Abstract
Background
Cheyne‐Stokes respiration (CSR) has been investigated primarily in outpatients with heart failure. In this study we compare CSR and periodic breathing (PB) between healthy and cardiac groups.
Methods
We compared CSR and PB, measured during 24 hr of continuous 12‐lead electrocardiographic (ECG) Holter recording, in a group of 90 hospitalized patients presenting to the emergency department with symptoms suggestive of acute coronary syndrome (ACS) to a group of 100 healthy ambulatory participants. We also examined CSR and PB in the 90 patients presenting with ACS symptoms, divided into a group of 39 (43%) with confirmed ACS, and 51 (57%) with a cardiac diagnosis but non‐ACS. SuperECG software was used to derive respiration and then calculate CSR and PB episodes from the ECG Holter data. Regression analyses were used to analyze the data. We hypothesized SuperECG software would differentiate between the groups by detecting less CSR and PB in the healthy group than the group of patients presenting to the emergency department with ACS symptoms.
Results
Hospitalized patients with suspected ACS had 7.3 times more CSR episodes and 1.6 times more PB episodes than healthy ambulatory participants. Patients with confirmed ACS had 6.0 times more CSR episodes and 1.3 times more PB episodes than cardiac non‐ACS patients.
Conclusion
Continuous 12‐lead ECG derived CSR and PB appear to differentiate between healthy participants and hospitalized patients.
Keywords: acute coronary syndrome, Cheyne‐Stokes, ECG derived respiration, Holter, periodic breathing
1. INTRODUCTION
Sleep apnea can be categorized as either obstructive (i.e., caused by upper airway collapse) or central (i.e., caused by brain dysfunction in sending signals to the muscles that control breathing) (Somers et al., 2008). Cheyne‐Stokes respiration (CSR) and periodic breathing (PB) are types of central sleep apnea (CSA) caused by brain dysfunction that impairs signals sent to the muscles that control breathing. In contrast to CSR and PB, obstructive sleep apnea is caused by a mechanical collapse or obstruction of the airway (Somers et al., 2008). Mechanisms that interplay in maintaining an adequate balance of carbon dioxide and oxygen in the blood and that may contribute to the development of CSA, CSR and PB include increased chemoreceptor sensitivity to fluctuating carbon dioxide and oxygen blood levels, circulatory delay and baseline oxygen and carbon dioxide levels (Leung et al., 2012). Sleep apnea has been associated with increased sympathetic nervous activity, heart rate, blood pressure, number of arrhythmias and poor outcomes in cardiac populations (Linz et al., 2015). CSR and PB have been associated with an increased risk for mortality in patients with heart failure (Brack et al., 2007; Hanly & Zuberi Khokhar, 1996; La Rovere et al., 2007; Lanfranchi et al., 1999). In prior studies CSR and PB were measured using the gold standard polysomnography (Hanly & Zuberi Khokhar, 1996; La Rovere et al., 2007), unattended sleep study (Lanfranchi et al., 1999), or portable systems (Brack et al., 2007; La Rovere et al., 2007), which are costly, and obtrusive.
Alternative noninvasive methods for identifying CSR and PB using continuous ECG waveform data have expanded in the past 20 years. The use of ECG waveforms to derive respiratory rate was first investigated by Moody et al. (1986) who used a two lead ECG arrhythmia detector and a pneumatic respiration transducer in patients during a sleep lab study to describe how changes in the cardiac electrical axis during respiration could be used to derive respiration and detect apnea. Moody et al. (1985), later used Holter monitors to derive ECG respiration and identify sleep apnea in patients referred to the sleep lab for sleep apnea and CSR in heart failure patients; and later described ECG algorithms to derive sleep apnea (Penzel et al., 2002).
Research has expanded in the past few years to include study of derivation of respiratory rate, tidal volume and apnea. A review by Helfenbein et al. (2014), described how changes in QRS amplitude, heart rate variability and electromyogram activity are three different techniques that utilize ECG data to derive respiratory rate. Recently, Weiss et al. (2014), used an algorithm that selected the best leads to derive respiratory rate from either intracardiac or body surface ECG in intubated and mechanically ventilated swine; furthermore, modulation in the ECG derived respiratory rate was used by the same group to derive tidal volume (Sayadi et al., 2014). Alternatively, ECG data routinely collected during polysomnography has been used to derive apnea and hypopnea (Pichot et al., 2015; Stein et al., 2003; Varon et al., 2015); using cyclic variations of the heart rate (Stein et al., 2003), or changes in the QRS complex (Pichot et al., 2015; Varon et al., 2015), and with an accuracy to detect the events of 84%, 89% and 85% respectively (Pichot et al., 2015; Stein et al., 2003; Varon et al., 2015).
An ECG based method to derive CSR and PB in a healthy and a cardiac heart failure group using 12‐lead Holter monitor data was published by Haigney et al. (2014). In a further assessment of this non‐invasive ECG method we designed a study to examine the following research aims: (1) to compare ECG‐derived CSR and PB between a group of healthy ambulatory community based participants to a group of patients presenting to the emergency department with symptoms suggestive of acute coronary syndrome (ACS); and (2) to compare the rate of CSR and PB in hospitalized patients specifically between those with a cardiac non‐ACS discharge diagnosis to those with an ACS discharge diagnosis.
We purposely chose and prospectively enrolled healthy individuals to determine the ability of the ECG to discriminate CSR and PB between a healthy group and a hospitalized group. We hypothesized the healthy group would have fewer CSR and PB episodes than the group of patients seeking emergency care for symptoms of ACS.
2. METHODS
2.1. Study design
Data from two studies were used. The data for the healthy control group came from a prospective descriptive study entitled Quantifying Novel Electrocardiographic Monitoring Measurements in Healthy Adults (T32 NR007088, F31 NR015196). The primary aim of this study was to determine the frequency of 12‐lead ECG‐derived CSR and PB in a group of healthy community based individuals. Data for the hospitalized patient group came from a subsample of patients enrolled in the prospective study entitled Ischemia Monitoring & Mapping in the Emergency Department in Appropriate Triage & Evaluation of Acute Ischemic Myocardium (IMMEDIATE AIM, RO1HL69753) (Schindler et al., 2007). The Institutional Review Board at the university approved both studies and research nurses obtained informed consent from all participants.
2.2. Study setting and population
2.2.1. Healthy group
The healthy control group included a convenience sample of 100 participants 18 years of age and older who were recruited between January and March, 2013. Potential participants were carefully queried about medical history to ensure they met the criteria for “healthy.” Exclusion criteria included skin allergy to electrode adhesive, current flu symptoms, or any of the following: coronary heart disease, angina pectoris, myocardial infarction, coronary bypass surgery, prior percutaneous coronary intervention (angioplasty, or stent), heart failure, heart transplantation, hypertension requiring medication, atrial fibrillation, pacemaker, implantable cardioverter defibrillator, ablation therapy, stroke, diabetes mellitus, chronic obstructive pulmonary disease, emphysema, chronic bronchitis, restrictive lung disease, asthma requiring year‐round inhaler, sleep apnea and/or treatment for sleep apnea with continuous positive airway pressure, cancer treatment in the past 12 months, end‐stage renal failure or renal dialysis. In addition, participants were excluded if they were taking medications routinely for the above stated diseases. Finally, potential participants were also screened for sleep apnea with the Geisinger Health Tool and excluded if they answered “yes” to two or more of the questions from this tool (Chung et al., 2008).
Healthy participants wore an H12 + Holter recorder (Mortara Instrument Inc, Milwaukee, WI, USA) for 24 hr, during which they were instructed to maintain routine daily activities. The H12 + Holter acquires all 12 ECG leads simultaneously using a digital sampling rate of 1,000 samples per second. Electrodes were placed by the primary investigator (AT) using a Mason‐Likar electrode configuration (Mason & Likar, 1966). Each electrode site was marked with ink and the participants were instructed on how to replace electrodes and lead wires (i.e., after showering or if electrodes came off).
2.2.2. Hospitalized group
The IMMEDIATE AIM study has been described in detail previously (Schindler et al., 2007). Briefly, patients presenting to the emergency department with symptoms suggestive of ACS from April 2002 to December 2004 were prospectively enrolled. Research nurses obtained verbal assent followed by written informed consent from 1,308 patients. This consent method was used so that the 12‐lead Holter recording (Mason‐Likar electrode configuration) could be applied as soon as possible following presentation to the emergency department. The median door‐to‐Holter initiation was 44 min (Schindler et al., 2007). Although the primary study enrolled 1,308 patients using a 12‐lead Holter recorder, only a subset of 188 (14%) patients enrolled at the end of the study were monitored with a H12 + device (Mortara Instrument Inc.), which records ECG at 1,000 samples per second, a requirement for deriving CSR and PB from the ECG. Of the 188 patients, 50 (26.6%) were excluded because a cardiac diagnosis was ultimately ruled out, 40 (21.3%) were excluded because they had <18 hr of ECG recording, and 8 (4.0%) were excluded because of an arrhythmia or waveform pattern that confounds the measurement of CSR and PB (i.e., atrial fibrillation, atrial flutter, or ventricular paced rhythm). Thus, the final sample included for the current analysis was 90 patients.
The final diagnosis in the 90 patients was cardiac non‐ACS (i.e., valvular heart disease, congestive heart failure, pericarditis, new onset arrhythmia, stable angina, hypertension crisis, aortic dissection or aneurysm) in 51 (56.7%), and ACS in 39 (43.3%). Of the 39 patients with ACS, 6 (15.4%) had ST elevation myocardial infarction, 8 (20.5%) had non‐ST elevation myocardial infarction, and 25 (64.1%) had unstable angina.
2.3. ECG analysis
The 12‐lead ECG Holter data was downloaded and analyzed with H‐Scribe software (4.34 software; Mortara Instrument Inc.). The high fidelity ECG data were then processed with research software (Super ECG; Mortara Instrument Inc.) to identify CSR and PB episodes during the recording period for each participant.
2.4. SuperECG software
The details of how SuperECG software identifies CSR and PB have been published previously by Haigney et al. (2014). Briefly, tidal volume increases during inspiration cause the heart to shift in the thorax which then leads to morphology changes to the QRS waveform. SuperECG software uses beat to beat changes in QRS morphology to derive tidal volume and calculate CSR and PB episodes from Holter ECG recordings. The software first calculates the QRS amplitude mean square variation over 15 s (area divided by width in 1/4 μV units); and then derives a waveform from the mean QRS amplitude (sample rate of 1 sample per second). The derived waveform is used to measure changes in tidal volume and calculate CSR and PB. Examples of SuperECG software technology to detect breathing are displayed in Figure 1. CSR is identified by the software when three or more consecutive cycles of hyperpnea/hypopnea/apnea respiration with a crescendo‐decrescendo breathing pattern occur (Figure 1, top). PB is identified by the software when three or more consecutive cycles of hyperpnea/hypopnea respiration with a crescendo‐decrescendo breathing pattern occur without apnea (Figure 1, bottom). Hence, the distinguishing feature of CSR and PB is that CSR has a period of apnea whereas PB does not.
Figure 1.
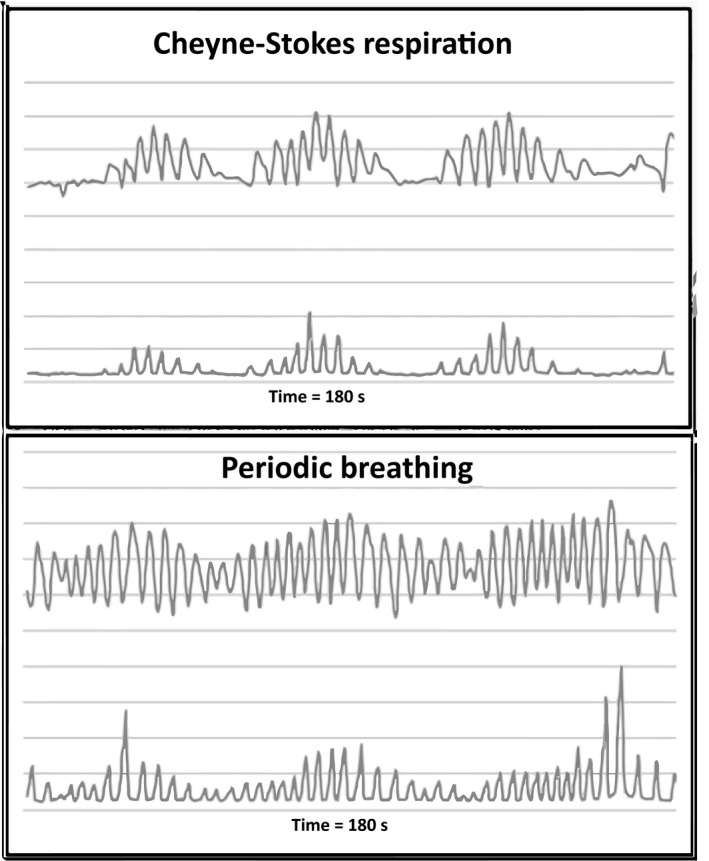
Top: Cheyne‐Stokes respiration; bottom: periodic breathing over 180 s. In each figure the top line is the mean QRS amplitude in 1/4 μV and is used to measure tidal volume. The bottom line is a myogram signal with arbitrary units used to detect tidal volume. In Cheyne‐Stokes respiration apnea is seen as a flat line in both the QRS amplitude and the myogram waveforms
2.5. Statistical analysis
Continuous data are reported as mean ± SD. The variables monitoring time, age and body mass index (BMI) were not normally distributed and the Wilcoxon rank‐sum test was used to compare group mean ranks for these variables. The Pearson chi‐square test was used to compare group frequencies for the categorical variables gender and ethnicity. The chi‐square test followed by post hoc pairwise comparisons was used for the variable race and the Fisher’s exact test was used when the assumptions for Pearson chi‐square test were not met. Spearman rank order correlations were used to test for a correlation between demographic variables (age and BMI) and the variables of interest, CSR and PB. Because CSR and PB data had the following characteristics; positive integer count variables, truncated at zero, skewed to the right and over‐dispersed, the incidence rate ratio between the two groups for each analysis was calculated using negative binomial regression (Hutchinson & Holtman, 2005). Estimation was carried out using a nonparametric, bias‐corrected bootstrap with 5,000 repetitions to reduce the potential influence of several cases with extreme outliers for both outcomes (CSR and PB) and bias corrected confidence intervals are reported (Erceg‐Hurn & Mirosevich, 2008; Wood, 2005; Zhu, 1997). Statistical analyses were conducted using Stata Release 14 (StataCorp, College Station, TX, USA). A p value <.05 was considered statistically significant.
3. RESULTS
3.1. Comparison of CSR and PB between healthy participants and hospitalized patients
As shown in Table 1, the mean age of healthy group was younger (34 years vs. 67 years; p < .001), and were predominantly white (70% vs. 41%; p < .001) as compared to the hospitalized group. As a group, the healthy individuals had a lower BMI (25 vs. 28; p < .001) than the hospitalized group.
Table 1.
Baseline characteristics comparing healthy group to hospitalized patients
| Variable | Healthy | Hospitalized | p Value |
|---|---|---|---|
| Number | 100 | 90 | |
| Age in years, mean ± SD/median | 34 ± 10/33 | 67 ± 14/68 | <.001a |
| Male, n (%) | 35 (35) | 42 (47) | .10b |
| Ethnicity | |||
| Hispanic, n (%) | 26 (26) | 16 (18) | .17b |
| Race | <.001b | ||
| African American, n (%) | 11 (11) | 20 (22) | .58c |
| American Indian/Alaskan Native, n (%) | 0 (0) | 13 (14) | h > Hc , d |
| Asian, n (%) | 19 (19) | 20 (22) | .04c |
| Native Hawaiian or other Pacific Islander, n (%) | 0 (0) | 0 (0) | NS |
| White, n (%) | 70 (70) | 37 (41) | H > hc |
| BMI, kg/m², mean ± SD/median | 25 ± 4/24 | 28 ± 7/27 | .001a |
BMI, body mass index; H, healthy group; h, hospitalized group; NS, not significant; SD, standard deviation.
Wilcoxon rank‐sum test.
Chi‐square test.
Post hoc pairwise comparison of each category for race against the remaining categories.
Fisher’s exact test.
Mean age was not only significantly different between the healthy and the hospitalized groups but age also correlated with both CSR (r s = +.29, p < .001) and PB (r s = +.36, p < .001). BMI was significantly different between the two groups and BMI was also a confounder; thus, both age and BMI were controlled for in all subsequent analyses. CSR and PB counts were adjusted in the negative binomial regression model to account for differences in the number of hours of monitoring for which data were collected for each patient (ranging between 18 and 24 hr).
The mean CSR count and daily PB counts were different between the healthy and hospitalized groups (1 vs. 9, p < .001) and (9 vs. 24, p < .001) (Table 2) respectively. Furthermore, regression analyses indicated hospitalized patients had 7.3 times more CSR (95% CI = 2.00–28.96 bias corrected) and 1.64 times more PB (95% CI = 1.15–2.38 bias corrected) than healthy individuals after controlling for age and BMI.
Table 2.
Comparison of Cheyne‐Stokes respiration and periodic breathing between healthy and hospitalized groups
| Variable | Healthy | Hospitalized | p Value |
|---|---|---|---|
| Number | 100 | 90 | |
| Monitoring time, mean hours/SD/median | 24 ± 1/24 | 23 ± 2/24 | .05a |
| Cheyne‐Stokes respiration during entire monitoring period mean/SD/median | 1 ± 1/1 | 9 ± 23/2 | <.001a |
| Cheyne‐Stokes respiration range | 0–7 | 0–165 | |
| Periodic breathing, entire monitoring period mean/SD/median | 9 ± 3/9 | 24 ± 27/14 | <.001a |
| Periodic breathing range | 0–18 | 3–170 |
SD, standard deviation.
Wilcoxon rank‐sum test.
3.2. Comparison of CSR and PB between hospitalized patients with cardiac non‐ACS versus ACS
The hospitalized group (n = 90) was further subdivided to determine whether patients who had a final diagnosis of cardiac non‐ACS diagnosis (n = 51) differed from those diagnosed with an ACS diagnosis (n = 39) in mean number of CSR and PB episodes. The two groups did not differ significantly with respect to patient's age, BMI, gender, race, ethnicity, heart failure diagnosis, or opiate administration during emergency department admission.
A review of patient's records showed that 15 out of the 90 hospitalized patients (16.7%) had history of heart failure: seven out of 39 (17.9%) with ACS diagnosis and eight out of 51 (15.7%) with non‐ACS diagnosis. Age and BMI were not significantly different between these two groups. Patients with heart failure and ACS diagnosis did not have a significantly different number of CSR or PB episodes than patients with heart failure and non‐ACS diagnosis.
Furthermore, 37.8% of the hospitalized patients received opiates in the emergency department: 17 out of 39 (43.6%) with ACS diagnosis and 17 out of 51 (33.3%) with non‐ACS diagnosis. Age and BMI were not significantly different between these two groups. Patients with ACS diagnosis who received opiates did not have a significantly different number of CSR or PB than patients with non‐ACS diagnosis who received opiates.
The cardiac non‐ACS group had a lower daily mean CSR count (3 vs. 17, p < .001) and a lower daily mean PB count (22 vs. 27, p < .001) than the ACS group (Table 3). Regression also showed that patients with a positive ACS diagnosis had 6.01 more CSR (CI = 2.78–13.73 bias corrected) than those with cardiac non‐ACS diagnosis.
Table 3.
Cheyne‐Stokes respiration and periodic breathing comparing hospitalized patients with acute coronary syndrome to hospitalized patients with a cardiac nonacute coronary syndrome diagnosis
| Variable | ACS | Cardiac non‐ACS | p Value |
|---|---|---|---|
| Number | 39 | 51 | |
| Monitoring time (hours), mean ± SD/median | 23 ± 1/24 | 23 ± 2/24 | .002a |
| Cheyne‐Stokes respiration, entire monitoring period mean ± SD/median | 17 ± 33/3 | 3 ± 7/1 | <.001a |
| Cheyne‐Stokes respiration, range | 0–165 | 0–38 | |
| Periodic breathing, entire monitoring period mean ± SD/median | 27 ± 22/18 | 22 ± 30/11 | .11a |
| Periodic breathing, range | 4–88 | 3–170 |
SD, Standard deviation; ACS, acute coronary syndrome.
Wilcoxon rank‐sum test.
4. DISCUSSION
This is the first study to investigate the use of ECG‐derived CSR and PB measurements in a group of patients in a hospital setting. Our study is unique in that we compared a healthy group, unlikely to have CSR or PB, to a group of hospitalized patients admitted for suspected ACS. Our findings indicate that ECG‐derived CSR rates are more than seven times higher in hospitalized cardiac patients compared with healthy individuals. Moreover, when comparing the subgroup of hospitalized patients by discharge diagnosis (noncardiac ACS vs. confirmed ACS), patients with ACS had the highest rate of CSR (average, 17.3 episodes per 24‐hr period.
Using ECG‐derived measurements, Haigney et al. (2014), reported an average of 2.6 CSR episodes per 24‐hr period in healthy subjects. We also observed a low number of CSR episodes (average, 1.04 per 24‐hr period) in our healthy subjects. In an extension of the Haigney et al. (2014), study, we found healthy subjects experienced PB (average, 9.24 per 24‐hr period). These findings support the knowledge that irregular breathing patterns such as PB exist naturally even in healthy individuals, which is believed to maintain physiologic stability (Dunai, Kleiman, & Trinder, 1999; Eckert et al., 2007). Haigney et al. (2014) also found patients with heart failure to have an average of 19 CSR episodes over a 24‐hr period which is congruent with our results in the ACS group but not our cardiac non‐ACS group (24‐hr CSR episode average of 17 and 3 respectively).
Researchers have used the gold standard polysomnography to measure sleep disordered breathing, CSA and obstructive sleep apnea in patients who have heart failure during sleep (Javaheri et al., 1998; Sharma, Owens, & Malhotra, 2010; Sin et al., 1999). Javaheri et al. (1998) reported a 51% CSA prevalence, and Sin et al. (1999) reported 32.9% CSA prevalence and 37.3% obstructive sleep apnea prevalence. Yet, few studies have evaluated CSA or obstructive sleep apnea in patients with ACS. Prior studies evaluated obstructive sleep apnea and reported rates of: 43.1%, 76%, 47%, and 63% respectively, (Areias et al., 2012; Correia et al., 2012; De Jesus et al., 2010; Leão et al., 2016) when using either the Berlin Questionnaire (Correia et al., 2012; De Jesus et al., 2010) or portable systems (Areias et al., 2012; Leão et al., 2016). Van den Broecke et al. performed a remotely conducted sleep study using a telemonitoring system to measure CSR and PB (Van den Broecke et al., 2014).
To our knowledge this is the first study to use 12‐lead ECG Holter data to measure CSR or PB in patients with developing ACS where 12‐lead ECG monitoring was initiated within a median of 44 min from patient presentation to the emergency department. Our results support those of Van den Broecke et al. (2014), who conducted sleep studies remotely in 27 patients within 72 hr (median 2 days) of admission to the coronary care unit. Van den Broecke et al. (2014) found that 82% of patients had at least one episode of CSR or PB. Correspondingly, 87% of patients in our ACS group had at least one episode of CSR. Research evaluating CSR in hospitalized patients is limited and needs to be studied further.
Our results, albeit in a small sample, support the notion that there is a link between sleep disordered breathing and cardiovascular disease. Further research is needed to assess for CSR and PB, and obstructive sleep apnea prevalence in patients with ACS and to evaluate if this disorder is associated with adverse events. Because there is treatment available to treat sleep disordered breathing, new research studies are needed to determine the impact of this pathology so timely treatment can be initiated and potentially improve patient outcomes.
4.1. Limitations
This study included a small sample consisting of 100 healthy participants and 90 hospitalized patients. A thorough interview was conducted to assess participants’ health status, however, inclusion in the healthy group was determined by patients’ self‐report. Patients in the hospitalized group were enrolled from a single emergency room. While target monitoring time was 24 hr, missing data led to the inclusion of cases that had at least 18 hr of continuous ECG recording. Variables of interest, CSR and PB, were not measured with the gold standard, polysomnography.
5. CONCLUSION
Continuous 12‐lead ECG data appears to identify CSR and PB patterns in hospitalized cardiac patients. CSR and PB counts were significantly different between healthy and hospitalized patients and also between patients with and without an ACS diagnosis. Hospitalized patients, especially those with an ACS discharge diagnosis had more CSR and PB than healthy individuals and cardiac non‐ACS patients. The Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) study (Cowie et al., 2015) reported that treating heart failure and CSA patients with continuous positive airway pressure did not decrease patient morbidity or mortality. However, testing and treating CSA might help clinicians screen for ACS before heart failure results.
Further research is needed to determine whether these ECG‐derived respiratory parameters change before and after ACS ensues, are linked to adverse patient outcomes and whether continuous measurement would be valuable in clinical practice to identify patients with sleep disordered breathing.
Tinoco A, Drew BJ, Hu X, Mortara D, Cooper BA, Pelter MM. ECG‐derived Cheyne‐Stokes respiration and periodic breathing in healthy and hospitalized populations. Ann Noninvasive Electrocardiol. 2017;22:e12462 10.1111/anec.12462
Funding information
This study was supported by grants from the National Institutes of Nursing Research (T32 NR007088, F31 NR015196) and a grant from the National Heart, Lung, & Blood Institute (RO1HL69753).
REFERENCES
- Areias, V. , Romero, J. , Cunha, K. , Faria, R. , Mimoso, J. , Gomes, V. , & Brito, U. (2012). Sleep Apnea‐Hypopnea syndrome and acute coronary syndrome—An association not to forget. Revista portuguesa de pneumologia, 18, 22–28. [DOI] [PubMed] [Google Scholar]
- Brack, T. , Thüer, I. , Clarenbach, C. F. , Senn, O. , Noll, G. , Russi, E. W. , & Bloch, K. E. (2007). Daytime Cheyne‐Stokes respiration in ambulatory patients with severe congestive heart failure is associated with increased mortality. Chest, 132, 1463–1471. [DOI] [PubMed] [Google Scholar]
- Chung, F. , Yegneswaran, B. , Liao, P. , Chung, S. A. , Vairavanathan, S. , Islam, S. , … Shapiro, C. M. (2008). STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology, 108, 812–821. [DOI] [PubMed] [Google Scholar]
- Correia, L. C. , Souza, A. C. , Garcia, G. , Sabino, M. , Brito, M. , Maraux, M. , … Esteves, J. P. (2012). Obstructive sleep apnea affects hospital outcomes of patients with non‐ST‐elevation acute coronary syndromes. Sleep, 35, 1241–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie, M. R. , Woehrle, H. , Wegscheider, K. , Sabino, M. , Brito, M. , Maraux, M. , … Esteves, J. P. (2015). Adaptive servo‐ventilation for central sleep apnea in systolic heart failure. New England Journal of Medicine, 373, 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus, E. V. , Dias‐Filho, E. B. , Mota Bde, M. , de Souza, L. , Marques‐Santos, C. , Rocha, J. B. , … Barreto‐Filho, J. A. (2010). Suspicion of obstructive sleep apnea by Berlin Questionnaire predicts events in patients with acute coronary syndrome. Arquivos Brasileiros de Cardiologia, 95, 313–320. [DOI] [PubMed] [Google Scholar]
- Dunai, J. , Kleiman, J. , & Trinder, J. (1999). Ventilatory instability during sleep onset in individuals with high peripheral chemosensitivity. Journal of Applied Physiology, 87, 661–667. [DOI] [PubMed] [Google Scholar]
- Eckert, D. J. , Jordan, A. S. , Merchia, P. , & Malhotra, A. (2007). Central sleep apnea: Pathophysiology and treatment. Chest, 131, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erceg‐Hurn, D. M. , & Mirosevich, V. M. (2008). Modern robust statistical methods: An easy way to maximize the accuracy and power of your research. American Psychologist, 63, 591–601. [DOI] [PubMed] [Google Scholar]
- Haigney, M. , Zareba, W. , La Rovere, M. T. , Grasso, I. , & Mortara, D. (2014). Assessing the interaction of respiration and heart rate in heart failure and controls using ambulatory Holter recordings. Journal of Electrocardiology, 47, 831–835. [DOI] [PubMed] [Google Scholar]
- Hanly, P. J. , Zuberi Khokhar, N. S. (1996). Increased mortality associated with Cheyne‐Stokes respiration in patients with congestive heart failure. American Journal of Respiratory and Critical Care Medicine, 153, 272–276. [DOI] [PubMed] [Google Scholar]
- Helfenbein, E. , Firoozabadi, R. , Chien, S. , Carlson, E. , & Babaeizadeh, S. (2014). Development of three methods for extracting respiration from the surface ECG: A review. Journal of Electrocardiology, 47, 819–825. [DOI] [PubMed] [Google Scholar]
- Hutchinson, M. K. , & Holtman, M. C. (2005). Analysis of count data using Poisson regression. Research in Nursing & Health, 28, 408–418. [DOI] [PubMed] [Google Scholar]
- Javaheri, S. , Parker, T. J. , Liming, J. D. , Corbett, W. S. , Nishiyama, H. , Wexler, L. , & Roselle, G. A. (1998). Sleep apnea in 81 ambulatory male patients with stable heart failure types and their prevalences, consequences, and presentations. Circulation, 97, 2154–2159. [DOI] [PubMed] [Google Scholar]
- La Rovere, M. T. , Pinna, G. D. , Maestri, R. , Barlera, S. , Bernardinangeli, M. , Veniani, M. , … for the GISSI‐HF Investigators . (2007). Clinical relevance of short‐term day‐time breathing disorders in chronic heart failure patients. European Journal of Heart Failure, 9, 949–954. [DOI] [PubMed] [Google Scholar]
- Lanfranchi, P. A. , Braghiroli, A. , Bosimini, E. , Mazzuero, G. , Colombo, R. , Donner, C. F. , & Giannuzzi, P. (1999). Prognostic value of nocturnal Cheyne‐Stokes respiration in chronic heart failure. Circulation, 99, 1435–1440. [DOI] [PubMed] [Google Scholar]
- Leão, S. , Conde, B. , Fontes, P. , Calvo, T. , Afonso, A. , & Moreira, I. (2016). Effect of obstructive sleep apnea in acute coronary syndrome. American Journal of Cardiology, 117, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Leung, R. S. T. , Comondore, V. R. , Ryan, C. M. , & Stevens, D. (2012). Mechanisms of sleep‐disordered breathing: Causes and consequences. Pflügers Archiv: European Journal of Physiology, 463, 213–230. [DOI] [PubMed] [Google Scholar]
- Linz, D. , Woehrle, H. , Bitter, T. , Fox, H. , Cowie, M. R. , Böhm, M. , & Olaf, O. (2015). The importance of sleep‐disordered breathing in cardiovascular disease. Clinical Research in Cardiology, 104, 705–718. [DOI] [PubMed] [Google Scholar]
- Mason, R. E. , & Likar, I. (1966). A new system of multiple lead exercise electrocardiography. American Heart Journal, 71, 196. [DOI] [PubMed] [Google Scholar]
- Moody, G. B. , Mark, R. G. , Bump, M. A. , Weinstein, J. S. , Berman, A. D. , Mietus, J. E. , & Goldberg, A. L. (1986). Clinical validation of the ECG‐derived respiration (EDR) technique. Computers in Cardiology, 13, 507–510. [Google Scholar]
- Moody, G. B. , Mark, R. G. , Zoccola, A. , & Mantero, S. (1985). Derivation of respiratory signals from multi‐lead ECGs. Computers in Cardiology, 12, 113–116. [Google Scholar]
- Penzel, T. , McNames, J. , De Chazal, P. , Raymond, B. , Murray, A. , & Moody, G. (2002). Systematic comparison of different algorithms for apnoea detection based on electrocardiogram recordings. Medical & Biological Engineering & Computing, 40, 402–407. [DOI] [PubMed] [Google Scholar]
- Pichot, V. , Chouchou, F. , Pepin, J. ‐L. , Tamisier, R. , Lévy, P. , Court‐Fortune, I. , … Roche, F. (2015). ECG‐derived respiration: A promising tool for sleep‐disordered breathing diagnosis in chronic heart failure patients. International Journal of Cardiology, 186, 7–9. [DOI] [PubMed] [Google Scholar]
- Sayadi, O. , Weiss, E. H. , Merchant, F. M. , Puppala, D. , & Armoundas, A. A. (2014). An optimized method for estimating the tidal volume from intracardiac or body surface electrocardiographic signals: Implications for estimating minute ventilation. American Journal of Physiology. Heart and Circulatory Physiology, 307, H426–H436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, D. M. , Lux, R. L. , Shusterman, V. , & Drew, B. J. (2007). Karhunen‐Loève representation distinguishes ST‐T wave morphology differences in emergency department chest pain patients with non–ST‐elevation myocardial infarction versus nonacute coronary syndrome. Journal of Electrocardiology, 40, S145–S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, B. , Owens, R. , & Malhotra, A. (2010). Sleep in congestive heart failure. Medical Clinics of North America, 94, 447–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin, D. D. , Fitzgerald, F. , Parker, J. D. , Newton, G. , Floras, J. S. , & Bradley, T. D. (1999). Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. American Journal of Respiratory and Critical Care Medicine, 160, 1101–1106. [DOI] [PubMed] [Google Scholar]
- Somers, V. K. , White, D. P. , Amin, R. , Abraham, W. T. , Costa, F. , Culebras, A. , … Young, T. (2008). Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Journal of the American College of Cardiology, 52, 686–717. [DOI] [PubMed] [Google Scholar]
- Stein, P. K. , Duntley, S. P. , Domitrovich, P. P. , Nishith, P. , & Carney, R. M. (2003). A simple method to identify sleep apnea using Holter recordings. Journal of Cardiovascular Electrophysiology, 14, 467–473. [DOI] [PubMed] [Google Scholar]
- Van den Broecke, S. , Jobard, O. , Montalescot, G. , Bruyneel, M. , Ninane, V. , Arnulf, I. , … Attali, V. (2014). Very early screening for sleep‐disordered breathing in acute coronary syndrome in patients without acute heart failure. Sleep Medicine, 15, 1539–1546. [DOI] [PubMed] [Google Scholar]
- Varon, C. , Caicedo, A. , Testelmans, D. , Buyse, B. , & Van Huffel, S. (2015). A novel algorithm for the automatic detection of sleep apnea from single‐lead ECG. IEEE Transactions on Biomedical Engineering, 62, 2269–2278. [DOI] [PubMed] [Google Scholar]
- Weiss, E. H. , Sayadi, O. , Ramaswamy, P. , Merchant, F. M. , Sajja, N. , Foley, L. , … Armoundas, A. A. (2014). An optimized method for the estimation of the respiratory rate from electrocardiographic signals: Implications for estimating minute ventilation. American Journal of Physiology. Heart and Circulatory Physiology, 307, H437–H447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, M. (2005). Bootstrapped confidence intervals as an approach to statistical inference. Organizational Research Methods, 8, 454–470. [Google Scholar]
- Zhu, W. (1997). Making bootstrap statistical inferences: A tutorial. Research Quarterly for Exercise and Sport, 68, 44–55. [DOI] [PubMed] [Google Scholar]


