Abstract
Cardiovascular disease remains a leading cause of morbidity and mortality in HIV positive patients, even in those whose viral loads are well controlled with antiretroviral therapy. However, the underlying molecular events responsible for the development of cardiac disease in the setting of HIV remain unknown. The HIV encoded Tat protein plays a critical role in the activation of HIV gene expression and profoundly impacts homeostasis in both HIV infected cells and uninfected cells that have taken up released Tat via a bystander effect. Since cardiomyocyte function, including excitation-contraction coupling, greatly depends on energy provided by the mitochondria, in this study we performed a series of experiments to assess the impact of Tat on mitochondrial function and bioenergetics pathways in a primary cell culture model derived from neonatal rat ventricular cardiomyocytes (NRVCs). Our results show that the presence of Tat in cardiomyocytes is accompanied by a decrease in oxidative phosphorylation, a decline in the levels of ATP, and an accumulation of reactive oxygen species (ROS). Tat impairs the uptake of mitochondrial Ca2+ ([Ca2+]m) and the electrophysiological activity of cardiomyocytes. Tat also affects the protein clearance pathway and autophagy in cardiomyocytes under stress due to hypoxia-reoxygenation conditions. A reduction in the level of ubiquitin along with dysregulated degradation of autophagy proteins including SQSTM1/p62 and a reduction of LC3 II were detected in cardiomyocytes harboring Tat. These results suggest that, by targeting mitochondria and protein quality control, Tat significantly impacts bioenergetics and autophagy resulting in dysregulation of cardiomyocyte health and homeostasis. This article is protected by copyright. All rights reserved
Keywords: HIV-1 Tat, mitochondrial bioenergetics, cardiomyocytes, autophagy, hypoxia/reoxygenation
INTRODUCTION
HIV positive patients have been reported to be more vulnerable to the development of various cardiovascular disorders such as atherosclerosis, myocardial fibrosis and myocardial infarction [Manga et al., 2017, Eugenin et al., 2008]. HIV-infected individuals also have a significantly increased risk of developing heart failure with reduced ejection fraction (HFrEF) even when adjusting for possible confounders [Freiberg et al 2017]. Although the direct infection of cardiomyocytes by HIV remains controversial, these cells can take up the HIV proteins released by adjacent infected cells [Fiala et al., 2004]. For example, HIV can lead to a chronic inflammatory response and subsequent cardiac dysfunction by infecting myocardial endothelial cells [Manga et al., 2017]. Moreover, HIV has been reported to infect vascular smooth muscle cells both in vivo and in vitro leading to the development of vascular disease [Eugenin et al., 2008]. Antiretroviral therapies have played an important role in suppressing HIV progression and reduced HIV mortality but they increase the risk of dyslipidemia and contribute to the development of heart disease [Domingo et al., 2008]. However, the precise molecular mechanisms through which HIV causes cardiac problems still remain to be elucidated.
Among the HIV-1 encoded proteins, Tat, a transcriptional activator, has received much attention due to its toxic effects on HIV infected as well as uninfected cells, which can take up Tat released by infected cells [Nath et al., 2014]. Tat is a highly toxic protein that leads to apoptosis activation and cell death in many cell types, including primary mouse striatal neurons [Singh et al., 2005]. In addition, Tat protein has been reported to be one of the key factors in the development of neurological complications in HIV-infected patients [Carey et al., 2012]. Tat also plays a critical role in the development of dilated cardiomyopathy [Duan et al., 2013, Fang et al., 2009]. Transgenic mice expressing Tat displayed dysregulated cardiac function with reduced heart rate as well as depressed systolic and diastolic function [Fang et al., 2009]. However, additional studies are needed to further understand the underlying pathways through which Tat results in cardiac dysfunction.
Mitochondrial abnormalities with impaired metabolic capacity are associated with the development of cardiac dysfunction as mitochondria are the major energy source for cardiac function [Parihar et al., 2017]. Therefore, it is important to determine whether Tat alters mitochondrial function and whether this leads to the development of HIV cardiomyopathy. Consistent with this possibility, in HIV-1 transgenic mice (Tg26), the stress of thoracic surgery resulted in significant reduction in both cardiac contractility and relaxation compared to wild-type littermates [Cheung et al., 2015]. However, the underlying mechanism through which HIV sensitizes cardiac cells to damage by stress remains unclear. Autophagy, an intracellular degradation pathway in which dysfunctional proteins and organelles are delivered to lysosomes for further degradation, has been reported to be targeted by HIV proteins [Fields et al., 2015]. For example, in a neuronal cell line, the HIV-1 Tat protein has been shown to interact directly with lysosomal-associated membrane protein 2A (LAMP2A), which then prevents autophagosome fusion with lysosomes and results in decreased autophagy [Fields et al., 2015]. Dysregulation of autophagy has been reported to be associated with left ventricular dilation as well as depressed contractility and cardiomyopathy [Gustafsson et al., 2008]. When impaired, mitophagy (selective autophagy degrading damaged mitochondria), led to significant cell death in primary cardiomyocytes [Tahrir et al., 2017]. These considerations prompted us to investigate the effect of HIV-1 Tat on the protein quality control machinery with a primary focus on autophagy following exposure of cardiomyocytes to various stressors.
In this study we demonstrate that HIV-1 Tat expression leads to a significant loss of mitochondrial metabolic function and an increase in the accumulation of toxic reactive oxygen species (ROS) in isolated rat cardiomyocytes. In addition, Tat dysregulated mitochondrial Ca2+ uptake by the mitochondria calcium uniporter (MCU) and electrophysiological activity of cardiomyocytes. Moreover, we found that when cardiomyocytes were exposed to hypoxia/reoxygenation, Tat interfered with the initiation of autophagy and the clearance of autophagic proteins.
MATERIALS AND METHODS
Cardiomyocyte Isolation and Culture Conditions
All animal experiments were performed in accordance with the guidelines of Temple University Institutional Animal Care and Use Committee. NRVCs were isolated from 1–2 day old Sprague-Dawley rats following the protocol previously described [Gupta et al., 2016]. Isolated NRVCs were then maintained in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies) supplemented with 2% fetal bovine serum (FBS, Denville Scientific Inc.) and 25 μg/mL gentamicin (Life Technologies) and maintained in a humidified incubator at 37°C and 5% CO2.
Adenoviral Transduction
NRVCs were transduced with Ad-Tat with a multiplicity of infection (MOI) of 1 in a reduced volume of FBS-free DMEM at 37°C for 2 hours after which medium was replaced with DMEM supplemented with 2% FBS and 25 μg/mL gentamicin (Life Technologies). Ad-Tat encodes for Tat protein with a molecular weight of 15 kDa. Experiments were performed 72 h post-transduction. Ad-null (Vector Biolabs) transduction was used as a control.
NRVCs were transduced with the autophagy reporter construct mRFP-EGFP-LC3 (Ad-ptfLC3) in the presence or absence of Tat transduction and analyzed 24 hr post transduction.
Western Blotting
NRVCs were washed with PBS and then scraped in RIPA lysis buffer [25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS] supplemented with fresh protease inhibitor cocktail (Sigma-Aldrich). Equal aliquots of cell lysates were loaded onto 10% or 12% SDS-polyacrylamide gels for electrophoresis using Bio-Rad’s western blotting system. The gels were transferred onto wet 0.1 μm nitrocellulose membranes (LI-COR, Inc., Lincoln, NE). Membranes were probed with primary antibodies (3 hr at RT) and washed with PBST (1 x, 5 min) containing 0.5% Tween 20 and PBS (3 x, 5 min ea.). Blots were then probed with appropriate secondary antibodies (1 hr at RT) followed by washing with PBST and PBS. Blots were scanned with ODYSSEY® CLx Imaging system (LI-COR, Inc., Lincoln, NE). Protein band intensities were quantified using Image Studio Software. The following antibodies were used for western blotting: BAG3 (Proteintech, 10599-1-AP), SQSTM1/p62 (Cell Signaling Technology, 5114), LC3 (Sigma, L8918), GAPDH (Santa Cruz, sc-32233), Cox-2 (Santa Cruz, sc-1745), Cytochrome c (Cell Signaling Technology, 4272), BAX (Santa Cruz, sc-493), Ubiquitin (Santa Cruz, sc-8017), NDUFA4L2 (Abcam, ab74138), Phospho-AKT (Cell Signaling Technology, 9271), ATG7 (Cell Signaling Technology, 2631) and Tat (NIH AIDS Reagent Program, R705).
RNA Isolation and Quantitative Real-Time RT-PCR (qRT-PCR)
RNA was extracted using RNAeasy kit (Ambion) followed by on-column DNase digestion (QIAGEN). The synthesis of cDNA was performed by utilizing 1 μg of the extracted RNA via reverse transcription process. The synthesized cDNA was used for qRT-PCR by using LightCycler® 480 SYBR Green I Master (Roche) in total reaction volume of 20 μL. β-actin primers were used as a housekeeping gene to normalize data. The following primers were used: SQSTM1/p62 FW: GAGTCATGCTGCACTCCACT; RV: TATCAGGCAGGAATGATGGA.
Immunocytochemistry
Immunocytochemistry analysis was described previously [Tahrir et al., 2017]. Briefly, fixed cells were permeabilized with 0.5% Triton-X 100 followed by incubation with glycine and blocking solutions. Afterwards, cells were incubated with primary and Alexa Fluor® secondary antibodies (Thermo Fisher Scientific). Cells were then mounted using VECTASHIELD Hard set medium (Vector Laboratories) and imaged using Leica fluorescent microscope.
Cell Cycle Assay
Transduced NRVCs were trypsinized, washed and resuspended in 1 mL PBS. The suspended cells were fixed immediately in 70% chilled ethanol followed by centrifugation (4000 rpm, 10 min) and PBS wash to eliminate ethanol. Cell pellets were resuspended in 300 μL of PBS supplemented with 0.5 mg/mL propidium iodide and 10 mg/mL RNase A. After incubation at 37°C for 30 min, cells were chilled at 4°C for 1 hr and analyzed with Flow Cytometry (FACS).
Cell Viability Assay
NRVCs were seeded in 96-well plates (10,000 cells/well) and incubated with CellTiter-Blue® viability assay reagent (Promega) at 37°C for 2 hr followed by measuring the fluorescence using a plate reader with excitation and emission maximums at 575 and 590 nm, respectively.
Annexin Apoptosis Assay
Transduced NRVCs were trypsinized, washed and resuspended in 500 μL DMEM 2% FBS. 100 μL of Guava Nexin® reagent were added to 100 μL of the cell suspension followed by incubation in the dark at RT for 20 minutes. Percentage of cells undergoing apoptosis or necrosis was determined with FACS analysis. Staurosporine-treated cells were used as a positive control.
ATP Assay
NRVCs were lysed and ATP levels were measured using an ATP determination kit (Molecular Probes) as per the manufacturer’s instructions. 10 μL of the cell lysate was added to the 90 μL of the standard reaction solution (8.9 mL dH2O, 0.5 mL 20X reaction buffer, 0.1 mL 0.1M DTT, 0.5 mL of 10 mM D-luciferin and 2.5 μL firefly luciferase (5 mg/ml)) and luminescence was measured using a luminometer (Femtomaster FB 12 luminometer, Zylux). CCCP-treated cells were used as a positive control for ATP depletion.
Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) Measurement
NRVCs were plated in XF96 cell culture microplates (Seahorse Bioscience) at a density of 45,000 cells/well. OCR was measured using a seahorse XF96 Analyzer (Seahorse Bioscience) according to the manufacturer’s protocol. The XFe96 extracellular flux assay kit (Seahorse Bioscience) was first calibrated using XF calibrant solution (Seahorse Bioscience) by overnight incubation in a non-CO2 incubator at 37°C. The XFe96 extracellular flux assay kit was loaded with various inhibitors of mitochondrial ETC complexes including oligomycin, FCCP, Rotenone and Antimycin A using the XF cell mito stress test kit (Seahorse Bioscience). Cells were incubated with XF Assay medium (Seahorse Bioscience) in a non-CO2 incubator at 37°C for 1 hr before recording. Mitochondrial complexes were inhibited by sequential adding of inhibitors and changes in OCR and ECAR were measured. OCR and ECAR were normalized to the number of cells.
ROS Determination Assay
NRVCs cultured on microscope cover glasses (Fisherbrand) were incubated with 1 mM MitoSOX red (Life Technologies) for 30 min. Cells were monitored using confocal microscopy and MitoSOX red signal was quantified with Image J.
Simultaneous Measurement of Mitochondrial Ca2+Uptake and Mitochondrial Membrane Potential (ΔΨm)
The measurement of Ca2+ uptake and ΔΨm was performed based on the protocol previously described [Tomar et al., 2016]. Briefly, transduced cells were trypsinized, washed with DMEM, and bathed in an intracellular medium (120 mM KCl, 10 mM NaCl, 1 mM KH2PO4, 20 mM Hepes-Tris, pH 7.2 and 2 μM thapsigargin). The cells were permeabilized with digitonin (40 μg/ml), loaded with bath Ca2+ indicator Fura2FF (1 μM) and ΔΨm indicator JC-1 (800 nM). Simultaneous measurement of extramitochondrial Ca2+ ([Ca2+]out) clearance and ΔΨ was measured using a multiwavelength excitation dual-wavelength emission fluorimeter (Delta RAM, PTI). After reaching steady state, series of extramitochondrial Ca2+ pulses (10 μM) were added and the rate of mitochondrial Ca2+ uptake was measured as a function of decrease in bath Ca2+ fluorescence.
Multi-electrode Array (MEA) Recording
NRVCs were plated on MEA plates (Multichannel Systems, Germany) pre-coated with EmbryoMax® 0.1% Gelatin Solution (Millipore) at a density of 1000 cells/mm2. Each MEA plate contains 60 titanium nitrate (TiN) electrodes with diameter of 30 μM positioned in a rectangular grid. Extracellular action potential was recorded at initial state before any transduction using MEA-1060 system (Multichannel Systems, Germany). Cells were then transduced with either Ad-Tat or Ad-null and extracellular action potential was recorded post transduction. The sampling frequency was set to 2000 kHz and an online built-in bandpass filter was utilized throughout the recording. Data were recorded using the MC_Rack software and further analyzed with MATLAB®.
Mitochondrial Mass
Transduced cardiomyocytes were incubated with 50 nM MitoTracker Red (Thermo Fisher Scientific) for 30 min at 37°C followed by fluorescence analysis using flow cytometry.
Statistical Analysis
Student’s t-test was used to assess statistical differences between two pairs of data. P<0.05 was considered significant.
RESULTS
HIV-1 Tat Dysregulates Mitochondrial Bioenergetics in Cardiomyocytes
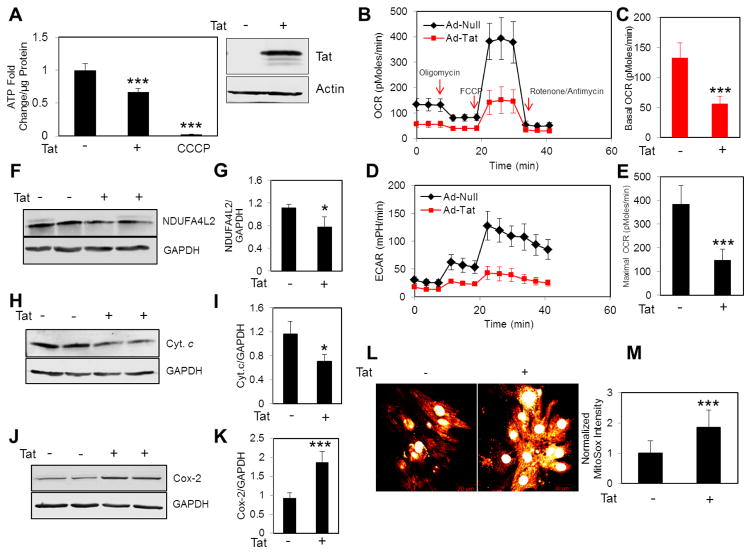
As a first step to investigate the impact of Tat on the bioenergetics pathway in cardiomyocytes, we measured cellular ATP levels in control and Tat expressing NRVCs using a luciferase-based ATP assay. Data showed that the expression of Tat in NRVCs significantly reduced cellular ATP levels (Fig. 1A). Next we asked whether the decrease in cellular ATP is due to impaired OXPHOS. We measured the oxygen consumption rate (OCR) in control and Tat expressing NRVCs and found a significant decrease in basal and maximal OCR as well as ECAR in cells expressing Tat compared to control (Fig. 1B–E). Additionally, we noticed that the impaired OXPHOS activity was accompanied by decrease in the levels of NDUFA4L2 (NADH dehydrogenase (ubiquinone) 1αsubcomplex 4-like 2)(a subunit of Complex I) and cytochrome c (complex III) and increase in the level of Cox-2 (Cytochrome c oxidase, complex IV) (Fig. 1F–K). Because complex I and complex III are the major source of mitochondrial ROS (mROS) production, one may expect increased leakage of electrons from complex I and III, and elevated levels of mROS production. Consistent with this notion that we observed a decrease in NDUFA4L2 and Cytochrome c abundance, mROS levels significantly increased in NRVCs expressing Tat (Fig. 1L–M). Together these data suggest that Tat transduced cardiomyocytes show reduced mitochondrial respiratory capacity leading to lower ATP level and elevated mROS.
Figure 1. Tat dysregulates mitochondrial bioenergetics.
NRVCs were transduced with either Ad-Tat or Ad-null for 3 days and then mitochondrial bioenergetics was assessed by various assays. (A) ATP levels were measured using luciferase-based assay in whole cell lysates. NRVCs were treated with CCCP (50 μM, 4 hr) as a positive control of ATP depletion. Data were normalized to ATP level in cells transduced with Ad-null. (B–E) NRVCs were subjected to subsequent treatments with mitochondrial inhibitors including Oligomycin, FCCP and Rotenone/Antimycin A and OCR and ECAR were measured using XF96 Seahorse. Data were normalized to the number of cells. Western blotting analysis showed that (F–G) NDUFA4L2 and (H–I) Cyt. c levels decreased and (J–K) Cox-2 level increased in the presence of Tat. (L–M) NRVCs were stained with MitoSOXred and imaged with a confocal fluorescent microscope. The mitochondrial red signal was quantified with Image J analysis software.*P<0.05; ***P<0.001.
HIV-1 Tat Dysregulates Ca2+ Uptake and Electrophysiological Activity of Cardiomyocytes
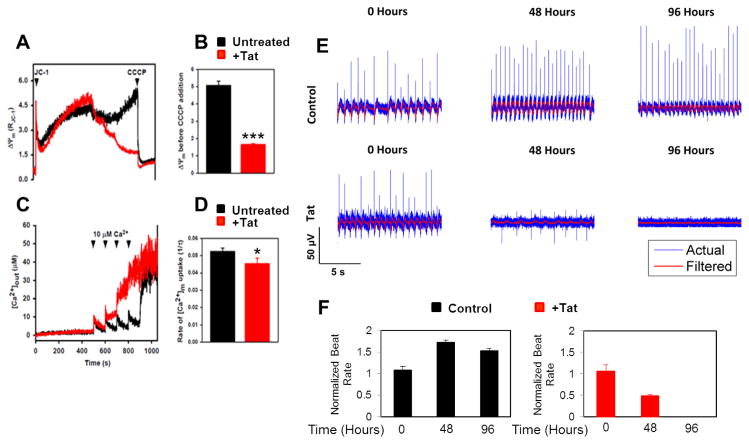
Because we observed increased mROS levels, next we assessed the mitochondrial membrane potential (ΔΨm) and mitochondrial Ca2+ uptake. Intracellular calcium flux is one of the key regulators of cardiac contractility. The mitochondria calcium uniporter (MCU) mediates Ca2+ uptake by mitochondria [Luongo et al., 2015]. As seen in Figure 2(A–D), expression of Tat in NRVCs significantly reduced the MCU-mediated mitochondrial Ca2+ uptake as a consequence of collapse in ΔΨm after a 10 μM Ca2+ bolus.
Figure 2. Tat dysregulates [Ca2+]m uptake and electrophysiological activity.
(A) Trypsin-detached NRVCs were resuspended in an intracellular-like media, permeabilized with digitonin (40 μg/ml), loaded with Ca2+and ΔΨm indicator Fura-2FF, and JC-1. After reaching steady state, 10 μM Ca2+ pulses were added and extramitochondrial Ca2+ ([Ca2+]out) clearance and changes in ΔΨm were recorded before adding CCCP (2 μM) to collapse ΔΨm and [Ca2+]m uptake. (A) ΔΨm decreased in the presence of Tat after addition of Ca2+ pulses. (B) ΔΨm was quantified based on the data shown in (A). (C) [Ca2+]out at each time point was recorded. (D) [Ca2+]m uptake rate was calculated as a function of bath Ca2+ clearance.(E) Electrophysiological activity of NRVCs was recorded based on the extracellular action potential measured by a multi-electrode array system in the presence or absence of Tat. (F) Number of spikes/min was calculated based on the data shown in (E). *P<0.05; ***P<0.001.
In another experiment we examined whether Tat impacts cardiac electrophysiology. To this end, we used the multi-electrode array (MEA) system to record electrical signals from beating NRVCs known as extracellular action potential over time. Data showed that Tat expression attenuated the amplitude and lowered the frequency of firing (number of spikes generated by cardiomyocytes per minute). 96 hr post transduction spikes were completely suppressed in Tat expressing cells whereas these reductions were not observed in control cells (Fig. 2E and F).
HIV-1 Tat Triggers Apoptosis in Cardiomyocytes
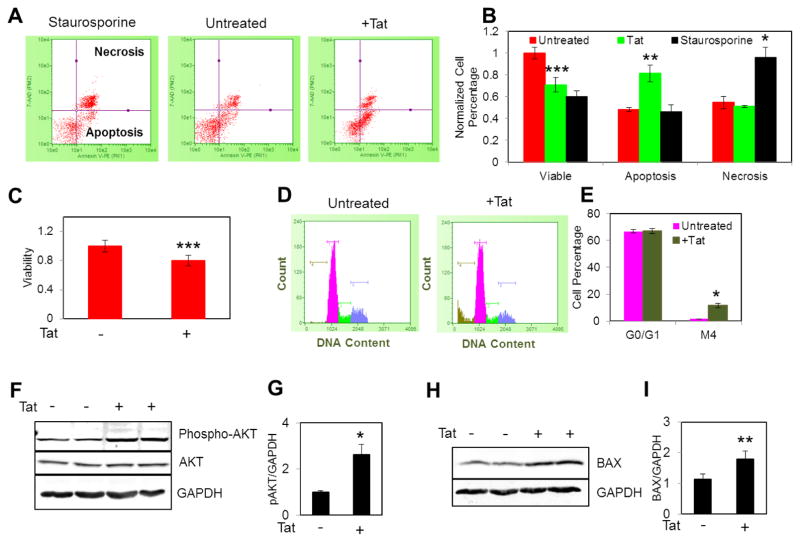
As the mitochondrial permeability transition pore (MPTP) can be sensitized by both mROS and mitochondrial Ca2+ overload, we anticipated that increased mROS production in Tat expressing NRVCs sensitized the opening of MPTP leading to cell death as evidenced from the collapse in ΔΨm (Fig 2A). To further verify whether Tat expressing NRVCs are prone to apoptotic cell death, we performed Annexin-V staining and flow cytometric analysis. Treatment with staurospoine (10 μM, 4 hr) was performed as a control. Results showed that 72 hr post transduction, Tat caused significant induction of apoptosis but not necrosis in cardiomyocytes. As expected, cellular viability in Tat expressing NRVCs was significantly reduced by 20% (Fig. 3A–C). Excessive ROS production results in the activation of cell death signaling pathways in which mitochondrial membrane permeability increases leading to the release of pro-apoptotic factors and activation of caspases. Caspase activation commits cells to death by promoting the cleavage of cellular DNA and proteins [Fulda et al., 2012]. Cell cycle analysis showed that Tat led to the significant fragmentation of cellular DNA (Fig. 3D and E).
Figure 3. Tat induces apoptosis.
(A–B) Transduced NRVCs were stained with Annexin-V and the percentage of apoptotic and necrotic cells was measured with FACS analysis. (C) NRVCs were plated in 96 well plates and 3 days post transduction were treated with CellTiter-Blue® viability assay reagent (ex/em of 575 nm/590 nm) and emission fluorescent was measured. (D–E) PI-based cell cycle assay showed that DNA fragmentation significantly increased by Tat expression. (F–G) Western blotting analysis showed that the level of anti-apoptotic protein phosphor-AKT significantly increased in the presence of Tat. (H–I) Western blotting analysis showed that the level of pro-apoptotic protein BAX significantly increased in the presence of Tat. *P<0.05; **P<0.001; ***P<0.001.
We then examined changes in the level of some important signaling proteins involved in apoptosis. AKT is one of the key anti-apoptotic proteins which inhibits caspase activation and promotes cell survival [Fulda et al., 2012]. The level of phospho-AKT increased in the presence of Tat (Fig. 3F and G); suggesting that protective pathways are activated to minimize cell death. On the other hand, the level of the pro-apoptotic protein, BAX, significantly increased in the presence of Tat (Fig. 3H and I).
HIV-1 Tat Interferes with Autophagy Initiation
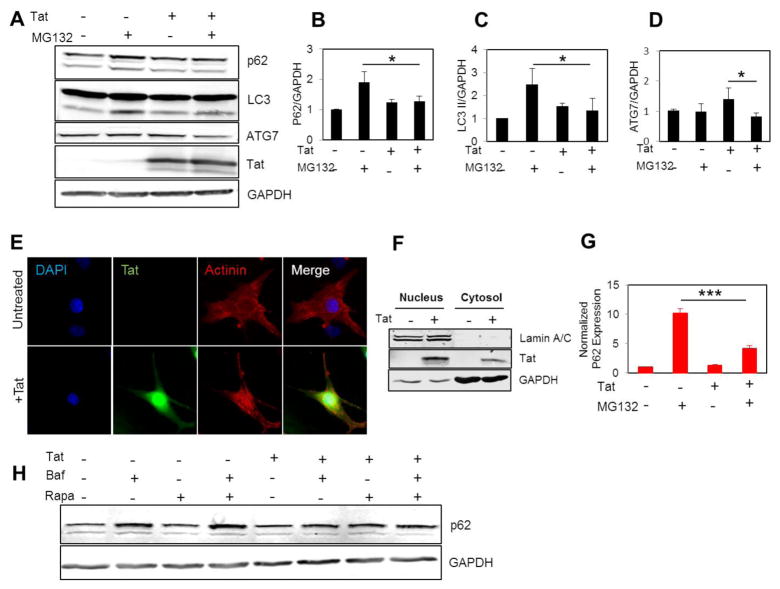
Autophagy is one of the key intracellular events which is upregulated during ATP reduction and plays a crucial role in maintaining cellular homeostasis and survival through degradation of nonessential components and macromolecules [Russel et al., 2014]. Autophagy dysregulation has been reported to be associated with cardiomyocyte death [Tahrir et al., 2017; Su et al., 2016]. Considering ATP reduction in Tat expressing cells, we next investigated how and through which mechanism the autophagy pathway is affected by Tat. Inhibition of the proteasome has been reported as an activator of the autophagy-lysosome pathway [Gamerdinger et al., 2011]. NRVCs were treated with the proteasome inhibitor, MG132, and expression of autophagy proteins such as LC3 and p62 was measured. Western blotting data showed that the level of the autophagy protein p62 increased in control cells but not in Tat-transduced cells after treating the cells with MG132 (5 μM, 12hr). In addition, LC3 II levels did not increase in Tat-transduced cells after MG132 treatment and the level of ATG7 was reduced in Tat-transduced cells after MG132 treatment, indicating that Tat-transduced cells were defective in activating the autophagy-lysosome pathway (Fig. 4A–D).
Figure 4. Tat interferes with autophagy initiation.
(A–D) NRVCs were transduced with either Ad-Tat or Ad-null for 72 hr in the presence or absence of MG132 (5 μM, 12 hr) and the level of autophagy proteins p62 and LC3 were quantified using western blotting analysis. (E) ICC data showed that Tat is highly expressed in cellular nucleus and cytosol. (F) Nuclear fractionation data showed that Tat is highly expressed in cellular nucleus. (G) RT-qPCR data showed that Tat impaired upregulation of p62 mRNA in the presence of MG132 (5 μM, 12 hr).(H) Cells were treated with autophagy activator Rapamycin (50 nM, 24 hr) and the level of p62 protein was analyzed in the presence or absence of Baf A1.*P<0.05; ***P<0.001.
Fluorescent microscopy showed that Tat localized to both the nucleus and the cytosol of cardiomyocytes (Fig. 4E). Western blotting data also showed that Tat protein was present in both nuclear and cytosolic fractions. Lamin A/C and GAPDH proteins were used as nuclear and cytosolic markers, respectively (Fig. 4F). Since levels of Tat were significant in cell nuclei, we evaluated the effect of Tat on the expression of the autophagy protein, p62. RT-qPCR results showed that p62 mRNA level was significantly reduced in Tat-transduced cells compared to the control cells after treatment with MG132 (Fig. 4G).
In another experiment we evaluated the effect of Tat on p62 levels when NRVCs were treated with the well-known autophagy activator, rapamycin, in the presence or absence of Baf A1 as an inhibitor of autophagolysosome formation. Data showed that the level of p62 increased in NRVCs after rapamycin treatment when autophagy flux was inhibited by Baf A1, while it did not change in Tat expressing cells (Fig. 4H). Taken together, these results indicate that Tat interferes with autophagy and upregulation of autophagy proteins, and p62 is one of the major targets for Tat.
HIV-1 Tat Dysregulates Mitochondrial Quality Control in Cardiomyocytes
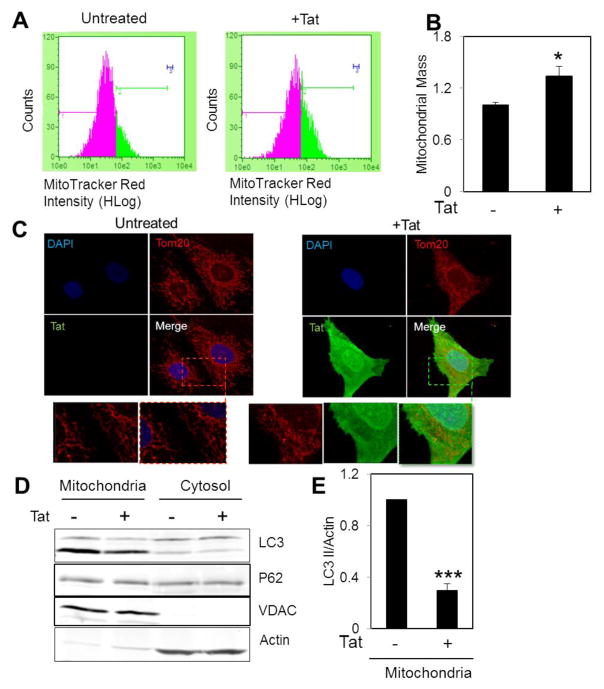
In order to measure mitochondrial mass, transduced NRVCs were stained with MitoTracker Red followed by measurement of fluorescent intensity using FACS analysis. Data showed that Tat transduction led to an increase in mitochondrial mass (Fig. 5A and B). Tat-induced mitochondrial hyperpolarization in neurons has been reported in previous studies [Norman et al., 2007; Perry et al., 2005]. Fluorescence microscopy showed that mitochondrial morphology changed from a filamentous network in control cells to an accumulated interconnected network in Tat expressing cells (Fig. 5C). In order to investigate whether an increase in mitochondrial mass resulted due to an impairment in mitophagy, a selective form of autophagy in which mitochondria are targeted for further degradation and removal, mitochondrial and cytosolic fractions were isolated and analyzed for autophagy proteins in the presence of Tat. Data showed that LC3 II recruitment to mitochondria was significantly reduced in Tat expressing cells compared to control cells (Fig. 5D and E).
Figure 5. Tat expression increases mitochondrial mass and impairs mitophagy.
(A–B) Transduced NRVCs were incubated with 50 nM MitoTracker Red for 30 min at 37°C and fluorescent intensity was analyzed using FACS analysis. (C) ICC by using antibody against Tom20 showed that Tat altered mitochondrial morphology. (D–E) Mitochondrial isolation indicated that LC3 II recruitment to mitochondria significantly reduced in the presence of Tat. *P<0.05, ***P<0.001.
HIV-1 Tat Dysregulates Autophagy after Hypoxia/Reoxygenation
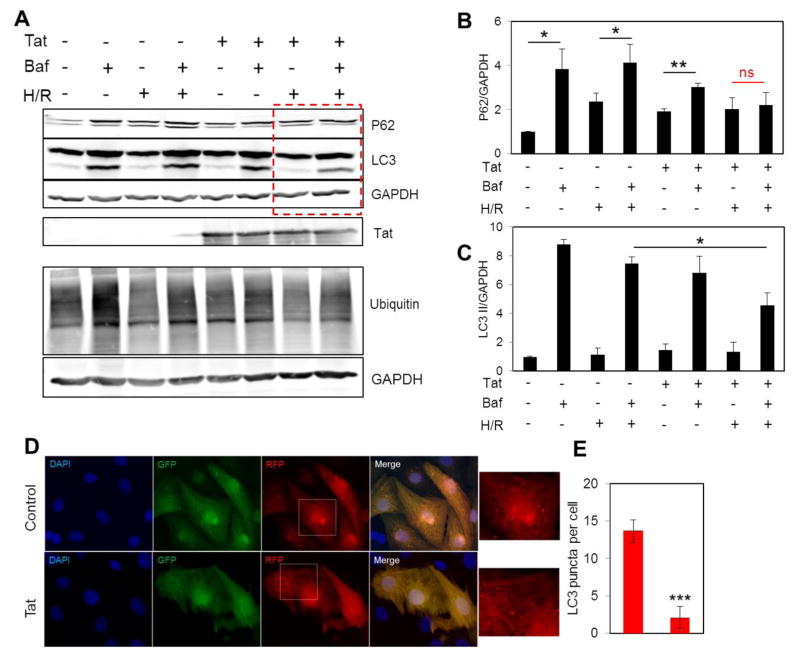
Hypoxia has previously been associated with fragmentation of the mitochondrial network and activation of autophagy machinery for clearance of damaged mitochondria [Liu et al., 2012]. During autophagy, dysfunctional organelles and damaged proteins are tagged by ubiquitin in a process called ubiquitination and targeted to lysosomes for further degradation. p62 functions as an autophagy receptor and binds to ubiquitin tags as well as LC3 on phagophore membrane and sequesters its substrate into autophagosomes [Fimia et al., 2013]. To investigate how the autophagy pathway is affected by Tat in cells under pathological stress, we incubated NRVCs under hypoxia/reoxygenation conditions in the presence or absence of Baf A1. Data showed that Tat transduction resulted in decreased levels of ubiquitination in NRVCs. When autophagy flux was inhibited by Baf A1, the levels of both LC3 and p62 proteins increased in control cells as degradation of these proteins occurs by the lysosome in the autophagy-lysosome pathway. Lysosomal degradation of p62 was reduced in Tat expressing cells under normal condition and no significant increase in p62 level was found in Tat expressing cells under hypoxia/reoxygenation when autophagy flux was inhibited. In addition, the autophagy markers, LC3 I and LC3 II, were significantly reduced under hypoxia/reoxygenation in the presence of Tat (Fig. 6A–C). Microscopic imaging after AdptfLC3 transduction also showed that LC3 puncta were reduced in Tat expressing cardiomyocytes compared to control cells when cells were exposed to H/R stress condition. These results indicate that Tat transduction significantly reduced autophagy and impaired lysosomal degradation of p62 (Fig. 6D and E).
Figure 6. Tat expression dysregulates autophagy under hypoxia/reoxygenation.
(A–C) NRVCs were subjected to hypoxic condition for 14 hr followed by reoxygenation for 4 hr in the presence or absence of Baf A1. The levels of autophagy proteins p62, LC3 and Ub were analyzed with western blotting. (D–E) NRVCs were transduced with Ad-ptfLC3 for 24 hr in the presence or absence of Tat transduction and subjected to H/R and monitored for LC3 puncta. *P<0.05; **P<0.01; ***P<0.001.
DISCUSSION
HIV-1 infection has been reported to be associated with the development of cardiomyopathy. In one study on patients with HIV cardiomyopathy, hearts were reported to be hypertrophic with increased average heart weight from 337 g (healthy) to 580 g (HIV positive) [Fiala et al., 2004]. Valvular heart disease and vasculopathy have also been reported in HIV infected patients [Manga et al., 2017]. Current antiretroviral therapies utilized in the treatment of HIV-infected patients may aggravate the development of peripheral and coronary artery diseases [Barbaro 2002]. Therefore, understanding the precise mechanisms by which HIV-1 infection causes cardiac dysfunction can make important contributions toward the design and development of novel and less toxic therapies. Herein, we targeted HIV-1 Tat for further studies due to its essential role as a transactivator controling viral replication [Jeang et al., 1999], as well its ability to alter host cell gene expression leading to changes organelle function to promote cell death [Rodríguez-Mora et al., 2015].
Our results unequivocally demonstrate that Tat significantly diminished cellular ATP levels, and reduced OCR and increased ROS accumulation in mitochondria. Lower levels of Cytochrome c and NDUFA4L2 in Tat-transduced NRVCs partly accounted for reduced electron transport chain activity and oxidative phosphorylation. ROS has been reported to play an important role in dysregulating cellular homeostasis contributing to the development of cardiac disease [Sugamura et al., 2011]. ROS accumulation causes mutations of mitochondrial DNA (mtDNA) and damages the respiratory chain system [Rodríguez-Mora et al., 2015]. In addition, our results showed that the level of Cox-2 significantly increased with Tat expression. Cox-2 is a component of complex IV and is encoded by mtDNA [Rodríguez-Mora et al., 2015]. Expression of genes encoded by mtDNA is regulated by the nuclear-encoded respiratory factor 1 (NRF-1) and mitochondrial transcription factor A (TFAM) [Rodríguez-Mora et al., 2015]. It is conceivable that HIV-1 Tat, by altering the expression of nuclear and mitochondrial genes, further exacerbated damage to the mitochondrial electron transport chain with a resultant reduction in oxidative phosphorylation. We previously reported that ATP depletion in NRVCs by the proton uncoupler CCCP enhanced the level of Cox-2 [Tahrir et al., 2017]. An increase in Cox-2 is indicative of higher cellular ATP demand which leads to an increase in the expression of ETC proteins for enhanced electron transfer within mitochondria. Our data showed that the mitochondrial reserve capacity was significantly suppressed in the presence of Tat, indicating that the capability of mitochondria to meet excess energy demands under stress was compromised, thus rendering cells to be less able to cope with stress conditions [Hill et al., 2009].
Ca2+ uptake by mitochondria is critically important for cellular signaling as well as for matching energy demand with energy production by the action of mitochondrial Ca2+-dependent dehydrogenases [Griffiths and Rutter 2009; McCormack and Denton, 1980]. In the presence of Tat, both Ca2+ uptake and ΔΨm were significantly reduced when 10 μM Ca2+ pulses were added. Extracellular action potential measurements over time were suppressed by Tat 96 hr post transduction.
Apoptosis has been suggested as an important component of HIV cardiomyopathy. [Maldarelli et al., 1995; Twu et al., 2002]. Consistent with earlier reports, our results showed that Tat significantly induced apoptosis but not necrosis in NRVCs. The level of the pro-apoptotic protein BAX significantly increased in the presence of Tat. AKT is important in the regulation of mitochondria-related apoptosis [Fulda 2012]. Upon activation by phosphorylation, pAKT phosphorylates key apoptotic proteins such as BAX and suppresses mitochondrial permeabilization and subsequent apoptosis [Fulda 2012]. Our results showed that the level of pAKT protein increased in Tat-transduced cells.
Autophagy promotes cell survival by recycling essential macromolecules through degradation of organelles and long-lived proteins [Gustafsson et al., 2008]. It was reported that the autophagy receptor p62 interacts with Tat and targets it for degradation and p62 knockdown led to an increase in Tat protein levels in Flag-Tat transfected HEK cells. In this system, treatment of cells with the autophagy inducer, torin 1, caused significant degradation of Tat, while this degradation was inhibited by silencing the autophagy gene, Atg7 [Sagnier et al., 2014]. Colocalization of the autophagy proteins LC3 and Beclin1 was observed with HIV proteins Gag and Nef, respectively [Kyei et al., 2009]. It was previously reported that MG132 treatment of cardiomyocytes increased the expression of autophagy proteins toward activation of the autophagy-lysosome pathway [Tahrir et al., 2017]. Consistent with these earlier reports, we found that Tat expression led to suppression of the autophagy-lysosome pathway since the level of autophagy proteins p62 and LC3 II was significantly reduced when the proteasome pathway was inhbited with MG132. Furthermore, Tat significantly suppressed mRNA expression of p62.
Mitophagy is selective degradation of defective mitochondria by the autophagy machinery. Mitochondrial mass significantly increased in Tat expressing cells. In addition, mitochondrial isolation indicated that LC3 II levels in the mitochondrial fraction of Tat transduced cells were reduced compared to control cells. Although microscopic imaging showed that Tat expression led to alterations in the morphology of the mitochondrial network, mitochondrial fragmentation as an essential component for mitophgay induction was not observed in the presence of Tat. Together, these data suggest that mitochondrial quality control was adversely affected by Tat.
In summary, the present study indicates that HIV-1 Tat significantly impacts mitochondrial function in NRVCs leading to significant ROS accumulation, respiration impairment and ATP reduction. Tat impairs calcium homeostasis and suppresses the action potential of cardiomyocytes and induces apoptosis. In addition, Tat interferes with autophagy initiation as well as proper degradation of autophagic proteins especially under stress conditions. Taken together, these findings support the idea that regulation of autophagy might be a promising target to reduce HIV toxicity in cardiac cells.
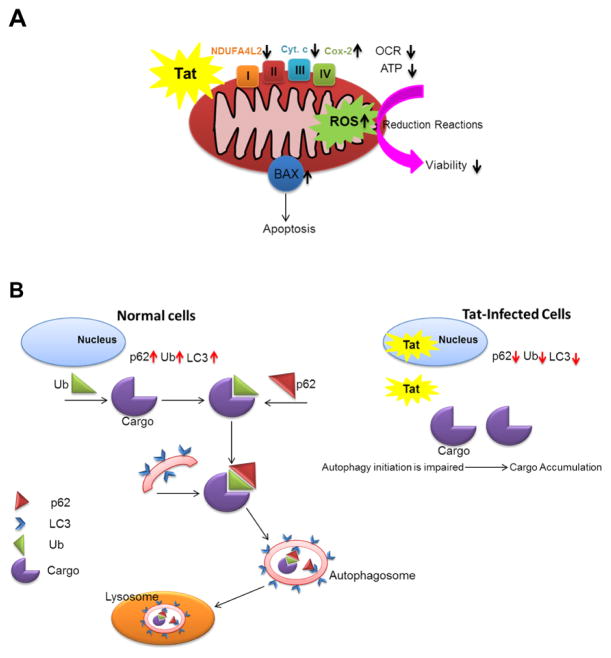
Figure 7. Schematic diagram of HIV-1 Tat impact on mitochondrial bioenergetics and autophagy in cardiomyocytes.
(A) Tat expressing cells indicated reduced viability and increased level of apoptosis. Tat expression led to the dysregulation of oxidative phosphorylation, reduced ATP level and enhanced ROS production. (B) HIV-1 Tat reduced the level of autophagy proteins as well as impaired proper degradation of autophagic protein, p62, under hypoxia/reoxygenation stress condition.
Acknowledgments
We thank past and present members of the Department of Neuroscience and Center for Neurovirology for their insightful discussion, and sharing of ideas and reagents. We are grateful to Dr. Ilker Sariyer for his time in reading the manuscript and his excellent suggests, which have greatly improved our presentation. We thank C. Papaleo for preparation of the mansucript and editorial assistance. This work was made possible by grants awarded by NIH to KK, AMF, JYC (R01HL123093), JMM (R01HL125695). The Comprehensive NeuroAIDS Center core facility (P30MH092177, awarded to KK) provided infrastructure for studies performed at Temple University.
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jcp.26002]
References
- Barbaro G. Cardiovascular manifestations of HIV infection. Circulation. 2002;106:1420–5. doi: 10.1161/01.cir.0000031704.78200.59. [DOI] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behavioural brain research. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JY, Gordon J, Wang J, Song J, Zhang XQ, Tilley DG, et al. Cardiac Dysfunction in HIV-1 Transgenic Mouse: Role of Stress and BAG3. Clinical and translational science. 2015;8:305–10. doi: 10.1111/cts.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo P, Suarez-Lozano I, Teira R, Lozano F, Terrón A, Viciana P, et al. Dyslipidemia and cardiovascular disease risk factor management in HIV-1-infected subjects treated with HAART in the Spanish VACH cohort. Open AIDS J. 2008;2:26–38. doi: 10.2174/1874613600802010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M, Yao H, Hu G, Chen X, Lund AK, Buch S. HIV Tat induces expression of ICAM-1 in HUVECs: implications for miR-221/-222 in HIV-associated cardiomyopathy. PLoS One. 2013;8:e60170. doi: 10.1371/journal.pone.0060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Morgello S, Klotman ME, Mosoian A, Lento PA, Berman JW, et al. Human immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: implications for the pathogenesis of HIV-mediated vascular disease. The American journal of pathology. 2008;172:1100–11. doi: 10.2353/ajpath.2008.070457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q, Kan H, Lewis W, Chen F, Sharma P, Finkel MS. Dilated cardiomyopathy in transgenic mice expressing HIV Tat. Cardiovascular toxicology. 2009;9:39–45. doi: 10.1007/s12012-009-9035-5. [DOI] [PubMed] [Google Scholar]
- Fiala M, Polik W, Qiao JH, Lossinsky AS, Alce T, Tran K, et al. HIV-1 induces cardiomyopathy by cardiomyocyte invasion and gp120, Tat, and cytokine apoptotic signaling. Cardiovascular toxicology. 2004;4:97–107. doi: 10.1385/ct:4:2:097. [DOI] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Elueteri S, Campos S, Serger E, Trejo M, et al. HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. The Journal of Neuroscience. 2015;35:1921–38. doi: 10.1523/JNEUROSCI.3207-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimia GM, Kroemer G, Piacentini M. Molecular mechanisms of selective autophagy. Cell death and differentiation. 2013;20:1. doi: 10.1038/cdd.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017 Apr 5; doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S. Shifting the balance of mitochondrial apoptosis: therapeutic perspectives. Frontiers in oncology. 2012;2:121. doi: 10.3389/fonc.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, Behl C. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO reports. 2011;12:149–56. doi: 10.1038/embor.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EJ, Rutter GA. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochimica et BiophysicaActa (BBA)-Bioenergetics. 2009;1787:1324–33. doi: 10.1016/j.bbabio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Gupta MK, Tahrir FG, Knezevic T, White MK, Gordon J, Cheung JY, et al. GRP78 Interacting Partner Bag5 Responds to ER Stress and Protects Cardiomyocytes From ER Stress-Induced Apoptosis. Journal of cellular biochemistry. 2016;117:1813–21. doi: 10.1002/jcb.25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson ÅB, Gottlieb RA. Autophagy in ischemic heart disease. Circulation research. 2009;104:150–8. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochemical Journal. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang KT, Xiao H, Rich EA. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. Journal of Biological Chemistry. 1999;274:28837–40. doi: 10.1074/jbc.274.41.28837. [DOI] [PubMed] [Google Scholar]
- Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. The Journal of cell biology. 2009;186:255–68. doi: 10.1083/jcb.200903070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nature cell biology. 2012;14:177–85. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, et al. The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell reports. 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manga P, McCutcheon K, Tsabedze N, Vachiat A, Zachariah D. HIV and nonischemic heart disease. Journal of the American College of Cardiology. 2017;69:83–91. doi: 10.1016/j.jacc.2016.09.977. [DOI] [PubMed] [Google Scholar]
- Maldarelli F, Sato H, Berthold E, Orenstein J, Martin MA. Rapid induction of apoptosis by cell-to-cell transmission of human immunodeficiency virus type 1. Journal of virology. 1995;69:6457–65. doi: 10.1128/jvi.69.10.6457-6465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JG, Denton RM. The activation of isocitrate dehydrogenase (NAD+) by Ca2+ within intact uncoupled rat brown adipose tissue mitochondria incubated in the presence and absence of albumin. Biochemical Society Transactions. 1980;8:339. doi: 10.1042/bst0080339. [DOI] [PubMed] [Google Scholar]
- Nath A, Steiner J. Synaptodendritic injury with HIV-Tat protein: what is the therapeutic target? Experimental neurology. 2014;251:112–4. doi: 10.1016/j.expneurol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA. HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. The Journal of Immunology. 2007;178:869–76. doi: 10.4049/jimmunol.178.2.869. [DOI] [PubMed] [Google Scholar]
- Parihar P, Parihar MS. Metabolic enzymes dysregulation in heart failure: the prospective therapy. Heart Failure Reviews. 2017;22:109–21. doi: 10.1007/s10741-016-9588-x. [DOI] [PubMed] [Google Scholar]
- Perry SW, Norman JP, Litzburg A, Zhang D, Dewhurst S, Gelbard HA. HIV-1 transactivator of transcription protein induces mitochondrial hyperpolarization and synaptic stress leading to apoptosis. The Journal of Immunology. 2005;174:4333–44. doi: 10.4049/jimmunol.174.7.4333. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Mora S, Mateos E, Moran M, Martín MÁ, López JA, Calvo E, et al. Intracellular expression of Tat alters mitochondrial functions in T cells: a potential mechanism to understand mitochondrial damage during HIV-1 replication. Retrovirology. 2015;12:78. doi: 10.1186/s12977-015-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell research. 2014;24:42–57. doi: 10.1038/cr.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagnier S, Daussy CF, Borel S, Robert-Hebmann V, Faure M, Blanchet FP, et al. Autophagy restricts HIV-1 infection by selectively degrading Tat in CD4+ T lymphocytes. Journal of virology. 2015;89:615–25. doi: 10.1128/JVI.02174-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, El-Hage N, Campbell ME, Lutz SE, Knapp PE, Nath A, et al. Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience. 2005;135:781–90. doi: 10.1016/j.neuroscience.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Myers VD, Knezevic T, Wang J, Gao E, Madesh M, et al. Bcl-2–associated athanogene 3 protects the heart from ischemia/reperfusion injury. JCI insight. 2016;1:19. doi: 10.1172/jci.insight.90931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radical Biology and Medicine. 2011;51:978–92. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahrir FG, Knezevic T, Gupta MK, Gordon J, Cheung JY, Feldman AM, et al. Evidence for the role of BAG3 in mitochondrial quality control in cardiomyocytes. Journal of Cellular Physiology. 2017;232:797–805. doi: 10.1002/jcp.25476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar D, Dong Z, Shanmughapriya S, Koch DA, Thomas T, Hoffman NE, et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell reports. 2016;15:1673–85. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu C, Liu NQ, Popik W, Bukrinsky M, Sayre J, Roberts J, et al. Cardiomyocytes undergo apoptosis in human immunodeficiency virus cardiomyopathy through mitochondrion-and death receptor-controlled pathways. Proceedings of the National Academy of Sciences. 2002;99:14386–91. doi: 10.1073/pnas.212327899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Grol J, Subauste C, Andrade RM, Fujinaga K, Nelson J, Subauste CS. HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3. PLoS One. 2010;5:e11733. doi: 10.1371/journal.pone.0011733. [DOI] [PMC free article] [PubMed] [Google Scholar]