Abstract
Pneumatosis intestinalis has been described as an incidental finding in domestic animals and humans where it is associated with human immunodeficiency virus infection among other comorbidities. This report describes emphysematous changes consistent with pneumatosis intestinalis in a simian immunodeficiency virus (SIV) infected rhesus macaque (Macaca mulatta).
Keywords: Pneumatosis cystoides intestinalis, intestinal emphysema, nonhuman primate
Case Report
An 11 month-old intact male Indian-Chinese hybrid rhesus macaque, born at the California National Primate Research Center (CNPRC), was housed indoors and was assigned to a terminal research protocol. All experimental procedures were approved by the University of California Davis (UCD) CNPRC’s Institutional Animal Care and Use Committee (IACUC). Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals, and it adhered to the principles stated in the 1996 and 2011 editions of the National Research Council’s guide for the Care and Use of Laboratory Animals.[1–2] The facility where this research was conducted is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. The animal was infected with virulent SIVmac251 for 12 weeks, had a high viral load (95 million viral RNA copies/ml plasma), reduced CD4+ T cell count (288 cells/μl blood)(mean CD4+ T cells for 22 uninfected juveniles is 703 cells/μl (range 362–1762)unpublished data from CNPRC) and was positive for cytomegalovirus. While on study the animal experienced few episodes of intermittent diarrhea and decreased appetite, and was treated with antimicrobials, probiotics, and fiber supplementation. Although the animal appeared to be clinically stable, it died unexpectedly overnight due to complications from left ventricular hypertrophic cardiomyopathy.
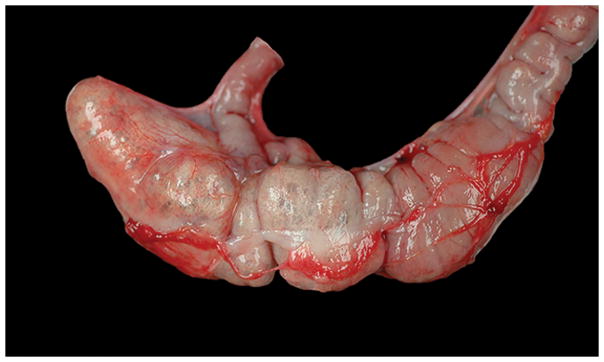
Gross lesions observed during necropsy, included the expansion of the parietal peritoneum of the ventral abdominal wall and to a lesser extent the mesentery by numerous clear, gas filled bullae, ~1–3mm in diameter (emphysema). Similarly, the walls of the cecum and colon were segmentally disrupted and expanded by numerous ~0.5mm to ~5mm diameter clear round variably coalescing emphysematous bullae, which were visible from the serosal surface (Figure 1). The overlying mucosa was severely thickened, with numerous pinpoint red foci. The mesenteric lymph nodes were moderately to severely enlarged (~0.3–1cm). Microbial cultures of the cecum and abdominal cavity were negative for pathogenic bacterial organisms. Additional significant gross lesions include severe concentric thickening of the wall of the left ventricle of the heart, consistent with the diagnosis of left ventricular hypertrophy. Representative samples of major organs were fixed in 10% neutral buffered formalin. Tissues were embedded in paraffin, routinely processed, sectioned at 4μm, and stained with hematoxylin and eosin (HE).
Figure 1.
Grossly the cecum and colon of this rhesus macaque are expanded by variably sized clear round coalescing emphysematous bullae visible from the serosal surface.
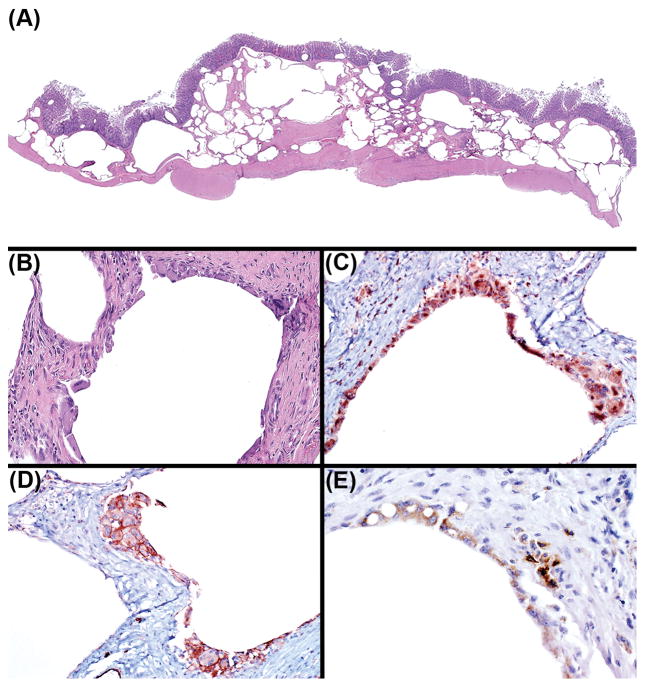
Light microscopy revealed that submucosa and muscularis mucosae and to a lesser extent the mucosa of the cecum and colon were segmentally and severely expanded by extensive coalescing clusters of dilated clear spaces (Figure 2A). Aggregates of histiocytes intermingled with low to moderate numbers of multinucleated giant cells bordered the periphery of the clear spaces (Figure 2B). The parietal peritoneum and mesenteric adipose were similarly affected by dilated clear spaces and cellular infiltrates. The adjacent muscularis mucosae was variably expanded by mixed inflammatory infiltrates including histiocytes and multinucleated giant cells. There was mild multifocal mucosal hyperplasia, with increased numbers of mitotic figures, particularly prominent in the elongated crypts. Low to moderate numbers of Cryptosporidia sp. were multifocally attached to the apical surface of the crypt epithelial cells. The lamina propria was multifocally infiltrated by mild pleocellular infiltrates, as well as rare multinucleated giant cells. Incidental findings included moderate numbers of intraluminal and to a lesser extent intra-crypt Balantidium coli.
Figure 2.
(A) Submucosa, muscularis mucosae, and mucosa were expanded by extensive coalescing clusters of dilated clear spaces. (B) Aggregates of histiocytes admixed with multinucleated giant cells bordered the periphery of the clear spaces. (C) Histiocytes and multinucleated giant cells have strong cytoplasmic immunoreactivity to CD68 immunohistochemistry (IHC). (D) Histiocytes and multinucleated giant cells have strong membranous immunoreactivity to CD31 IHC. (E) In situ hybridization for SIV revealed large numbers of immunoreactive histiocytes and multinucleated giant cells.
Ancillary diagnostics including immunohistochemistry, in situ hybridization, and special stains were employed to further characterize the cellular infiltrates and cystic spaces. Microscopically, the round cells, and multinucleated cells that bordered the cystic spaces demonstrated strong cytoplasmic immunoreactivity to the histiocyte marker CD68 (Figure 2C), as well as membranous immunoreactivity to CD31 (Figure 2D), expressed on both histiocytes and endothelia. Often emphysematous spaces were solely bordered by compressed muscularis mucosae, and lacked endothelial lining cells, indicating that some of these cystic spaces were not dilated lymphatic or blood vessels. In situ hybridization for SIV revealed large numbers of immunoreactive intralesional histiocytes and multinucleated giant cells (Figure 2E). Giemsa, Gram, acid-fast, and GMS stains were negative for intralesional pathogenic organisms.
Discussion
Pneumatosis intestinalis (PI) is defined as the presence of gas expanding the bowel wall. [3] It is an uncommon diagnosis in human and veterinary medicine and to our knowledge has never been reported in a rhesus macaque. PI is referred to by many different names, including pneumatosis cystoides intestinalis, and intestinal emphysema.[3] In domestic animals, PI is considered uncommon but it is described as an incidental lesion in intestines of hogs at time of slaughter.[4] While also considered rare in humans, PI is sporadically identified as an incidental finding during diagnostic imaging and primarily affects the small and large intestines depending on the underlying etiology.[3,5] The prevalence of PI within the human population is difficult to establish due to asymptomatic and subclinical cases. [3] When apparent, symptoms of PI may include diarrhea, melena, abdominal distension, and varying degrees of abdominal pain. [5] There are numerous reports of PI, in patients with Human Immunodeficiency Virus (HIV), Acquired Immunodeficiency Syndrome (AIDS), as well as immunodeficiency associated gastrointestinal opportunistic infections. [6] Occasionally, ruptured emphysematous bullae can result in pneumoperitoneum and pneumoretroperitoneum, without septic peritonitis. [5] PI is rarely fatal, with the exception of cases of ischemic colitis, where intestinal contents leak from the devitalized intestine resulting in a septic abdomen. [6–7]
PI can be classified as primary (idiopathic) without any obvious cause or secondary to a wide variety of conditions. [8] Secondary PI is associated with numerous disease processes, including necrotizing enterocolitis, gastrointestinal obstruction, collagen vascular disorders, and immunosuppression either due to immunosuppressive therapy or infection with HIV or AIDS-associated enterocolitides. [3] PI is further classified into four pathogenic categories denoting the underlying pathogenesis: 1) Bowel necrosis, 2) mucosal disruption, 3) increased mucosal permeability, and 4) pulmonary disease. [3,6] PI resulting from increased mucosal permeability has been associated with organ transplantation, steroid and chemotherapeutic administration, and patients with immunodeficiency disease. [3] Lymphoid depletion results in the shrinkage and collapse of Peyer’s patches leading to a subsequent loss of structural integrity and increased mucosal permeability. [6,9] Increased mucosal permeability allows for the translocation and extension of gas through the affected segment of intestine resulting in gas filled spaces expanding depleted Peyer’s patches. [6,9] In cases with concurrent HIV or AIDS infection the translocation of gas may occur via lymphoid depletion or increased mucosal permeability arising from opportunistic infections. [3] This case represents secondary PI associated with increased mucosal permeability resulting from lymphoid depletion and or widespread cryptosporidiosis, both secondary to this animal’s SIV associated immunodeficiency.
Gross lesions in humans recognized endoscopically are described as polypoid or variably raised mucosal folds. [10] Histopathology of these foci are categorized as microvesicular, cystic, or diffuse. [3] Microvesicular PI is characterized by the presence of small gas filled spaces expanding the lamina propria, and the mucosa is otherwise unremarkable, unless concurrent disease is identified. [3] Cystic PI is characterized by the presence of submucosal or subserosal cysts ranging from a few millimeters to several centimeters, which are lined by macrophages and foreign body type multinucleated giant cells. [3] These cysts are surrounded by loosely arranged fibrous connective tissue, with little to no inflammation, and no intralesional bacteria. [3] The histopathologic features of the case presented most closely align with the cystic category of PI. Lastly, diffuse PI is recognized as a diffusely spongy bowel wall. [3] Microscopically, these cystic spaces lack any lining cells, with or without an inflammatory response or intralesional bacteria. [3]
Conclusion
This case represents an incidental finding of secondary cystic pneumatosis intestinalis in an SIV infected rhesus macaque; with concurrent wide spread cryptosporidiosis and primary cardiac left ventricular hypertrophy (LVH). Cases of LVH have been identified in the breeding colony at CNPRC, where it can sometimes be a cause of sudden death as seen in this case. [11] Based on the experimental inoculation with SIV, this case of PI speculatively resulted from the translocation of gas as a consequence of increased mucosal permeability secondary to immunosuppression. It is not clear if the increased mucosal permeability resulted from severe lymphoid depletion or the concurrent cryptosporidiosis. To our knowledge this is the first report of pneumatosis intestinalis in a rhesus macaque.
Acknowledgments
The authors would like to thank Anne Gibbons, Mark Allen, and Sarah Lockwood for their technical support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the primate center base grant P51OD011107 from the National Institutes of Health.
The animal study was supported by a research agreement from the Katholieke Universiteit Leuven (Belgium).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
References
- 1.Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
- 2.Animal Welfare Regulations. 2013. 9 CFR § 3.129.
- 3.Heng Y, Schuffler MD, Haggitt RC, Rohrmann CA. Pneumatosis intestinalis: a review. Am J Gastroentero. 1995;90:1747–1758. [PubMed] [Google Scholar]
- 4.Gelberg HB. Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal Cavity. In: Zachary JF, editor. Pathologic basis of veterinary disease. 6. St. Lois: Elsevier Health Sciences; 2017. p. 405. [Google Scholar]
- 5.Jamart J. Pneumatosis cystoides intestinalis a statistical study of 919 cases. Acta Hepatogastroenterol (Stuttg) 1979;26:419–422. [PubMed] [Google Scholar]
- 6.Pear BL. Pneumatosis intestinalis: a review. Radiology. 1998;207:13–19. doi: 10.1148/radiology.207.1.9530294. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson JK, Graham CB, Oliver TK, Jr, Goldenberg VE. Neonatal necrotizing enterocolitis. A report of twenty-one cases with fourteen survivors. Am J Surg. 1967;118:260–272. doi: 10.1016/0002-9610(69)90129-9. [DOI] [PubMed] [Google Scholar]
- 8.Koss LG. Abdominal gas cysts (pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53:523–549. [PubMed] [Google Scholar]
- 9.Smith BH, Welter LH. Pneumatosis intestinalis. Am J Clin Pathol. 1967;48:455–465. doi: 10.1093/ajcp/48.5.455. [DOI] [PubMed] [Google Scholar]
- 10.Koreishi A, Lauwers GY, Misdraji J. Pneumatosis intestinalis: a challenging biopsy diagnosis. Am J Surg Pathol. 2007;31:1469–1475. doi: 10.1097/PAS.0b013e318032c473. [DOI] [PubMed] [Google Scholar]
- 11.Reader JR, Canfield DR, Lane JF, Kanthaswamy S, Ardeshir A, et al. Left Ventricular Hypertrophy in Rhesus Macaques (Macaca mulatta) at the California National Primate Research Center (1992–2014) Comp Med. 2016;66:162–9. [PMC free article] [PubMed] [Google Scholar]