Abstract
Females have a higher prevalence of most autoimmune diseases; however, the mechanism is unknown. In this study, we examined the expression of tight junction protein zonula occludens 1 (ZO-1) and estrogen receptor (ER)-α/β in human primary gut tissues by immunohistochemistry, immunofluorescence and qPCR. The expression of ZO-1 and ER-β but not ER-α was present in both male and female gut tissues. There was no sex difference in ER-β expression, but ZO-1 expression was decreased in females compared to males. In vitro, estrogen treatment decreased ZO-1 mRNA and protein expression, ZO-1 promoter activity, IL-6 production, and NF-κB activation in human primary gut tissues or the Caco-2 cells, but increased the ER-β expression in Caco-2 cells. Consistently, plasma IL-6 levels in females were reduced relative to males in vivo. Our finding indicates that estrogen may play a role in gut tight junction expression and permeability.
Keywords: Sex differences, estrogen, ZO-1, gut tissues
Introduction
There is accumulating evidence that sex has profound effects on the immune system [1–4]. The fundamental differences between males and females, including the number of specific immune cell types and their activation and function in response to immunological challenge following vaccination or exposure to a pathogen, are likely to result in the different susceptibility to a variety of pathogens and incidence of autoimmune diseases [5–7]. In general, males are more susceptible and females are more resistant to pathogenic infections, which are especially prominent following from puberty until menopause [8–10]. In contrast, females have a higher prevalence of most autoimmune diseases after adolescence [11, 12]. Several mechanisms may take part in these sex-biased immune responses, including sex hormones, genetic and environmental factors [13–15]. However, the exact mechanisms underlying this phenomenon are largely unknown.
The prevalence of various autoimmune diseases in females after puberty suggests that female sex hormones, like estrogen, may play a major role in autoimmune responses [14, 16]. 17β-estradiol may protect females from viral-mediated pathogenesis by suppressing inflammatory responses [17]. Estrogen has also been reported to mediate immune activation and trigger different immune responses through estrogen receptor (ER) dependent or independent pathways [1, 18]. There are two functional ER subtypes ER-α and ER-β in the ER dependent pathway. However, ER-α and ER-β exhibit differential expression among immune cell subsets; ER-α was expressed at higher levels in CD4+ T cells rather than B cells. Instead, B cells expressed high levels of ER-β [19, 20]. Furthermore, IL-6 expression is downregulated by estrogen receptor through a NF-κB dependent pathway [21]. The ER independent pathway also is implicated in immune cell responses, enabling protein-protein interactions between ERs and ER independent transcription factors, including NF-κB, specific protein 1 (SP1) and activator protein 1 (AP-1) [22].
In addition to sex-biased immune responses, the loss of the protective function of mucosal barriers that interact with the environment (mainly the gastrointestinal mucosa) is necessary for development of autoimmunity [23]. As we know, tight junctions are composed of occludin, claudins, and zonula occludens (ZO)-1, -2, -3, which govern the paracellular permeability of endothelial and epithelial cells, and provide a barrier function such as inhibit bacterial invasion [24]. Several autoimmune diseases, including celiac disease, type 1 diabetes, multiple sclerosis, and rheumatoid arthritis, are characterized by increased intestinal permeability that allow the translocation of antigens (e.g., microbial products) from the intestinal flora, challenging the immune system to produce an aberrant immune responses and inflammation [25–28]. For example, both ZO-1 protein levels and mRNA were clearly reduced in patients with active celiac disease, which may be responsible for the increased paracellular permeability [29, 30]. A study has showed that altered expression of claudin lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease [31]. Specifically, in some female-preponderant autoimmune diseases such as Sjögren’s syndrome and autoimmune thyroid diseases, disrupted tight junction integrity is an important contributor to the dysfunction of immune system [32, 33].
Although the importance of tight junction that influences the autoimmune systems of female and male differently, it is presently unclear about the role of sex hormones (e.g., estrogen) on tight junction protein expression in human primary gut tissues. Therefore, this study sought to determine the expression of ER-α/β and tight junction protein ZO-1 in primary gut tissues of both male and female. Moreover, we further investigated the inhibitory effect of estrogen on ZO-1 expression, IL-6 production and NF-κB activation in vitro.
Materials and Methods
Study subjects
This study was approved by IRB from the Medical University of South Carolina (MUSC, Pro00037761), and all participants provided written informed consents. Human fresh colon biopsies of non-cancer tissues from males and postmenopausal females with an annual intestinal microscopy test were obtained at Hollings Cancer Center, biorepository at MUSC.
Immunohistochemical analysis of ZO-1, ER-α and ER-β under normal physiological conditions
Immunohistochemical assay was performed according to the previous description with little modification [34]. Gut samples from 8 males and 7 females (table 1) were fixed in 10% formalin at room temperature for 24 h, faded in 70% ethanol 2 h for four times, and dehydrated in 80%, 95% and 100% ethanol sequentially. Then the samples were embedded in paraffin wax, sectioned (5 µm) and mounted on slides. The slides were deparaffinated in xylene, rehydrated in diluted ethanol series from 95% up to distilled water. After antigens were refolded in sodium citrate-hydrochloric acid buffer, the slides were blocked with 3% BSA in PBST at 37°C for 30 min. To examine whether ER-α and ER-β were expressed in human primary gut tissues, mouse anti-human ER-α antibody (diluted 1:500 in 3% BSA, Dako, Glostrup, Denmark) and mouse anti-human ER-β antibody (diluted 1:500 in 3% BSA, Invitrogen, Carlsbad, CA, USA) were added to the slide. The slide was incubated at 37°C for 1 h. After washing with PBST, the sheep anti-mouse second antibody conjugated with horseradish peroxidase (1/1000 dilution, Jackson ImmunoResearch, West Grove, PA, USA) was incubated with the sections at 37°C for 1 h. Proteins were visualized using DAB staining with a fluorescence microscopy (Zeiss Axio Vet. A1, Germany). To examine ZO-1 expression in human primary gut tissues, rabbit anti-human ZO-1 antibody (diluted 1:500 in 3% BSA, Invitrogen), rabbit anti-human cytokeratin antibody (diluted 1:500 in 3% BSA, Invitrogen) or mouse anti-human ER-β antibody (diluted 1:500 in 3% BSA, Invitrogen) was added to the slide. The slide was incubated at 37°C for 1 h and washed in PBST. DyLight 594 labeled goat anti-rabbit second antibody (KPL, Gaithersburg, MD, USA) and Alexa Flour 488 labeled sheep anti-mouse second antibody (1/1000 dilution, Jackson ImmunoResearch) were added to the slide. The slide was incubated at 37°C. After 1 h, the slide was washed with PBST three times and observed under a fluorescence microscope (Zeiss Axio Vet. A1).
Table 1.
Clinical characteristics
| Male | Female | P value a | |
|---|---|---|---|
| Immunohistochemistry assay | |||
| Numbers of subjects | 8 | 7 | |
| Age (years) median b | 67.5 (45–71) | 61 (57–74) | 0.71 |
| Ethnicity (No.) b | > 0.99 | ||
| White | 4 | 3 | |
| Africa American | 2 | 2 | |
| East Asian | 2 | 2 | |
| Tissue | Normal colon | Normal colon | |
| Phase of menstrual cycle | Post-menopausal | ||
| ELISA for Plasma IL-6 levels test | |||
| Numbers of subjects | 10 | 10 | |
| Age (years) median b | 47 (43–59) | 49 (45–58) | 0.75 |
| Ethnicity (No.) b | > 0.99 | ||
| White | 5 | 5 | |
| Africa American | 5 | 5 | |
| Phase of menstrual cycle | Post-menopausal | ||
| Hormone replacement therapies | No | No | |
| Systemic healthy and no smokers | Yes | Yes | |
| Systemic antibiotic treatment (past 6 months) | No | No |
P values compared between the two study groups were analyzed by Mann Whitney U test (non-paired).
Data are median (interquartile range) values.
qPCR analysis of ZO-1 mRNA expression under estrogen treatment
Gelfoam (Gelfoam absorbable gelatin sponge, Pfizer Inc., NY, USA) were distributed into 12-well tissue culture plates. Gut samples from postmenopausal females including 3 whites and 3 Africa Americans (3 mm × 3 mm) were placed on the Gelfoam and then cultured at 37°C with RPMI-1640 medium (Gibco) containing penicillin (100 unit/ml), streptomycin (100 µg/ml) and 10% charcoal stripped FBS (Gibco). The tissues were treated with different concentrations (0, 0.2, 2 and 20 ng/ml) of 17β-estradiol (Alfa Aesar, Tewksbury, USA) for 24 h. Samples were extracted total RNA by EZNA Total RNA Kit (Omega Bio-tek, Doraville, GA, USA). cDNA was synthesized from 5 µg of each RNA sample using the SuperScript III First-Strand Synthesis System (Invitrogen) followed by PCR. Primer sequences, reaction conditions, and optimal cycle numbers of PCR were described previously [35]. The expression level of ZO-1 was analyzed using comparative threshold cycle method (2−ΔΔCT) with GAPDH as an internal reference [36]. The expression level of medium without estrogen treatment was set as 1. The experiment was performed three times.
Cell culture
Caco-2 cell line, a human epithelial colorectal cancer cell line, was purchased from ATCC (Manassas, VA, USA). Caco-2 cells were maintained in Dulbecco’s Modified Eagle’s medium (DMEM, phenol red-free, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 20 % charcoal stripped fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA), 2 mM L-glutamine, 10mM HEPES, 100 unit/ml penicillin and 100 µg/ml streptomycin (Gibco) in a humidified 37°C, 5% CO2 incubator.
Effect of estrogen on ZO-1 expression determined by Western blotting
Caco-2 cells were cultured as above and then incubated with different concentrations (0, 0.2, 2, 20 and 200 ng/ml) of 17β-estradiol for 24 h. The cells were harvested for preparation of whole-cell extracts. The whole-cell extracts were loaded in each lane for 10% SDS-PAGE and then transferred onto a nitrocellulose membrane. The membrane was blocked with (10 mM Tris-HCl, 100 mM NaCl, 0.1% (w/v) Tween 20, pH 8.0) containing 5% skim milk for 2 h, and incubated with rabbit anti-human ZO-1 antibody (1:1000, v/v, Invitrogen) at room temperature for 1 h. The rabbit anti-β-actin antibody (1:1000, v/v, Santa Cruz, CA, USA) was employed as a negative control. After washing with TBST for three times, the membranes were incubated with 1: 2000 (v/v) horseradish peroxidase-conjugated goat anti-rabbit IgG (Invitrogen) for 1 h, followed by thoroughly washing. The target proteins were developed with TMB 1-component membrane solution (KPL). The experiment was performed three times.
Immunofluorescence analysis of ZO-1 and ER-β expression under estrogen treatment
Caco-2 cells were cultured as above and treated with or without 20 ng/ml of 17β-estradiol for 24 h. The cells were washed with PBST and incubated with 4 % paraformaldehyde for 30 min. After incubation, rabbit anti-human ZO-1 antibody (1/1000, v/v, as described above) was added and incubated at 37°C for 2 h, and washed 3 × with PBST. FITC labeled goat anti-rabbit antibody (1/1000, v/v, KPL) was added to the cells and incubated at 37°C for 1 h. To examine ER-β expression, Caco-2 cells were cultured as above and treated with different concentrations (0, 2 and 20 ng/ml) of 17β-estradiol for 24 h. After incubation, mouse anti-human ER-β antibody and Alexa Flour 488 labeled sheep anti-mouse second antibody were added to the slide as described above. The cells were washed twice with PBST and stained for 5 min at room temperature in DAPI (Invitrogen). After washing, the cells were observed with fluorescent microscope (Zeiss Axio Vet. A1). The experiment was performed three times.
Fluorescence intensity analysis
Images were taken as above with the ×20 objective using a red fluorescence filter for each staining, whereas their corresponding ER-β images were taken using a green fluorescence filter. All images were taken using identical camera, microscope lens, and light settings. Fluorescence intensity analysis was performed using ImageJ software (ImageJ, Bethesda, USA). The mean intensity values were corrected for background differences by dividing the measured intensities with the average intensity of a cell-free region in each section. Briefly, images were inverted to gray-scales and were drawn a region of interest at first. Next, images were calibrated the optical density and then measured the areas and integrated densities. The same method was used for analyzing the intensities of background regions. The percentages of changes in immunofluorescent intensity were shown after subtracting values in background (%fluorescence intensity = integrated densities/areas).
ZO-1 reporter activity in response to estrogen treatment
For ZO-1 promoter assay, Caco-2 cells were cultured in 6-wells plate (5 × 105 cells/well) with Eagle’s minimal essential medium (MEM) and transfected with TJP1 reporter plasmid (s709631, Switchgear Genomics, CA, USA) or control reporter plasmid (s790001, Switchgear Genomics) using Lipofectamine™ 2000 (Invitrogen) according to the instructions of the manufacturer. After transfection, the cells were divided into 96-wells plate and then treated with or without 20 ng/ml 17β-estradiol for 24 h. The chemiluminescence assay was performed using LightSwitch Assay Reagent (Switchgear Genomics). The experiment was performed three times.
ELISA analysis of IL-6 expression in Caco-2 cell culture supernatants and human plasma
Caco-2 cells were cultured as above and incubated with 17β-estradiol for 24 h. IL-6 protein was measured in supernatants by ELISA according to the manufacturers’ instruction (eBioscience, San Diego, CA, USA). To measure plasma IL-6 level in healthy people, plasmas from 10 healthy males and 10 healthy females (table 1) were isolated and stored in a 1.5-ml microtube at 80°C until thawing once for study. IL-6 protein was measured by ELISA.
NF-κB reporter activity in response to estrogen treatment
Caco-2 cells were resuspended in MEM medium and distributed into 96-well culture plates (1 × 104 cells/well). Cells were transfected with 0.5 µg pNFκB-SEAP-1 (Clontech, Mountain View, CA, USA) and 10 ng pMet-Luc plasmid (an internal control, Clontech). After transfection, the cells were treated with or without 20 ng/ml 17β-estradiol for 24 h. The activities of SEAP and Met-Luc in the supernatant were analyzed by chemiluminescence as previously described [37]. The experiment was performed three times.
Statistical analysis
Data from repeated experiments were averaged and expressed as means ± standard deviations. Statistical analysis was performed by GraphPad Prism 6.0 (GraphPad, San Diego, USA) using the Mann Whitney U test (non-paired). P values of < 0.05 were considered statistically significant.
Results
Immunohistochemical analysis of ZO-1, cytokeratin, ER-α and ER-β in human gut tissues
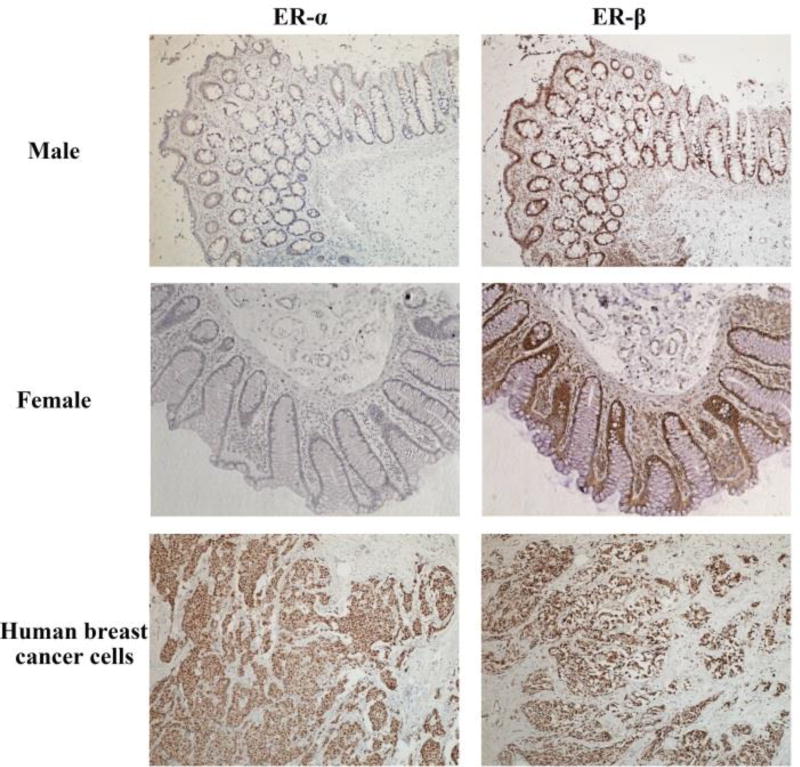
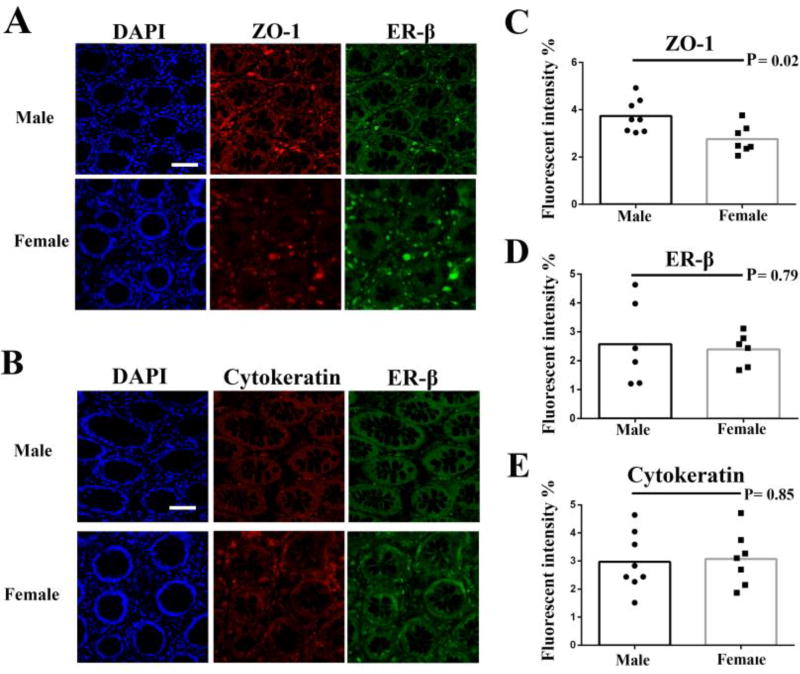
The human intestinal epithelium is formed by epithelial cells and connected by tight junctions, which play an important role in the integrity of epithelium formed mucosal barrier. The ER-α expression was essentially negative while ER-β is the predominant ER subtype in the human colon by immunohistochemistry assays (Fig. 1), which is in agreement with others [38, 39]. Thus, to explore sex difference in the expression of tight junction related protein (ZO-1), we examined the expression of ZO-1 as well as ER-β in male and female primary gut tissues by immunofluorescence assays. The cytoskeleton protein cytokeratin was used as an epithelial cell marker. The results showed that ZO-1, ER-β and cytokeratin were all detected in male and female primary gut tissues (Fig. 1 and Fig. 2).
Figure 1.
Estrogen receptor (ER) α and β expression in human primary gut tissues. ER-α and ER-β in male and female primary gut tissues were detected by immunohistochemistry (100 ×). ER-α and ER-β staining in primary human breast cancer cells as positive controls were detected by immunohistochemistry (100 ×).
Figure 2.
Immunofluorescence analysis of the distribution of ZO-1, cytokeratin and ER-β in male and female gut tissues. The nuclei of cells were stained blue by DAPI in single section. ZO-1 and cytokeratin were detected by DyLight 594-labeled antibody. ER-β was detected by Alexa Flour 488-labeled antibodies (A and B). Bar: 100 µm. Images were then taken with a fluorescence microscopy; Fluorescence intensity of ZO-1 (C), cytokeratin (D) and ER-β (E) was analyzed using ImageJ software. P values were analyzed by Mann Whitney test (non-paired).
Fluorescence intensity analysis of ZO-1, cytokeratin and ER-β
Next, we analyzed the expression levels by fluorescence intensities. Of interest, the fluorescence intensity of ZO-1 in male gut tissues was significantly higher than those in female gut tissues (P = 0.02, Fig. 2C). No sex differences in the fluorescence intensities of ER-β (P = 0.79, Fig. 2D) and cytokeratin (P = 0.85, Fig. 2E) were found.
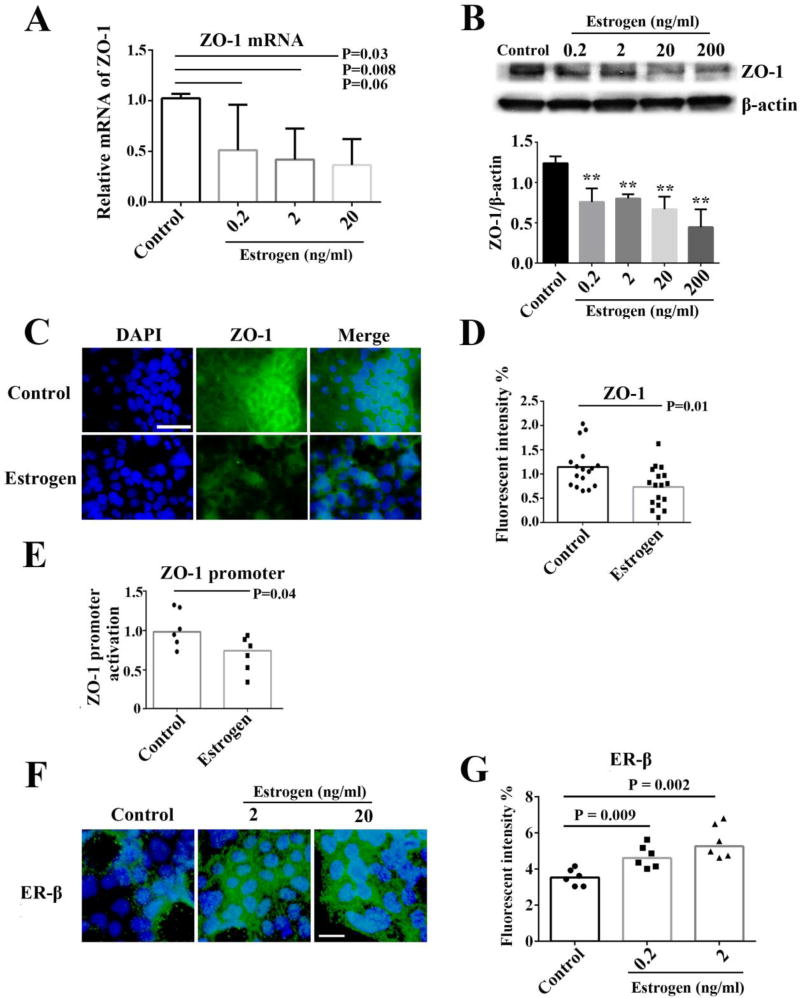
Effect of estrogen on ZO-1 expression
To determine the effect of estrogen on ZO-1 expression, primary female gut tissues were incubated with different concentrations of 17β-estradiol, and the ZO-1 mRNA expression relative to GAPDH was subsequently determined by qPCR. Dose dependent decreases of ZO-1 expression were observed in gut tissues by 17β-estradiol treatment in vitro (Fig. 3A). To further verify the effect of estrogen on ZO-1 expression, Caco-2 cells were treated with 17β-estradiol and examined for ZO-1 expression by western blotting. Consistently, dose dependent decreases of ZO-1 expression were observed in Caco-2 cells by 17β-estradiol in vitro (Fig. 3B). Moreover, ZO-1 protein expression was decreased by 17β-estradiol treatment in Caco-2 cells by fluorescence microscopy in vitro (Fig. 3C and 3D). ZO-1 promoter activity was significantly decreased by 17β-estradiol in Caco-2 cells, confirming the inhibitory effect of 17β-estradiol on the transcription of ZO-1 expression (Fig. 3E). Interestingly, we also found that ER-β expression was increased after estrogen treatment in Caco-2 cells (Fig. 3F and 3G).
Figure 3.
Effect of estrogen on ZO-1 expression. Female primary gut tissues or Caco-2 cells were treated with medium alone (control) or different concentrations of estrogen and examined for ZO-1 expression. ZO-1 mRNA expression was analyzed by qPCR in human postmenopausal female gut tissues (n = 6) (A). Western blot analysis and densitometry quantification of ZO-1 expression normalized to total β-actin in Caco-2 cells, repeated three times (B). ZO-1 expression and nuclei were stained by FITC-labeled antibody and DAPI respectively in Caco-2 cells (C). Bar: 50 µm. After immunofluorescence assay in Caco-2 cells, images were taken with a fluorescence microscopy, and fluorescence intensity of ZO-1 was then analyzed using ImageJ software (D). Caco-2 cells were transiently transfected with TJP1 reporter plasmid, and then treated with or without estrogen; ZO-1 promoter activation was examined by luminescent assay (E). ER-β expression and nuclei were stained by Alexa Flour 488-labeled antibody and DAPI respectively in Caco-2 cells (F). Bar: 25 µm. The fluorescence intensity of ER-β was then analyzed using ImageJ software as above (G). P values were analyzed by Mann Whitney test (non-paired).
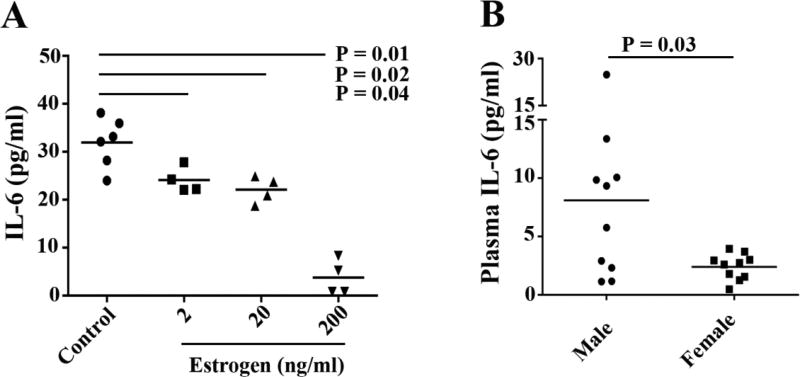
Effect of estrogen on IL-6 expression
IL-6 promoter has been reported to be inhibited by estrogen through NF-κB [40]. To determine the effect of estrogen on IL-6 production, Caco-2 cells were treated with estrogen in vitro. We found that IL-6 production in Caco-2 cell culture supernatants was reduced by 17β-estradiol compared to medium alone (Fig. 4A). To verify our finding in vivo, we measured plasma IL-6 levels in healthy males and females. Consistent with previous studies [41], plasma levels of IL-6 were decreased in females compared to males (P = 0.03, Fig. 4B).
Figure 4.
Measurement of IL-6 levels. Caco-2 cells were treated with or without different concentrations of estrogen and then examined for IL-6 in cell culture supernatants by ELISA (A). Plasma IL-6 levels in healthy males and females were examined by ELISA (B). P values were analyzed by Mann Whitney test (non-paired).
Effect of estrogen on NF-κB activation
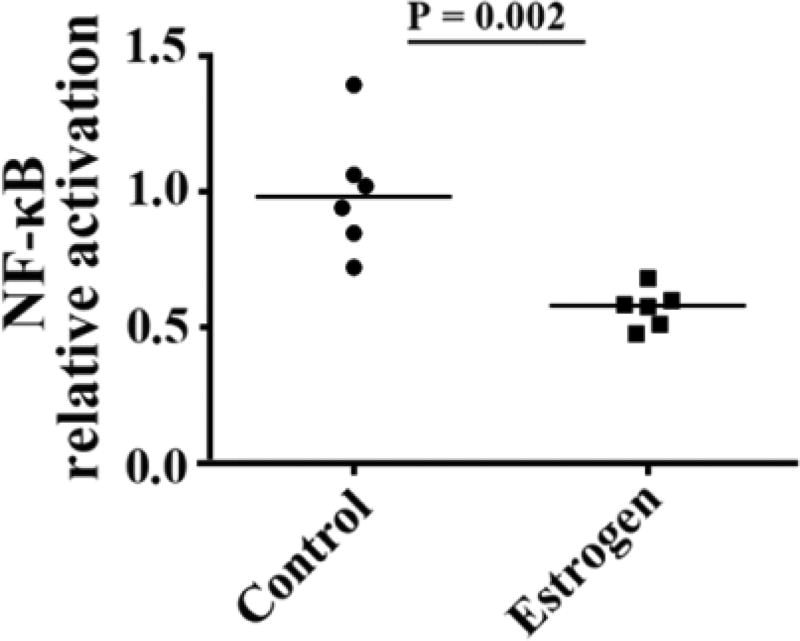
Since NF-κB activation is essential for the regulation of IL-6 production by estrogen, a NF-κB reporter was employed to assess the impact of estrogen on NF-κB activation. Luciferase activity was analyzed in Caco-2 cells after the NF-κB reporter pNFκB-SEAP-1 transfection. We found significantly decreased luciferase activity in Caco-2 cells incubated with 17β-estradiol compared to medium alone (P = 0.002, Fig. 5). These results suggest that estrogen reduces NF-κB activity.
Figure 5.
Effect of estrogen on NF-κB activation. Caco-2 cells were transiently transfected with pNFκB-SEAP-1 and then treated with or without estrogen. NF-κB activation in the estrogen-treated group relative to the control group was examined by luminescent assay. P values were analyzed by Mann Whitney test (non-paired).
Discussion
Tight junctions are essential for establishing a mucosal barrier, and they act as a selective gate to control paracellular diffusion of ions and solutes [24]. Mutations in genes encoding tight junction proteins have been linked to a range of human diseases [23, 31–33, 42]. In addition, studies examined the impact of estrogen on vascular permeability in mammals suggest that 17β-estradiol has an effect on vascular endothelial growth factor (VEGF) through ER-α and ER-β, resulting in the increased permeability of blood vessels and tumor metastasis [43–45]. To investigate the role of estrogen in human gut permeability, we analyzed the expression of ZO-1 in male and female primary gut tissues. We found that ZO-1 expression in females was significantly lower compared to males, indicating that sex hormones may be involved in the regulation of gut tight junction. Moreover, estrogen inhibited ZO-1 mRNA and protein expression and ZO-1 promoter activity in human primary gut tissues and the Caco-2 cell line in vitro. Although the effect of estrogen on tight junctions of epithelial cells has been studied previously [46–48], our study is the first one to show the inhibitory effect of estrogen on ZO-1 expression in primary human gut tissues.
Previous studies reported that estrogen reduces IL-6 production and therefore inhibits carcinogenesis and osteoporosis [41, 49–51]. More importantly, estrogen may have an anti-inflammatory effect due to inhibition of NF-κB activation [52]. These studies are consistent with our results that estrogen-mediated inhibition of IL-6 production and NF-κB activation in gut epithelial cells, which may contribute to anti-inflammation immune responses at local sites.
The fact that the expression of ZO-1 is inhibited by estrogen indicates estrogen may increase gut permeability and as a consequence of systemic microbial translocation and its associated inflammation. If so, multiple microbial products such as RNA, DNA, peptidoglycan and flagellin derived from bacteria, viruses, fungi and other gastrointestinal residents may translocate into systems and cause systemic immune activation [53–55]. Indeed, our recent work shows that monocytes were activated in vivo in women compared to men, and plasma level of soluble CD14, a marker of monocyte activation by LPS, was higher in women relative to men [56], suggesting a role of systemic bacterial products in immune activation and inflammation. In addition, our recent studies have revealed that the heightened plasma level of LPS play an important role on T cell and B cell activation in HIV pathogenesis [57, 58]. Therefore, women should have overall higher levels of certain inflammation (e.g., sCD14) and increased proportions of activated monocyte subset (CD14−CD16+) compared to men [56]. Paradoxically, in the current study, we found that the plasma IL-6 level in female was reduced compared to male in vivo. This result is in line with previous studies, which showed that decreased plasma IL-6 levels are due to the estrogen treatment [59, 60]. Given the results we observed that IL-6 production and NF-κB activation were inhibited by estrogen, we propose that plasma IL-6 may be impacted through a direct estrogen-mediated mechanism in vivo, which appears to be different from monocyte activation and its associated inflammation in women through an indirect mechanism of estrogen-mediated gut permeability and microbial translocation.
The main limitation of our study is that all female gut tissues were from postmenopausal women, given the difficulty to get the normal gut tissues from premenopausal women, we are unable at this time to identify the expression of ZO-1 and ER-β in primary gut tissues from premenopausal women. After menopause in females, follicles are depleted and estrogen concentration reduces to very low levels [1, 42, 61]. Therefore, the expression of ZO-1 in primary gut tissues from premenopausal women may be different from postmenopausal women.
In conclusion, the strengths of this study are the demonstration of ZO-1 expression on human primary gut tissues, and the inhibitory effect of estrogen on ZO-1 expression in gut tissues and human gut epithelial cell line. The in vitro inhibitory effect of estrogen on IL-6 and NF-κB activation supports a role for estrogen in the regulation of host immune response in the gut. These observations provide the first insights to the sex-based differences of gut ZO-1 expression and inflammation.
Acknowledgments
The funding support was from the National Institute of Allergy and Infectious Diseases grant AI091526 (Jiang) and AI128864 (Jiang), National Institute of Arthritis and Musculoskeletal and Skin Diseases grant P60 AR062755 (Gilkeson), UL1 RR029882, the Medical Research Service at the Ralph H. Johnson VA Medical Center Merit grant VA CSRD MERIT (CX001211, Gilkeson), BLRD MERIT (BX000470, Gilkeson), the Biorepository & Tissue Analysis Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313), and Beijing Natural Science Foundation (Grant No. 7152063) and Scientific Research Common Program of Beijing Municipal Commission of Education (KM2016100250017).
We thank that Dr. Alter Harvey kindly provide NF-κB reporter plasmid and reagents.
References
- 1.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Re. Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 2.Giron-Gonzalez JA, et al. Consistent production of a higher TH1: TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol. 2000;143(1):31–36. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- 3.Hewagama A, et al. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes. Immun. 2009;10(5):509–516. doi: 10.1038/gene.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdullah M, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell. Immunol. 2012;272(2):214–219. doi: 10.1016/j.cellimm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Furman D, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. 2014;111(2):869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aomatsu M, et al. Gender difference in tumor necrosis factor-α production in human neutrophils stimulated by lipopolysaccharide and interferon-γ. Biochem. Biophys. Res. Commun. 2013;441(1):220–225. doi: 10.1016/j.bbrc.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 7.Asai K, et al. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock. 2001;16(5):340–343. doi: 10.1097/00024382-200116050-00003. [DOI] [PubMed] [Google Scholar]
- 8.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 2001;167(4):2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 9.Lin AA, et al. Androgens suppress antigen-specific T cell responses and IFN-γ production during intracranial LCMV infection. J. Neuroimmunol. 2010;226(1):8–19. doi: 10.1016/j.jneuroim.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccinni MP, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J. Immunol. 1995;155(1):128–133. [PubMed] [Google Scholar]
- 11.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci. Biobehav. Rev. 2000;24(6):627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 12.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012;62(3):263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro I, et al. X-chromosome-located microRNAs in immunity: Might they explain male/female differences? Bioessays. 2011;33(11):791–802. doi: 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- 14.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J. Autoimmun. 2012;38(2):282–291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Griesbeck M, et al. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-α production in women. J. Immunol. 2015;195(11):5327–5336. doi: 10.4049/jimmunol.1501684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karpuzoglu E, Ahmed SA. Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide. 2006;15(3):177–186. doi: 10.1016/j.niox.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DP, et al. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7(7):e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deroo BJ, Korach KS. Estrogen receptors and human disease. J. Clin. Invest. 2006;116(3):561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phiel KL, et al. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 2005;97(1):107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin. Rev. Allergy. Immunol. 2011;40(1):66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 21.Galien R, Garcia T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-κB site. Nucleic Acids Res. 1997;(12):2424–2429. doi: 10.1093/nar/25.12.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015;294(2):63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fasano A. Leaky gut and autoimmune disease. Clinic. Rev. Allergy Immunol. 2012;42(1):71–78. doi: 10.1007/s12016-011-8291-x. [DOI] [PubMed] [Google Scholar]
- 24.Zihni C, et al. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell. Biol. 2016;17(9):564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 25.Abreu MT. Toll-like receptor signaling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010;10(2):131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 26.Fasano A. Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. Am. J. Pathol. 2008;173(5):1243–1252. doi: 10.2353/ajpath.2008.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009;124(1):3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011;91(1):151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 29.Montalto M, et al. Immunohistochemical analysis of ZO-1 in the duodenal mucosa of patients with untreated and treated celiac disease. Digestion. 2002;65(4):227–233. doi: 10.1159/000063817. [DOI] [PubMed] [Google Scholar]
- 30.Pizzuti D, et al. Transcriptional downregulation of tight junction protein ZO-1 in active coeliac disease is reversed after a gluten-free diet. Dig. Liver Dis. 2004;36(5):337–341. doi: 10.1016/j.dld.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Zeissig S, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56(1):61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker OJ, et al. Proinflammatory cytokines tumor necrosis factor-α and interferon-γ alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am. J. Physiol. Cell Physiol. 2008;295(5):1191–1201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rebuffat SA, et al. IL-1β and TSH disturb thyroid epithelium integrity in autoimmune thyroid diseases. Immunobiology. 2013;218(3):285–291. doi: 10.1016/j.imbio.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, et al. A cytokine-like factor astakine accelerates the hemocyte production in Pacific oyster Crassostrea gigas. Dev. Comp. Immunol. 2016;55(2016):179–187. doi: 10.1016/j.dci.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Babkair H, et al. Aberrant expression of the tight junction molecules claudin-1 and zonula occludens-1 mediates cell growth and invasion in oral squamous cell carcinoma. Hum. Pathol. 2016;57:51–60. doi: 10.1016/j.humpath.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Miao YS, et al. MiR-18a increased the permeability of BTB via RUNX1 mediated down-regulation of ZO-1, occludin and claudin-5. Cell. Signal. 2015;27(1):156–167. doi: 10.1016/j.cellsig.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, et al. The modulation of hepatitis C virus 1a replication by PKR is dependent on NF-kB mediated interferon beta response in Huh7.5.1 cells. Virology. 2013;438(1):28–36. doi: 10.1016/j.virol.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiorelli G, et al. Functional estrogen receptor β in colon cancer cells. Biochem. Biophys. Res. Commun. 1999;261(2):521–527. doi: 10.1006/bbrc.1999.1062. [DOI] [PubMed] [Google Scholar]
- 39.Looijer-van Langen M, et al. Estrogen receptor-β signaling modulates epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300(4):621–626. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 40.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol. Cell. Biol. 1995;15(9):4971–4799. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 42.Sawada N. Tight junction - related human diseases. Pathol. Int. 2013;63(1):1–12. doi: 10.1111/pin.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niklaus AL, et al. Effect of estrogen on vascular endothelial growth/permeability factor expression by glandular epithelial and stromal cells in the baboon endometrium. Biol. Reprod. 2003;68(6):1997–2004. doi: 10.1095/biolreprod.102.011288. [DOI] [PubMed] [Google Scholar]
- 44.Cullinan-Bove K, Koos RD. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology. 1993;133(2):829–837. doi: 10.1210/endo.133.2.8344219. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura J, et al. Estrogen regulates vascular endothelial growth/permeability factor expression in 7, 12-dimethylbenz (a) anthracene-induced rat mammary tumors. Endocrinology. 1996;137(12):5589–5596. doi: 10.1210/endo.137.12.8940388. [DOI] [PubMed] [Google Scholar]
- 46.Gorodeski GI. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodeling of occludin. Endocrinology. 2007;148(1):218–231. doi: 10.1210/en.2006-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Na W, et al. 17β-Estradiol ameliorates tight junction disruption via repression of MMP transcription. Mol. Endocrinol. 2015;29(9):1347–1361. doi: 10.1210/ME.2015-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin JA, et al. Activation of classical estrogen receptor subtypes reduces tight junction disruption of brain endothelial cells under ischemia/reperfusion injury. Free Radic. Biol. Med. 2016;92:78–89. doi: 10.1016/j.freeradbiomed.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Kurebayashi S, et al. Characterization of mechanisms of interleukin-6 gene repression by estrogen receptor. J. Steroid. Biochem. Mol. Biol. 1997;60(1):11–17. doi: 10.1016/s0960-0760(96)00175-6. [DOI] [PubMed] [Google Scholar]
- 50.Kassem M, et al. Estrogen inhibits interleukin-6 production and gene expression in a human osteoblastic cell line with high levels of estrogen receptors. J. Bone Miner. Res. 1996;11(2):193–199. doi: 10.1002/jbmr.5650110208. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, et al. Estrogen suppresses metastasis in rat hepatocellular carcinoma through decreasing interleukin-6 and hepatocyte growth factor expression. Inflammation. 2012;35(1):143–149. doi: 10.1007/s10753-011-9299-3. [DOI] [PubMed] [Google Scholar]
- 52.Straub RH. The complex role of estrogens in inflammation. Endocr. Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 53.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21(1):335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 54.Netea MG, Wijmenga C, O'Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nat. Immunol. 2012;13(6):535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Z, et al. Toll-like receptor-mediated immune responses in intestinal macrophages; implications for mucosal immunity and autoimmune diseases. Clin. Immunol. 2016;173:81–86. doi: 10.1016/j.clim.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang W, et al. Sex differences in monocyte activation in systemic lupus erythematosus (SLE) PloS One. 2014;9(12):e114589. doi: 10.1371/journal.pone.0114589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang W, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 2009;199(8):1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, et al. Plasmacytoid dendritic cells mediate synergistic effects of HIV and lipopolysaccharide on CD27+ IgD− memory B cell apoptosis. J. Virol. 2014;88(19):11430–11441. doi: 10.1128/JVI.00682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogers A, Eastell R. Effects of estrogen therapy of postmenopausal women on cytokines measured in peripheral blood. J. Bone Miner. Res. 1998;13(10):1577–1586. doi: 10.1359/jbmr.1998.13.10.1577. [DOI] [PubMed] [Google Scholar]
- 60.Rachon D, et al. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J. Endocrinol. 2002;172(2):387–395. doi: 10.1677/joe.0.1720387. [DOI] [PubMed] [Google Scholar]
- 61.Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am. J. Obstet. Gynecol. 2000;182(2):432–438. doi: 10.1016/s0002-9378(00)70235-3. [DOI] [PubMed] [Google Scholar]