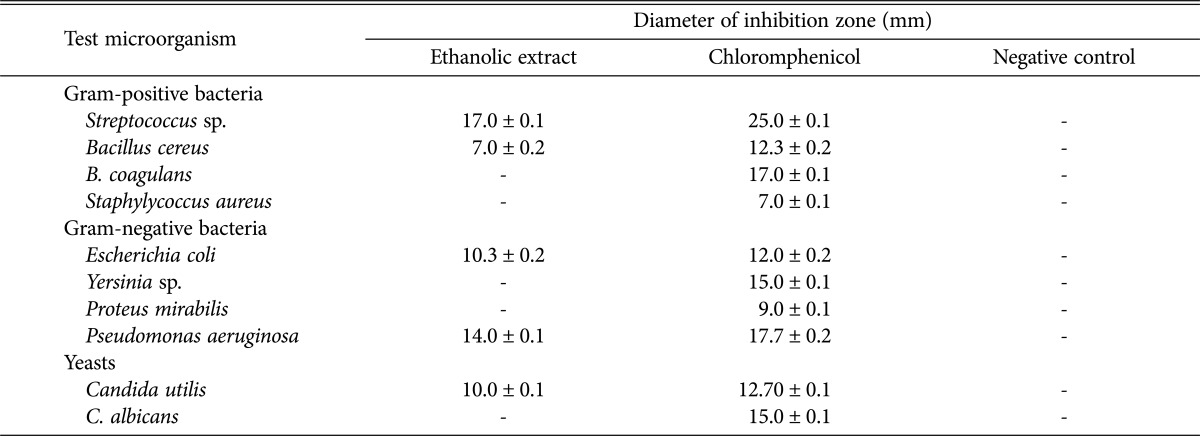
Microbial infections of foot ulcers are the major cause of amputation among diabetic patients. In present study, we assess the antimicrobial potential of P. amestolkiae elv609 against microorganisms isolated from diabetic wound. In this study, we reported that P. amestolkiae elv609 exhibited significant antimicrobial activity against 5 out of 10 test microorganisms (Table 1). Even so, the size of inhibition zones varied among all microbes, indicating different susceptibility of the test microorganism to the extract. Among the microbial species, the largest clear zone was shown by Streptococcus sp. which is a gram-positive bacteria. The results obtained are in agreement with previous studies that most of the fungal extracts are generally exhibit lower antimicrobial activity against gram-negative bacteria compared to gram-positive bacteria [17]. It has been reported that gram-negative bacteria have an outer membrane containing lipopolysaccharide that can protect peptidoglycan cell wall which makes them more resistant to antimicrobial compounds [18,19].
Table 1. Antimicrobial activity of Penicillium amestolkiae elv609 extract on disc diffusion assay .
-, no inhibitory activity.
P. amestolkiae elv609 exhibited broad spectrum antimicrobial activity, it also exhibited significant inhibitory effect on C. utilis. Bacterial infections of diabetic ulcers are commonly polymicrobials, hence a broad spectrum antibacterial agent is effective to reduce the bacterial population present on the ulcer. No data is available for comparison as this is the first report on the antimicrobial activity of P. amestolkiae.
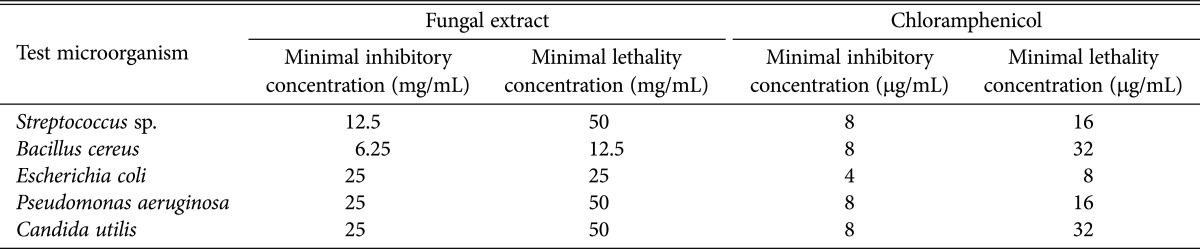
The MIC of the extract was ranged from 6.25 to 25 mg/mL. The results indicate the different susceptibility levels of test microorganisms to the extract. The lowest MLC value obtained in this study was 12.5 mg/mL on B. cereus. Based on the results presented Table 2, the MLC was significantly higher than MIC, as a higher concentration of extract was needed to kill the test microorganisms, instead of inhibiting the growth. Therefore, the results showed that the ethanolic extract was concentration-dependent. The extract exhibited microbialcidal effect to all test microorganisms, based on the MLC obtained [20]. The results showed that P. amestolkiae elv609 are potential source of antimicrobial compounds to combat microbial infection in diabetic wound.
Table 2. Minimal inhibitory concentration and minimal lethality concentration of test microorganisms to Penicillium amestolkiae elv609 extract on broth microdilution assay.
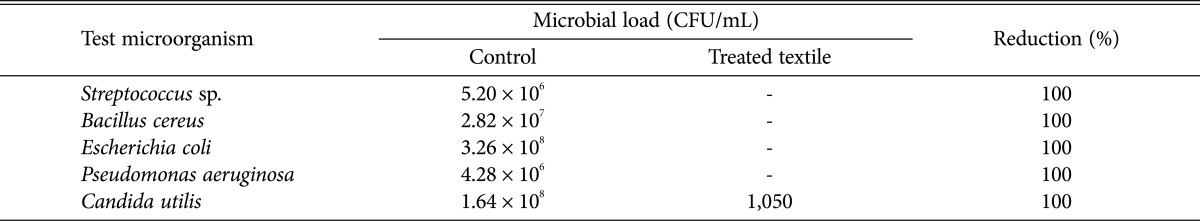
Hohenstein challenge test was widely applied to evaluate the antimicrobial efficacy of antimicrobial textile. The results revealed that the reduction of bacterial colonies was significant on all test microorganisms (Table 3). The textile with P. amestolkiae elv609 extract finishing significantly reduced the microbial load of all test microorganisms. One hundred percent microbial load reductions were observed for all test microorganisms. The results showed excellent potential of the textile in inhibiting the growth of the diabetic wound microorganisms. Besides that, the textile finishing technique also affects the antimicrobial efficiency of antimicrobial textile. Jothi [21] also applied the same finishing technique on medical grade cotton with Aloe vera extract, they also reported a 99.9% growth reduction of S. aureus.
Table 3. Growth reduction of microbial cultures treated with medical cotton finished with Penicillium amestolkiae elv609 ethanolic extract.
CFU, colony-forming unit.
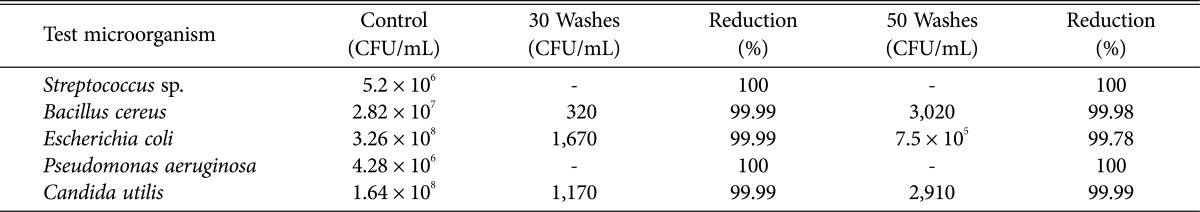
The developed textile also showed excellent wash durability, by using citric acid as mordant. The textile sample showed significant bacterial reduction, even after 50 washes with standard detergent. However, the microbial load significantly increased compared to 30 cycles (p ≤ 0.05) (Table 4). The good wash durability of the developed textile can be due to the presence of the Van der Waals and hydrogen bonds that exists between fungal extract and textile cellulosic material, that allows the slow release of the extract from the textile [22]. According to Nithya et al. [23], there are a few factors that contribute to the good result for wash durability test which are cross linking of extract, antimicrobial finish by binding agent and due to hydrophilicity. In this study, citric acid was used as mordant to act as cross linking agent. Based on previous studies citric acid can improve the tensile strength and thermal stability [24]. So, this study suggested that treated textile can be use in the textile application.
Table 4. The wash durability of medical cotton finished with Penicillium amestolkiae elv609 ethanolic extract.
The wash durability of the textile was determined based on the percentage reduction in the growth of test microorganisms.
CFU, colony-forming unit.
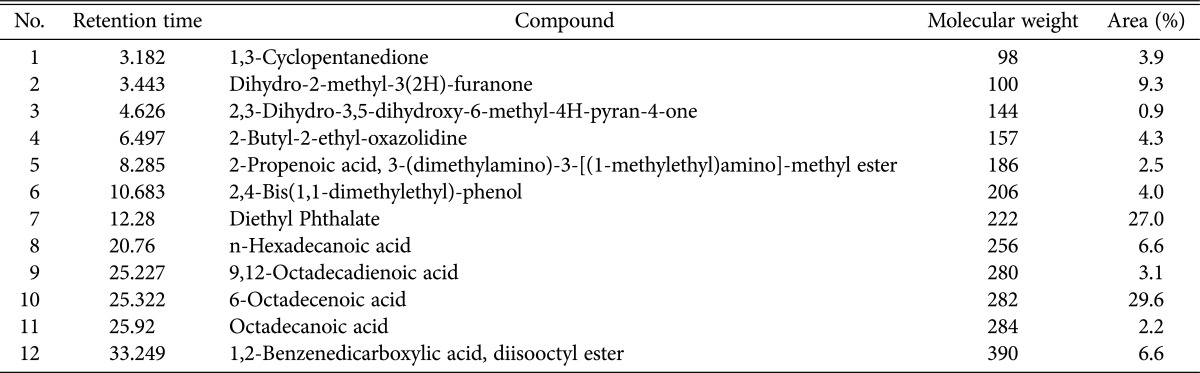
A total of 12 compounds were detected in P. amestolkiae elv609 ethanolic extract via gas chromatography mass spectrometry analysis. The retention time, molecular weight, and peak area were depicted in Table 5. In general, 2 major compounds were predominant in the extract, they are diethyl phthalate and 6-octadecenoic acid, based on their percentage of peak area. 6-Octadecenoic or known as stearic acid was found to be present as major constituent with highest peak area of 29.6% and retention time 25.322. It has been reported that 6-octadecenoic acid exhibited significant antimicrobial and antioxidant activity, particularly on gram-positive bacteria [25,26]. The results obtained are in agreement with the antimicrobial activity screening results, where the extract exhibited better inhibitory effect on gram-positive bacteria. Premjanu and Jayanthy [27] reported the antimicrobial and antioxidant activity of diethyl phthalate, which is one of the main constituent in P. amestolkiae elv609 ethanolic extract. The compound was widely used as cosmetic ingredients for topical application [27].
Table 5. GC-MS analysis of Penicillium amestolkiae elv609 ethanolic extract.
GC-MS, gas chromatography mass spectrometry.
In conclusion, P. amestolkiae elv609 showed significant inhibitory effect on diabetic wound microorganisms. The GCMS of the bioactive extract also showed the presence of diethyl phthalate and 6-octadecenoic acid. We also successfully incorporated the extract into medical grade cotton, and the finished textile showed excellent antimicrobial activity on in vitro assays, with good wash durability.
ACKNOWLEDGEMENTS
The authors are thankful to Universiti Kuala Lumpur and Universiti Sains Malaysia.
References
- 1.Ying C, Kuay LK, Huey TC, Hock LK, Hamid HA, Omar MA, Ahmad NA, Cheong KC. Prevalence and factors associated with physical inactivity among Malaysian adults. Southeast Asian J Trop Med Public Health. 2014;45:467–480. [PubMed] [Google Scholar]
- 2.Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3:4. doi: 10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Y, Xie B, Ben D, Lv K, Zhu S, Lu W, Tang H, Cheng D, Ma B, Wang G, et al. Pathogenic alteration in severe burn wounds. Burns. 2012;38:90–94. doi: 10.1016/j.burns.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Green B. Understanding infection in wound care. Prof Nurs Today. 2012;16:48–55. [Google Scholar]
- 5.Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, Woo K, Boeni T, Ayello EA, Kirsner RS. Diabetic foot ulcers: Part II. Management. J Am Acad Dermatol. 2014;70:21.e1–24. doi: 10.1016/j.jaad.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 6.Kavitha KS, Baker S, Rakshith D, Kavitha HU, Yashwantha Rao HC, Harini BP, Satish S. Plants as green source towards synthesis of nanoparticles. Int Res J Biol Sci. 2013;2:66–76. [Google Scholar]
- 7.Fouda MM. Antibacterial modification of textiles using nanotechnology. In: Bobbarala V, editor. A search for antibacterial agents. Rijeka: InTech; 2014. pp. 50–51. [Google Scholar]
- 8.Nisa H, Kamili AN, Nawchoo IA, Shafi S, Shameem N, Bandh SA. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: a review. Microb Pathog. 2015;82:50–59. doi: 10.1016/j.micpath.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Tan RX, Zou WX. Endophytes: a rich source of functional metabolites. Nat Prod Rep. 2001;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 10.Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol. 2014;78:175–341. doi: 10.1016/j.simyco.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang MH, Li TX, Wang Y, Liu RH, Luo J, Kong LY. Antimicrobial metabolites from the plant endophytic fungus Penicillium sp. Fitoterapia. 2017;116:72–76. doi: 10.1016/j.fitote.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Tong WY, Ang SN, Darah I, Latiffah Z. Antimicrobial activity of Penicillium minioluteum ED24, an endophytic fungus residing in Orthosiphon stamineus Benth. World J Pharm Pharm Sci. 2014;3:121–132. [Google Scholar]
- 13.Powthong P, Jantrapanukorn B, Thongmee A, Suntornthiticharoen P. Screening of antimicrobial activities of the endophytic fungi isolated from Sesbania grandiflora (L.) Pers. J Agric Sci Technol. 2013;15:1513–1522. [Google Scholar]
- 14.Jantas R, Gorna K. Antibacterial finishing of cotton fabrics. Fibres Text East Eur. 2006;14:88–91. [Google Scholar]
- 15.Thilagavathi G, Rajendrakumar K, Rajendran R. Development of ecofriendly antimicrobial textile finish using herbs. Indian J Fibre Text Res. 2005;30:431–436. [Google Scholar]
- 16.Sarkar RK, Purushottam D, Chauhan PD. Bacteria-resist finish on cotton fabrics using natural herbal extracts. Indian J Fibre Text Res. 2003;28:322–331. [Google Scholar]
- 17.Mahadevamurthy M, Puttaswamy H, Channappa TM, Sidappa M, Madegowda P, Chikkamanchegowda JS, Nagaraj AK. Antibacterial potential of fungal endophytes isolated from Boerhaavia diffusa L. J Appl Pharm Sci. 2016;6:216–221. [Google Scholar]
- 18.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Supaphon P, Phongpaichit S, Rukachaisirikul V, Sakayaroj J. Antimicrobial potential of endophytic fungi derived from three seagrass species: Cymodocea serrulata, Halophila ovalis and Thalassia hemprichii. PLoS One. 2013;8:e72520. doi: 10.1371/journal.pone.0072520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang CG, Hah DS, Kim CH, Kim YH, Kim E, Kim JS. Evaluation of antimicrobial activity of the methanol extracts from 8 traditional medicinal plants. Toxicol Res. 2011;27:31–36. doi: 10.5487/TR.2011.27.1.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jothi D. Experimental study on antimicrobial activity of cotton fabric treated with aloe gel extract from Aloe vera plant for controlling the Staphylococcus aureus (bacterium) Afr J Microbiol Res. 2009;3:228–232. [Google Scholar]
- 22.Rathinamoorthy R, Udayakumar S, Thilagavathi G. Antibacterial efficacy analysis of Punica granatum L leaf, rind and Terminalia chebula fruit extract treated cotton fabric against five most common human pathogenic bacteria. Int J Pharm Life Sci. 2011;2:1147–1153. [Google Scholar]
- 23.Nithya E, Radhai R, Rajendran R, Shalini S, Rajendran V, Jayakumar S. Synergetic effect of DC air plasma and cellulase enzyme treatment on the hydrophilicity of cotton fabric. Carbohydr Polym. 2011;83:1652–1658. [Google Scholar]
- 24.Reddy N, Yang Y. Citric acid cross-linking of starch films. Food Chem. 2010;118:702–711. [Google Scholar]
- 25.Elezabeth DV, Arumugam S. GC-MS analysis of bioactive constituents of Indigofera suffruticosa leaves. J Chem Pharm Res. 2014;6:294–300. [Google Scholar]
- 26.da Silva LL, Nascimento M, Silva DH, Furlan M, da Silva Bolzani V. Antibacterial activity of a stearic acid derivative from Stemodia foliosa. Planta Med. 2002;68:1137–1139. doi: 10.1055/s-2002-36346. [DOI] [PubMed] [Google Scholar]
- 27.Premjanu N, Jayanthy C. Antimicrobial activity of diethyl phthalate: an in silico approach. Asian J Pharm Clin Res. 2014;7:141–142. [Google Scholar]