Abstract
Background
ADAMTS13 test distinguishes thrombotic thrombocytopenic purpura (TTP) from other thrombotic microangiopathies (TMAs). PLASMIC score helps determine the pretest probability of ADAMTS13 deficiency. Due to inherent limitations of both tests, and potential adverse effects and cost of unnecessary treatments, we performed a cost-effectiveness analysis (CEA) investigating the benefits of incorporating an in-hospital ADAMTS13 test and/or PLASMIC score into our clinical practice.
Methods
A CEA model was created to compare 4 scenarios for a patient with TMAs, utilizing either an in-house vs. send-out ADAMTS13 assay with or without prior risk stratification using PLASMIC scoring. Model parameters, including probabilities and costs, were gathered from the medical literature, except for the ADAMTS13 send-out and in-house tests, which were obtained from our institutional data.
Results
If only the cost is considered, in-house ADAMTS13 test for patients with intermediate-high risk PLASMIC score is the least expensive option ($4,732/patient). If effectiveness is assessed as measured by the number of averted deaths, send-out ADAMTS13 test is the most effective. Considering the cost/effectiveness ratio, the in-house ADAMTS13 test in patients with intermediate-high risk PLASMIC score is the best option, followed by the in-house ADAMTS13 test without the PLASMIC score.
Conclusions
In patients with clinical presentations of TMAs, having an in-hospital ADAMTS13 test to promptly establish the diagnosis of TTP appears to be cost-effective. Utilizing the PLASMIC score increases the cost-effectiveness of the in-house ADAMTS13 test. Our findings indicate the benefit of having a rapid and reliable in-house ADAMTS13 test, especially in the tertiary medical center.
Keywords: ADAMTS13, assay evaluation, cost effectiveness, thrombotic microangiopathy, TTP
Introduction
Thrombotic thrombocytopenic purpura (TTP) is one of the major prototypes of thrombotic microangiopathies (TMAs), characterized by microangiopathic hemolytic anemia and thrombocytopenia.1,2 A key laboratory test to distinguish TTP from other TMAs is the plasma ADAMTS13 activity.3 PLASMIC score, which utilizes both clinical and basic laboratory results, has demonstrated its efficacy in stratifying the likelihood of having a severe deficiency of plasma ADAMTS13 activity.4,5 Bendapudi et al. reported a clinical score based on 7 clinical components with 1 point assigned to each variable: platelet count <30,000/μL, hemolysis variables (reticulocyte count >2.5%, undetectable haptoglobin, or indirect bilirubin > 2 mg/dL), no active cancer, no history of solid or hematopoietic progenitor cell transplant, mean corpuscular volume <90 fL, international normalized ration (INR) <1.5, and creatinine <2 mg/dL.4,5 If the score is less than 4, the patient is less likely to have severe deficiency of ADAMTS13 (defined as level <10%).4,5 However, neither ADAMTS13 assay nor PLASMIC score has an optimal sensitivity and/or specificity for diagnosing and distinguishing TTP from other TMAs.4,6 Furthermore, the treatment for acquired TTP is different from other TMAs.1,7 Specifically, therapeutic plasma exchange (TPE) is indicated for acquired TTP,1,2,8,9 which reduces the mortality rate to 10–30%.3,10 However, TPE has little efficacy in treating other forms of TMAs.11,12 For example, eculizumab, an anti-complement therapy, is the treatment of choice for atypical hemolytic uremic syndrome (aHUS).1 Nonetheless, both therapies are costly and have potential adverse effects including transfusion- and procedure-related risks for TPE13,14 and infection for eculizumab.15
Given that an in-house ADAMTS13 assay may be completed within hours, TPE could be initiated emergently once TTP is confirmed. Previous studies have suggested that in-house testing may reduce the costs compared to the send-out test with TPE initiated while awaiting the results.16–18 Additionally, the use of PLASMIC score may reduce the number of unnecessary ADAMTS13 tests for patients with low likelihood of TTP.19 No study, thus far, has critically examined the cost effectiveness of these different testing options, particularly when stratified by the PLASMIC score. To assist in developing a global policy for caring and testing patients with TMAs, we used a decision analytic model to rigorously determine the cost-effectiveness of having an in-house vs. send-out ADAMTS13 test with or without the PLASMIC score for risk stratification after accounting for limitations (i.e. both the positive and negative predictive values of the ADAMTS13 assay and PLASMIC score) including costs of the assay, adverse events associated with TPEs, and the mortality associated with both treated and untreated TTP and other TMAs.
Materials and Methods
Patient population
The hypothetical patient cohort was modeled using the profile of actual patients. An extensive literature search and data extraction were performed to identify the clinical trials and observational studies with actual TMA patients, suspected of having TTP, in order to provide the input data for the model parameters for hypothetical patients (see Tables 1 and S1 and below for further details). TMA was suspected based on the following signs/symptoms: anemia with schistocytes, undetectable haptoglobin, high lactate dehydrogenase, and thrombocytopenia.
Table 1.
Model parameters and estimates
| Model parameters | Estimate (SE or α/β for sensitivity analysis) | Source | |
|---|---|---|---|
| Probability estimates | Probability of TTP in patients with TMA | 0.342 (137/264) * | Bendapudi et al.4,5,11 |
| Sensitivity of ADAMTS13 assay | 0.890 (0.313) | Groot et al.6 and Smock23 | |
| Specificity of ADAMTS13 assay | 1.00 (0.313) | ||
| Sensitivity of PLASMIC score assessment | 0.985 (0.014) | Bendapudi et al.4,5 | |
| Specificity of PLASMIC score assessment | 0.610 (0.032) | ||
| Mortality in TTP patients receiving TPE treatment | 0.017 (0.87/50.13) * | Rock et al.10 | |
| Mortality in TTP patients without TPE treatment | 0.950 (0.026) | ||
| Mortality in patients without TTP receiving TPE treatment | 0.035 (2.07/56.94) * | Li et al.12 | |
| Mortality in patients without TTP not receiving TPE treatment | 0.070 (4.13/54.87) * | ||
| Mortality due to TPE complications | 1.3×10−5 (3.9×10−3/302)* | Som et al.14 | |
| Infection complication due to TPE | 3.6×10−6 (1.8×10−3/302) * | ||
| Catheter thrombosis complication due to TPE | 3.2×10−5 (9.6×10−3/302) * | ||
| Venous thrombosis complication due to TPE | 9.1×10−6 (3.0×10−3/302) * | ||
| Bleeding/Major complications due to TPE | 1.1×10−4 (3.3×10−2/302) * | ||
| Cost estimates after adjusted for medical inflation | In-house ADAMTS13 assay | $350.00 (44.4/7.88) ** | UABH projected institutional cost |
| Send-out ADAMTS13 assay | $461.00 (44.4/10.37) ** | UABH institutional cost | |
| Therapeutic Plasma Exchange | $4,503.01 (44.44/101.32) ** | Combined 3-day cost of TPE procedures (Heatwole et al.26), plasma products (Li et al.12 and Pham et al.27), catheter placement (Heatwole et al.26), and professional service fee (UABH institutional cost) | |
| Infection | $1,800.37 (44.44/40.51) ** | Heatwole et al.26 | |
| Catheter Thrombosis | $2,005.17 (44.44/45.12) ** | ||
| Venous Thrombosis | $4,437.90 (44.44/106.74) ** | ||
| Bleeding/Major complications | $13,509.00 (44.44/303.95) ** | Average 3 ICU days cost (Okerberg et al.28) |
Values in the parentheses that only have one value are derived from a normal distribution
Values in the parentheses are derived from a β distribution
Values in the parentheses are derived from a γ distribution
Model structure
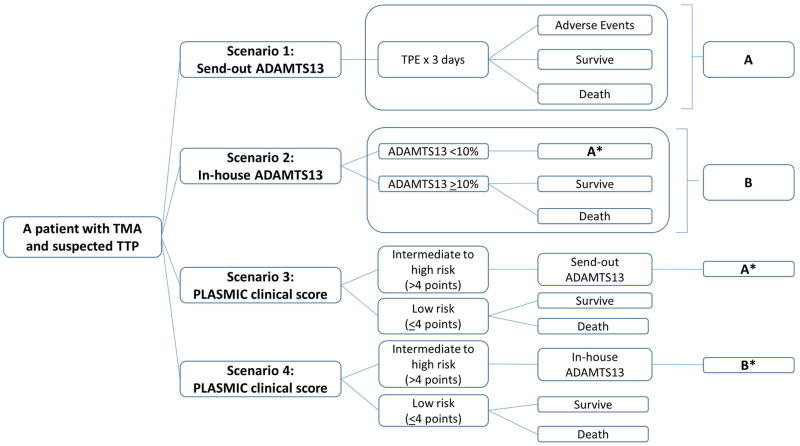
A decision analytic model (Figure 1) was developed using Microsoft Excel (Microsoft Corporation, Redmond, WA) to simulate a hypothetical cohort of adult patients admitted to the hospital with TMA or suspected TTP. Patients were stratified into 4 different clinical scenarios related to testing.
Figure 1.
Decision model – a decision tree structure for suspected TTP patients undergoing 4 different clinical scenarios in a 3-day analysis. The data for each clinical and cost parameter used in the model is in Tables 1 and S1.
(*) denotes divergent branches (i.e. the mortality rate used in the model is different between patients who received TPEs who had true positive ADAMTS13 results (i.e. true TTP) and false positive ones (i.e. non-TTP), Table 1)
The base (or reference) case was defined as scenario (4). The 4 scenarios and their rationale for a patient with TMA and suspected TTP are as follows (Figure 1):
A send-out ADAMTS13 to a reference laboratory with a TPE performed for 3 consecutive days while awaiting results: this scenario may potentially result in TPE related complications and/or mortality from either TPE or the underlying disease process.9 Based on institutional experience and the literature, the turnaround time for this test from a reference laboratory is approximately 3 days.18 After 3 days, using the result of the ADAMTS13 assay, the subsequent management for this scenario is assumed to be identical to the scenarios involving the in-house ADAMTS13 testing (i.e. the only difference is the 3-day TPE).
An in-hospital ADAMTS13 analysis with TPE initiated only if the ADAMTS13 is <10% (i.e. confirmed TTP): When positive (for both true and false positive cases), the patient undergoes TPE for 3 days, resulting in the same pathways as scenario (1) with appropriate mortality rate adjustment for true and false positive cases (Table 1).12 In cases when the patient’s test result is negative (i.e. ADAMTS13 is ≥10%), TPE is not performed as TTP has been excluded. The survival probabilities for the true negative cases (patients without TTP); however, are much higher than those for patients who represent the false negative cases (patients with TTP).10–12 This fact has been accounted for in the model (Table 1).
Performing scenario (1) if the PLASMIC score was shown to be at an intermediate or high risk for a severe ADAMTS13 deficiency: The PLASMIC score is calculated first based on 7 clinical components with 1 point assigned to each variable (as stated in the “Introduction” section) to stratify the patients into a low-risk (≤4 points) vs. an intermediate-high risk group (>4 points) for severe ADAMTS13 deficiency. Based on the PLASMIC score validation, a score of more than 4 points has a sensitivity of 98.5% of detecting an ADAMTS13 < 10% and thus, this is the cutoff we used to stratify hypothetical patients into low risk vs. intermediate-high risk group.4,5 If the patient falls in the low risk category, then no ADAMTS13 test is conducted and no TPE is performed. Otherwise, the ADAMTS13 sample is sent out and 3-day TPE is initiated for the same rationale as explained in scenario (1). Again, appropriate adjustment in mortality rates for both true/false positive and true/false negative cases from PLASMIC score were performed (Table 1).10–12
Utilizing option (2) if the PLASMIC score was shown to be at an intermediate or high risk for severe deficiency of ADAMTS13: Similar to scenario (2), all patients undergo the PLASMIC score assessment and those patients with an intermediate-high risk score undergo scenario (2) (i.e. utilizing an in-house ADAMTS13 assay). Those that do not have an intermediate-high risk PLASMIC score do not undergo further procedures. In this scenario, we accounted for the difference in mortality rates due to the true/false positive and true/false negative rates of both the PLASMIC score and ADAMTS13 assay results (Table 1).10–12
A time horizon of 3 days was used for the analysis to estimate the immediate 3-day cost-effectiveness of each scenario since the management options converge once the ADAMTS13 result is obtained. We did not adjust for inflation with regards to costs and outcomes throughout the time horizon due to the short analysis period.
Model outcomes
Similar to many conventional cost-effectiveness analysis (CEA) studies, the outcomes of the model are estimates of health care resource utilization, which are (a) 3-day payer costs (in US dollars [USD]; defined as the cost outcome), (b) deaths averted within 3 days (effectiveness outcome), and (c) incremental cost-effectiveness ratios (ICER, defined as ) using both cost and effectiveness outcomes.20,21 Typically, the ICER outcome is the composite outcome and is the one used to determine if an alternative method is cost-effective compared to a reference method for policy-making purposes. For example, we used a conventional cutoff of ICER <$50,000/death averted to conclude an alternative method is cost-effective.20,21
We only investigated the immediate 3-day outcomes since 3 days is typically the turnaround time for an ADAMTS13 level from a reference laboratory and this is the only difference between the 4 scenarios defined in the “Model Structure” subsection in “Methods” since after the ADAMTS13 result is available, the clinical management and how the hypothetical cohort of patients responds or is managed is based on such result (identical between scenarios [i.e. the only difference is the 3-day period]). Furthermore, since 3 days is a short period, our model is not set up to investigate the relapse, exacerbation, and/or remission from TPE since these important outcomes require a longer follow-up.
Clinical parameters
Clinical parameter estimates, were derived from the best available medical literature based on the clinical trials and observational studies conducted on patients with TMA with (or without) ADAMTS13 deficiency receiving (or not receiving) TPE (Tables 1 and S1). Depending on the parameters, either data from a subgroup of patients in the studies or all patients will be used. For example, since the PLASMIC score is a major component of our model, we chose to estimate the prevalence of TTP in TMA patients by combining data from 2 studies performed by Bendapudi et al. leading to the derivation and validation of the PLASMIC score.4,5,11 Their group reported that there were 68 out of 254 in the Harvard TMA Research Collaborative 11 and 69 out of 147 patients from a major Southern institution had ADAMTS13 <10%4,5; thus, the average probability of a patient with TMA having TTP (defined as having an ADAMTS13 <10%) was calculated as (68+69)/(254+147) or 34.2%.4,5,11
There are several different types of ADAMTS13 assays on the market22 – in this study, we assumed that both the in-house and send-out assays were FRETS (fluorescent resonance energy transfer)-based assays and thus, the sensitivity and specificity estimates in this model were for this assay.6,23 We used 10% as the decision point for the ADAMTS13 assay since it is the value that is commonly used to distinguish TTP from other forms of TMAs.6,11,12 Furthermore, it allowed for consistency since less than 10% activity was also used as the cutoff point to define a severe ADAMTS13 deficiency in the process of creating and validating the PLASMIC score.5
Major adverse events occurring during and/or after TPE are not very common and thus, the data in the model were derived from the 15-year data in the Oklahoma TTP registry since it is one of the largest and comprehensive TMA registries in the US, and it included the following complications: infection, catheter/venous thrombosis, bleeding/major complications, and death.14 Mortality probabilities were derived from the medical literature for patients with TTP with and without treatment10 as well as for TMA patients without TTP with and without TPE procedure.12 Furthermore, the probabilities for each decision nodes represented the probability at 3 days with the assumption that the daily rate of events is constant over the 3-day analysis. For instance, in 15 years, Som et al. reported that there were 17 catheter thromboses in 302 patients.14 Using the equations converting between rate and probability by Fleurence et al.24, the daily probability was calculated as or 1.06 × 10−5. Then, the 3-day probability of catheter thrombosis is estimated to be 1 − e(−1.06*10−5)*3 or 3.2 × 10−5. Similar calculations were performed to approximate other probability estimates (Tables 1 and S1).
Since the clinical trial in Rock et al. was conducted prior to the availability of ADAMTS13 test,10 this group may have some TMA patients who did not have TTP. Therefore, in the supplemental section, we also tested the model using the mortality rate of TTP patients (as suggested by severe deficiency of ADAMTS13 level) receiving TPE from a retrospective multi-institutional study (Table S1).11
All clinical parameters were subject to sensitivity analyses due to the variability and heterogeneity of patients and procedures
Cost parameters
All costs were expressed in 2016 USD; those not expressed in 2016 USD were adjusted for medical care inflation (Table 1).25 Estimated costs for the ADAMTS13 assay (in-house and send-out) and professional service costs were derived from the actual costs at the University of Alabama at Birmingham Hospital. The cost of TPE included costs for the procedures,26 plasma products (assuming each patient with TMA weighs 70 kg with an average hematocrit of 26%12 and that each unit of plasma is 250 mL and costs $5027), catheter placement,26 and professional service fees. Major adverse event costs such as infection,26 catheter/venous thrombosis,26 bleeding, and other major complications (with the assumption that cost is equivalent to the cost of a 3-day intensive care unit admission)28 were also derived from published literature. There was no extra cost to perform the PLASMIC score since it is only based on basic clinical and laboratory parameters that would be performed in all TMA patients,4 and we assumed that a hematologist would be consulted on a patient with TMA regardless of performance of PLASMIC scoring. Only direct institutional costs were included in the model. Similar to clinical parameters, all cost variables were subject to sensitivity analyses due to the variability and heterogeneity of patients and procedures.
Sensitivity analyses
One-way and probabilistic sensitivity analyses (PSA) were performed to estimate the influence that the range of input values had on the ICER variable. For one-way sensitivity analyses, published literature and expert opinions informed the range of possible input values for the probability of TTP in TMA patients,22 sensitivity/specificity of ADAMTS13 assay,6,23 and sensitivity/specificity of PLASMIC score assessment (Bendapudi BK, personal communication) (Table S2).
In contrast to the above one-way sensitivity analysis which only examines the variation on one parameter at a time, PSA is also conducted using Markov Chain Monte Carlo method of stimulation to investigate the effects of multiple variations occurring at once in the model and to provide a single global analysis of uncertainty in decision.29 We performed PSAs with 1,000 simulations to estimate the influence of the range of input values (i.e. 95% confidence interval for each parameter estimate) on the ICER variable (Table 1). Within the PSAs, we assumed the uncertainty in each variable followed the typical statistical distribution as conventionally done in health economic analysis. Specifically, the uncertainties in the sensitivity and specificities of the ADAMTS13 assay and PLASMIC clinical score assessment were addressed using a normal distribution; the uncertainty in the probability of TTP as well as the mortality for those with TTP given TPE and for those without TTP and not given TPE were managed using a β distribution whereas the probability of death without TPE was assumed to follow a normal distribution. Moreover, uncertainty regarding the major adverse events was evaluated using β distributions. For cost, uncertainty was assessed using γ distributions.
Results
Model results
The total 3-day costs per patient for scenarios (1) through (4) were $15,567, $4,943, $9,423, and $4,732, respectively. Thus, in term of costs alone, scenario (4) is the cheapest, while scenario (1) is the most expensive (Table 2). If the number of deaths averted is considered as an effective outcome, then scenario (1) is most effective, followed by scenarios (3), (2), and (4). Overall, regarding the ratio of cost to effectiveness (i.e. cost/death averted), scenario (4) prevailed over the other scenarios (at $5,207/death averted). Hence, when setting scenario (4) as the base case, scenarios (1), (2), and (3) had an ICER of $146,242, $49,644, and $107,793 per death averted, respectively. Using the conventional cutoff <$50,000/death averted as the cost-effective treatment,20,21 scenario (2) 4 is still cost effective. Similar results were obtained when a more recent TTP mortality rate was used in the model (Tables S1 and S3).10–12
Table 2.
Deterministic base-case analysis result
| Scenario | Cost, $ | Death averted | Cost/Death averted | ICER |
|---|---|---|---|---|
| (1) | 15,567 | 0.98 | 15,838 | 146,242 |
| (2) | 4,943 | 0.91 | 5,414 | 49,644 |
| (3) | 9,423 | 0.95 | 9,895 | 107,793 |
| (4) | 4,732 | 0.91 | 5,207 | Reference |
Sensitivity analysis results
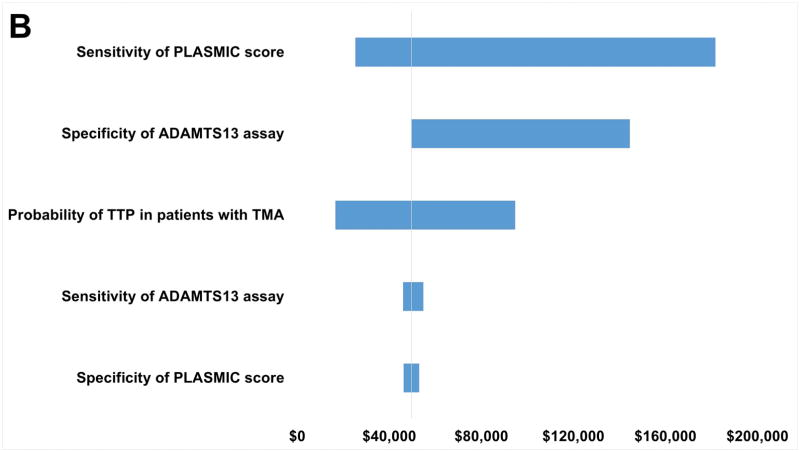
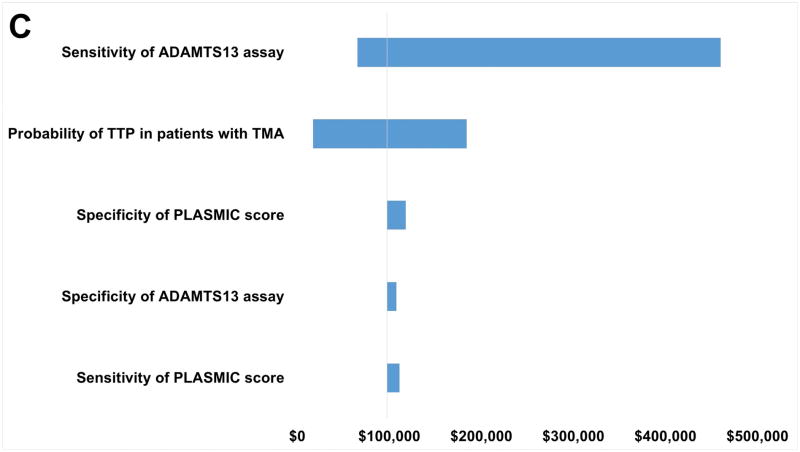
One-way sensitivity analysis indicates that ICER for scenarios (1) through (3) compared to scenario (4) are sensitive to the range of possible values for the probability of TTP in patients with TMA. This is not surprising since the input range is wide (18–100%) (Figure 2 and Table S2). When the pretest probability for TTP is high, comparing to scenario (4), the ICER values for scenarios (1), (2), and (3) are $17,218, $16,612, and $17,341 per death averted, respectively. Sensitivity of the ADAMTS13 assay over the tested range also has a major impact on the robustness of the model for all scenarios, while specificity of the ADAMTS13 assay has an impact in scenario (2). The sensitivity of the PLASMIC clinical score only has a substantial impact on the ICER in scenario (2). Nonetheless, in all one-way sensitivity analyses over the tested range, scenario (4) is always the best option in term of cost-effectiveness. This conclusion is further strengthened from the PSA results, showing the scenario (4) being the most cost effective (Table 3 and Figure 3).
Figure 2.
One-way sensitivity analysis results. Variables represented on the y-axis are varied to determine the robustness of each variable with regards to the incremental cost-effectiveness ratio (ICER) variable, which is represented on the x-axis. The wider the range of the ICER, the less robust the result is with regards to the range of possible values the variable takes. A: Scenario (1) compared with Scenario (4). B: Scenario (2) compared with Scenario (4). C: Scenario (3) compared with Scenario (4).
Table 3.
Probabilistic sensitivity analysis results
| Scenario | Cost, $ | Death averted | Cost/Death averted | ICER |
|---|---|---|---|---|
| (1) | 15,473 | 0.98 | 15,741 | 105,906 |
| (2) | 5,558 | 0.89 | 6,280 | 214,213 |
| (3) | 9,359 | 0.94 | 9,923 | 75,308 |
| (4) | 4,681 | 0.88 | 5,313 | Reference |
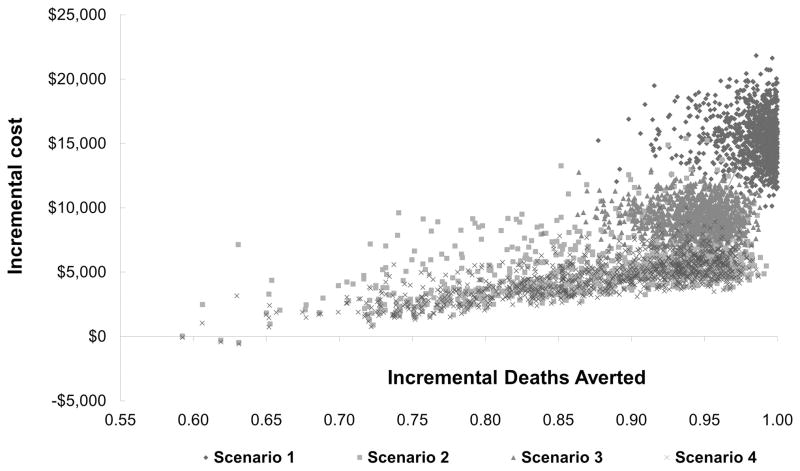
Figure 3.
Probabilistic sensitivity analysis – One-thousand simulation samples were used to confirm the deterministic (base-case) analysis. Y-axis represents the incremental cost while the x-axis represents the incremental deaths averted.
Discussion
We determined the cost-effectiveness of in-house vs. send-out ADAMTS13 test with or without PLASMIC score utilization in management of patients with presumptive diagnosis of TMAs. To our knowledge, this is the first such kind of health economic evaluation on this topic using data and costs from multiple studies in the literature, as well as our own institutional data. Our results are consistent with other studies describing that an in-house rapid ADAMTS13 assay is more cost-effective than to send-out the test to a reference laboratory.16–18 Our results also suggest that the use of PLASMIC score to stratify patients before testing can further improve the cost-effectiveness of an in-house assay. These findings emphasize the importance of having an experienced TMA diagnostic and management team for the care of these patients. In today’s healthcare environment, cost effectiveness analysis will become an important part of the medical decision-making process.27,28,30–33
Although sensitivity analyses demonstrate that the model is robust to dramatic variation in many parameter estimates, our model has some limitations.
First, TTP is a rare disease, affecting only 4 to 11 cases for every million people in the US.34 The estimates for some parameters in the model may be inaccurate. In particular, TTP mortality rates used in the model are from the era prior to the discovery of ADAMTS13 and test availability.10 Thus, the data available may contain a mixture of TMA patients (both TTP and non-TTP patients). This is even more important considering the treatments of TTP with TPE and aHUS with eculizumab are highly effective if used corrected. Having a rapid in-house test and the use of PLASMC score may help clinicians to select right regimen for the right patient early on to reduce the morbidity and mortality. Nonetheless, PSA as well as the model using the different mortality rates from both recent and previous studies (Tables 1 and S1) has shown that the model is still robust.
Second, errors may occur in the pre-analytical phase of obtaining and preparing the samples for send-out (such as wrong blood in tube) as well as in the post-analytical phase (such as delay in result reporting). Hence, these may cause further unnecessary TPE treatments, resulting in higher costs to scenarios (1) and (3). However, the dramatic variation in cost has been accounted for through the PSA and the resulting cost-effectiveness estimates are, therefore, robust.
Third, to simplify the approach, we modeled an ideal situation with several theoretical assumptions. For example, we assumed that both the in-house and send-out ADAMTS13 tests were FRETS-based 23 and thus, had identical assay performance characteristics. This may not be true since there are other methods measuring ADAMTS13 and/or using different cutoff points from 10% ADAMTS13 activity (as in this evaluation) for distinguishing TTP from other TMAs, which may lead to different sensitivity/specificity parameters between laboratories.16,22,35 It must also be emphasized that the ADAMTS13 results cannot be interpreted correctly without clinical information. Hemolyzed blood and hyperbilirubinemia may result in falsely low ADAMTS13 activity.36,37 Furthermore, the probabilities for each decision node in the model were converted to 3 days with the assumption that the daily rate of events was constant over the 3-day analysis. However, since these probabilities are extremely small, the impact on the model may be minimal. Another assumption in the model was that patients would complete 3 days of TPE regardless of the adverse events and that if death occurred, it would occur, at the end of 3 days. In reality, some patients may die prior to the end of 3-day treatment. However, this assumption should have minimal impact on the model as this assumption was inherent for all the modeled scenarios.
Fourth, the patients with TMA may be admitted to any hospital, both community-based and large academic centers. Depending on the number of patients to be tested, the center may choose to bring in a different test other than the FRETS-based ADAMTS13 assay.23 This could impact the cost and time to receive the results. Additionally, if they are at large tertiary care hospitals, then these centers will likely have other patients requiring urgent massive transfusions and/or other emergent apheresis procedures. Thus, the technologists in the transfusion service will be busy preparing blood products and the apheresis nurses may be performing other indicated apheresis procedures. Hence, any time saved from not having to thaw and re-label plasma or not having to perform TPE for patients who do not have TTP would benefit the health system overall. The community hospitals would also likely experience similar benefit as they could avoid mobilizing an outside apheresis service (usually very costly) to perform unnecessary emergent TPE for patients who do not have TTP. This indirect benefit was not estimated or included in our model. Additionally, we did not account for the initial investment of bringing a new test in-house, which in the long run would be offset by the financial benefit of the in-house assay. We also could not account for the competency maintenance of the technologist(s) given that this test may be performed relatively infrequently at many community hospitals; hence, another limitation of our model. Furthermore, in the model, we did not quantify the effect of early initiation of other appropriate therapeutics for patients with alternative TMAs.
Fifth, if the patient receives TPE and ultimately did not have TTP, then this patient was unnecessarily exposed to donor plasma and as such is at risk for alloimmunization, and transfusion-transmitted disease.38,39 The plasma units used for TPE are then considered inappropriate usage – we did not include the psychological effect of inappropriate utilization of plasma. Every time we inappropriately use 1 unit of plasma, more than just monetary value is discarded because we have wasted scarce blood resources as well as the donors’ altruism that went into the production of these plasma units. The broader societal outcome may be improved by having a more efficient blood supply chain. This overall perspective is outside the scope of our evaluation.
Finally, CEA study is designed to examine the costs and different trade-offs, such as death averted in this case, of various treatment options from a global policy making perspective given each treatment has its distinct benefits and disadvantages. Using a probabilistic method, it may help to provide rational direction to achieve the goal of allocating limited resources to places where benefits can be maximized.20 However, each patient may be different and thus, a CEA study result cannot be substituted for clinical judgement when providing care for each individual patient.
In conclusion, this is the first pharmacoeconomic model to demonstrate that in patients with clinical presentations of TMAs, an in-hospital ADAMTS13 test to establish the prompt diagnosis of TTP appears to be cost-effective, and thus, providing evidence to support the need for establishing a rapid and reliable in-house ADAMTS13 test to aid in the diagnosis and management of patients with TMA, especially at large academic medical centers in the US. Utilizing the PLASMIC score in conjunction with the in-house assay further increases the cost-effectiveness of the test.
Supplementary Material
Acknowledgments
Source of support
This study is supported in part by NIH R01-HL126724 and R01-HL115187 to XLZ.
Footnotes
Authorship statement
LAW, EMS, XLZ, and HPP conceptualized and designed the study; CHK, SS, and LAW were responsible for data abstraction; CHK and HPP was responsible for economic modeling; CHK, SS, EMS, and HPP wrote the first draft of the manuscript; and all authors critically reviewed, revised, and approved the final manuscript for scientific content.
Conflicts of interest: XLZ is a consultant for Ablynx and a speaker for Alexion. In addition, XLZ received grant support from Alexion and Lee’s Pharmaceutical. All other authors declared no conflict of interest.
References
- 1.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654–66. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 2.Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nature Reviews Disease Primers. 2017;3:17020. doi: 10.1038/nrdp.2017.20. [DOI] [PubMed] [Google Scholar]
- 3.Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211–25. doi: 10.1146/annurev-med-061813-013241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendapudi PK, Li A, Hamdan A, Fry AM, Uhl L, Marques M, Kaufman R, Stowell CP, Dzik WS, Makar RS. Derivation and Prospective Validation of a Predictive Score for the Rapid Diagnosis of Thrombotic Thrombocytopenic Purpura: The Plasmic Score. Blood. 2014;124:231. [Google Scholar]
- 5.Bendapudi PK, Hurwitz S, Fry A, Marques MB, Waldo SW, Li A, Sun L, Upadhyay V, Hamdan A, Brunner AM, Gansner JM, Viswanathan S, Kaufman RM, Uhl L, Stowell CP, Dzik WH, Makar RS. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017 doi: 10.1016/S2352-3026(17)30026-1. [DOI] [PubMed] [Google Scholar]
- 6.Groot E, Hulstein JJ, Rison CN, de Groot PG, Fijnheer R. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4:698–9. doi: 10.1111/j.1538-7836.2005.01767.x. [DOI] [PubMed] [Google Scholar]
- 7.Pham HP, Schwartz J. New apheresis indications in hematological disorders. Curr Opin Hematol. 2016;23:581–7. doi: 10.1097/MOH.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 8.Pham HP, Schwartz J. How to approach an apheresis consultation using the American Society for Apheresis guidelines for therapeutic apheresis procedures. ISBT Science Series. 2015;10:79–88. [Google Scholar]
- 9.Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, Dunbar NM, Witt V, Wu Y, Shaz BH. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher. 2016;31:149–62. doi: 10.1002/jca.21470. [DOI] [PubMed] [Google Scholar]
- 10.Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spasoff RA. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–7. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 11.Bendapudi PK, Li A, Hamdan A, Uhl L, Kaufman R, Stowell C, Dzik W, Makar RS. Impact of severe ADAMTS13 deficiency on clinical presentation and outcomes in patients with thrombotic microangiopathies: the experience of the Harvard TMA Research Collaborative. Br J Haematol. 2015;171:836–44. doi: 10.1111/bjh.13658. [DOI] [PubMed] [Google Scholar]
- 12.Li A, Makar RS, Hurwitz S, Uhl L, Kaufman RM, Stowell CP, Dzik WS, Bendapudi PK. Treatment with or without plasma exchange for patients with acquired thrombotic microangiopathy not associated with severe ADAMTS13 deficiency: a propensity score-matched study. Transfusion. 2016;56:2069–77. doi: 10.1111/trf.13654. [DOI] [PubMed] [Google Scholar]
- 13.Delaney M, Wendel S, Bercovitz RS, Cid J, Cohn C, Dunbar NM, Apelseth TO, Popovsky M, Stanworth SJ, Tinmouth A, Van De Watering L, Waters JH, Yazer M, Ziman A Biomedical Excellence for Safer Transfusion C. Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;388:2825–36. doi: 10.1016/S0140-6736(15)01313-6. [DOI] [PubMed] [Google Scholar]
- 14.Som S, Deford CC, Kaiser ML, Terrell DR, Kremer Hovinga JA, Lammle B, George JN, Vesely SK. Decreasing frequency of plasma exchange complications in patients treated for thrombotic thrombocytopenic purpura-hemolytic uremic syndrome, 1996 to 2011. Transfusion. 2012;52:2525–32. doi: 10.1111/j.1537-2995.2012.03646.x. quiz 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kistler AD. Eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;369:1378. doi: 10.1056/NEJMc1308826. [DOI] [PubMed] [Google Scholar]
- 16.Barrows BD, Teruya J. Use of the ADAMTS13 Activity Assay Improved the Accuracy and Efficiency of the Diagnosis and Treatment of Suspected Acquired Thrombotic Thrombocytopenic Purpura. Arch Pathol Lab Med. 2014;138:546–9. doi: 10.5858/arpa.2013-0170-OA. [DOI] [PubMed] [Google Scholar]
- 17.Connell NT, Cheves T, Sweeney JD. Effect of ADAMTS13 activity turnaround time on plasma utilization for suspected thrombotic thrombocytopenic purpura. Transfusion. 2016;56:354–9. doi: 10.1111/trf.13359. [DOI] [PubMed] [Google Scholar]
- 18.Martin IW, Katus MC, Martin CL, Szczepiorkowski ZM, Gorham JD, Dunbar NM. Rapid ADAMTS13 availability impacts treatment for microangiopathic hemolytic anemia and thrombocytopenia. J Clin Apher. 2016;31:419–22. doi: 10.1002/jca.21419. [DOI] [PubMed] [Google Scholar]
- 19.Upadhyay V, Geisler B, Sun L, Makar RS, Bendapudi P. Utilization and Cost Effectiveness of a Risk Stratified Diagnostic Approach to Patients with Suspected Thrombotic Thrombocytopenic Purpura. Blood. 2016;128:1456. [Google Scholar]
- 20.Cohen DJ, Reynolds MR. Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol. 2008;52:2119–26. doi: 10.1016/j.jacc.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med. 1998;13:716–7. doi: 10.1046/j.1525-1497.1998.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peyvandi F, Palla R, Lotta LA, Mackie I, Scully MA, Machin SJ. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J Thromb Haemost. 2010;8:631–40. doi: 10.1111/j.1538-7836.2010.03761.x. [DOI] [PubMed] [Google Scholar]
- 23.Smock KJ. The Role of ADAMTS13 Testing in the Work up of Suspected Thrombotic Thrombocytopenic Purpura [monograph on the internet] American Association for Clinical Chemistry; 2016. Available from: https://www.aacc.org/publications/cln/articles/2016/april/the-role-of-adamts13-testing-in-the-work-up-of-suspected-thrombotic-thrombocytopenic-purpura. [Google Scholar]
- 24.Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25:3–6. doi: 10.2165/00019053-200725010-00002. [DOI] [PubMed] [Google Scholar]
- 25.Halfhill TR. Tom’s Inflation Calculator [monograph on the internet] 2016 Available from: http://www.halfhill.com/inflation_js.html.
- 26.Heatwole C, Johnson N, Holloway R, Noyes K. Plasma exchange versus intravenous immunoglobulin for myasthenia gravis crisis: an acute hospital cost comparison study. J Clin Neuromuscul Dis. 2011;13:85–94. doi: 10.1097/CND.0b013e31822c34dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham HP, Sireci AN, Kim CH, Schwartz J. Cost-Effectiveness Analysis of Plasma Versus Recombinant Factor VIIa for Placing Intracranial Pressure Monitors in Pretransplant Patients With Acute Liver Failure. Clin Appl Thromb Hemost. 2014;20:607–14. doi: 10.1177/1076029614524621. [DOI] [PubMed] [Google Scholar]
- 28.Okerberg CK, Williams LA, 3rd, Kilgore ML, Kim CH, Marques MB, Schwartz J, Pham HP. Cryoprecipitate AHF vs. fibrinogen concentrates for fibrinogen replacement in acquired bleeding patients - an economic evaluation. Vox Sang. 2016;111:292–8. doi: 10.1111/vox.12417. [DOI] [PubMed] [Google Scholar]
- 29.Ades AE, Claxton K, Sculpher M. Evidence synthesis, parameter correlation and probabilistic sensitivity analysis. Health Econ. 2006;15:373–81. doi: 10.1002/hec.1068. [DOI] [PubMed] [Google Scholar]
- 30.Pham HP, Kim CH, Schwartz J. Phenotypically matched vs. traditional screen method for preparing red blood cell units in patients with abnormal placentation: a decision analysis approach. Vox Sang. 2014;107:399–406. doi: 10.1111/vox.12176. [DOI] [PubMed] [Google Scholar]
- 31.Pham HP, Muller MC, Williams LA, 3rd, Juffermans NP. Mathematical model and calculation to predict the effect of prophylactic plasma transfusion on change in international normalized ratio in critically ill patients with coagulopathy. Transfusion. 2016;56:926–32. doi: 10.1111/trf.13447. [DOI] [PubMed] [Google Scholar]
- 32.Aljabri A, Huckleberry Y, Karnes JH, Gharaibeh M, Kutbi HI, Raz Y, Yun S, Abraham I, Erstad B. Cost-effectiveness of anticoagulants for suspected heparin-induced thrombocytopenia in the United States. Blood. 2016;128:3043–51. doi: 10.1182/blood-2016-07-728030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryan AW, Jr, Staley EM, Kennell T, Jr, Feldman AZ, Williams LA, 3rd, Pham HP. Plasma Transfusion Demystified: A Review of the Key Factors Influencing the Response to Plasma Transfusion. Lab Med. 2017 doi: 10.1093/labmed/lmx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terrell DR, Williams LA, Vesely SK, Lammle B, Hovinga JA, George JN. The incidence of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS-13 deficiency. J Thromb Haemost. 2005;3:1432–6. doi: 10.1111/j.1538-7836.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- 35.Tripodi A, Peyvandi F, Chantarangkul V, Palla R, Afrasiabi A, Canciani MT, Chung DW, Ferrari S, Fujimura Y, Karimi M, Kokame K, Kremer Hovinga JA, Lammle B, de Meyer SF, Plaimauer B, Vanhoorelbeke K, Varadi K, Mannucci PM. Second international collaborative study evaluating performance characteristics of methods measuring the von Willebrand factor cleaving protease (ADAMTS-13) J Thromb Haemost. 2008;6:1534–41. doi: 10.1111/j.1538-7836.2008.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu RN, Yang S, Wu HM, Zheng XL. Unconjugated bilirubin inhibits proteolytic cleavage of von Willebrand factor by ADAMTS13 protease. J Thromb Haemost. 2015;13:1064–72. doi: 10.1111/jth.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer SC, Sulzer I, Lammle B, Kremer Hovinga JA. Hyperbilirubinemia interferes with ADAMTS-13 activity measurement by FRETS-VWF73 assay: diagnostic relevance in patients suffering from acute thrombotic microangiopathies. J Thromb Haemost. 2007;5:866–7. doi: 10.1111/j.1538-7836.2007.02438.x. [DOI] [PubMed] [Google Scholar]
- 38.Harvey AR, Basavaraju SV, Chung KW, Kuehnert MJ. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion. 2015;55:709–18. doi: 10.1111/trf.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pham HP, Williams LA., 3rd Plasma vs. cryoprecipitate for fibrinogen replacement in therapeutic plasma exchange procedures. J Clin Apher. 2015;30:382–3. doi: 10.1002/jca.21392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.