Abstract
This study was done to produce γ-aminobutyric acid (GABA) from wild yeast as well as investigate its anti-hyperglycemic effects. Among ten GABA-producing yeast strains, Pichia silvicola UL6-1 and Sporobolomyces carnicolor 402-JB-1 produced high GABA concentration of 134.4 µg/mL and 179.2 µg/mL, respectively. P. silvicola UL6-1 showed a maximum GABA yield of 136.5 µg/mL and 200.8 µg/mL from S. carnicolor 402-JB-1 when they were cultured for 30 hr at 30℃ in yeast extract-peptone-dextrose medium. The cell-free extract from P. silvicola UL6-1 and S. carnicolor 402-JB-1 showed very high anti-hyperglycemic α-glucosidase inhibitory activity of 72.3% and 69.9%, respectively. Additionally, their cell-free extract-containing GABA showed the anti-hyperglycemic effect in streptozotocin-induced diabetic Sprague-Dawley rats.
Keywords: Anti-hyperglycemic effects, Gamma-aminobutyric acid, Pichia silvicola UL6-1, Sporobolomyces carnicolor 402-JB-1, Wild yeast
Generally, yeasts are heterotrophic, facultative anaerobes with relatively simple nutritional needs. They are wildly distributed in natural habitats such as in flowers, fruits, and cereals as well as in plant debris found on the surface area of soils. Most yeasts were isolated from various fermentation foods or their raw materials including meju [1] and more recently from flowers and soil samples from the mountains, islands and inlands of Korea [2,3,4,5].
Yeasts have long been used to prepare alcoholic beverages [6], soy sauces [7], etc. Recently, they have received much attention because of their various physiological activities such as anti-gout, anti-hypertensive, and anti-diabetic activities as well as other activities [8,9,10,11,12,13].
Gamma-aminobutyric acid (GABA) is a non-protein amino acid that is widely distributed in plants and animals [14] and also produced by microorganisms [15,16,17].
GABA is produced by decarboxylation through glutamate decarboxylase with the cofactor pyridoxal-5-phosphate. It acts as a major neurotransmitter in the mammalian central nervous system. Additionally, GABA has hypotensive, tranquilizing and diuretic effects and can prevent diabetes [18,19,20,21]. Furthermore, GABA may improve the concentration of plasma growth hormones and the rate of protein synthesis in the brain [22] and inhibit small airway-derived lung adenocarcinoma [23]. Therefore, GABA has potential as a bioactive component in foods and pharmaceuticals.
The GABA are produced from various microorganisms including Saccharomyces cerevisiae [15], Rhodotorula mucilaginosa and Debaryomyces hansenii [16], and Lactobacillus buchneri, L. brevis, and L. sakei from Kimchi [17,24,25,26,27], etc. However, their GABA productivity was low, and the physiological activities of the GABA in those studies were not investigated for the preparation of functional foods and biomedicines.
In a previous paper, ten GABA-producing yeast strains were screened and their microbiological characteristics were investigated [28]. In this study, a potent yeast strain with a high GABA content with anti-hyperglycemic effects was finally selected for further investigation. Moreover, the optimal conditions for GABA production in this potent strain were determined, and the anti-hyperglycemic action of GABA was also investigated.
MATERIALS AND METHODS
Yeast strains, rats, and chemicals
Ten yeast strains that were screened as GABA-producing yeasts in a previous paper [28] were used in this study.
Sprague-Dawley (SD) male rats, weighing 180–200 g and 7 weeks old, were purchased from Orientbio Co., Seongnam, Korea.
Angiotensin 1-converting enzyme from rabbit lung acetone powder, tyrosinase, xanthine oxidase, and γ-aminobutyric acid transaminase (GABase) from Pseudomonas fluorescens were purchased from Sigma-Aldrich (St. Louis, MO, USA). β-NADP+, hippuric acid-histidine-leucine, pyrogallol, and 2,2-diphenyl-1-picrylhydrazy were also purchased from Sigma-Aldrich. Unless otherwise specified, all chemicals were analytical grade.
Determination of GABA contents
Quantitative determination of GABA with GABase was done as follows. The reaction mixture (cell-free extract from yeast 10 µL, GABase 0.02 units 10 µL, 10 mM β-NADP+ 70 µL, 0.1M potassium pyrophosphate [pH 8.6] 240 µL, and 0.1M α-ketoglutarate 10 µL) was kept at 37℃ for 60 min after which the absorbance was measured at 340 nm with a enzyme-linked immunosorbent assay reader. The GABA contents were calculated with a GABA standard curve.
Assay of physiological functionalities
The physiological activities of the cell-free extracts containing GABA from the selected yeast strains were determined as follows. Antihypertensive angiotensin I-converting enzyme (ACE) inhibitory activity was assayed by the method of Cushman and Cheung [29] using ACE from rabbit lung. Antioxidant activity was assayed with DPPH as the substrate [30], and superoxide dismutase-like activity was assayed by the method of Lee et al. [31] using pyrogallol. Tyrosinase inhibitory activity was measured by conversion of L-DOPA to a red-colored oxidation product dopachrome spectrophotometrically [32]. Xanthine oxidase inhibitory activity was determined by the modification method of Noro et al. [33]. α-Glucosidase inhibitory activity was assayed using α-glucosidase and p-nitrophenyl α-D-glucopyranoside [10].
In vivo test for anti-hyperglycemic effects
The anti-hyperglycemic effects of the cell-free extracts containing GABA from the selected yeast strains were tested with SD rats following the Guidelines on Animal Breeding for Animal Experiments - Ethics Committee of Paichai University (registration No. 2015. pcu-001).
Male SD rats (age, 6 weeks; weight, 180–200 g) were maintained on a 12-hr light/dark cycle in a temperature and humidity-controlled room for 1 wk. All rats were randomly distributed into experimental groups (n = 5/group). A diabetes inducer streptozotocin was used to induce hyperglycemia in rats. The rats were injected intraperitoneally with streptozotocin (60 mg/kg). Then, various concentrations of the cell-free extract containing GABA from Pichia silvicola UL6-1 (1,000 mg/kg and 500 mg/kg) and the commercial anti-diabetic agent acarbose (15 mg/kg) were administered orally.
Each experiment was performed at least three times, and all quantitative data are expressed as the mean ± standard deviation values.
RESULTS AND DISCUSSION
Selection of potent GABA-producing yeast strains and production of GABA
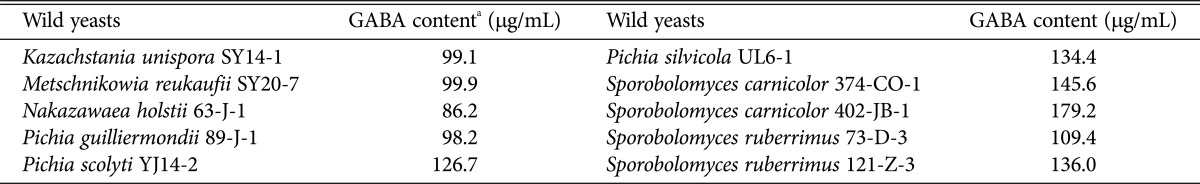
The GABA contents of ten yeast strains including Kazachstania unispora SY14-1, were determined with GABase (Table 1). The cell-free extracts of asporogenous Sporobolomyces carnicolor 402-JB-1 had the highest GABA content of 179.2 µg/mL. Ascosporogenous P. silvicola UL6-1 was also produced high content of GABA (134.4 µg/mL) even though lower than that of S. carnicolor 402-JB-1. Finally, P. silvicola UL6-1 and S. carnicolor 402-JB-1 were selected as potent GABA-producing yeasts. These GABA contents also were similar or higher than that of L. plantarum K74 from Kimchi (134.52 µg/mL) [34] while they were lower than that of Bokbunja wine (330 µg/mL) [15], and L. sakei A156 (15.81 ± 0.98 mg/mL) and Lactobacillus zymae GU240 (16.94 ± 1.14 mg/mL) [35].
Table 1. Quantitative GABA contents of the first 10 screened yeast strains.
aDetermined with γ-aminobutyric acid (GABA)-transaminase.
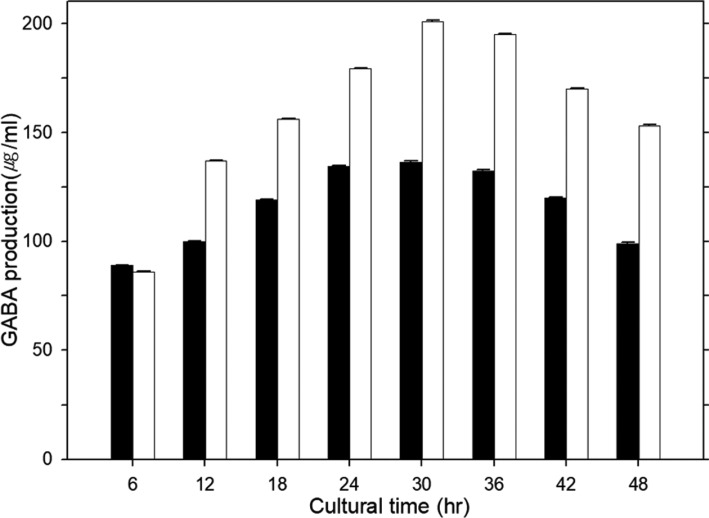
Meanwhile, the effect of the culture time on GABA production in P. silvicola UL6-1 and S. carnicolor 402-JB-1 was investigated (Fig. 1). The maximum yield of GABA (200.8 µg/mL, 136.5 µg/mL) from S. carnicolor 402-JB-1 and P. silvicola UL6-1 were achieved when their wild yeast strains were cultured for 30 hr at 30℃ in yeast extract-peptone-dextrose media, respectively. Asporogenous S. carnicolor 402-JB-1 was higher produced GABA than ascosporogenous P. silvicola UL6-1, even though its production condition was very similar.
Fig. 1. Effect of the culture time on γ-aminobutyric acid (GABA) production in Pichia silvicola UL6-1 (black bar) and Sporobolomyces carnicolor 402-JB-1 (white bar).
Physiological functionality of GABA-producing yeasts
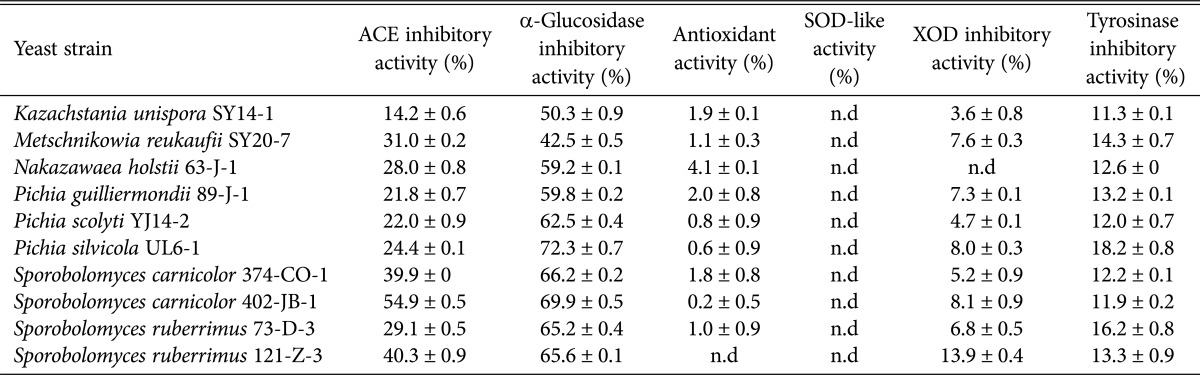
To investigate the application of GABA from yeasts in medicinal foods, several physiological funtionalities of the cell-free extracts from the 1st screened ten yeasts were investigated (Table 2). The cell-free extract from P. silvicola UL6-1 had the highest anti-hyperglycemic α-glucosidase inhibitory activity at 72.3%, and S. carnicolor 402-JB-1 had high anti-hyperglycemic α-glucosidase inhibitory activity at 69.9% and anti-hypertensive angiotensin 1-converting enzyme inhibitory activity at 54.9%.
Table 2. Physiological activities of the cell-free extracts from the first 10 screened yeast strains.
ACE, angiotensin I-converting enzyme; SOD, superoxide dismutase; XOD, xanthine oxidase; n.d, not detected.
These α-glucosidase inhibitory activities were higher than that of Makgeolli made by Saccharomyces cerevisiae Y111-5 (42.0%) [36] while they were lower than those of Bullera coprosmaensis JS00600 (94.7%) [37] and P. burtonii Y257-7 (90.9%) [10].
Finally, Pichia silvicola UL6-1 and S. carnicolor 402-JB-1, which had high GABA contents as well as a high anti-hyperglycemic effect, were selected as potent yeast strains for the medicinal foods industry.
Anti-hyperglycemic effect of GABA from Pichia silvicola UL6-1 and Sporobolomyces carnicolor 402-JB-1
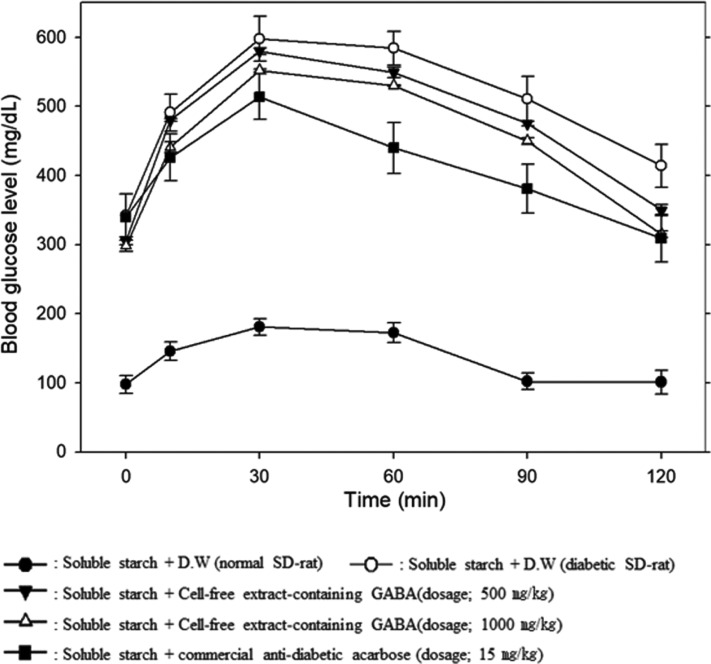
The anti-hyperglycemic action of the cell-free extract-containing GABA from P. silvicola UL6-1 and S. carnicolor 402-JB-1 were investigated in normal rats and streptozotocin-induced diabetic rats.
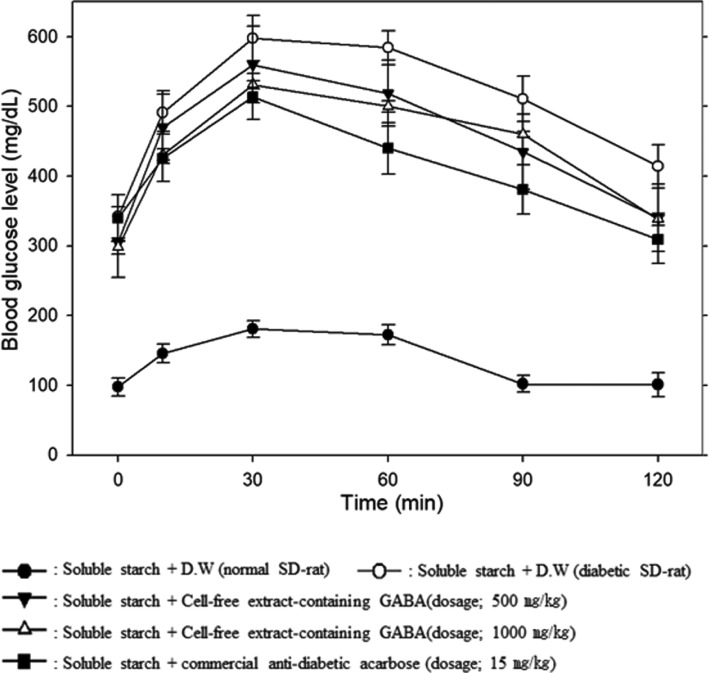
As shown in Figs. 2 and 3, the blood glucose level at 30 min after the administration of soluble starch (3 g/kg) was significantly increased to 495–600 mg/dL from 300–330 mg/dL in the streptozotocin-induced diabetic rats. However, the blood glucose level decreased to 335–350 mg/dL dose-dependently at 120 min after administered the cell-free extract from P. silvicola UL6-1. Anti-hyperglycemic effect of the cell-free extract from S. carnicolor 402-JB-1 was also very similar tendency that of P. silvicola UL6-1.
Fig. 2. Changes in blood glucose levels up to 120 min after administration of 3 g/kg soluble starch and various concentrations of the cell-free extract containing the α-glucosidase inhibitor from Pichia silvicola UL6-1 in streptozotocin-induced diabetic Sprague-Dawley (SD) rats and normal SD rats. GABA, γ-aminobutyric acid.
Fig. 3. Changes in blood glucose levels up to 120 min after administration of 3 g/kg soluble starch and various concentrations of the cell-free extract containing the α-glucosidase inhibitor from Sporobolomyces carnicolor 402-JB-1 in streptozotocin-induced diabetic Sprague-Dawley (SD) rats and normal SD rats. GABA, γ-aminobutyric acid.
From these results, we concluded that the cell-free extract-containing GABA from P. silvicola UL6-1 and S. carnicolor 402-JB-1 have anti-hyperglycemic effects although higher dose were required than that of the commercial anti-diabetic acarbose. Therefore, these two wild yeasts would be very useful in the healthy food industry for development of new anti-diabetic foods.
ACKNOWLEDGEMENTS
This work was supported by the research grant of PaiChai University in 2017.
References
- 1.Kim JH, Kim NM, Lee JS. Physiological characteristics and ethanol fermentation of thermotolerant yeast Saccharomyces cerevisiae OE-16 from traditional Meju. Korean J Food Nutr. 1999;12:490–495. [Google Scholar]
- 2.Min JH, Ryu JJ, Kim HK, Lee JS. Isolataion and identification of yeasts from wild flowers in Gyejoksan, Oseosan and Beakamsan of Korea. Korean J Mycol. 2013;41:47–51. [Google Scholar]
- 3.Hyun SH, Min JH, Kim SA, Lee JS, Kim HK. Yeasts associated with fruits and blossoms collected from Hanbat arboretum, Daejeon, Korea. Korean J Mycol. 2014;42:178–182. [Google Scholar]
- 4.Hyun SH, Lee JG, Park WJ, Kim HK, Lee JS. Isolation and diversity of yeasts from fruits and flowers of orchard in Sinam-myeon of Yesan-gun, Chungcheongnam-do, Korea. Korean J Mycol. 2014;42:21–27. [Google Scholar]
- 5.Hyun SH, Han SM, Lee JS. Isolation and physiological functionality of yeasts from wild flowers in Seonyudo of Gogunsanyeoldo, Jeollabuk-do, Korea. Korean J Mycol. 2014;42:201–206. [Google Scholar]
- 6.Yi SH, Ann YG, Choi JS, Lee JS. Development of peach fermented wine. Korean J Food Nutr. 1996;9:409–412. [Google Scholar]
- 7.Lee TS, Lee SK. Studies on the yeasts for the brewing of soy sauce (I). Isolation, identification and classification of the yeasts in the soy sauce koji. J Korean Soc Appl Biol Chem. 1970;13:97–103. [Google Scholar]
- 8.Han SM, Hyun SH, Kim NM, Lee JS. Antioxidant activity and inhibitory activities of xanthine oxidase and tyrosinase of yeasts from wild flowers in Korea. Korean J Mycol. 2015;43:99–103. [Google Scholar]
- 9.Kim JH, Lee DH, Jeong SC, Chung KS, Lee JS. Characterization of antihypertensive angiotensin I-converting enzyme inhibitor from Saccharomyces cerevisiae. J Microbiol Biotechnol. 2004;14:1318–1323. [Google Scholar]
- 10.Kim YH, Shin JW, Lee JS. Production and anti-hyperglycemic effects of α-glucosidase inhibitor from yeast, Pichia burtonii Y257-7. Korean J Microbiol Biotechnol. 2014;42:219–224. [Google Scholar]
- 11.Lee DH, Lee DH, Lee JS. Characterization of a new antidementia β-secretase inhibitory peptide from Saccharomyces cerevisiae. Enzyme Microb Technol. 2007;42:83–88. [Google Scholar]
- 12.Lee DH, Lee JS, Yi SH, Lee JS. Production of the acetylcholinesterase inhibitor from Yarrowia lipolytica S-3. Mycobiology. 2008;36:102–105. doi: 10.4489/MYCO.2008.36.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong SC, Lee DH, Lee JS. Production and characterization of an anti-angiogenic agent from Saccharomyces cerevisiae K-7. J Micriobiol Biotechnol. 2006;16:1904–1911. [Google Scholar]
- 14.Ueno H. Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B Enzym. 2000;10:67–79. [Google Scholar]
- 15.Kim JH. Isolation and characterization of high GABA producing yeast isolated from fermented Bokbunja wine [dissertation] Jeonju: Chonbuk National University; 2014. [Google Scholar]
- 16.Lee SH. Study on a gamma-aminobutyric acid (GABA) producing yeast isolated from Korean fermented soybean product, meju and optimal condition for GABA synthesis [dissertation] Jeonju: Chonbuk National University; 2014. [Google Scholar]
- 17.Cho YR, Chang JY, Chang HC. Production of γ-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol. 2007;17:104–109. [PubMed] [Google Scholar]
- 18.Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, Yamori Y. Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Nutr. 2004;92:411–417. doi: 10.1079/bjn20041221. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H. Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr. 2003;57:490–495. doi: 10.1038/sj.ejcn.1601555. [DOI] [PubMed] [Google Scholar]
- 20.Jakobs C, Jaeken J, Gibson KM. Inherited disorders of GABA metabolism. J Inherit Metab Dis. 1993;16:704–715. doi: 10.1007/BF00711902. [DOI] [PubMed] [Google Scholar]
- 21.Wong CG, Bottiglieri T, Snead OC., 3rd GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol. 2003;54(Suppl):S3–S12. doi: 10.1002/ana.10696. [DOI] [PubMed] [Google Scholar]
- 22.Tujioka K, Ohsumi M, Horie K, Kim M, Hayase K, Yokogoshi H. Dietary gamma-aminobutyric acid affects the brain protein synthesis rate in ovariectomized female rats. J Nutr Sci Vitaminol (Tokyo) 2009;55:75–80. doi: 10.3177/jnsv.55.75. [DOI] [PubMed] [Google Scholar]
- 23.Schuller HM, Al-Wadei HA, Majidi M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis. 2008;29:1979–1985. doi: 10.1093/carcin/bgn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park KB, Oh SH. Isolation and characterization of Lactobacillus buchneri strains with high γ-aminobutyric acid producing capacity from naturally aged cheese. Food Sci Biotechnol. 2006;15:86–90. [Google Scholar]
- 25.Park KB, Oh SH. Cloning, sequencing and expression of a novel glutamate decarboxylase gene from a newly isolated lactic acid bacterium, Lactobacillus brevis OPK-3. Bioresour Technol. 2007;98:312–319. doi: 10.1016/j.biortech.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Ueno Y, Hayakawa K, Takahashi S, Oda K. Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Biosci Biotechnol Biochem. 1997;61:1168–1171. doi: 10.1271/bbb.61.1168. [DOI] [PubMed] [Google Scholar]
- 27.Nomura M, Kimoto H, Someya Y, Suzuki I. Novel characteristic for distinguishing Lactococcus lactis subsp. lactis from subsp. cremoris. Int J Syst Bacteriol. 1999;49(Pt 1):163–166. doi: 10.1099/00207713-49-1-163. [DOI] [PubMed] [Google Scholar]
- 28.Han SM, Jeon SJ, Lee HB, Lee JS. Screening of γ-aminobutyric acid (GABA)-producing wild yeasts and their microbiological characteristics. Korean J Mycol. 2016;44:87–93. [Google Scholar]
- 29.Cushman DW, Cheung HS. Spectrophotometic assay and properties of angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee SE, Seong NS, Bang JK, Kang SW, Lee SW, Chung TY. Inhibitory effect against angiotensin converting enzyme and antioxidant activity of Panax ginseng C. A. Meyer extracts. Korean J Med Crop Sci. 2003;11:236–245. [Google Scholar]
- 31.Lee JS, Yi SH, Kown SJ, Ahn C, Yoo JY. Enzyme activities and physiological functionality of yeasts from traditional Meju. Korean J Microbiol Biotechnol. 1997;25:448–453. [Google Scholar]
- 32.Kim JK, Cha WS, Park JH, Oh SL, Cho YJ, Chun SS, Choi C. Inhibition effect against tyrosinase of condensed tannins from Korean green tea. Korean J Food Sci Technol. 1997;29:173–177. [Google Scholar]
- 33.Noro T, Oda Y, Miyase T, Ueno A, Fukushima S. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem Pharm Bull (Tokyo) 1983;31:3984–3987. doi: 10.1248/cpb.31.3984. [DOI] [PubMed] [Google Scholar]
- 34.Park SY, Shim HY, Kim KS, Lim SD. Physiological characteristics and GABA production of Lactobacillus plantarum K74 isolated from Kimchi. J Korean Dairy Technol Sci Assoc. 2013;31:143–152. [Google Scholar]
- 35.Sa HD, Park JY, Jeong SJ, Lee KW, Kim JH. Characterization of glutamate decarboxylase (GAD) from Lactobacillus sakei A156 isolated from Jeot-gal. J Microbiol Biotechnol. 2015;25:696–703. doi: 10.4014/jmb.1412.12075. [DOI] [PubMed] [Google Scholar]
- 36.Jang IT, Kang MG, Yi SH, Lim SI, Kim HR, Ahn BH, Lee JS. Physiological functionality of Nuruk, Makgeolli and Cheonggukjang made with fungi and bacteria isolated from Korean traditional fermented foods. Korean J Mycol. 2012;40:164–173. [Google Scholar]
- 37.Han SM, Hyun SH, Lee HB, Lee HW, Kim HK, Lee JS. Isolation and identification of yeasts from wild flowers collected around Jangseong lake in Jeollanam-do, republic of Korea, and characterization of the unrecorded yeast Bullera coprosmaensis. Mycobiology. 2015;43:266–271. doi: 10.5941/MYCO.2015.43.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]