Abstract
Background and Aims
Chronic alcohol use is associated with lower gray matter volume, and we recently reported that alcohol use showed negative associations with widespread gray matter (GM) volume even among young adults. The current study aimed to test the strength of association between (1) alcohol use and GM volume; (2) alcohol use and white matter (WM) integrity; (3) cannabis use and GM volume; and (4) cannabis use and WM integrity among adults and adolescents.
Design and Setting
General linear models within large pooled cross-sectional samples of adolescents and adults who had participated in studies collecting substance use and neuroimaging data in the southwestern United States.
Participants
The current analysis included adults ages 18–55 years (N=853) and adolescents ages 14–18 years (N=439) with a range of alcohol and cannabis use.
Measurements
The dependent variable was GM volume or WM integrity, with key predictors of alcohol use (AUDIT score) and cannabis use (past 30-day use).
Findings
Alcohol use showed large clusters of negative associations (ηp2=.028 to .145, p<.001) with GM volume among adults, and to a lesser extent (one cluster; ηp2=.070, p<.05) among adolescents. Large clusters showed significant associations (ηp2=.050 to .124, p<.001) of higher alcohol use with poorer WM integrity, whereas adolescents showed no significant associations between alcohol use and WM. No associations were observed between structural measures and past 30-day cannabis use in adults or adolescents.
Conclusions
Alcohol use severity is associated with widespread lower gray matter volume and white matter integrity in adults, and with lower gray matter volume in adolescents.
Keywords: alcohol use, cannabis use, neuroimaging, voxel-based morphometry, diffusion tensor imaging
Introduction
Neuroimaging studies provide strong evidence of deleterious effects of chronic alcohol use on brain structure in adults and adolescents [1–2]. Chronic alcohol consumption is associated with lower gray matter (GM) volume globally [3–5] and in specific cortical structures [6–7]. Results of our recent large-scale study support a negative correlation between alcohol use severity and global GM volume in adults as young as 18 years [8]. Studies among adolescents suggest regional reductions in frontal [9–11], temporal and parietal [10], and cerebellar volume [12], or differences localized to specific structures such as the hippocampus [13]. Recent longitudinal research has reflected that even minor or moderate alcohol use may have teratogenic effects [14]; and further, that initiation of regular drinking in late adolescence dose-dependently disrupts GM development [15–16]. These findings support potential alcohol-associated GM reductions in widely distributed brain areas among adults and adolescents.
Similarly, widespread regional associations are found between alcohol and white matter (WM) measures in adults, although findings are less consistent among adolescents. Reduced WM integrity has been demonstrated in adult alcohol users compared to age-matched low or non-drinkers [1, 17–18] in frontal and temporal tracts, cortico-striatal tracts, and corpus callosum [18–21]. Among adolescents, some studies suggest reduced WM integrity in drinkers in long-range tracts spanning posterior to frontal regions [14, 22–23], while others have suggested small areas of increased WM integrity [24]. However, it remains unclear whether areas of positive association reflect premorbid risk or a causal association with alcohol use [24–25].
In contrast with the neuroimaging literature on alcohol consumption, studies present inconsistent recreational or chronic cannabis use associations with structural brain measures [26]. Much of the extant research employed region of interest analyses, and contradictory results can be found in orbitofrontal cortex, hippocampus, and amygdala [27–31]. In some cases, cannabis-using and non-cannabis-using groups differed on alcohol use [31]. Studies of WM integrity and cannabis use are likewise inconsistent. Where deficits are reported, their locations vary [32–34]. Adolescent literature on the relationship between GM or WM and cannabis use is sparse, partly due to frequent use of alcohol and cannabis in this age group [35–36]. Researchers therefore tend to include control groups who either use alcohol alone or are substance-naïve [25, 37–39]. Results suggest that combined cannabis- and alcohol-using adolescents exhibit GM and WM differences compared to alcohol-only and substance-naïve controls [25, 37–39], but the regions of difference vary among studies, and generally do not match adult findings. While these studies often employ sizable samples, it remains difficult to distinguish relative impacts of alcohol and cannabis in group-based analyses, and when considered overall, cannabis findings in GM average to a null effect [40].
We aimed to test the strength of association between (1) alcohol use and GM volume; (2) alcohol use and WM integrity; (3) cannabis use and GM volume; and (4) cannabis use and WM integrity among adults and adolescents. We examined general linear models (GLM) in GM and WM that included terms for alcohol and cannabis use, with follow up cannabis models comprising participants reporting weekly or greater cannabis use. Based on our previous work [8], we hypothesized that negative associations between alcohol use and GM would be observed in adults throughout frontal and parietal regions and cerebellum, and similar but less widespread associations would be observed in adolescents. We also expected that alcohol use would show widespread negative association with WM integrity among adults, and to a lesser extent among adolescents. Finally, based on previous work [40], we expected that no significant associations between GM or WM and cannabis use would emerge in adults or adolescents.
Methods
Design
The current study was cross-sectional and pooled data from existing studies that recruited substance-using adults, particularly alcohol, and collected neuroimaging data [41–42]. Data for adolescents were pooled from two existing neuroimaging studies among high-risk adolescents who were recruited to participate in a sexual health intervention [44–46]. Importantly, subsamples within the pooled dataset have been reported upon previously [8, 42], and were included here to maximize sample sizes and include a wide range of substance use. Key predictors of alcohol and cannabis use were treated as continuous measures, and participants with a wide range of use were included to test strengths of associations in GLM.
Sample and Procedures
Adult Participants
Participants were recruited from a southwestern metropolitan region of the United States through print and radio advertisements and online media. Exclusionary criteria across studies included traumatic brain injury with loss of consciousness >5 minutes, history of bipolar disorder or a psychotic disorder, or MRI contraindications (e.g., a positive pregnancy test, irremovable metal implants or piercings, claustrophobia). Subjects were asked to stop drinking 24 hours and abstain from smoking cigarettes 2 hours before scanning, and had to demonstrate a blood alcohol concentration of 0 prior to participation. Written informed consent, approved by the participating Institutional Review Board, was obtained from all participants.
Adolescent Participants
Research assistants recruited adolescents from juvenile justice partner programs to participate in interventions targeting risky health behaviors. All participants were assented and parental/legal guardian consent was obtained prior to study participation. Participants were between the ages of 14–18 years, and had no MRI contraindications. The participating Institutional Review Board approved the study and a federal certificate of confidentiality was obtained. Participants completed behavioral measures and a single neuroimaging session prior to participation in interventions.
Final Samples
Data were included for all available participants with complete measures needed for analyses. Among adult samples, 914 participants had anatomical neuroimaging data, and 904 participants had complete data for main variables across questionnaires. Following exclusions during initial processing, the final sample of adult participants for VBM models was N=853. Fewer participants completed diffusion tensor imaging (DTI), such that 850 participants had completed both a DTI scan and questionnaire data. Following exclusions during initial processing (n=37), N=813 adults were included in DTI models.
Among adolescent samples, 526 participants completed an anatomical scan, but n=66 were missing substance use data. An additional n=21 participants were excluded during initial processing, for a total sample of N=439 adolescents in VBM models. Similar to adults, fewer participants had available diffusion tensor imaging (n=406), and n=3 participants were excluded during initial processing for a total sample size of N=403 in DTI models.
Measures
In addition to demographic information, participants responded to substance use questionnaires. All participants in both the adult and the adolescent studies completed the Alcohol Use Disorders Identification Test (AUDIT) [47–48] to assess alcohol use severity. Cannabis use was derived from the Time-line Follow-back (TLFB) [49], and was computed as days of use out of the past 30 days for both adult and adolescent samples. Both AUDIT score and days of cannabis use were subjected to square root transformation (i.e., due to positive skew) prior to inclusion in statistical analyses.
Image Acquisition
MRI was performed on a 3T Siemens Trio (Erlangen, Germany) whole body scanner with a 12-channel radio frequency coil. A high-resolution T1-weighted structural image was acquired with a 5-echo multi-echo MPRAGE sequence with TE=1.64, 3.50, 5.36, 7.22, and 9.08 ms, TR=2.53 s, TI=1.20 s, flip angle=7°, NEX=1, slice thickness=1 mm, 192 sagittal slices, FOV=256×256 mm, resolution = 256×256×176, voxel size=1×1×1 mm, and pixel bandwidth=650 Hz.
DTI scans were acquired using a single-shot spin-echo echo planar imaging (EPI) sequence with a twice-refocused balanced echo to reduce eddy current distortions. Sequence parameters were: FOV=256×256 mm, 128×128 matrix, slice thickness=2 mm, NEX=1, TE=84 ms, and TR=9000 ms. A 12-channel radiofrequency (RF) head-phased array coil was used, with GRAPPA (X2), 30 gradient directions, and b=800 s/mm2.
Voxel-Based Morphometry
Voxel-based morphometry (VBM) analyses were performed using FMRIB’s Software Library’s (FSL; v5.0.1) [50] FSLVBM analysis pipeline following standard automated processing [51–52], as in other publications [40, 53]. This pipeline uses modulation to incorporate the volumetric changes during normalization in the analysis for optimized VBM. The raw T1-weighted images were brain-extracted (i.e., removal of non-brain tissue and skull) using the FSL default BET brain extraction process. The resulting GM images were aligned to Montreal Neurological Institute (MNI) standard space using the affine registration tool FMRIB’s Linear Image Registration Tool (FLIRT), followed by nonlinear registration using FMRIB’s Nonlinear Image Registration Tool (FNIRT). Automated calculations for exclusion due to motion were used to accommodate the large sample sizes of pooled data. For T1 data, participants were excluded if the correlation between the spatially normalized image and the MNI template was <.93. Remaining images were then averaged into a study-specific template (separate templates for adolescents and adults). Native GM images were then non-linearly re-registered to this template using FNIRT. The registered partial volume images were then modulated by dividing the Jacobian of the warp field. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 3, yielding full-width half-maximum (FWHM) 3×2.3 mm = 6.9 mm.
Tract-Based Spatial Statistics
For DTI data, motion exclusion for a single volume occurred if motion was greater than 4mm of root mean square displacement, and a participant was not considered for further analysis if more than 10% of gradient directions were dropped [42]. DTI data were preprocessed using FSL’s Diffusion Toolbox [54]. Data were corrected for eddy current distortion and then all images were registered to a b=0 s/mm2 image using 6 degrees of freedom affine transformation using FSL’s linear registration algorithm (FLIRT). Diffusion tensor and index maps were calculated using Dtifit.
Fractional anisotropy (FA) and mean, axial, and radial diffusivity (MD, AD, and RD, respectively) values were obtained using FSL Tract-Based Spatial Statistics (TBSS) [55]. A nonlinear registration algorithm (FNIRT) aligned each FA image to Montreal Neurological Institute (MNI) standard space. All transformed FA images were merged into a single 4D image file, and a mean image was created and then skeletonized (separate skeletons for adolescents and adults). Finally, a threshold value of 0.2 was applied to the mean skeleton image, and all aligned FA data were projected onto the mean skeleton for use in voxelwise statistics. The nonlinear warps and projection vectors from the FA processing were then applied to AD, RD, and MD images to obtain a single skeletonized 4D image for each diffusivity index.
Data Analyses
GLMs conducted using FSL’s Randomise program [56] evaluated relationships between brain structure (i.e., dependent variables of GM volume or WM integrity) and key predictors of AUDIT score and TLFB-derived cannabis use days (e.g., in order to examine each predictor while controlling for the other), with additional covariates for age, sex, and study cohort, as well as intracranial volume (ICV) in VBM models. Multiple comparison correction used voxelwise thresholding applied through FSL’s Randomise permutation-based non-parametric testing with Monte Carlo simulations. A total of 5000 simulations were run for each permutation test, and threshold-free cluster enhancement [57] was used to identify clusters of significant association. Clusters are reported of sizes ≥1000 contiguous voxels in VBM models and ≥200 contiguous voxels in DTI models. In order to obtain partial eta squared values for each cluster, average values were extracted for inclusion in univariate GLMs in SPSS [58]. In order to further test possible associations between brain structure and cannabis use, the samples were restricted to participants who reported using cannabis at least once per week. Analyses were repeated but excluding the AUDIT predictor.
Results
Sample Characteristics
Sample characteristics are presented in Table 1. Based on AUDIT score, adults reported a mild to moderate degree of alcohol problems on average, while adolescents reported alcohol use below clinical thresholds on average. Across substance use measures (alcohol use in last 6 months, and cannabis use in last 30 days), 487 adult participants reported using only alcohol, 5 reported using only cannabis, and 28 reported using neither substance. Similarly, among adolescents, 113 participants reported using only alcohol, 35 reported using only cannabis, and 60 reported using neither substance. AUDIT score and TLFB cannabis use days were not correlated in adults [r(851)=.04], but were significantly correlated in adolescents [r(437)=.30, p<.01].
Table 1.
Sample Characteristics.
| Adults | Adolescents | |||
|---|---|---|---|---|
|
| ||||
| Whole Sample | Weekly or Greater Cannabis Users |
Whole Sample | Weekly or Greater Cannabis Users |
|
| N | 853 | 191 | 439 | 201 |
| Ethnicity | ||||
| Caucasian | 474 (56%) | 111 (58%) | 66 (15%) | 29 (14%) |
| Latino | 136 (16%) | 19 (10%) | 285 (65%) | 133 (66%) |
| Native American | 60 (7%) | 13 (7%) | 32 (7%) | 17 (8%) |
| African American | 25 (3%) | 7 (4%) | 28 (6%) | 11 (5%) |
| Asian/Pacific Islander | 10 (1%) | 0 (0%) | 6 (1%) | 3 (1%) |
| Mixed | 120 (14%) | 30 (16%) | 21 (5%) | 8 (4%) |
| Unknown/Declined | 28 (3%) | 11 (6%) | 1 (<1%) | 0 (0%) |
| Females:Males | 326:527 (62% Male) | 64:131 (69% Male) | 134:305 (69% Male) | 52:149 (74% Male) |
| Age | 31.64 (9.64) | 28.81 (8.44) | 15.97 (1.17) | 16.00 (1.08) |
| AUDIT Total Score | 13.14 (8.47) | 14.00 (7.79) | 6.39 (6.67) | 8.48 (6.65) |
| TLFB Alcohol Drinking Days | 12.78 (8.95) | 13.81 (8.89) | 2.17 (3.78) | 3.39 (4.48) |
| TLFB Cannabis Smoking Days | 4.33 (8.66) | 18.19 (9.24) | 9.69 (11.94) | 20.60 (9.51) |
AUDIT: Alcohol Use Disorders Identification Test; TLFB: Timeline Follow-Back (30 days).
Percentages approximate due to rounding error.
Alcohol Use Models
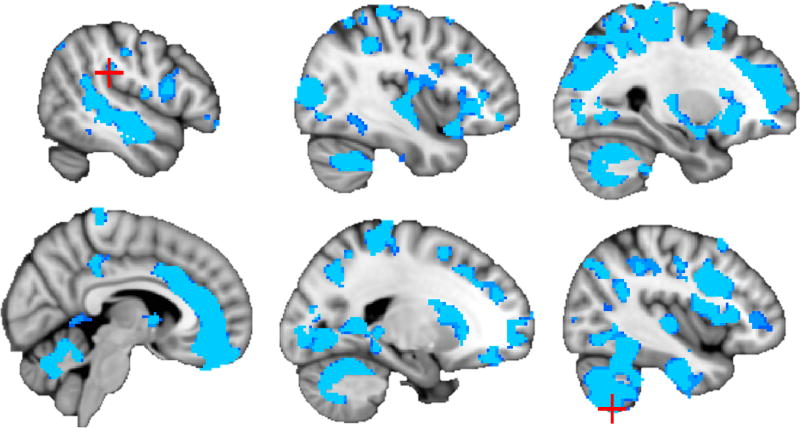
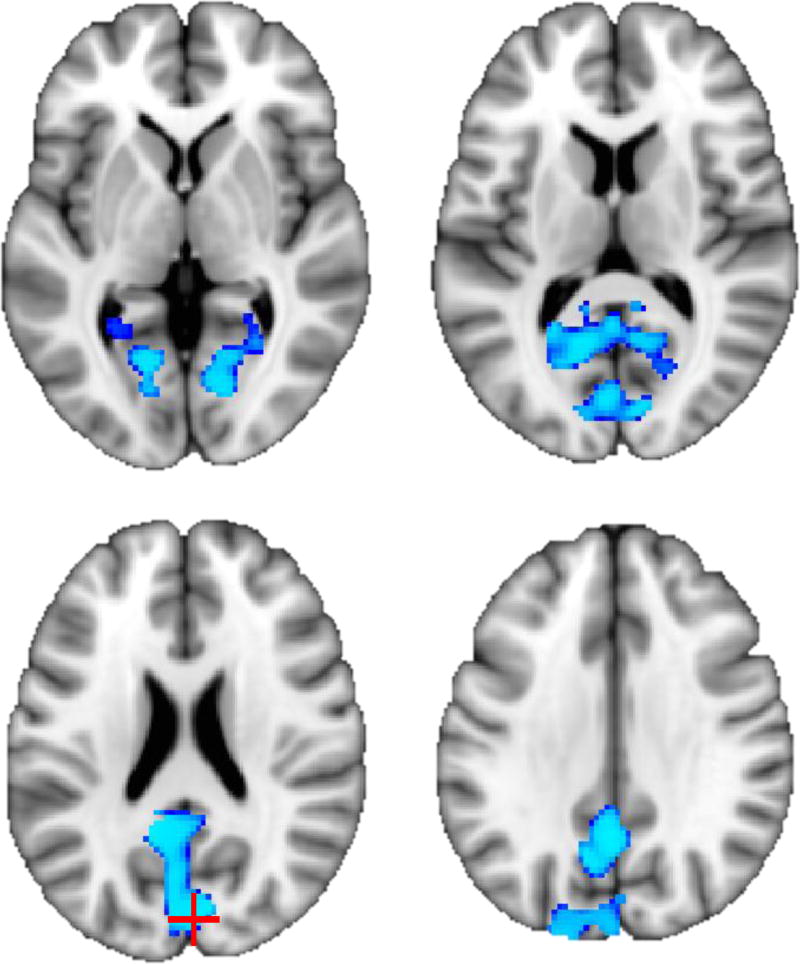
Consistent with previous results, AUDIT scores showed large clusters of negative association (ηp2=.028–.145, p<.001) with GM volume among adults above and beyond cannabis use and covariates (see Table 2, Figure 1). Peak effects were observed in cerebellum, insula, caudate, and putamen. Among adolescents, negative association (ηp2=.070, p<.05) between GM and AUDIT score was observed in one large cluster, with peak effects in the cuneus, precuneus, and posterior cingulate gyrus (see Table 2, Figure 2).
Table 2.
Clusters (≥ 1000 voxels) of significant negative association between Voxel-Based Morphometry (VBM) and AUDIT scores.
| Regiona | Cluster Size (voxels) |
MNI Coordinatesa | Peak tb | Partial Eta Squaredc |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Adults (N = 853; p < .001) | ||||||
|
| ||||||
| Cerebellum VIIb L; Insula R; Caudate L, Central opercular cortex L, Putamen L | 348256 | 130 | 66 | 12 | 8.06 | .145 |
| Parietal operculum cortex R; Anterior supramarginal gyrus R | 5168 | 38 | 96 | 98 | 5.01 | .028 |
|
| ||||||
| Adolescents (N = 439; p < .05) | ||||||
|
| ||||||
| Cuneus R; Precuneus L, Posterior cingulate gyrus L | 27872 | 86 | 42 | 94 | 4.05 | .070 |
Corresponding to peak t; followed by local maxima
FSL Randomise output
SPSS GLM on extracted average cluster values
L = Left; R = Right
Figure 1.
Negative association (p < .001) between AUDIT score and gray matter volume among adults (N = 853). Peak voxels within each cluster are marked with red crosshairs (see Table 2; slices from top left: x = 38, 50, 62, 84, 110, and 130).
Figure 2.
Negative association (p < .05) between AUDIT score and gray matter volume among adolescents (N = 439). Peak voxel is marked with red crosshair (see Table 2; slices from top left: z = 74, 84, 94, and 104).
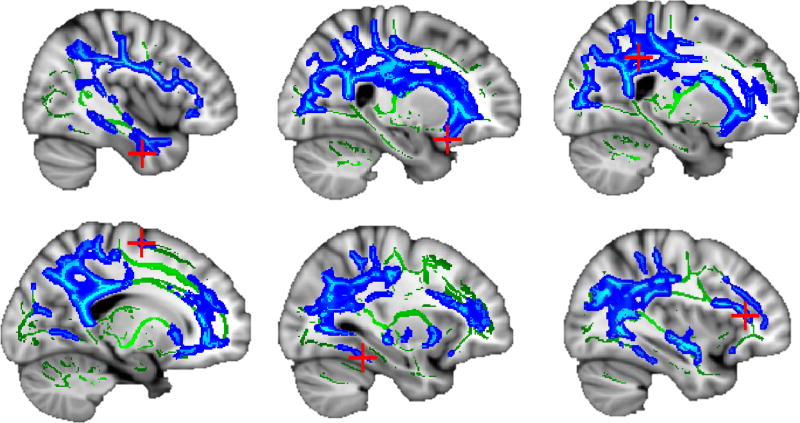
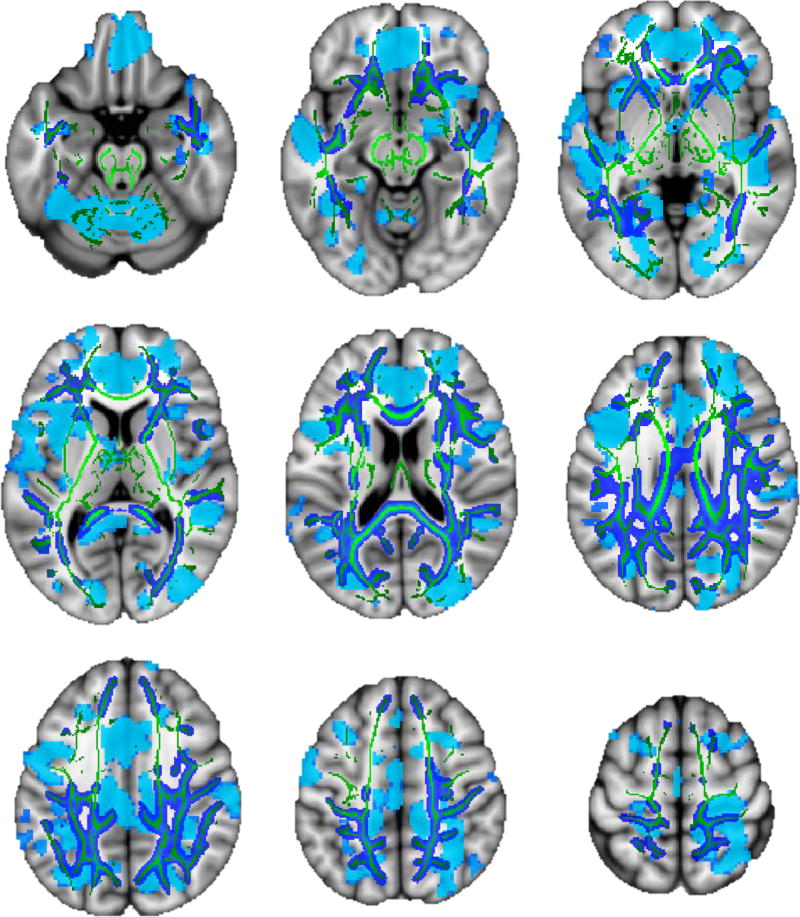
A similar pattern was observed among WM indices in adults, such that large clusters showed significant associations (ηp2=.050–.124, p<.001) of higher AUDIT scores with poorer WM integrity (i.e., negative association with FA and positive association with diffusivity; see Table 3 and Figures 3–4). In particular, higher AUDIT scores were associated with large clusters (i.e., up to 37% of the entire WM skeleton in a single cluster) of greater diffusivity (MD, AD, and RD) with peak effects observed in inferior, superior, and inferior fronto-occipital fasciculi. Among adolescents, no associations were observed between WM and AUDIT score.
Table 3.
Clusters (≥ 200 voxels) of significant association (p < .001) between white matter integrity and AUDIT scores in adults (N = 813).
| Regiona | Cluster Size (voxels) |
MNI Coordinatesa | Direction | Peak tb | Partial Eta Squaredc |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Fractional Anisotropy | |||||||
|
| |||||||
| Cingulum (cingulate gyrus) R; Inferior fronto-occipital fasciculus R | 528 | 67 | 83 | 108 | − | 6.16 | .072 |
|
| |||||||
| Mean Diffusivity | |||||||
|
| |||||||
| Inferior longitudinal fasciculus R; Superior longitudinal fasciculus L, Superior longitudinal fasciculus R | 45154 | 48 | 120 | 38 | + | 7.80 | .079 |
|
| |||||||
| Radial Diffusivity | |||||||
|
| |||||||
| Inferior longitudinal fasciculus L; Superior longitudinal fasciculus R, Superior longitudinal fasciculus L, Inferior fronto-occipital fasciculus R | 39029 | 121 | 86 | 53 | + | 7.51 | .079 |
|
| |||||||
| Axial Diffusivity | |||||||
|
| |||||||
| Inferior fronto-occipital fasciculus R; Superior longitudinal fasciculus L, Superior longitudinal fasciculus R | 21352 | 65 | 143 | 52 | + | 8.01 | .124 |
| Inferior fronto-occipital fasciculus L; Superior longitudinal fasciculus L, Anterior thalamic radiation L | 988 | 125 | 158 | 82 | + | 5.74 | .073 |
| Superior longitudinal fasciculus L | 430 | 107 | 119 | 136 | + | 6.01 | .050 |
Corresponding to peak t; followed by local maxima
FSL Randomise output
SPSS GLM on extracted average cluster values
L = Left; R = Right
Figure 3.
Negative association (p < .001) between AUDIT score and white matter integrity among adults (N = 813). Mean diffusivity clusters (blue) overlaid on white matter skeleton (green); peak voxels marked with red crosshairs (see Table 3; slices from top left: x = 48, 65, 67, 107, 121, and 125).
Figure 4.
Overlay of gray matter (light blue) and white matter mean diffusivity (dark blue, with mean white matter skeleton in green) associations with AUDIT score among adults (p < .001; slices from top left: z = 50, 60, 70, 80, 90, 100, 110, 120, and 130).
Cannabis Use Models
In adults and adolescents, no associations were observed between cannabis use and GM above and beyond other predictors, in either the full samples or when limited to weekly or greater users and excluding the AUDIT predictor. Further, among adults and adolescents, no associations were observed between cannabis use and WM indices above and beyond other model predictors, in either the full sample or restricted sample of weekly or greater cannabis users and excluding the AUDIT predictor.
Discussion
The current study sought to expand previous work on associations between alcohol use and GM structure [8] by further examining WM microstructure; and by examining these associations among adults and adolescents. Given the inconclusive prior evidence regarding the possible relationship between cannabis use and brain structure [26], we also tested associations between structural measures and recent cannabis use. Our previous results suggested widespread negative associations of medium effect size between alcohol use and GM throughout the brain and cerebellum, above and beyond important confounding variables such as age. Negative associations between alcohol use and GM volume were observed even among the youngest age group of the sample (ages 18 to 25 years) [8]. The current study supported previous VBM results among adults, such that large clusters of negative association between problem drinking and GM volume (e.g., accounting for approximately 15% of variance) were observed. Further, a large cluster of similar effect as adults was observed among adolescents. Additionally, we examined similar models among adults and adolescents for WM. Similar to VBM results, very large clusters showed negative association between alcohol use severity and WM integrity among adults (e.g., accounting for approximately 12% of variance), although no WM associations were observed in adolescents. No significant associations were observed between cannabis use and structural measures across any sample, even when limited to participants reporting weekly or greater use and removing the influence of the alcohol predictor.
Negative associations between GM volume and alcohol use were expected among the adult sample, and are consistent with existing literature [4, 59–60]. In adolescents, the cluster of negative association was larger than expected, but did not survive increasing the significance threshold above p<.05. While no causal conclusions may be drawn, adolescents showed lower cuneus, precuneus, and posterior cingulate volume associated with alcohol use, with about 4 years of drinking on average. These regions are more commonly reported among adult alcohol use disorder (AUD) patients [6], but overall this finding is comparable to results from a large consortium project, in which adolescent drinkers showed smaller cortical volumes and thickness than nondrinkers [61]. Several longitudinal projects have been able to extend these findings to examine predictors of future alcohol use or structural changes over time, and results may suggest a dose-dependent relationship between alcohol use and changes in GM emerging even during adolescence. Future binge drinking was predicted by lower GM volume in superior frontal gyrus but greater GM volume in middle and precentral gyrus [63]. Further, in another longitudinal study [16], adolescents who progressed from negligible to heavy drinking over 3 years had smaller baseline volumes of anterior cingulate and inferior frontal gyrus, and reduced temporal gyri and subcortical volumes at follow up compared to non-drinkers. These volume reductions appeared to be dose dependent, in that they positively correlated with lifetime alcohol use [16]. Another study found that individuals who started drinking regularly at approximately 18 years of age exhibited over-thinning of the middle frontal gyrus, an area key to executive processing, compared to non-drinkers at follow up after 2 years [15]. Taken together, these results suggest that initiation of regular alcohol use in adolescence may disrupt typical GM development [15–16], which has been associated with important functional changes in risk taking and reward responding [62–63].
Adult alcohol users consistently show lower WM volume and integrity compared to age-matched low- or non-drinkers [1, 17–18, 59], which exceed normal age-related decline [61]. These impairments have been found in widespread brain regions, including frontal and temporal tracts, corticostriatal tracts, and corpus callosum [18–21]. The current results suggest a pervasive association between reduced WM integrity and alcohol use. Consistent with these results, a meta-analysis concluded that AUDs are associated with significant WM deficits with a small-to-moderate effect size [65]. Among adolescent populations, however, associations between WM measures and alcohol use are less conclusive. Greater number of lifetime drinks was associated with smaller subcortical WM volume [61], but another study using the same sample found no differences in WM integrity measures between adolescent drinkers and non- or low-drinkers [66]. Several cross-sectional studies of adolescent alcohol use and WM measures have found lower FA in corpus callosum, corona radiata, inferior and superior longitudinal fasciculi [14, 22–23, 67]. Other studies found greater FA among adolescents with AUDs compared to their non-drinking peers in limbic tracts even when matching groups for age [24], or that a higher number of lifetime drinking occasions was associated with increased superior longitudinal fasciculus integrity in adolescents [67]. Similar to these findings, the results of the current study did not indicate any regions of negative association between WM integrity and heavy alcohol use, and longitudinal studies will be important for clarifying alcohol effects on brain structural development.
The current study did not observe any associations of past 30-day cannabis use with GM or WM among adults or adolescents beyond other model predictors. This is consistent with our previous work suggesting that regionally specific differences between cannabis users and non-users are often inconsistent across studies and that some of the observed associations may actually be related to comorbid alcohol use [40]. The present results are also consistent with a recent study from a large consortium project that found no relationship between cannabis use and cortical GM (N=466) [68] and a large twin study (N=483) that found the association between cannabis use and GM volumes was explained by genetics rather than cannabis use [69]. While the analyses reported herein are consistent with the effects reported in studies with large sample sizes, future longitudinal studies will be important to clarify the effects of cannabis and alcohol use on brain structure.
Several limitations should be considered when interpreting the current results. Participants were pooled from several studies to maximize sample size, and the current study used a similar model for VBM and AUDIT score as a previous paper [8]. The AUDIT was selected as the primary measure of alcohol use due to its inclusion across adult and adolescent studies, and because it provides an estimate of behavior for a slightly longer period than other available measures (i.e., 30 days via the TLFB). The AUDIT offers high reliability and validity in terms of measuring risk of alcohol problems [47], but does not provide a detailed history. This could lead to an underestimation of long-term alcohol effects, which is particularly relevant for older participants. Similarly, the TLFB is a limited measure of cannabis use, and lacks detailed information on history of cannabis use and quantity of consumption. It was selected as the only available common metric of cannabis consumption across adult and adolescent samples, and the average use of the current samples was relatively low. We attempted to address this limitation by examining potential associations within weekly or greater users and without the influence of an alcohol use predictor (i.e., as in many other studies in the existing literature), but results are still limited to recent use. Future prospective studies should carefully select measures of cannabis use representing history, frequency, and quantity of use. Finally, collecting comparable measures of substance use could enhance interpretability of findings.
In addition, the present analyses do not account for psychopathology (other than excluding participants with history of bipolar disorder or psychosis) or use of substances other than alcohol and cannabis. Studies have demonstrated GM reductions with tobacco smoking [70–71], which has also been associated with exacerbated age-related brain atrophy [72–73]. Future studies primarily focusing on tobacco and cannabis use should control for alcohol use history [40]. Further, these data are cross-sectional, which prevents consideration of the contributions of preexisting conditions or causality. Although we do not believe the adolescents in this study represent a fundamentally different population than other adolescents given their justice involvement, the question of causality is particularly relevant for adolescents, and ongoing large consortium projects will inform whether any observed associations are likely premorbid or result from heavy alcohol use [74].
The current results extend previous findings on the significant, widespread associations between alcohol use severity and alterations in brain structure. These results were expected for GM [8], but the global nature of associations between alcohol use and WM integrity was surprising; even at an increased significance threshold, approximately 30% of voxels in the WM skeleton showed negative association between AUDIT score and WM integrity. WM damage in adult hazardous alcohol users may be partially reversed with extended abstinence [75–77], but the current results underline the importance of increasing efforts for early and effective treatments for AUDs. Further, identification of specific brain regions impacted by alcohol use throughout the lifespan may aid in the development of more efficacious pharmacological treatment options.
Acknowledgments
This work was supported by NIH grants from the National Institute on Drug Abuse (NIDA) R01DA025074 to KEH and R36DA040020 to RET; the National Institute on Alcohol Abuse and Alcoholism (NIAAA) R01AA012238 to KEH and R01AA017390 to ADB; and the National Institute of Nursing Research (NINR) R01NR013332 to ADB and SWFE.
Footnotes
Conflicts of interest: None
References
- 1.Bühler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35(10):1771–93. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 2.Welch KA, Carson A, Lawrie SM. Brain structure in adolescents and young adults with alcohol problems: systematic review of imaging studies. Alcohol Alcoholism. 2013;48(4):433–44. doi: 10.1093/alcalc/agt037. [DOI] [PubMed] [Google Scholar]
- 3.Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158(2):198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- 4.Paul CA, Au R, Fredman L, Massaro JM, Seshadri S, Decarli C, et al. Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol. 2008;65(10):1363–7. doi: 10.1001/archneur.65.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16(6):1078–89. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 6.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26(4):558–64. [PMC free article] [PubMed] [Google Scholar]
- 7.Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64(3):192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thayer RE, Hagerty SL, Sabbineni A, Claus ED, Hutchison KE, Weiland BJ. Negative and interactive effects of sex, aging, and alcohol abuse on gray matter morphometry. Hum Brain Mapp. 2016;37(6):2276–92. doi: 10.1002/hbm.23172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29(9):1590–600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- 10.Fein G, Greenstein D, Cardenas VA, Cuzen NL, Fouche JP, Ferrett H, et al. Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Res. 2013;214(1):1–8. doi: 10.1016/j.pscychresns.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina KLMT, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. 2008:386–94. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139(3):181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewing SW, Sakhardande A, Blakemore SJ. The effect of alcohol consumption on the adolescent brain: a systematic review of MRI and fMRI studies of alcohol-using youth. Neuroimage Clin. 2014;5:420–37. doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abuse. 2013;39(6):345–55. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, et al. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–25. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanraud S, Reynaud M, Wessa M, Penttila J, Kostogianni N, Cachia A, et al. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology. 2009;34(5):1223–32. doi: 10.1038/npp.2008.101. [DOI] [PubMed] [Google Scholar]
- 18.Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173(1):22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65:680–90. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbloom MJ, Sassoon SA, Fama R, Sullivan EV, Pfefferbaum A. Frontal callosal fiber integrity selectively predicts coordinated psychomotor performance in chronic alcoholism. Brain Imaging Behav. 2008;2(2):74–83. doi: 10.1007/s11682-007-9017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, Harris GJ. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcoholism: Clinical and Experimental Research. 2013;37(1):110–22. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–85. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thayer RE, Callahan TJ, Weiland BJ, Hutchison KE, Bryan AD. Associations between fractional anisotropy and problematic alcohol use in juvenile justice-involved adolescents. Am J Drug Alcohol Abuse. 2013;39(6):365–71. doi: 10.3109/00952990.2013.834909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardenas VA, Greenstein D, Fouche JP, Ferrett H, Cuzen N, Stein DJ, et al. Not lesser but greater fractional anisotropy in adolescents with alcohol use disorders. Neuroimage Clin. 2013;2:804–9. doi: 10.1016/j.nicl.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, et al. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32(3):395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, et al. Neuroimaging in cannabis use: a systematic review of the literature. Psych Med. 2010;40(03):383–98. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- 27.Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014;39(9):2041–8. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U S A. 2014;111(47):16913–8. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzetti V, Solowij N, Whittle S, Fornito A, Lubman DI, Pantelis C, et al. Gross morphological brain changes with chronic, heavy cannabis use. Br J Psychiatry. 2015;206(1):77–8. doi: 10.1192/bjp.bp.114.151407. [DOI] [PubMed] [Google Scholar]
- 30.Mashhoon Y, Sava S, Sneider JT, Nickerson LD, Silveri MM. Cortical thinness and volume differences associated with marijuana abuse in emerging adults. Drug Alcohol Depend. 2015;155:275–83. doi: 10.1016/j.drugalcdep.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price JS, McQueeny T, Shollenbarger S, Browning EL, Wieser J, Lisdahl KM. Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology (Berl) 2015;232(16):2939–50. doi: 10.1007/s00213-015-3931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M. Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cogn Neurosci. 2015;16:23–35. doi: 10.1016/j.dcn.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shollenbarger SG, Price J, Wieser J, Lisdahl K. Poorer frontolimbic white matter integrity is associated with chronic cannabis use, FAAH genotype, and increased depressive and apathy symptoms in adolescents and young adults. Neuroimage Clin. 2015;8:117–25. doi: 10.1016/j.nicl.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zalesky A, Solowij N, Yucel M, Lubman DI, Takagi M, Harding IH, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135(Pt 7):2245–55. doi: 10.1093/brain/aws136. [DOI] [PubMed] [Google Scholar]
- 35.Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res. 2009;43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173(3):228–37. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J Stud Alcohol Drugs. 2014;75(5):729–43. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, et al. Cortical thickness in adolescent marijuana and alcohol users: a three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci. 2015;16:101–9. doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015;172(6):531–42. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J Neurosci. 2015;35(4):1505–12. doi: 10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Claus ED, Kiehl KA, Hutchison KE. Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcohol Clin Exp Res. 2011;35(7):1209–19. doi: 10.1111/j.1530-0277.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monnig MA, Thayer RE, Caprihan A, Claus ED, Yeo RA, Calhoun VD, et al. White matter integrity is associated with alcohol cue reactivity in heavy drinkers. Brain Behav. 2014;4(2):158–70. doi: 10.1002/brb3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houck JM, Bryan AD, Feldstein Ewing SW. Functional connectivity and cannabis use in high-risk adolescents. Am J Drug Alcohol Abuse. 2013;39(6):414–23. doi: 10.3109/00952990.2013.837914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Feldstein Ewing SW. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci. 2015;16:5–15. doi: 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magnan RE, Callahan TJ, Ladd BO, Claus ED, Hutchison KE, Bryan AD. Evaluating an integrative theoretical framework for HIV sexual risk among juvenile justice involved adolescents. J AIDS Clin Res. 2013;4(6):217. [PMC free article] [PubMed] [Google Scholar]
- 46.Thayer RE, Feldstein Ewing SW, Dodd AB, Hansen NS, Mayer AR, Ling JM, et al. Functional activation during the Stroop is associated with recent alcohol but not marijuana use among high-risk youth. Psychiatry Res. 2015;234(1):130–6. doi: 10.1016/j.pscychresns.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT - The alcohol use disorders identification test: Guidelines for use in primary care: World Health Organization. 2001 Available from: http://www.who.int/substanceabuse/publications/alcohol/en/index.html.
- 48.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 49.Sobell LC, Sobell MB. Time-line follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption. Totowa, New Jersey: Humana Press; 1992. pp. 73–98. [Google Scholar]
- 50.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 51.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 52.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 53.Smolker HR, Depue BE, Reineberg AE, Orr JM, Banich MT. Individual differences in regional prefrontal gray matter morphometry and fractional anisotropy are associated with different constructs of executive function. Brain Struct Funct. 2015;220(3):1291–306. doi: 10.1007/s00429-014-0723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 56.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage. 2014;92:381–97. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 58.IBM Corp. IBM SPSS Statistics for Mac, Version 24.0. Armonk, NY: IBM Corp.; Released 2016. [Google Scholar]
- 59.Wang J, Fan Y, Dong Y, Ma M, Ma Y, Niu Y, et al. alterations in brain structure and functional connectivity in alcohol dependent patients and possible association with impulsivity. PLoS One. 2016;11(8):e0161956. doi: 10.1371/journal.pone.0161956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X, Tian F, Zhang H, Zeng J, Chen T, Wang S, et al. Cortical and subcortical gray matter shrinkage in alcohol-use disorders: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2016;66:92–103. doi: 10.1016/j.neubiorev.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 61.Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, et al. Adolescent development of cortical and white matter structure in the NCANDA sample: role of sex, ethnicity, puberty, and alcohol drinking. Cereb Cortex. 2016;26(10):4101–21. doi: 10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, et al. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am J Psychiatry. 2012;169(1):39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- 63.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185–9. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–32. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- 65.Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, McCrady BS. White matter volume in alcohol use disorders: a meta-analysis. Addict Biol. 2013;18(3):581–92. doi: 10.1111/j.1369-1600.2012.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pohl KM, Sullivan EV, Rohlfing T, Chu W, Kwon D, Nichols BN, et al. Harmonizing DTI measurements across scanners to examine the development of white matter microstructure in 803 adolescents of the NCANDA study. Neuroimage. 2016;130:194–213. doi: 10.1016/j.neuroimage.2016.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31(6):349–55. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orr JM, Paschall CJ, Banich MT. Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin. 2016;12:47–56. doi: 10.1016/j.nicl.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pagliaccio D, Barch DM, Bogdan R, Wood PK, Lynskey MT, Heath AC, et al. Shared predisposition in the association between cannabis use and subcortical brain structure. JAMA Psychiatry. 2015;72(10):994–1001. doi: 10.1001/jamapsychiatry.2015.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55(1):77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- 71.Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29(8):1484–95. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- 72.Akiyama H, Meyer JS, Mortel KF, Terayama Y, Thornby JI, Konno S. Normal human aging: factors contributing to cerebral atrophy. J Neurol Sci. 1997;152(1):39–49. doi: 10.1016/s0022-510x(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 73.Hayee A, Haque A, Anwarullah AK, Rabbani MG. Smoking enhances age related brain atrophy--a quantitative study with computed tomography. Bangladesh Med Res Counc Bull. 2003;29(3):118–24. [PubMed] [Google Scholar]
- 74.Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, et al. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): a multisite study of adolescent development and substance use. J Stud Alcohol Drugs. 2015;76(6):895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, et al. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res. 2012;36(11):1922–31. doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain. 2010;133(Pt 4):1043–53. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, et al. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry. 2014;1(3):202–12. doi: 10.1016/S2215-0366(14)70301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]