Abstract
Background
Outcomes in blast phase CML (CML-BP) are historically dismal. We sought to analyse the characteristics, prognostic factors and survival outcomes in patients with CML-BP in the TKI era.
Methods
All patients with CML-BP (n=477) were treated with a TKI at some point during the course of their CML. Cox proportional hazard models identified characteristics which predicted for survival. Overall survival (OS) and failure free survival (FFS) were assessed. Optimal cut off points for specific parameters, were identified using CART (classification and regression tree).
Results
Median age was 53 years (range, 16 to 84); 64% were male. Eighty percent were initially diagnosed in chronic phase CML (CML-CP) median 41 months (0.7 to 298 months) before transformation to CML-BP. De-novo CML-BP occurred in 71 patients. Seventy two percent patients received TKI therapy prior to CML-BP. Initial therapy for CML-BP included, TKI alone (35%), TKI with chemotherapy (46%) and non-TKI therapies (19%). The median OS was 12 months and median FFS was 5 months. In multivariate analysis, myeloid immunophenotype, prior TKI, age ≥58 years, LDH ≥1227 IU/L, platelet count <102 K/μL, no stem cell transplantation (SCT), blast phase from CP/AP and presence of chromosome 15 aberrations predicted for a significantly increased risk of death. Achievement of major hematologic response and/or complete cytogenetic response to first line treatment predicted for better survival. Combination of TKI with intensive chemotherapy followed by stem cell transplant confer the best outcome.
Conclusions
Patients with CML-BP continue to pose a therapeutic challenge, have dismal outcomes and require newer treatment approaches.
Keywords: Chronic myeloid leukemia in blast phase, CML, CML-BP, Tyrosine kinase inhibitors (TKI), imatinib, nilotinib, dasatinib, ponatinib, bosutinib
Introduction
With the widespread use of tyrosine kinase inhibitors (TKI) for the treatment of chronic myeloid leukemia (CML), the risk of transformation to blast phase has markedly decreased. However a significant minority of patients transform to blast phase; this is less frequent in patients treated with second generation TKI as initial therapy.1 The median survival in patients with CML-BP is less than 12 months.2–4,5 The pathobiology of CML-BP involves multiple intermingling pathways with genetic and epigenetic aberrations involved in transformation, but is not fully understood.6
Although the poor prognosis of patients with CML-BP is well recognized, the predictive prognostic features in the era of TKI have been scantily addressed. One study with 51 patients in CML-BP (only a minority of whom were treated with TKI) suggested that low platelet count and age >60 years predict for poor survival.2 Generally, patients with CML-BP are treated with TKI combined with intensive chemotherapy followed by allogenic stem cell transplantation (SCT); survival has improved with combined modality treatment as compared to that seen with TKI alone.7–9 To the best of our knowledge, this is the largest analysis of CML-BP, describing the clinical characteristics, prognostic factors, survival outcomes and treatments for patients with CML-BP, who received a TKI at some point during the course of CML.
Patients and Methods
Four hundred seventy seven patients with blast phase CML (defined as ≥30% blasts in peripheral blood and/or bone marrow, or extramedullary disease) who were seen at MDACC from 1997 to 2016 were included in this analysis. A retrospective chart review protocol was approved by the institutional review board (IRB) and waiver of informed consent was obtained. Response to treatment was assessed after initial treatment for CML-BP as defined previosuly.10 Major hematologic response (MHR) encompasses both complete hematologic response (CHR) and no evidence of leukemia (NEL). Definitions of cytogenetic and molecular response were as previously described.11 The pattern of clonal evolution was classified as previously described.12 Only patients with available treatment and response information and who received frontline therapy for CML-BP were included in the final analysis. Overall survival (OS) was assessed from the date of diagnosis of BP until death or the date of last follow up. Failure free survival (FFS) was assessed from the time start of first line treatment for CML-BP to the date of first relapse, date of switch to second line therapy, date of death, or date of last follow up.
Statistical analysis
Univariate and multivariate Cox proportional hazard models were performed to identify specific characteristics of CML-BP which can predict for survival outcomes. Variables with p-value ≤0.25 in the univariate analysis were entered into a multivariate model. Median survival and survival probabilities were analyzed using the Kaplan-Meier methods and differences calculated by the log-rank test. We used classification and regression tree (CART) analysis to identify the optimal cut off points for specific parameters associated with survival, subsequently we identified prognostic factors which could independently predict survival in CML-BP. A p-value of <0.05 was considered significant. Statistical analyses were carried out using STATA/SE version 14.1 statistical software (Stata Corp. LP, College Station, Texas).
Results
Patients and disease characteristics
Patients with CML-BP (n=477) who were treated with TKI at some point during the course of their disease were included in this analysis. Among these patients, 343 (72%) were treated with TKI prior to developing CML-BP and 195 (57%) of them were evaluable for response. Response to prior TKI therapy was CHR in 97%, CCyR in 31% and MMR in 11%. The majority (n=381; 80%) of patients were in CML-CP at the time of initial CML diagnosis, with a median time from initial diagnosis of CML-CP to BP of 41 months (range 0.7 to 298 months). Twenty five patients (5%) were in accelerated phase at the time of initial diagnosis of CML; their median time from initial diagnosis of CML-AP to BP was 14 months (range 0.4 to 160 months). De-novo CML-BP was seen in 71 patients (15%). Types of first line treatment received by the patients at the time of initial diagnosis of CML, included – interferon based (37% and 16%), imatinib (26% and 48%), miscellaneous (21% and 16%), dasatinib (6% and 4%), chemotherapy alone (5%) and nilotinib (5% and 16%) for patients in CP and AP, respectively. For patients in CP who were initially treated with imatinib (n=99) and transformed to BP, 24 received imatinib as part of their initial therapy for CML-BP, while 31 received dasatinib, 7 nilotinib, 3 ponatinib and 2 bosutinib.
Patient and disease characteristics at the time of diagnosis of CML-BP are summarized in Table 1 and in supplemental figure-1. Overall, median age was 53 years (range, 16 to 84); 64% were male and 65% Caucasian. Extramedullary BP was identified in 128 patients [27%; isolated extramedullary BP in 50 (39%)]. Distribution of patients according to immunophenotype was myeloid in 67%, lymphoid in 28%, mixed lineage 4%, megakaryocytic 0.5% and undifferentiated 0.5% immunophenotype occurred less frequently; the immunophenotype was unknown in 19 patients. The most common transcript type was e13a2 in 38% patients. Distribution of ABL mutations is shown in Table-1. Forty three percent of patients (n=200) had clonal evolution, of which 18% (n=85) were group 1 (trisomy 8, -Y and extra Philadelphia.), 15% (n=68) were group 2 (iso17, chromosome 3 aberration and -7/-7p), 10% (n=47) were an overlap of both groups, and 57% (n=263) did not belong to either group (Table-2 and supplemental figure-2). Six percent of patients had a variant Philadelphia chromosome. Using CART analysis, we identified the optimal cut off values for different continuous variables which correlated with survival outcomes including age (<58 years vs ≥58 years), hemoglobin (<13 gm/dL years vs ≥13 gm/dL), platelet count (<102 vs ≥102 ×109/L), bone marrow blast % (<5% vs ≥5%) and serum lactate dehydrogenase (LDH) (<1227 IU/L vs ≥1227 IU/L).
Table-1.
Patient characteristics at blast phase CML (CML-BP)
| Characteristic | No. (%), or Median [Range] |
|---|---|
| N | 477 |
| Age at BP, years [range]* | 53 [16–84] |
| Gender (Male/Female) | 305 (64)/172 (36) |
| Ethnicity (Caucasian/Others) | 312 (65)/165 (35) |
| Hemoglobin [g/dL]* | 10 (0–16) |
| WBC (K/μL)* | 19 (0–768) |
| Platelet (K/μL)* | 77 (0–2750) |
| Peripheral blood blast % | 21 (0–145) |
| Bone Marrow blast %* | 40 (0–99) |
| Serum LDH (IU/L)* | 1242 (0–21874) |
| Serum Uric Acid (mg/dL) | 5.6 (0.7–20) |
| Myeloid/Lymphoid immunophenotype | 308 (67)/128 (28) |
| Prior TKI (Yes/No) | 343 (72)/134 (28) |
| Transcript Type | |
| e13a2 | 182 (41) |
| e14a2 | 170 (39) |
| e13a2 +e14a2 | 74 (17) |
| e1a2 | 14 (3) |
| Extramedullary disease | 128 (27) |
| Isolated extramedullary disease | 50 (39) |
| CNS involvement at any point (Yes/No) | 50 (13)/327 (87) |
| SCT after BP (Yes/No) | 104 (22)/373 (78) |
| Characteristic | No. (%), or Median [Range] |
| Patients with ABL mutation testing | 187 (39) |
| T315I | 28 (15) |
| E255K | 15 (8) |
| F317L | 12 (6) |
| Miscellaneous# | 39 (21) |
| Negative | 93 (50) |
| Initial Treatment Type** | 426 (89) |
| TKI Alone | 149 (35) |
| TKI with chemotherapy | 195 (46) |
| Others Non-TKI therapies | 82 (19) |
| Type of TKI’s used | |
| Imatinib | 189 (55) |
| Dasatinib | 110 (32) |
| Nilotinib | 26 (7) |
| Bosutinib | 5 (1) |
| Ponatinib | 15 (4) |
| CML phase at initial diagnosis | |
| Chronic Phase (CP) | 381 (80) |
| Accelerated phase (AP) | 25 (5) |
| Blast phase (BP) | 71 (15) |
| Time from initial diagnosis to BP (months) | 26 [0–298] |
| CP to BP | 381 (80), 41 [0.7–298] |
| AP to BP | 25 (5), 14 [0.4–160] |
| Median follow-up (months) | 11.5 (0–195) |
| Last follow up status (Alive/Died) | 95 (20)/382 (80) |
Optimal cut off values identified by CART analysis for different variables were – age (<58 years vs ≥ 58 years), hemoglobin (<13 gm/dL years vs ≥ 13 gm/dL), white blood cell count (WBP) none identified, platelet count 102 K/μL, bone marrow blast % (<5% vs ≥ 5%), serum lactate dehydrogenase (LDH) (<1227 IU/L vs ≥ 1227 IU/L),
* ABL mutations (n) – Y253H (8), G250E (4), Q252H (3), E255V (3), M351T (3), V299L (2), F311L (2), H396R (2), other mutations (12, 1 each),
Among those patients who had initial treatment information available
Table-2.
Distribution of various chromosomal aberrations at the time of CML-BP
| Chromosomal abnormalities | N | % |
|---|---|---|
| No. of non-Ph+ chromosomal abnormalities n [Median (range)] | 463 [1.0 (0–19)] | 97 |
| Classic Philadelphia Chromosome (Ph+) alone | ||
| No | 356 | 76.7 |
| Yes | 108 | 23.3 |
| Double Ph+ | ||
| No | 382 | 82.5 |
| Yes | 81 | 17.5 |
| iso17 (i17) | ||
| No | 429 | 92.5 |
| Yes | 35 | 7.5 |
| Trisomy 8 | ||
| No | 386 | 83.2 |
| Yes | 78 | 16.8 |
| Trisomy 19 | ||
| No | 447 | 96.3 |
| Yes | 17 | 3.7 |
| -Y aberration | ||
| No | 448 | 96.6 |
| Yes | 16 | 3.4 |
| Trisomy 21 | ||
| No | 446 | 96.1 |
| Yes | 18 | 3.9 |
| Trisomy 17 | ||
| No | 457 | 98.5 |
| Yes | 7 | 1.5 |
| Del7 | ||
| No | 424 | 91.4 |
| Yes | 40 | 8.6 |
| Chromosome 3 aberrations | ||
| No | 410 | 88.4 |
| Yes | 54 | 11.6 |
| Variant Ph | ||
| No | 435 | 93.8 |
| Yes | 29 | 6.3 |
| Chromosome 15 aberrations | ||
| No | 454 | 97.8 |
| Yes | 10 | 2.2 |
| Chromosome 17 aberrations | ||
| No | 390 | 88.4 |
| Yes | 70 | 11.6 |
| Type of clonal evolution, n (%) | 200 | 43 |
| Group 1 (Trisomy 8, -Y and Double Ph.) | 85 | 18 |
| Group 2 (iso17, chr. 3 aberration and -7/-7p) | 68 | 15 |
| Group 1 + 2 | 47 | 10 |
| Neither group | 263 | 57 |
Type of treatments and response to first line therapy in patients with CML-BP
We then analysed the initial treatment for CML-BP and response to therapy in 426 (89%) patients with available information (Summarized in table-3). Treatment modalities were divided into TKI alone (n=149; 35%), TKI plus chemotherapy (n=195; 46%), and non-TKI based therapies (n=82; 19%). Non-TKI based therapies included ara-C-based regimens (non HyperCVAD; n=23), troxacitabine (n=13), Hyper-CVAD (n=6), omacetaxine (n=6), gemtuzumab-based (n=4) and miscellaneous (including investigational) therapies (n=30). Distribution of patients according to the type of TKI was imatinib (n=189; 55%), dasatinib (n=110; 32%), nilotinib (n=26; 7%), ponatinib (n=15; 4%) and bosutinib (n=5; 1%). The overall rates of MHR, CCyR and MMR were 50%, 21% and 12%, respectively. Patients treated with a combination of TKI with chemotherapy had significantly higher MHR compared to other modalities (p=0.0001). Similarly, rates of CCyR and MMR were higher with TKI combined with chemotherapy. Patient treated with dasatinib or ponatinib had a numerical trend for better response rates. MHR was not significantly higher with dasatinib compared to imatinib (p=0.08). Since few patients were treated with other TKI’s, we could not compare dasatinib with other TKI’s. Of the 149 patients who were treated with TKIs alone as a first line treatment for CML-BP, 80 patients (54%) were treated with imatinib and 76/80 (95%) progressed.
Table-3.
Summary of responses and type of frontline treatments for CML-BP
| Overall Response with frontline therapies (ITT) | N (%) | |||
|---|---|---|---|---|
| Major hematologic response (MHR) | 206 (50) | |||
| Complete cytogenetic response (CCyR) | 88 (21) | |||
| Major Molecular response (MMR) | 48 (12) | |||
| Type of therapies and responses according to type of therapy | ||||
| Initial treatment | 426 (89) | % Response | ||
|---|---|---|---|---|
| MHR | CCyR | MMR | ||
| TKI alone | 149 (35) | 43 | 17 | 10 |
| TKI with chemotherapyˆ | 195 (46) | 64 | 29 | 16 |
| Others (non-TKI) therapies | 82 (19) | 29 | 13 | 4 |
| TKIs used for CML-BP | ||||
| Imatinib | 189 (55) | 53 | 17 | 10 |
| Dasatinib‡ | 110 (32) | 64 | 34 | 22 |
| Nilotinib* | 26 (7) | 35 | 15 | 15 |
| Bosutinib | 5 (1) | 40 | – | – |
| Ponatinib | 15 (4) | 67 | 33 | 27 |
MHR p=0.001,
MHR p=0.08,
No. of patients with available response data was <5
One hundred and four patients (22%) underwent stem cell transplantation (SCT) at some point after the diagnosis of CML-BP. The proportion of patients who received a SCT was higher among those treated with TKI-based combinations (21%) than those treated with TKI alone or non-TKI therapy (3% and 10%, respectively). Twenty five patients (35%) with de novo CML-BP underwent SCT compared to 79 (19%) of those who had transformed from CML-CP/AP. At the time of SCT, 12 patients remained in blast phase, 79 patients (76%) were in MHR and 45 (43%) in CCyR. The proportion of patients who underwent SCT according to the type of responses achieved were: MHR/no MHR (33% vs 16%), CCyR/no CCyR (40% vs 26%), and MMR/no MMR (43% vs 15%). Additional reasons for not undergoing SCT in 373 patients were disease progression/disease related complications in 46%, unknown and/or lost to follow up in 50%, and non-CML associated comorbidities in 4%. Of note, 56 (54%) of the patients who received a SCT required 2 or more therapies for CML-BP to be able to proceed to SCT. Overall, 56 patients (54%) needed second or subsequent therapies to get to stem cell transplant.
Prognostic factors and survival outcomes in patients with CML-BP
The median follow up was 11.5 months (range 0–195). The median overall survival was 12 months with 79% deaths; median FFS was 5 months with 87% failing first line treatment (Figure-1 A–B). We then analysed the impact of various patient characteristics at the time of CML-BP on OS and FFS. Patients with de novo blast phase had longer OS when compared to patients who transformed from CML-CP/CML-AP (p<0.0001) (Figure-1C). As shown in figure 1-D, overall survival has progressively improved since 2000 (i.e., the advent of imatinib) to the present date. Patients with lymphoid immunophenotype and patients who were not treated with TKI prior to CML-BP, had longer survival compared to patients with myeloid immunophenotype (p<0.001) and those who had received TKI prior to transformation to blast phase (p<0.0001) (Figure-1 E–F). We also identified a difference in overall survival with optimal cut off values determined by recursive partitioning for different variables. Patients age ≥58 years and those with LDH ≥1227IU/L (p<0.001 in both) had significantly inferior survival (Figure-1 G–H). Other variables (not shown) which showed significant correlation with inferior survival outcome were hemoglobin <13 g/dL, male gender, bone marrow blast at the time of blast phase ≥5% (compared to extramedullary disease only), platelet count <102 ×109/L, lack of isolated EMD, higher number of prior TKI and no SCT after CML-BP. OS was similar in patients exhibiting different transcript types, extramedullary disease (with or without blood/bone marrow disease) and patients with different ABL mutations (not shown). The grouping of chromosomal abnormalities per the classification of Wang et al had no bearing in OS (not shown). However, when survival was analyzed according to individual chromosomal aberrations, patients with trisomy 8, trisomy 19, trisomy 17, or Philadelphia chromosome with other cytogenetic abnormalities, or with chromosome 3 or chromosome 15 aberrations, had significantly inferior OS compared to patients without these aberrations (not shown).
Figure-1. Survival outcome in patients with CML-BP – Overall and according to patient characteristics.
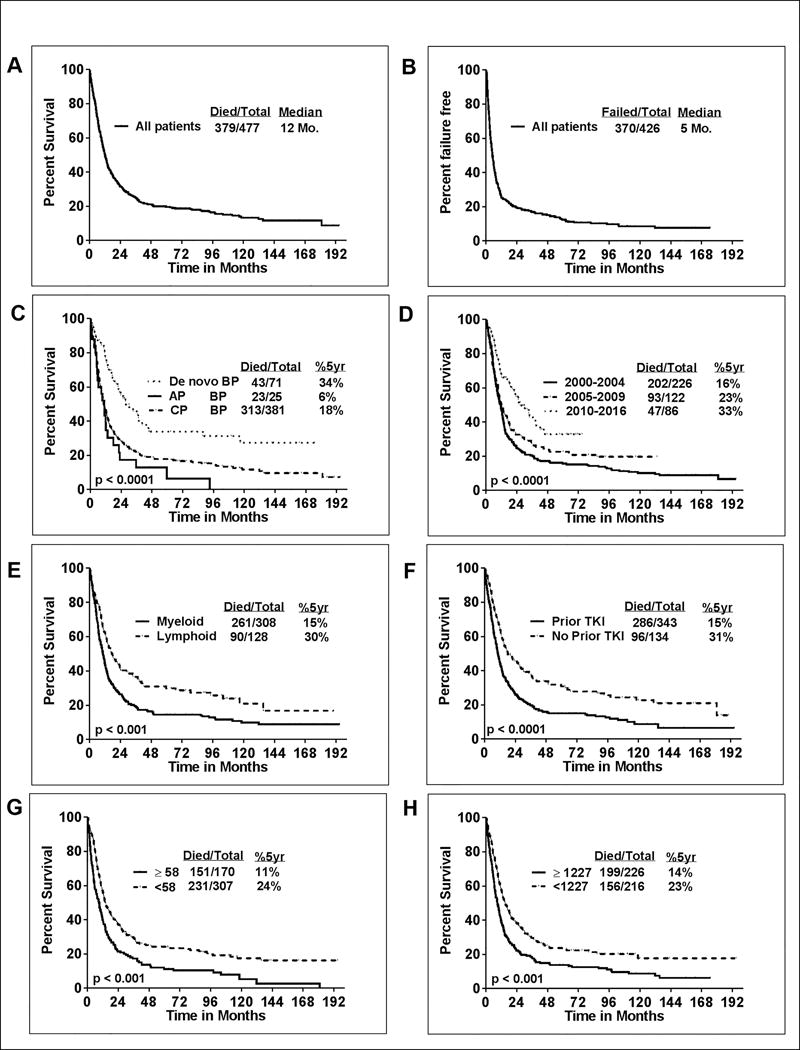
A) Overall survival (OS) for all patients. The median overall survival was 12 months with 79% deaths. B) Failure free survival (FFS) for all patients. The median FFS was 5 months with 87% failing first line treatment C) Patients with de novo blast phase had better OS compared to patients who transformed from chronic/accelerated phase CML; p<0.0001 D) Survival has progressively improved after the advent of imatinib in 2000 and is significantly better in the current era; p<0.0001 E) Patients with myeloid immunophenotype of CML-BP have inferior survival compared to CML-BP with lymphoid immunophenotype (p<0.001; 5 year survival % is 15% vs 30%) F) Patients previously treated with tyrosine kinase inhibitor (TKI) had poor survival (p<0.0001; 5 year survival % is 15% vs 31%) G) Patients age ≥ 58 years had poor survival (p<0.001; 5 year survival % is 11% vs 24%). The cut off of 58 years is derived from recursive partitioning H) Patients with LDH levels ≥ 1227 IU/L had poor survival (p<0.001; 5 year survival % is 14% vs 23%). The cut off of 1227 for serum LDH is derived from recursive partitioning method
We then looked at the impact of type of treatment and responses achieved in CML-BP. Patients treated with a combination of TKI with chemotherapy had superior outcome compared to TKI alone (p=0.005) (Figure-2A) or with non-TKI based treatments. This correlates with a more frequent use of combination of TKI with chemotherapy (74% of patients treated in 2010–2016 compared to 36% in 2000–2004). Because of the small numbers of patients treated with some individual TKI, no firm conclusions can be drawn about the impact of individual TKI, but there was a suggestion of a better survival for patients treated with dasatinib compared to imatinib (p=0.06) (Figure-2B). Other TKI cohorts have too few patients to evaluate. Patients who received SCT after their initial treatment for CML-BP had a significantly longer survival than patients who did not receive SCT after their initial therapy (Figure-2C). Moreover, patients who achieved MHR or CCyR or MMR after initial blast phase treatment had better survival outcome (Supplemental figure- 3A–C).
Figure-2. Overall survival (OS) in patients with CML-BP according to treatment modality.
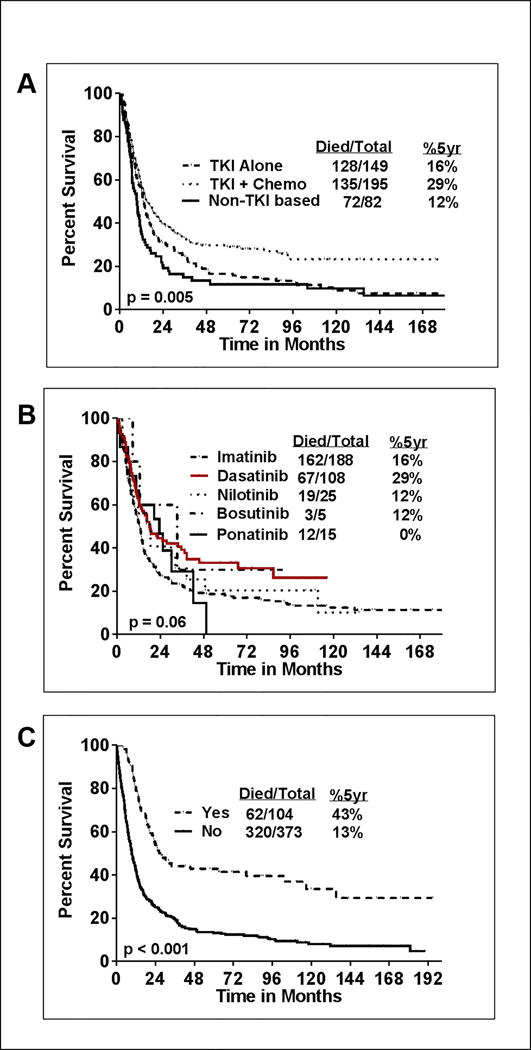
A) Patients who are treated with frontline tyrosine kinase inhibitor (TKI) in combination with chemotherapy had superior survival (p=0.005; 5 year survival % is 30% vs 14% vs 9%) compared to patients who were treated with TKI alone and non-TKI based therapies as the first line treatment for CML-BP B) Patients treated with various TKI’s are shown. Dasatinib therapy appears to have a better survival compared to other TKI’s (p=0.06; 5 year survival % with dasatinib is 29%. Too few patients to evaluate in nilotinib, ponatinib and bosutinib cohorts. C) Patients who are not treated with stem cell transplantation (SCT) after the diagnosis of CML-BP had inferior survival (p<0.001; 5 year survival % is 13% vs 43%)
We also analysed the impact of patient characteristics on FFS. Patients with myeloid immunophenotype, prior TKI therapy, age ≥58 years and LDH ≥1227 IU/L (p<0.001 in both) had significantly inferior FFS. (Supplemental figure-4 A–D). Chromosomal aberrations which showed significant correlation with inferior FFS were chromosome 3 aberrations, trisomy 8, Philadelphia chromosome with other cytogenetic abnormalities, chromosome 15, chromosome 17 aberrations and minus Y (not shown). Patients age ≥58 years, those with LDH ≥1227 IU/L hemoglobin <13 g/dL, bone marrow blast ≥5%, platelet count <102 ×109/L, lack of isolated EMD, or with no SCT after CML-BP had a significantly inferior FFS. Patients who achieved MHR or CCyR or MMR after initial blast phase treatment had significantly longer FFS (p<0.0001) (Supplemental figure 3 D-F). Furthermore, patients treated with a combination of TKI with chemotherapy had superior FFS compared to patients treated with TKI alone (p<0.001) (Figure-3A) or with non-TKI based treatments. Similar to OS, there was a trend for longer FFS with dasatinib compared to imatinib (p=0.06) (Figure-3B). Patients who received SCT after their initial treatment for CML-BP had a significantly longer FFS then those patients who did not receive SCT after their initial therapy (Figure-3C).
Figure-3. Failure free survival (FFS) in patients with CML-BP according to treatment.
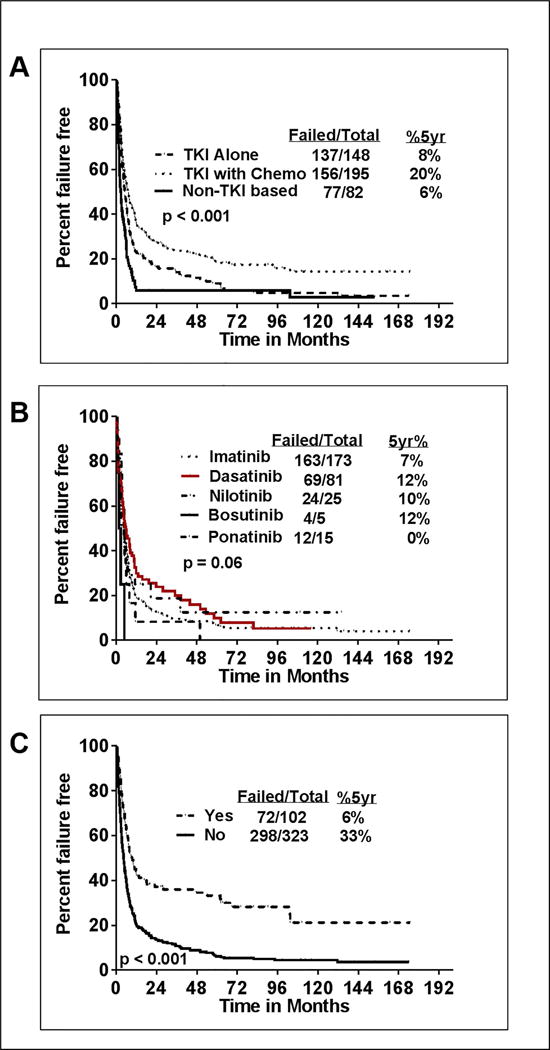
A) Patients who are treated with frontline tyrosine kinase inhibitor (TKI) in combination with chemotherapy had superior FFS (p<0.001; 5 year FFS % is 20% vs 8% vs 6%) compared to patients who were treated with TKI alone and non-TKI based therapies as the first line treatment for CML-BP B) Patients treated with dasatinib therapy had better survival compared to other TKI’s, imatinib, nilotinib, ponatinib, bosutinib (p=0.06; 5 year FFS % with dasatinib is 20%. C) Patients who are not treated with stem cell transplantation (SCT) after the diagnosis of CML-BP had inferior outcome (p<0.001; 5 year FFS % is 6% vs 33%)
First-line treatment for patients with de novo CML-BP included TKI alone (n=24; 34%), TKI plus chemotherapy (n=41; 58%), non-TKI based therapies (n=2; 3%) and 4 unknown. Outcomes by type of first line therapy for denovo BP and in patients who progressed from CP/AP is shown in supplemental figure-5 (A–D).
Factors predictive for survival outcomes – multivariate analysis (MVA)
We then created cox regression models for OS (Table-4) and FFS (Supplemental table-1) and identified factors which were associated with survival. In multivariate analysis (MVA) for OS, adjusting for different variables, age ≥58 years (HR=1.41, p=0.004), platelet count ≥102 ×109/L (HR=0.65, p=0.001), treatment with TKI prior to transformation to CML-BP (HR=1.51, p=0.004), LDH ≥1227 IU/L (HR 1.32, p=0.01), myeloid immunophenotype (HR=1.67, p<0.001), chromosome 15 aberrations (HR 2.20, p=0.02), transformation from CML-CP/AP (vs. de novo CML-BP; HR 1.43, p=0.06), and treatment with SCT after CML-BP (HR 0.40, p<0.001) were significantly associated with OS. In a separate MVA, we noted that the achievement of MHR and/or CCyR emerged as the most significant independent predictor for survival while isolated EMD was no longer associated with survival (not shown). Type of clonal evolution by group 1, group 2, both or neither groups did not predict for OS.
Table-4.
Factors predictive for overall survival (OS) in patients with blast phase CML (CML-BP) - Cox proportional hazard model
| N | Events | Log-rank | HR | 95% CI HR | P-value | |
|---|---|---|---|---|---|---|
| # ˆ $ Univariate | ||||||
| Age at transformation | 477 | 382 | 1.02 | (1.01–1.02) | <0.001 | |
| Hemoglobin (g/dL) | 447 | 358 | 0.93 | (0.89–0.97) | 0.001 | |
| Serum LDH (IU/L) | 442 | 355 | 1.00 | (1.00–1.00) | <0.001 | |
| BP Immunophenotype | <0.001 | |||||
| Lymphoid | 128 | 90 | ||||
| Myeloid | 308 | 261 | 1.57 | (1.23–1.99) | <0.001 | |
| CML phase at initial diagnosis | <0.001 | |||||
| De Novo BP | 71 | 43 | ||||
| AP | 25 | 23 | 2.56 | (1.54–4.25) | <0.001 | |
| CP | 381 | 316 | 2.01 | (1.46–2.77) | <0.001 | |
| Chromosome 15 aberration | 0.125 | |||||
| No | 454 | 363 | ||||
| Yes | 10 | 9 | 1.67 | (0.86–3.24) | 0.129 | |
| Prior TKI | <0.001 | |||||
| No | 134 | 96 | ||||
| Yes | 343 | 286 | 1.68 | (1.33–2.12) | <0.001 | |
| SCT after BP | <0.001 | |||||
| No | 373 | 320 | ||||
| Yes | 104 | 62 | 0.39 | (0.30–0.51) | <0.001 | |
| Type of initial treatment | 0.001 | |||||
| Non-TKI based | 82 | 72 | ||||
| TKI Alone | 149 | 128 | 0.78 | (0.58–1.04) | 0.085 | |
| TKI with Chemotherapy | 195 | 135 | 0.58 | (0.43–0.77) | <0.001 | |
| Age≥58 years* | <0.001 | |||||
| No | 307 | 231 | 1.64 | (1.34–2.02) | <0.001 | |
| Yes | 170 | 151 | ||||
| LDH≥1227 IU/L* | <0.001 | |||||
| No | 216 | 156 | 1.56 | (1.26–1.92) | <0.001 | |
| Yes | 226 | 199 | ||||
| Hemoglobin≥13 g/dL* | <0.001 | |||||
| No | 404 | 332 | 0.48 | (0.32–0.72) | <0.001 | |
| Yes | 43 | 26 | ||||
| BM blast %≥5* | <0.001 | |||||
| No | 46 | 24 | 2.13 | (1.41–3.23) | <0.001 | |
| Yes | 394 | 327 | ||||
| Platelet count ≥102 K/μL* | <0.001 | |||||
| No | 257 | 220 | ||||
| Yes | 192 | 141 | 0.68 | (0.55–0.84) | <0.001 |
| # Multivariate | N | Events | Log-rank | HR | 95% CI HR | P-value |
|---|---|---|---|---|---|---|
| BP Immunophenotype | ||||||
| Lymphoid | 128 | 90 | ||||
| Myeloid | 308 | 261 | 1.70 | 1.30–2.22 | <0.001 | |
| CML phase at initial diagnosis | ||||||
| De Novo BP | 71 | 43 | ||||
| AP | 25 | 23 | 1.83 | 1.01–3.32 | 0.047 | |
| CP | 381 | 316 | 1.43 | 0.97–2.11 | 0.069 | |
| Chromosome 15 aberration | ||||||
| No | 454 | 363 | ||||
| Yes | 10 | 9 | 2.20 | 1.12–4.32 | 0.021 | |
| Prior TKI | ||||||
| No | 134 | 96 | ||||
| Yes | 343 | 286 | 1.51 | 1.14–2.00 | 0.004 | |
| SCT after BP | ||||||
| No | 373 | 320 | ||||
| Yes | 104 | 62 | 0.40 | 0.29–0.55 | <0.001 | |
| Age≥58 years* | ||||||
| No | 307 | 231 | ||||
| Yes | 170 | 151 | 1.41 | 1.11–1.78 | 0.004 | |
| LDH≥1227 IU/L* | ||||||
| No | 216 | 156 | ||||
| Yes | 226 | 199 | 1.32 | 1.05–1.65 | 0.018 | |
| Platelet count ≥102 K/μL* | ||||||
| No | 257 | 220 | ||||
| Yes | 192 | 141 | 0.65 | 0.51–0.83 | 0.001 |
Optimal cut off values identified by CART (classification and regression tree) for different variables were – age (<58 years vs ≥ 58 years), hemoglobin (<13 gm/dL years vs ≥ 13 gm/dL), platelet count (<102 vs ≥ 102 K/μL), bone marrow blast % (<5% vs ≥ 5%), serum lactate dehydrogenase (LDH) (<1227 IU/L vs ≥ 1227 IU/L);
Platelet count, white blood cell count, peripheral blood blast %, BCR-ABL transcript type, spleen size, gender type, race, type of treatments, type of TKI used, number of prior TKI, extramedullary disease, LDH levels, bone marrow blast % were not significant in multivariate analysis;
Chromosomal aberrations which were significantly predictive of overall survival in univariate but not in multivariate analysis were trisomy 8, trisomy 19, trisomy 17, Philadelphia chromosome with other cytogenetic abnormalities, chromosome 3 aberrations;
Variables which were significantly predictive for longer survival in univariate analysis but not included in this MVA model due to >20% missing values were achievement of major hematologic response (MHR), complete cytogenetic response (CCyR) and isolated extramedullary disease (EMD). Inclusion of only patients with available MHR or CCyR to the baseline characteristics model predicted for longer survival while isolated EMD did not predict for longer survival in MVA (not shown). Type of clonal evolution by group 1, group 2, both or neither groups did not predict for OS.
We then analysed FFS in patients on which we had the available information for their frontline therapy for CML-BP (n=426). The following factors significantly predicted for the increased risk of failure in MVA: myeloid immunophenotype, prior TKI therapy, presence of chromosome 3 aberrations, lack of SCT after CML-BP, non-TKI based therapy and platelet count <102 ×109/L (Supplemental Table-1). Similar to the OS, achievement of MHR and/or CCyR predicted for longer FFS, while isolated EMD did not predict for FFS after adjusting for response. In addition, a higher number of TKI therapies prior to progressing to CML-BP was also associated with inferior survival in MVA (OS, p=0.055; FFS p=0.019).
Discussion
Despite the marked progress in therapy for CML, patients who transform to CML-BP have a dismal outcome, particularly if previously treated with TKI. To the best of our knowledge, this is the largest study describing the characteristics and outcome of patients with CML-BP treated with TKI. We have identified that myeloid immunophenotype, treatment with TKI prior to transformation to CML-BP, age ≥58 years, LDH ≥1227 IU/L, platelet count <102 ×109/L, no treatment with SCT after blast phase, transformed CML from CP/AP and the presence of chromosome 15 aberrations were predictive for poor survival in CML-BP.
The incidence of CML-BP has markedly decreased after the introduction of TKI for the treatment of CML, particularly with the use of second and third generation inhibitors.5,13 However, our data suggests that although the outcome is poor with a median survival of only 12 months, the survival has progressively improved over time. As shown in Figure-1D, there is an improvement in outcome after the introduction of TKI and after 2005, when second generation TKI became increasingly available, first through clinical trials and later through standard of care, it has further improved. This could potentially be due to the increased use of TKI combined with chemotherapy followed by SCT after diagnosis of CML-BP.9,14 Our results are similar to those reported in previous smaller series treated mostly with imatinib.7,15 This is despite more patients having received prior TKI before transformation, an adverse prognostic factor identified in our analysis. Our data also suggests that treatment with dasatinib may confer a survival benefit when compared to treatment with imatinib. This is perhaps not unexpected considering the higher potency, wider coverage of abl mutants, and the reported significant albeit modest success for patients treated with dasatinib after imatinib failure.10 Although it would be expected that similar benefit would be observed with other newer agents, our cohort included too few patients with each individual TKI to draw firm conclusions in this regard. Similarly, we cannot make any inferences regarding the relative benefit of the different second generation TKI. One of the caveats of our data is that the study is retrospective and the treatment, kinase domain mutation data and the response data were not available for all patients. Furthermore, in our analysis, as therapy became more effective, the rate of transformation decreased over time. For example, only 36 patients were diagnosed with CML-BP after 2013. This affected the exposure to newer TKI such as ponatinib which was approved in December 2012. Only 8 of these 36 patients were treated with ponatinib-based regimen as a first line therapy of CML-BP (including both patients with documented T315I), as combination therapies with other TKI were better known and preferred over single agent TKI by that time. Eventually, 2 additional patients received ponatinib for CML-BP as this therapy and combinations based on ponatinib became more established.16 We further identified that use of non-TKI based therapies was independently predictive of poor failure free survival compared to TKI based treatments. In our analysis, the median time from initial diagnosis of CML-CP to BP was 41 months (range 0.7 to 298 months) which is perhaps longer than would be expected. The precise reasons for these longer than expected time to transformation is not clear, but we hypothesize that the availability of TKIs and other treatment options, many of them in clinical trials before they were widely available, allowed patients to remain in CP longer even if not having an optimal response.
The pathogenesis of CML-BP in the TKI era has received little attention17 and precise molecular mechanisms/mutational/epigenetic changes delineating the transition from chronic phase CML to accelerated phase and blast phase CML are still unclear. It is possible that treatment with TKI exerts a selection pressure over CML cells which can then evolve over time and develop into an overt BP.18 Our data could be viewed as supportive of this hypothesis since we have shown that prior TKI therapy was a poor prognostic factor in CML-BP. Whether this transition is dependent upon exposure to TKI or is secondary to an inherent mutation profile of various sub clones which evolve over time is unclear.19 Existence of these sub clones which could predispose to an overt BP is supported by one study which showed that the presence of cells with aberrant immunophenotype - myeloid or lymphoid in CML-CP patients can predispose CML patients to CML-BP.20 Whether the sub clones give rise to a more resistant disease is not known, but since we show that denovo CML-BP had better outcome than those who transformed from CP/AP (supplemental figure-5), our results would be compatible with this hypothesis. Our study also shows a very high incidence of clonal evolution, more frequently observed than ABL kinase domain mutations, suggesting that transformation is the result of complex molecular processes rather than the mere development of resistance to TKI. One recent study suggested the presence of novel fusions in MLL gene and in ANKRD11 gene in patients with CML-BP, using RNA sequencing.21 Previously, ABL, RUNX1, ASXL122 and IDH1 mutations were described in patients with CML-BP,23 however paired sample analysis from several patients on TKI therapy was not reported. In contrast, the presence and type of ABL kinase domain mutations had minimal prognostic consequences as we had previously reported.24
Our analysis generated some novel findings. We identified that chromosome 15 aberrations, which are uncommon in CML, constitutes a poor prognostic risk factor in CML-BP. Chromosome 15 abnormalities are identified as a minor route abnormality in CML.25 The long arm of chromosome 15 contains a tumor suppressor gene CCNDBP126 which is down regulated in different cancers, but its role in CML is unknown. In addition we identified the optimal cut off points for various parameters which indicated high disease burden in CML-BP such as elevated serum LDH levels, low platelet count and low hemoglobin level. Advanced age and low hemoglobin were shown to predict for poor survival in another study from 51 patients with CML-BP, most of them not treated with TKI.2 Some chromosomal abnormalities were significantly correlated to survival in univariate but not in MVA such as trisomy 8, trisomy 19. Interestingly, the type of clonal evolution12 and chromosome 17 abnormalities did not predict for survival. In addition, age ≥58 years and lack of SCT after the diagnosis of BP were also predictive for poor OS. Our analysis also showed that prognostic factors for FFS were similar to those for OS, except that non-TKI based therapy and chromosome 3 aberrations significantly predicted for increased risk of failure of first line therapy. Chromosome 3 aberrations were previously reported to have poor prognostic impact in patients with CML.27 Importantly, response to therapy is incorporated into the MVA, achievement of MHR or CCyR after first line treatment for CML-BP was a stronger predictor for longer survival compared to other prognostic factors. We also identified isolated EMD as a favorable factor for survival compared to patients with bone marrow involvement with or without EMD.
In summary, this study provides the most comprehensive analysis of the disease characteristics and outcome of patients with CML-BP in the TKI era. It identifies novel leads into the prognostication and survival outcomes of patients with CML-BP in the TKI era. Treatment with a combination of TKI and chemotherapy followed by SCT remains the backbone in the management of these patients. New approaches to treat patients with CML-BP that address the molecular complexity of CML-BP are needed.28,29,30
Supplementary Material
Condensed Abstract.
Myeloid immunophenotype, prior TKI use, older age, higher LDH, low platelet count, stem cell transplantation (SCT) after blast phase, transformed CML from CP/AP and presence of chromosome 15 abnormalities were predictive for poor survival in CML-BP. Patients with CML-BP treated with a combination of TKI with chemotherapy have superior survival then TKI alone.
Acknowledgments
Funding – Funding for these studies was provided in part by the MD Anderson Cancer Center Support Grant CA016672 (PI: Dr. Ronald DePinho) and Award Number P01 CA049639 (PI: Dr. Richard Champlin) from the National Cancer Institute. None of the authors are employed by NIH.
Footnotes
Presented at: Oral session in The Annual Meeting of the American Society of Hematology, 2016
Authorship Contributions –
P.J. and J.C. designed the study.
G. N. G., P.J, and J.C. analyzed results.
P.J., A.G., G.N.G., K.S., D.K.C. and J.C. wrote the paper.
P.J., G.N.G., R.L., I.G., S.P., S.D., R.K.S., H.K., and J.C. did clinical correlation.
H.K., S.O.B., F.R., E.J., W.W., S.V., Z.E., N.D., T.K., G.B. and J.C. contributed patient samples.
All authors reviewed and gave the final approval for the paper.
Conflicts of Interest Disclosures: J.C - Consultant: BMS, Pfizer, Ariad, Novartis. Research support: Pfizer, Ariad, Teva, Bristol Myers Squibb (BMS), Novartis, F.R. - research funding from BMS and honoraria from BMS, Novartis, and Pfizer. The remaining authors declare no competing financial interests.
References
- 1.Hoffmann VS, Baccarani M, Hasford J, et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015;29(6):1336–1343. doi: 10.1038/leu.2015.73. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Jacobo F, Tuna-Aguilar E, Demichelis-Gomez R, et al. Prognostic Factors, Response to Treatment, and Survival in Patients With Chronic Myeloid Leukemia in Blast Phase: A Single-Institution Survey. Clin Lymphoma Myeloma Leuk. 2015;15(12):778–784. doi: 10.1016/j.clml.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Hehlmann R, Saussele S, Voskanyan A, Silver RT. Management of CML-blast crisis. Best Pract Res Clin Haematol. 2016;29(3):295–307. doi: 10.1016/j.beha.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Keating MJ, Talpaz M, et al. Chronic myelogenous leukemia in blast crisis. Analysis of 242 patients. Am J Med. 1987;83(3):445–454. doi: 10.1016/0002-9343(87)90754-6. [DOI] [PubMed] [Google Scholar]
- 5.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34(20):2333–2340. doi: 10.1200/JCO.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbour EJ, Hughes TP, Cortes JE, Kantarjian HM, Hochhaus A. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia. Leuk Lymphoma. 2014;55(7):1451–1462. doi: 10.3109/10428194.2013.845883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Xu LP, Liu DH, et al. Allogeneic hematopoietic SCT in combination with tyrosine kinase inhibitor treatment compared with TKI treatment alone in CML blast crisis. Bone Marrow Transplant. 2014;49(9):1146–1154. doi: 10.1038/bmt.2014.146. [DOI] [PubMed] [Google Scholar]
- 8.Kruger P, Cooney J, Nivison-Smith I, et al. All is not lost in accelerated phase/blast crisis and after tyrosine kinase inhibitors fail in chronic myeloid leukaemia: a retrospective study of allogeneic stem cell transplant outcomes in Australia and New Zealand. Bone Marrow Transplant. 2016;51(10):1400–1403. doi: 10.1038/bmt.2016.143. [DOI] [PubMed] [Google Scholar]
- 9.Strati P, Kantarjian H, Thomas D, et al. HCVAD plus imatinib or dasatinib in lymphoid blastic phase chronic myeloid leukemia. Cancer. 2014;120(3):373–380. doi: 10.1002/cncr.28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109(8):3207–3213. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 11.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Cortes JE, Tang G, et al. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016;127(22):2742–2750. doi: 10.1182/blood-2016-01-690230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyekunle A, Zander AR, Binder M, et al. Outcome of allogeneic SCT in patients with chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Ann Hematol. 2013;92(4):487–496. doi: 10.1007/s00277-012-1650-8. [DOI] [PubMed] [Google Scholar]
- 15.Saussele S, Lauseker M, Gratwohl A, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115(10):1880–1885. doi: 10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki K, Jabbour EJ, Ravandi F, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A propensity score analysis. Cancer. 2016;122(23):3650–3656. doi: 10.1002/cncr.30231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120(7):2254–2264. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt M, Rinke J, Schafer V, et al. Molecular-defined clonal evolution in patients with chronic myeloid leukemia independent of the BCR-ABL status. Leukemia. 2014;28(12):2292–2299. doi: 10.1038/leu.2014.272. [DOI] [PubMed] [Google Scholar]
- 19.Kim T, Tyndel MS, Kim HJ, et al. Spectrum of somatic mutation dynamics in chronic myeloid leukemia following tyrosine kinase inhibitor therapy. Blood. 2016 doi: 10.1182/blood-2016-04-708560. [DOI] [PubMed] [Google Scholar]
- 20.El Rassi F, Bergsagel JD, Arellano M, et al. Predicting early blast transformation in chronic-phase chronic myeloid leukemia: is immunophenotyping the missing link? Cancer. 2015;121(6):872–875. doi: 10.1002/cncr.29142. [DOI] [PubMed] [Google Scholar]
- 21.Marum JE, Wang PP, Stangl D, et al. Novel Fusion Genes at CML Diagnosis Reveal a Complex Pattern of Genomic Rearrangements and Sequence Inversions Associated with the Philadelphia Chromosome in Patients with Early Blast Crisis. Blood. 2016;128(22):1219–1219. [Google Scholar]
- 22.Menezes J, Salgado RN, Acquadro F, et al. ASXL1, TP53 and IKZF3 mutations are present in the chronic phase and blast crisis of chronic myeloid leukemia. Blood Cancer J. 2013;3:e157. doi: 10.1038/bcj.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossmann V, Kohlmann A, Zenger M, et al. A deep-sequencing study of chronic myeloid leukemia patients in blast crisis (BC-CML) detects mutations in 76.9% of cases. Leukemia. 2011;25(3):557–560. doi: 10.1038/leu.2010.298. [DOI] [PubMed] [Google Scholar]
- 24.Jabbour E, Jones D, Kantarjian HM, et al. Long-term outcome of patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors after imatinib failure is predicted by the in vitro sensitivity of BCR-ABL kinase domain mutations. Blood. 2009;114(10):2037–2043. doi: 10.1182/blood-2009-01-197715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin CC, Abruzzo LV, Qiu X, et al. del(15q) is a recurrent minor-route cytogenetic abnormality in the clonal evolution of chronic myelogenous leukemia. Cancer Genet Cytogenet. 2009;192(1):18–23. doi: 10.1016/j.cancergencyto.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma W, Stafford LJ, Li D, et al. GCIP/CCNDBP1, a helix-loop-helix protein, suppresses tumorigenesis. J Cell Biochem. 2007;100(6):1376–1386. doi: 10.1002/jcb.21140. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Cortes JE, Lin P, et al. Clinical and prognostic significance of 3q26.2 and other chromosome 3 abnormalities in CML in the era of tyrosine kinase inhibitors. Blood. 2015;126(14):1699–1706. doi: 10.1182/blood-2015-05-646489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietarinen PO, Pemovska T, Kontro M, et al. Novel drug candidates for blast phase chronic myeloid leukemia from high-throughput drug sensitivity and resistance testing. Blood Cancer J. 2015;5:e309. doi: 10.1038/bcj.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed W, Van Etten RA. Alternative approaches to eradicating the malignant clone in chronic myeloid leukemia: tyrosine-kinase inhibitor combinations and beyond. Hematology Am Soc Hematol Educ Program. 2013;2013:189–200. doi: 10.1182/asheducation-2013.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter BZ, Mak PY, Mu H, et al. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci Transl Med. 2016;8(355):355ra117. doi: 10.1126/scitranslmed.aag1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


