Abstract
Aims
There are no treatments for the extreme hyperphagia and obesity in Prader-Willi syndrome (PWS). The bestPWS trial assessed the efficacy, safety, and tolerability of the methionine aminopeptidase 2 (MetAP2) inhibitor, beloranib.
Materials and Methods
Participants with PWS (12–65 years) were randomly assigned (1:1:1) to biweekly placebo, 1.8 mg beloranib, or 2.4 mg beloranib injection for 26 weeks at 15 US sites. Coprimary endpoints were the changes in hyperphagia (measured by Hyperphagia Questionnaire for Clinical Trials [HQ-CT]; possible score 0–36) and weight by intention-to-treat. ClinicalTrials.gov registration: NCT02179151.
Results
107 were included in the intention-to-treat analysis: placebo (n=34), 1.8 mg beloranib (n=36), or 2.4 mg beloranib (n=37). Improvement (reduction) in HQ-CT total score was greater in the 1.8 mg (mean difference −6.3, 95% CI −9.6 to −3.0; p=0.0003) and 2.4 mg beloranib groups (−7.0, 95% CI −10.5 to −3.6; p=0.0001) vs placebo. Compared to placebo, weight change was greater with 1.8 mg (mean difference −8.2%, 95% CI −10.8 to −5.6; p<0.0001) and 2.4 mg beloranib (−9.5%, 95% CI −12.1 to −6.8; p<0.0001). Injection site bruising was the most frequent adverse event with beloranib. Dosing was stopped early due to an imbalance in venous thrombotic events in beloranib-treated participants (two fatal events of pulmonary embolism and two events of deep vein thrombosis) compared to placebo.
Conclusions
MetAP2 inhibition with beloranib produced statistically significant and clinically meaningful improvements in hyperphagia-related behaviors and weight loss in participants with PWS. Although investigation of beloranib has ceased, inhibition of MetAP2 is a novel mechanism for treating hyperphagia and obesity.
Introduction
Prader-Willi syndrome (PWS) is characterized by motor and cognitive delays, significant behavioral disturbances, low muscle tone, slow metabolism, and extreme unrelenting hunger, resulting in hyperphagia and early childhood-onset obesity[1–3] that significantly contribute to morbidity and early mortality.[4–6] Without aggressive external control of eating behavior, individuals with PWS develop severe obesity and are highly susceptible to obesity-related morbidities, including type 2 diabetes, obstructive sleep apnea, and hypertension.[1, 7] Furthermore, obesity-related complications such as respiratory and cardiac failure, pulmonary embolism (a previously under-reported complication), and renal failure are leading causes of death in PWS, followed by deaths related to food seeking (gastrointestinal perforation, choking, and accidents).[8] Moreover, the obsessive and compulsive behaviors displayed by individuals with PWS that are often directed toward obtaining and consuming food also have a profoundly deleterious impact on social function and quality of life.[9, 10]
To our knowledge, there are no pharmacological treatment options for hyperphagia or obesity in PWS. Clinical experience with a variety of diets, supplements, and other approaches has been disappointing and the long-term risk-benefit of bariatric surgery[11–13] in PWS is unclear. The current management of hyperphagia and obesity in PWS focuses on lifelong food restriction, placing a substantial burden on individuals with PWS and their families.[9, 14, 15]
Beloranib inhibits methionine aminopeptidase 2 (MetAP2), preventing removal of the proximal (amino-terminus) methionine residue from proteins cleaved by MetAP2.[16] Beloranib and other MetAP2 inhibitors reduce food intake, body weight, fat content, and adipocyte size in animal models.[16–20] Weight loss caused by beloranib and other MetAP2 inhibitors is hypothesized to be due to direct effects on adipose tissue that reduce fat synthesis and increase fat oxidation as well as metabolic and hormonal changes that reduce appetite and food intake, while maintaining basal metabolic rate.[19, 21] In prior clinical trials in individuals with obesity (without PWS), beloranib produced clinically significant and sustained weight loss of 0.5 to 1.0 kg per week and also reduced subject-reported hunger.[21, 22]
Given the significant contribution of hyperphagic behaviors to obesity in PWS, the efficacy of beloranib on hyperphagia-related behavior and body weight in PWS was tested in an unpublished randomized, placebo-controlled, proof-of-concept trial in 17 adults. Beloranib treatment for up to eight weeks reduced body weight, fat mass, and plasma lipids and attenuated hyperphagia-related behavior. The aim of the current trial was to investigate the efficacy of beloranib in the treatment of hyperphagia and obesity as well as safety and tolerability over 26 weeks in adolescent and adult participants with PWS and comorbid obesity.
Materials and Methods
Study design and participants
The bestPWS study was a Phase 3, randomized, placebo-controlled, double-blind trial conducted at 15 sites in the United States between September 8, 2014 and December 11, 2015. Eligible participants had PWS (genetically confirmed), were between the age of 12–65 years, had elevated BMI (age 12–17: BMI ≥95th percentile for age and sex; age 18–65: BMI 27–60 kg/m2), total score ≥13 (out of a possible 0–36) on the Hyperphagia Questionnaire for Clinical Trials (HQ-CT), were weight stable (gain/loss <10%) for ≥3 months, and met vital sign parameters: systolic blood pressure 90–160 mmHg, diastolic blood pressure 50–100 mmHg, pulse 40–100 bpm. Individuals with type 2 diabetes were accepted if they had hemoglobin A1C <10% (86 mmol/mol), fasting glucose <240 mg/dL (<13.3 mmol/L), no history of ketoacidosis or hyperosmolar coma, and no insulin therapy. Glucose-, lipid-, and blood pressure-lowering medications were allowed if the participant’s condition was stable and treatment with growth hormone was allowed if a stable dose was prescribed for ≥3 months. Participants were also required to have at least one primary consistent and reliable caregiver to evaluate changes in the participant’s eating behavior, mood, quality of life, adverse events, and overall behavior throughout the study. The HQ-CT relies on reporting by a single observer who is with the subject every day for a minimum of 4 hours. Participants living in a group home ≥50% of the time were excluded because this level of interaction is not consistently achieved in this type of setting.
The institutional review boards at all study sites approved the protocol prior to study initiation. Adult participants provided written informed consent. Participants under the age of 18 years or who were not able to understand and provide written informed consent instead provided written assent; written consent was provided by a guardian. The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice and was registered with ClinicalTrials.gov, number NCT02179151.
Randomization and Masking
Participants were randomized via computer using a centralized Interactive and/or Web Response System (IxRS) in a 1:1:2:2 ratio to lower-dose placebo (0.45 mL), higher-dose placebo (0.6 mL), 1.8 mg beloranib (0.45 mL), or 2.4 mg beloranib (0.6 mL; Appendix Figure 1). Randomization was stratified based on recombinant human growth hormone use (yes/no) and age (≥16/<16 y). Pharmacy personnel not involved in the conduct of the study dispensed study medication in containers that differed only by participant code number. To ensure adequate blinding of the dosing volume (0.45 mL or 0.6 mL for 1.8 mg or 2.4 mg beloranib), syringes were supplied with a mark (no numbers or graduation) to indicate the appropriate dosing volume. Except for a Safety Review Committee that convened every three months to review safety and tolerability data, all participants and personnel remained blinded for the duration of the study.
Procedures
Following a 2-week single-blind placebo lead-in, participants were randomized to study treatment. Participants in the 2.4 mg beloranib dose group received 1.8 mg beloranib for the first four weeks followed by the maintenance dose of 2.4 mg beloranib for the remaining 22 weeks. Doses were selected based on previous experience in an unpublished Phase 2 trial of 17 participants with PWS. Study drug or placebo was supplied as a sterile lyophilized powder in single-use glass vials that were reconstituted with supplied diluent. Study drug was administered twice-weekly by either site staff or by a home health nurse. There was no diet/exercise intervention. Study drug administration could be stopped at any time at the request of the participant or caregiver or at the discretion of the Investigator. The study included an optional 26-week open-label extension in which all participants received four weeks of 1.8 mg beloranib followed by 2.4 mg beloranib for 22 weeks.
Medical history and adverse events were coded using The Medical Dictionary for Regulatory Activities (MedDRA, Version 17.1). Chemistry and hematology were analyzed centrally by PPD Central Labs (Highland Heights, KY) and biomarker analyses were conducted at Myriad RBM (Austin, TX). Collection, evaluation, and analysis of dual-energy absorptiometry (DXA) images (obtained using GE Lunar or Hologic instruments under standardized protocols) were conducted by BioClinica, Inc. (Newtown, PA).
Maladaptive behavior and quality of life were assessed using the Repetitive Behavior Scale-Revised (RBS-R) and Pediatric Quality of Life Inventory-Family Impact Module (PedsQL™-FIM). HQ-CT responders were classified by a predefined anchor-based method utilizing the Caregiver Global Impression of Change (CGIC) question where a score of “moderately better” was selected as a threshold for clinically meaningful change (questionnaire provided in Appendix Figure 2). The mean reduction in HQ-CT total score for all participants who were associated with a CGIC rating of moderately better was 7.7. Thus, HQ-CT responders were defined as those with a reduction in HQ-CT total score of at least 7.7.
Safety evaluations included assessment of incidence and severity of adverse events with onset on or after the first day of treatment, physical examination, electrocardiogram, vital signs, concomitant medications, and laboratory parameters. Retrospective testing was performed for the coagulation marker, D-dimer.
The randomized double-blind portion of the trial was stopped early due to an imbalance of venous thromboembolic events, including two participant deaths, in beloranib-treated participants compared to placebo. After the first of these deaths, dosing was stopped and all participants were screened for venous thromboembolism by assessing bilateral lower extremity ultrasonography and D-dimer concentrations. Participants with evaluations that were negative for venous thrombosis were eligible to enter the open-label extension; consenting participants received open-label beloranib and additional safety visits were conducted every two months. Dosing was again stopped after the second death but safety monitoring continued.
Outcomes
The prespecified coprimary endpoints were the change in hyperphagia-related behaviors (indicated by HQ-CT total score) and the percent change in body weight from baseline to Week 26. Hyperphagia-related behaviors were assessed by the HQ-CT, an instrument designed to measure symptoms of food-related preoccupations and behaviors that was completed by the caregiver; caregiver assessment was required owing to cognitive deficits in participants with PWS. The HQ-CT consists of nine items with responses ranging from 0–4 units each (possible total score range: 0–36; questionnaire provided in Appendix Figure 2).
Key secondary endpoints included the change from baseline in body fat mass (measured by DXA), and low- and high-density lipoprotein cholesterol. Other endpoints included the change from baseline in body weight (measured in kilograms), body mass and lean mass (measured by DXA), total cholesterol, triglycerides, cardiometabolic biomarkers (high-sensitivity C-reactive protein [hsCRP], adiponectin, and leptin), the percentage of participants classified as HQ-CT responders, the percentage of participants with ≥5% weight loss, maladaptive behavior, and quality of life.
Statistical analysis
We estimated that a sample size of 28 participants per group would provide 94% power to detect a between-group difference in each of the coprimary endpoints. To account for a dropout rate of up to 18%, the planned total sample size was 102 participants (34 per group). The power for the coprimary endpoints was calculated using a 2-sided, 2-sample t-test at a 5% significance level.
The prespecified efficacy analyses used data from the intent-to-treat population, which included all participants who underwent randomization, received at least one dose of study drug, and had baseline coprimary efficacy endpoint measurements. The safety analysis set included all participants who underwent randomization and received at least one dose of study drug. The coprimary and key secondary endpoints were analyzed in a hierarchical order to control for overall type I error that first compared the 2.4 mg beloranib group with placebo at the 5% significance level, followed by the 1.8 mg beloranib group. Both coprimary endpoints had to be statistically significant to deem the 2.4 mg beloranib dose superior to placebo. Unless described otherwise, efficacy endpoints were analyzed separately using a mixed model for repeated measures (MMRM) with treatment group, growth hormone use, age (≥16 or <16 years), visit, and treatment-by-visit interaction as fixed effects, the baseline value of the relevant variable as a covariate, and participant as a random effect. Missing data were not imputed in the primary efficacy analyses that used a MMRM analysis. Sensitivity analyses were performed to assess the robustness of the primary efficacy analyses; analysis of covariance (ANCOVA) models were fit using various imputation strategies for missing data, including multiple imputation with retrieve dropouts (MI-RD), retrieve dropouts weighted analysis (RD-weighted), baseline observation carried forward (BOCF) and last observation carried forward (LOCF). Additionally, efficacy endpoints were evaluated for the per protocol population (all participants who received at least 67% of doses of study drug and who completed Week 26 without any notable protocol violations) and predefined subgroup analyses of the coprimary endpoints were conducted based on sex, baseline HQ-CT (dichotomized at median value), PWS genetic subtype, growth hormone use, BMI tertiles, and age (≥16 or <16 years and ≥18 or <18 years). Adverse events were summarized by treatment group, system organ class, and preferred term.
Results
Out of 126 screened individuals, 108 participants were randomized, and 107 received study drug and were included in the intent-to-treat and safety analysis populations (Appendix Figure 1). Demographic and baseline characteristics were well matched across treatment groups for age, sex, growth hormone use, weight, BMI, fat mass, and genetic subtype (Table 1). The study population was primarily White and otherwise generally representative of participants with PWS and obesity. Vital signs, lipids, and cardiometabolic biomarkers were generally within normal range. Nearly all participants, 99/107 (93%), had a history of psychiatric disorders and behavior problems common in PWS.[15] These were primarily dermatillomania (skin-picking) 79/107 (74%), aggression 67/107 (63%), self-injurious behavior 26/107 (24%), and anxiety 16/107 (15%). Few participants 10/107 (9%) had type 2 diabetes.
Table 1.
Demographics and Baseline Characteristics*
| Placebo (N=34) |
1.8 mg Beloranib (N=36) |
2.4 mg Beloranib (N=37) |
|
|---|---|---|---|
| Age (years) | 20.9 (7.8) | 19.2 (5.2) | 19.5 (5.8) |
| Sex, male | 15 (44%) | 19 (52%) | 22 (60%) |
| Proportion <18 years | 15 (44%) | 15 (42%) | 17 (46%) |
| Race (% White/Black/Other) | |||
| White | 31 (91%) | 30 (83%) | 35 (95%) |
| Black or African American | 2 (6%) | 5 (14%) | 1 (3%) |
| Other | 1 (3%) | 1 (3%) | 1 (3%) |
| Weight (kg) | 100.9 (25.5) | 97.5 (24.1) | 105.7 (29.1) |
| Body mass index (kg/m2) | 40.3 (9.4) | 38.2 (8.9) | 41.4 (11.7) |
| Fat mass (kg) | 51.5 (15.5) | 47.6 (14.6) | 53.2 (19.4) |
| Body fat (%) | 53.1 (4.8) | 51.5 (6.3) | 53.0 (7.6) |
| HQ-CT total score, possible total score range: 0–36 (units) | 15.0 (5.8) | 17.4 (6.2) | 18.3 (7.3) |
| Growth hormone use (% yes) | 15 (44%) | 15 (42%) | 15 (41%) |
| Intelligence quotient | 71.6 (23.3) | 69.6 (23.1) | 67.1 (25.1) |
| PWS genetic subtype | |||
| Chromosome 15q11-13 region deletion | 25 (74%) | 24 (67%) | 27 (73%) |
| Maternal uniparental disomy | 7 (21%) | 11 (31%) | 9 (24%) |
| Imprinting defect | 2 (6%) | 1 (3%) | 1 (3%) |
| History of psychiatric disorder | 33 (97%) | 31 (86%) | 35 (95%) |
| Lipids | |||
| Total cholesterol (mmol/L) | 4.4 (0.6) | 4.3 (1.0) | 4.8 (1.0) |
| LDL cholesterol (mmol/L) | 2.5 (0.6) | 2.5 (0.8) | 2.9 (0.8) |
| HDL cholesterol (mmol/L) | 1.3 (0.2) | 1.3 (0.4) | 1.3 (0.3) |
| Triglycerides (mmol/L)† | 1.2 (0.1) | 1.1 (0.1) | 1.2 (0.1) |
| Vital Signs | |||
| Systolic blood pressure (mm Hg) | 118.7 (16.4) | 118.3 (11.5) | 119.5 (13.3) |
| Diastolic blood pressure (mm Hg) | 70.3 (8.1) | 73.5 (10.5) | 74.6 (8.6) |
| Heart rate (beats per minute) | 75.3 (9.7) | 76.3 (15.1) | 79.4 (14.6) |
| Cardiometabolic Biomarkers | |||
| hsCRP (µg/mL)† | 8.4 (1.9) | 6.9 (1.7) | 7.5 (1.7) |
| Adiponectin (µg/mL) | 4.9 (1.9) | 4.7 (2.5) | 4.3 (1.6) |
| Leptin (ng/mL) | 56.2 (24.6) | 57.3 (30.8) | 60.2 (37.8) |
Data are mean (SD), n (%), or geometric mean (SE) for the Safety Population (N=107). Percentages may not add up to 100 due to rounding. HDL denotes high-density lipoprotein, hsCRP high-sensitivity C-reactive protein, HQ-CT Hyperphagia Questionnaire for Clinical Trials, LDL low-density lipoprotein, PWS Prader-Willi syndrome.
To account for skewness, data for triglycerides and hsCRP were log transformed and values are geometric mean (SE).
When the double-blind treatment was stopped, 74/107 (69%) participants had completed 26 weeks of treatment (placebo n=24, 1.8 mg beloranib n=26, 2.4 mg beloranib n=24) and six participants had stopped treatment due to adverse events (placebo n=0, 1.8 mg beloranib n=4; 2.4 mg beloranib n=2). The remaining 27 participants had completed at least 75% of the 26-week double-blind treatment period and were not removed from the primary efficacy analysis. It was deemed that there were sufficient data to allow for a robust assessment of efficacy.
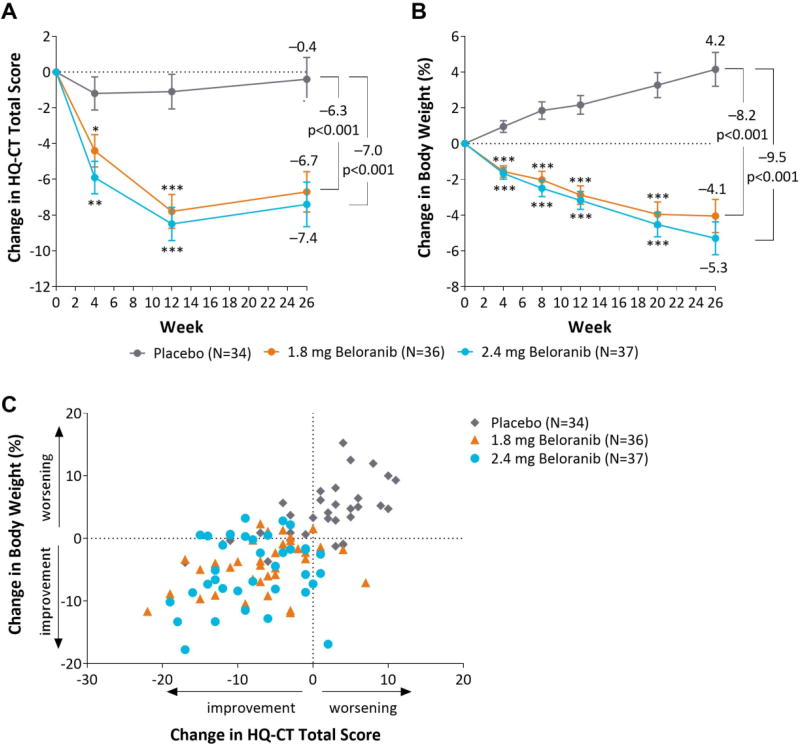
Beloranib resulted in statistically significant reductions from baseline to Week 26 compared to placebo for both coprimary endpoints (Figure 1A and 2B). After 26 weeks, the least squares mean change in HQ-CT total score was −6.7 (SE 1.1) and −7.4 (1.3) in the 1.8 mg and 2.4 mg beloranib treatment groups, respectively, compared to −0.4 (1.2) in the placebo group, indicating a greater improvement in hyperphagia-related behaviors with beloranib treatment than placebo (Table 2, Figure 1A). The improvement in hyperphagia-related behaviors with beloranib was statistically significant compared to placebo at the earliest time point measured (Week 4) and continued for the duration of treatment (Figure 1A).
Figure 1.
Change in coprimary endpoints and scatterplot of change in weight and HQ-CT total score. Least squares mean change (SE) in HQ-CT total score (A) or least squares mean percent change in body weight (B) from baseline to Week 26 for the intent-to-treat population. Estimated treatment differences are from a mixed model repeated measures model with no imputation for missing data. *p<0.05, **p<0.01, ***p<0.001 for change from baseline for beloranib vs placebo. (C) Percent change in weight and absolute change in HQ-CT total score from baseline to Week 26 for each participant in the intent-to-treat population with missing data imputed using last-observation-carried-forward.
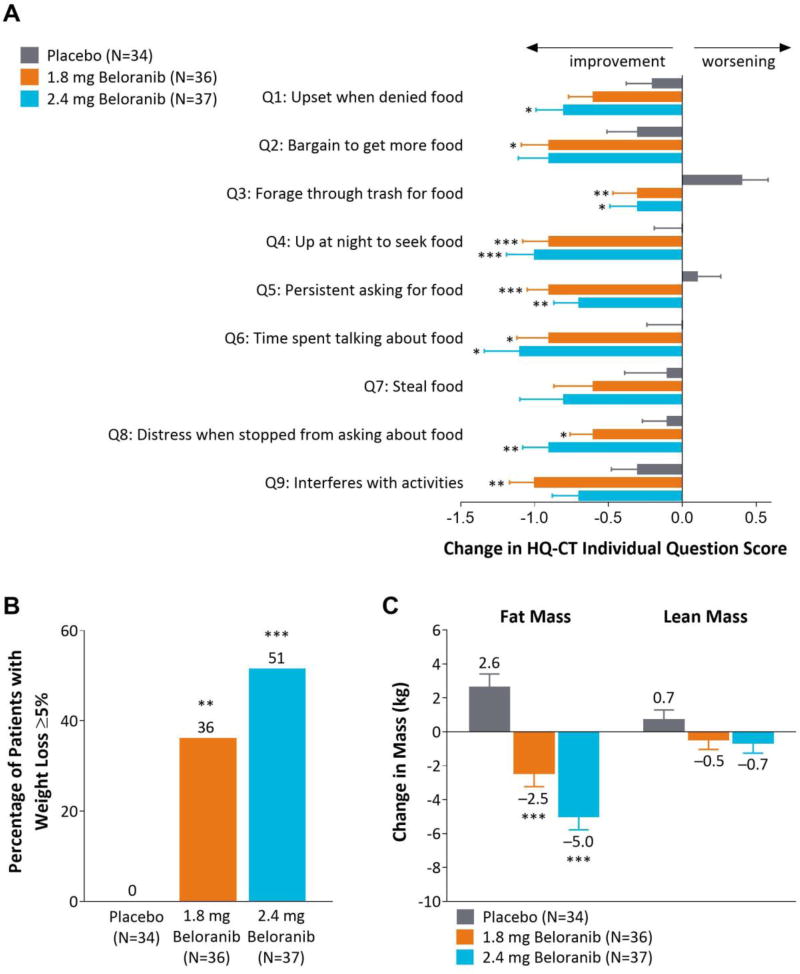
Figure 2.
Change in HQ-CT individual item scores, participants with ≥5% weight loss, and change in body mass. (A) Least squares mean change (SE) in individual question scores for the HQ-CT at Week 26. Each item had five possible responses with a score ranging from 0 to 4 (Appendix Figure 2). (B) Percentage of participants categorized as body weight responders, i.e. with at least 5% weight loss. (C) Least squares mean change (SE) in body mass from baseline to Week 26 as measured by dual-energy absorptiometry. Data are for the intent-to-treat population. *p<0.05, **p<0.01, ***p<0.001 for beloranib vs placebo.
Table 2.
Change from Baseline in Coprimary Endpoints, and Cardiometabolic Risk Factors, and Other Assessments from Baseline to Week 26*
| Placebo (N=34) |
1.8 mg Beloranib (N=36) |
Treatment effect, 1.8 mg beloranib vs placebo [95% CI]† |
P value | 2.4 mg Beloranib (N=37) |
Treatment effect, 2.4 mg beloranib vs placebo [95% CI]† |
P value | |
|---|---|---|---|---|---|---|---|
| Coprimary endpoints | |||||||
| Body weight (%) | 4.2 (0.9) | −4.1 (0.9) | −8.2 [−10.8 to −5.6] | <0.0001 | −5.3 (0.9) | −9.5 [−12.1 to −6.8] | <0.0001 |
| 95% CI | [2.3 to 6.0] | [−5.9 to −2.2] | [−7.1 to −3.5] | ||||
| Body weight (kg) | 3.8 (0.9) | −4.1 (0.9) | −8.0 [−10.5 to −5.4] | <0.0001 | −5.4 (0.9) | −9.2 | <0.0001 |
| 95% CI | [2.0 to 5.7] | [−5.9 to −2.3] | [−7.2 to −3.6] | [−11.8 to −6.6] | |||
| Change in HQ-CT total score | −0.4 (1.2) | −6.7 (1.1) | −6.3 [−9.6 to −3.0] | 0.0003 | −7.4 (1.3) | −7.0 | 0.0001 |
| 95% CI | [−2.8 to 2.0] | [−8.9 to −4.4] | [−9.9 to −4.9] | [−10.5 to −3.6] | |||
| Body composition | |||||||
| Fat mass (kg) | 2.6 (0.8) | −2.5 (0.8) | −5.1 [−7.2 to −3.0] | <0.0001 | −5.0 (0.8) | −7.6 [−9.8 to −5.5] | <0.0001 |
| Lean mass (kg) | 0.7 (0.6) | −0.5 (0.6) | −1.2 [−2.8 to 0.4] | 0.1272 | −0.7 (0.6) | −1.4 [−3.0 to 0.2] | 0.0814 |
| Lipids | |||||||
| Total cholesterol (mmol/L) | 0.0 (0.1) | −0.4 (0.1) | −0.5 [−0.8 to −0.2] | 0.0008 | −0.5 (0.1) | −0.5 [−0.8 to −0.2] | 0.0008 |
| LDL cholesterol (mmol/L) | 0.0 (0.1) | −0.4 (0.1) | −0.5 [−0.7 to −0.3] | <0.0001 | −0.5 (0.1) | −0.5 [−0.7 to −0.3] | <0.0001 |
| HDL cholesterol (mmol/L) | 0.0 (0.0) | 0.0 (0.0) | 0.0 [−0.1 to 0.1] | 0.7725 | 0.0 (0.0) | 0.0 [−0.1 to 0.2] | 0.7039 |
| Triglycerides (mmol/L) ‡ | 1.00 (0.05) | 0.97 (0.05) | 0.99 (0.06) | ||||
| Triglyceride ratio ‡ | 0.97 [0.83 to 1.13] | 0.7153 | 1.00 [0.86 to 1.16] | 0.9667 | |||
| Cardiometabolic Biomarkers | |||||||
| hsCRP (µg/ml) ‡ | 1.03 (0.14) | 0.44 (0.06) | 0.47 (0.06) | ||||
| hsCRP ratio ‡ | 0.43 [0.30 to 0.62] | <0.0001 | 0.46 [0.32 to 0.65] | <0.0001 | |||
| Adiponectin (µg/ml) | −0.5 (0.3) | 1.7 (0.3) | 2.2 [1.4 to 2.9] | <0.0001 | 1.9 (0.3) | 2.4 [1.6 to 3.1] | <0.0001 |
| Leptin (ng/ml) | 7.6 (2.9) | −20.6 (2.9) | −28.1 [−36.0 to −20.3] | <0.0001 | −23.8 (2.9) | −31.4 [−39.2 to −23.6] | <0.0001 |
Data are least squares mean (SE) and [95% confidence interval], for change from baseline to Week 26 for the intent-to-treat population. HDL denotes high-density lipoprotein, hsCRP high-sensitivity C-reactive protein, HQ-CT Hyperphagia Questionnaire for Clinical Trials, LDL low-density lipoprotein.
Estimated treatment differences are from a mixed model repeated measures model with no imputation for missing data. To account for skewness, data for triglycerides and hsCRP were log transformed for analysis and treatment effects are presented as relative to baseline (ratio of Week 26 to baseline value).
Values are geometric least squares means (SE) for change from baseline to Week 26 and treatment effect refers to the ratio of the baseline and Week 26 geometric least squares means.
The least squares mean percent change in body weight from baseline to Week 26 was −4.1% (SE 0.9) and −5.3% (0.9) for the 1.8 mg and 2.4 mg beloranib groups, respectively, compared to weight gain of 4.2% (0.9) with placebo (Table 2, Figure 1B). Consistent with the PWS phenotype and natural history, placebo-treated participants exhibited gradual weight gain while beloranib-treated participants exhibited weight loss that was statistically significant compared to placebo at the earliest time point measured (Week 4) and continued for the duration of treatment (Figure 1B).
The change in weight and HQ-CT total score for each participant is shown in Figure 1C. Most beloranib-treated participants (28/36 [78%] and 24/37 [65%] of participants in the 1.8 mg and 2.4 mg beloranib groups, respectively) exhibited an improvement in both hyperphagia-related behaviors and body weight (defined as change in HQ-CT <0 and change in body weight <0%). In contrast, many (19/34 [56%]) placebo-treated participants exhibited weight gain and a worsening in HQ-CT total score. A superior effect of beloranib compared to placebo for both coprimary efficacy endpoints was further supported by various sensitivity and subgroup analyses (Appendix Figure 4 and Appendix Figure 5). Results of these analyses were generally comparable to the primary efficacy analyses.
More participants achieved improvement in HQ-CT total score with beloranib than with placebo across the full spectrum of responses observed with very few subjects treated with beloranib having a worsening of response (Appendix Figure 6A). The improvement in HQ-CT total score with beloranib treatment was driven by improvements in each of the nine individual questions (Figure 2).
HQ-CT responders were classified by an anchor-based method in which a score of moderately better on the CGIC item was selected as a meaningful change; this corresponded to a mean reduction in HQ-CT total score of 7.7. Therefore, participants achieving a threshold reduction of ≥7.7 in HQ-CT total score were classified as responders. Compared to 4/34 (12%) of placebo participants, 13/36 (36%) and 19/37 (51%) of participants in the 1.8 mg and 2.4 mg beloranib groups, respectively, were classified as HQ-CT responders (p=0.0175 and p=0.0005, Appendix Figure 6B).
No placebo-treated participants had weight loss of at least 5%, however, 13/36 (36%) and 19/37 (51%) of participants in the 1.8 mg and 2.4 mg beloranib treatment groups achieved this benchmark (p=0.0001 and p<0.0001, Figure 2B). Almost all weight loss was attributed to a reduction in fat mass (Figure 2C). Placebo-treated participants gained 2.6 kg fat mass at Week 26 while 1.8 mg and 2.4 mg beloranib-treated participants had a mean change in fat mass of −2.5 kg and −5.0 kg, respectively and a small change in lean mass that was not statistically significant. In beloranib-treated participants, fat mass accounted for approximately 85% of weight loss.
Baseline lipids were generally within normal limits (Table 1). Beloranib-treated participants had substantial and statistically significant reductions in LDL cholesterol that contributed to the reduction in total cholesterol (Table 2). There were no significant changes in HDL cholesterol or triglycerides. High-sensitivity CRP was reduced with both doses of beloranib compared to placebo (Table 2). Both doses of beloranib were also associated with statistically significant salutary changes in the adipokines, leptin and adiponectin (Table 2).
Adverse events were more common among beloranib-treated participants compared to placebo during the 26-week randomized treatment phase (Table 3). Most were mild to moderate in severity. The most frequent adverse event in beloranib-treated participants was injection site bruising (Table 3). All injection site-related adverse events were mild, except for one moderate adverse event of injection site pain (in a participant in the 2.4 mg beloranib group) that resulted in withdrawal of study drug. Six beloranib and no placebo participants experienced events that led to withdrawal of study drug during the 26-week double-blind treatment period. These were: abnormal behavior, anxiety, mental status changes, and fatal pulmonary embolism (1.8 mg beloranib), injection site pain or psychotic disorder (2.4 mg beloranib). Five participants experienced serious adverse events of ankle fracture or aggression (placebo), mental status change or pulmonary embolism (1.8 mg beloranib), or aggression (2.4 mg beloranib). Four participants experienced venous thrombotic events (fatal pulmonary embolism n=2, deep vein thrombosis [DVT] n=2); these are described in detail in the Appendix. All venous thrombotic events occurred in participants receiving beloranib treatment; one event of fatal pulmonary embolism occurred during randomized treatment, the other three events occurred during the open-label extension.
Table 3.
Adverse Events*
| Placebo (N=34) |
1.8 mg Beloranib (N=36) |
2.4 mg Beloranib (N=37) |
Beloranib Combined (N=73) |
|
|---|---|---|---|---|
| Any adverse event | 22 (65%)/ 104 | 30 (83%)/ 130 | 29 (78%)/ 125 | 59 (81%)/ 255 |
| Adverse events in ≥5% of participants in Combined Beloranib groups | ||||
| Injection site bruising | 1 (3%) | 4 (11%) | 6 (16%) | 10 (14%) |
| Aggression | 5 (15%) | 5 (14%) | 4 (11%) | 9 (12%) |
| Hyperphagia | 3 (9%) | 6 (17%) | 2 (5%) | 8 (11%) |
| Fatigue | 0 (0%) | 5 (14%) | 1 (3%) | 6 (8%) |
| Injection site pain | 0 (0%) | 3 (8%) | 3 (8%) | 6 (8%) |
| Anxiety | 0 (0%) | 3 (8%) | 2 (5%) | 5 (7%) |
| Diarrhea | 0 (0%) | 3 (8%) | 2 (5%) | 5 (7%) |
| Irritability | 0 (0%) | 3 (8%) | 2 (5%) | 5 (7%) |
| Nasopharyngitis | 3 (9%) | 3 (8%) | 2 (5%) | 5 (7%) |
| Alopecia | 0 (0%) | 2 (6%) | 2 (5%) | 4 (6%) |
| Headache | 5 (15%) | 2 (6%) | 2 (5%) | 4 (6%) |
Data are n (%) or number of participants (%)/ number of events.
Adverse events that occurred from baseline to Week 26 in the Safety population (N=107). Adverse events are included if they had an onset date on or after the first dose of study drug.
D-dimer levels were measured retrospectively on existing samples. Mean baseline D-dimer was within the upper limit of the normal range (82–506 ng/mL) in all treatment groups (placebo: 447 ng/mL, 1.8 mg beloranib: 386 ng/mL, 2.4 mg beloranib: 366 ng/mL) and was not correlated with BMI, weight, age, or PWS genetic subtype. At Week 26, the least squares mean D-dimer was 393 (SE 209) ng/mL for placebo compared to 895 (201) ng/mL and 467 (215) ng/mL for 1.8 mg and 2.4 mg beloranib, respectively (not significant). However, D-dimer was significantly increased from baseline for both 1.8 mg and 2.4 mg beloranib compared to placebo at Week 12 (least squares mean: 583 [SE 41] ng/mL and 562 [40] ng/mL vs 413 [39]; both p<0.01).
Aggression, anxiety, and irritability were the most commonly reported psychiatric adverse events (Table 3). Of these, anxiety and irritability were more frequent with beloranib than placebo. Most of these adverse events occurred in participants with a relevant medical history. Vital signs were generally unchanged in all treatment groups and there were no notable safety signals or laboratory findings.
Discussion
The bestPWS trial is the first large randomized clinical trial to demonstrate a statistically significant and clinically meaningful improvement in hyperphagia-related behaviors and weight loss in individuals with PWS. In this population, the only effective treatment options currently available involve continuous restriction of access to food that is typically combined with a rigorous exercise routine.[1–3] Because individuals with PWS exhibit a pathological lack of satiety and thus feel intense hunger almost constantly, food restriction and implementation of exercise routines requires continuous supervision of the individual with PWS as well as extensive manipulation of the environment.[9, 14, 15] This has a significant negative impact on the household and usually results in challenging behavior as the individual with PWS struggles against these limitations. Furthermore, food restriction and exercise do nothing to address the underlying physiological abnormality of unrelenting hunger and are only moderately effective. Treatment with the MetAP2 inhibitor, beloranib, produced clinically important reductions in hyperphagia and body weight. The bestPWS trial is the first of its kind to show that hyperphagia and obesity in PWS can be successfully treated with a pharmacological approach.
Although other pharmacological approaches to the management of weight and hyperphagia in participants with PWS have been investigated,[23, 24] none has been effective in reducing hyperphagia. Only growth hormone has been shown to produce a moderate improvement in weight and body composition in some patients.[25] Extant behavioral treatments for obesity in individuals with PWS fail to address the extreme hunger and food-seeking drive these individuals experience and instead rely on reducing energy intake by limiting access to food.[9, 14, 15] This places a substantial burden on families and caretakers and causes most adult individuals with PWS to live in group homes with a strictly controlled environment.[3]
Hyperphagia-related behaviors were assessed by the HQ-CT, an instrument designed to measure symptoms of food-related preoccupations and behavior. In the present study, more participants achieved improvement in HQ-CT total score with beloranib than with placebo across the full spectrum of responses observed, with few beloranib-treated subjects having a change in score indicating a worsening. The individual change in HQ-CT total score also correlated with an improvement in the CGIC item and supported the use of the CGIC as an anchor. These data indicate that the HQ-CT is an appropriate measure of hyperphagia-related behaviors in participants with PWS.
Participants in the placebo group experienced weight gain (especially adolescent participants) over the 26-week study while those treated with beloranib had weight loss that continued for the duration of the study. Consistent with the participant population, participants had very high fat mass at baseline (approximately 50%)[26] and weight loss was mostly due to loss of body fat. There were also improvements in obesity-related markers of metabolic and cardiovascular risk. C-reactive protein (CRP) is elevated in a wide range of acute and chronic inflammatory conditions and is an independent predictor of coronary events and stroke in adults.[27] Consistent with previous observations in participants with PWS,[28, 29] mean hsCRP concentrations were elevated at baseline (>3 µg/mL).
Beloranib-treated participants exhibited statistically significant changes in the adipokines, adiponectin and leptin, compared to placebo. These changes are in keeping with the hypothesized effects of beloranib on adipose tissue. Adiponectin is normally abundant but concentrations are reduced in obesity, diabetes, and cardiovascular disease as well as in adults (but not children) with PWS.[26, 30, 31] Circulating leptin concentrations are correlated with adiposity and participants with PWS have elevated leptin, consistent with increased fat mass.[31–33] The reduction in leptin and increase in adiponectin observed with beloranib treatment are consistent with weight loss and concomitant decrease in fat mass.[34]
The mechanisms for weight loss and improvement in cardiometabolic markers with MetAP2 inhibitors is attributed to two primary effects: increased fat mobilization and oxidation and decreased caloric intake.[17, 18, 22] Although the specific mechanisms that produce these effects continue to be investigated, increased stability of MetAP2 is expected to attenuate activity of extracellular signal regulated kinases 1 and 2 (ERK1/2), resulting in a cascade of downstream effects.[35] Some of these effects include suppression of sterol regulatory element binding protein (SREBP) activity resulting in reduced lipid and cholesterol biosynthesis[36, 37] and effects on other signaling cascades, including retinoic acid-related orphan receptor α (RORα), which is implicated in fat biosynthesis, insulin action, and inflammation.[38] Reductions in hunger and caloric intake are likely the result of an integrated effect of changes in circulating fatty acids, lipids, adipokines, and other peripheral signals on CNS regions that regulate appetite.[39] Whether MetAP2 inhibition normalizes the hyperactivation evident in brain regions that drive eating behavior that has been observed in patients with PWS remains to be determined.[40]
Beloranib has been studied in participants with obesity, type 2 diabetes, and hypothalamic injury-associated obesity.[21, 22] The effects of beloranib on weight loss, appetite-related measures, lipids, and cardiometabolic biomarkers (hsCRP, leptin, and adiponectin) were similar across populations with some variation in the effects on lipids. Thus, the effects of beloranib on obesity do not depend on the presence of chromosome 15 abnormalities and reinforces the hypothesis that beloranib influences peripheral and extrahypothalamic mechanisms regulating energy balance.
After the first fatal pulmonary embolism, the sponsor stopped administration of study drug and screened all participants for venous thrombosis. Participants who were free of venous thrombosis were permitted to resume beloranib treatment in the open-label extension. At the time, it was unclear if the death should be attributed to beloranib treatment. After the second death, administration of study drug was again stopped and beloranib development has since ceased. However, overall, adverse events of venous thrombosis were more common with beloranib treatment in this trial and across the beloranib clinical program. Evaluation of clinical data, along with extensive preclinical studies conducted after the bestPWS clinical trial was halted, is currently underway to elucidate the mechanism(s) that contributes to these events. Preliminary results lead us to postulate that beloranib slows endothelial cell proliferation, which influences pro- and anti-coagulant factors on the cell surface.[41] This effect is expected to be exacerbated by the extended exposure produced by the suspension formulation, which results in drug exposure lasting at least 12 hours after each dose, and is hypothesized to contribute to the imbalance of thrombotic events in clinical studies of beloranib.
Compared to placebo, beloranib was associated with a greater number of adverse events of anxiety and irritability. These types of psychiatric disorders are common in PWS[15] and most of these events occurred in individuals with a relevant medical history. The mechanisms through which beloranib might increase the prevalence of such events is unclear.
The major study limitation was the early halting of the trial. Not all participants received 26 weeks of treatment, though the majority completed the trial and those that did not complete the trial completed at least 75% of the treatment period. Nevertheless, the effect size was large enough that robust statistical significance could be ascertained even with early halting of the study. Additionally, the low number of participants with PWS and diabetes did not allow for a separate assessment of an effect of beloranib on glycemic control in these participants.
The bestPWS trial is the first study to demonstrate that treatment with a pharmacological agent can reduce the pathological hunger experienced by participants with PWS and produce clinically meaningful weight loss. The mechanism for weight loss is currently under investigation but appears to be due to reduced energy intake (due to reduced hyperphagia) as well as reduced adipocyte mass and improved adipocyte function. Beloranib is no longer being investigated as a potential treatment for hyperphagia and obesity in PWS due to the occurrence of serious venous thrombotic events. However, alternative MetAP2 inhibitors without an effect on thrombotic parameters, as well as the proteins whose actions are altered by MetAP2 inhibition offer promising targets for treating the hyperphagia and obesity in PWS.
Supplementary Material
Acknowledgments
We thank study volunteers for their participation and supporting investigators and key trial staff, including David Arendt, Carrie Bailey, Christine Benoit, Sheila M. Brady, Ananth Chandrasekaran, Ovidiu A. Galescu, Andrea Hale, Hailee Hunt-Hawkins, Susan Kearns, Lalani Khetani, Audrey Lynn, Brittany Machus, Humaira Masoud, M. Richardson, Ira L. Tigner Jr., M. Tran, Kimberly Wallis, and R. Winograd. We also thank Alice Chen and Joyce M. Simpauco for assistance with clinical operations (funded by Zafgen, Inc.) and Sonja K. Billes for writing assistance (funded by Zafgen, Inc.). JAY is a Commissioned Officer in the United States Public Health Service and is supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the Public Health Service or the U.S. Department of Health and Human Services.
Disclosure
MJA, MAA, SEB, MGB, CLC, EMD, CF, JMiller, SEMyers, ER, PS, AS, DStafford, DStyne, DV, BYW, and JAY have received institutional grant support from Zafgen. LMB has received personal fees, non-financial report, and institutional support from Zafgen. SEMcCandless has received institutional grant support and personal consulting fees from Zafgen. JMalloy, DZ, KT, and DDK are employees of and hold stock in Zafgen. TEH is an employee of and holds stock in Zafgen and has patents issued to Zafgen.
Footnotes
Data from this study were presented at ENDO 2016, the annual meeting of the Endocrine Society, in Boston, MA, ICO 2016, the XIII International Congress on Obesity, in Vancouver, Canada, and IPWSO, the 9th Congress of the International Prader-Willi syndrome Organization in Toronto, Canada.
Contributors
EMD, ER, JAY, JMiller, KT, KT, TEH, and DDK designed the study. MJA, MAA, ER, DStafford, BYW, JMalloy, and KT coordinated the study. MJA, MAA, SEB, LMB, MGB, CLC, SEMcCandless, JMiller, SEMyers, ER, PS, AS, DStafford, DStyne, DV, BYW, JAY, and KT were responsible for screening and enrollment of participants and arranged informed consent. MJA, MAA, SEB, LMB, MGB, CLC, CF, SEMcCandless, JMiller, SEMyers, ER, PS, AS, DStafford, DStyne, DV, BYW, and JAY provided participant care and/or took samples. EMD and ER contributed to HQ-CT validation and interpretation. DZ had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. DZ and JMalloy contributed to the statistical analysis. MJA, LMB, MGB, EMD, TEH, ER, JAY, DZ, JMalloy, KT, and DDK participated in data review and/or interpretation. MGB, SEMcCandless, JAY, JMalloy, DZ, and KT contributed to the writing of the report. All authors critically reviewed the report and approved the final version.
References
- 1.Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. 2015;38:1249–1263. doi: 10.1007/s40618-015-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012;14:10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 3.Butler MG. Single Gene and Syndromic Causes of Obesity: Illustrative Examples. In: Tao YX, editor. Genetics of Monogenetic and Syndromic Obesity. London: Academic Press; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, Cassidy SB, Schrander JJ, Fryns JP. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A. 2004;124A:333–338. doi: 10.1002/ajmg.a.20371. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson DA, Heinemann J, Angulo M, et al. Gastric rupture and necrosis in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2007;45:272–274. doi: 10.1097/MPG.0b013e31805b82b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevenson DA, Heinemann J, Angulo M, et al. Deaths due to choking in Prader-Willi syndrome. Am J Med Genet A. 2007;143A:484–487. doi: 10.1002/ajmg.a.31502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurance BM, Brito A, Wilkinson J. Prader-Willi Syndrome after age 15 years. Arch Dis Child. 1981;56:181–186. doi: 10.1136/adc.56.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med. 2016 doi: 10.1038/gim.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCandless SE, Committee on G Clinical report-health supervision for children with Prader-Willi syndrome. Pediatrics. 2011;127:195–204. doi: 10.1542/peds.2010-2820. [DOI] [PubMed] [Google Scholar]
- 10.Mazaheri MM, Rae-Seebach RD, Preston HE, et al. The impact of Prader-Willi syndrome on the family's quality of life and caregiving, and the unaffected siblings' psychosocial adjustment. J Intellect Disabil Res. 2013;57:861–873. doi: 10.1111/j.1365-2788.2012.01634.x. [DOI] [PubMed] [Google Scholar]
- 11.Scheimann AO, Butler MG, Gourash L, Cuffari C, Klish W. Critical analysis of bariatric procedures in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2008;46:80–83. doi: 10.1097/01.mpg.0000304458.30294.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alqahtani AR, Elahmedi MO, Al Qahtani AR, Lee J, Butler MG. Laparoscopic sleeve gastrectomy in children and adolescents with Prader-Willi syndrome: a matched-control study. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2016;12:100–110. doi: 10.1016/j.soard.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheimann AO, Miller J, Glaze DG. Laparoscopic sleeve gastrectomy in children and adolescents with Prader-Willi syndrome: a matched control study. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2016 doi: 10.1016/j.soard.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M, speakers contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93:4183–4197. doi: 10.1210/jc.2008-0649. [DOI] [PubMed] [Google Scholar]
- 15.Dykens E, Shah B. Psychiatric disorders in Prader-Willi syndrome: epidemiology and management. CNS Drugs. 2003;17:167–178. doi: 10.2165/00023210-200317030-00003. [DOI] [PubMed] [Google Scholar]
- 16.Kim YM, An JJ, Jin YJ, et al. Assessment of the anti-obesity effects of the TNP-470 analog, CKD-732. J Mol Endocrinol. 2007;38:455–465. doi: 10.1677/jme.1.02165. [DOI] [PubMed] [Google Scholar]
- 17.Rupnick MA, Panigrahy D, Zhang CY, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lijnen HR, Frederix L, Van Hoef B. Fumagillin reduces adipose tissue formation in murine models of nutritionally induced obesity. Obesity (Silver Spring) 2010;18:2241–2246. doi: 10.1038/oby.2009.503. [DOI] [PubMed] [Google Scholar]
- 19.White HM, Acton AJ, Considine RV. The angiogenic inhibitor TNP-470 decreases caloric intake and weight gain in high-fat fed mice. Obesity (Silver Spring) 2012;20:2003–2009. doi: 10.1038/oby.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brakenhielm E, Cao R, Gao B, et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 21.Kim DD, Krishnarajah J, Lillioja S, et al. Efficacy and safety of beloranib for weight loss in obese adults: a randomized controlled trial. Diabetes Obes Metab. 2015;17:566–572. doi: 10.1111/dom.12457. [DOI] [PubMed] [Google Scholar]
- 22.Hughes TE, Kim DD, Marjason J, Proietto J, Whitehead JP, Vath JE. Ascending dose-controlled trial of beloranib, a novel obesity treatment for safety, tolerability, and weight loss in obese women. Obesity (Silver Spring) 2013;21:1782–1788. doi: 10.1002/oby.20356. [DOI] [PubMed] [Google Scholar]
- 23.Salehi P, Hsu I, Azen CG, Mittelman SD, Geffner ME, Jeandron D. Effects of exenatide on weight and appetite in overweight adolescents and young adults with Prader-Willi syndrome. Pediatr Obes. 2016 doi: 10.1111/ijpo.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motaghedi R, Lipman EG, Hogg JE, Christos PJ, Vogiatzi MG, Angulo MA. Psychiatric adverse effects of rimonobant in adults with Prader Willi syndrome. Eur J Med Genet. 2011;54:14–18. doi: 10.1016/j.ejmg.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deal CL, Tony M, Hoybye C, et al. GrowthHormone Research Society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013;98:E1072–1087. doi: 10.1210/jc.2012-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy L, Bittel DC, Kibiryeva N, Kalra SP, Torto R, Butler MG. Circulating adiponectin levels, body composition and obesity-related variables in Prader-Willi syndrome: comparison with obese subjects. Int J Obes (Lond) 2006;30:382–387. doi: 10.1038/sj.ijo.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S, Harmer JA, Loughnan G, Skilton MR, Steinbeck K, Celermajer DS. Characteristics of cardiac and vascular structure and function in Prader-Willi syndrome. Clin Endocrinol (Oxf) 2007;66:771–777. doi: 10.1111/j.1365-2265.2007.02808.x. [DOI] [PubMed] [Google Scholar]
- 29.Butler MG, Bittel DC, Kibiryeva N, Garg U. C-reactive protein levels in subjects with Prader-Willi syndrome and obesity. Genet Med. 2006;8:243–248. doi: 10.1097/01.gim.0000204469.30913.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoybye C, Bruun JM, Richelsen B, Flyvbjerg A, Frystyk J. Serum adiponectin levels in adults with Prader-Willi syndrome are independent of anthropometrical parameters and do not change with GH treatment. Eur J Endocrinol. 2004;151:457–461. doi: 10.1530/eje.0.1510457. [DOI] [PubMed] [Google Scholar]
- 31.Haqq AM, Muehlbauer MJ, Newgard CB, Grambow S, Freemark M. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J Clin Endocrinol Metab. 2011;96:E225–232. doi: 10.1210/jc.2010-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weigle DS, Ganter SL, Kuijper JL, Leonetti DL, Boyko EJ, Fujimoto WY. Effect of regional fat distribution and Prader-Willi syndrome on plasma leptin levels. J Clin Endocrinol Metab. 1997;82:566–570. doi: 10.1210/jcem.82.2.3761. [DOI] [PubMed] [Google Scholar]
- 33.Butler MG, Moore J, Morawiecki A, Nicolson M. Comparison of leptin protein levels in Prader-Willi syndrome and control individuals. American journal of medical genetics. 1998;75:7–12. [PMC free article] [PubMed] [Google Scholar]
- 34.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 35.Datta B, Majumdar A, Datta R, Balusu R. Treatment of cells with the angiogenic inhibitor fumagillin results in increased stability of eukaryotic initiation factor 2-associated glycoprotein, p67, and reduced phosphorylation of extracellular signal-regulated kinases. Biochemistry. 2004;43:14821–14831. doi: 10.1021/bi049172p. [DOI] [PubMed] [Google Scholar]
- 36.Kotzka J, Knebel B, Avci H, et al. Phosphorylation of sterol regulatory element-binding protein (SREBP)-1a links growth hormone action to lipid metabolism in hepatocytes. Atherosclerosis. 2010;213:156–165. doi: 10.1016/j.atherosclerosis.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 37.Raghow R, Yellaturu C, Deng X, Park EA, Elam MB. SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol Metab. 2008;19:65–73. doi: 10.1016/j.tem.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Jetten AM, Kang HS, Takeda Y. Retinoic acid-related orphan receptors alpha and gamma: key regulators of lipid/glucose metabolism, inflammation, and insulin sensitivity. Frontiers in endocrinology. 2013;4:1. doi: 10.3389/fendo.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 40.Holsen LM, Zarcone JR, Brooks WM, et al. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring) 2006;14:1028–1037. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.