Abstract
Objective
To identify implementation challenges associated with conducting a randomized controlled trial (RCT) of group prenatal care (PNC) and report outcomes of the pilot.
Methods
A multi-site randomized pilot was conducted in Malawi and Tanzania between July 31, 2014, and June 30, 2015. Women aged at least 16 years with a pregnancy of 20–24 weeks were randomly assigned using sealed envelopes (1:1) to individual or group PNC. Structured interviews were conducted at baseline, in the third trimester and 6–8 weeks after delivery. The primary outcomes were attendance at four PNC visits and attendance at the postnatal visit.
Results
The pilot showed that an RCT with individual randomization can be conducted in these two low-resource settings. Significantly more women in group PNC than in individual PNC completed at least four PNC visits (96/102 [94.1%] vs 53/91 [58.2%]) and attended a postnatal visit (76/102 [74.5%] vs 45/90 [50.0%]; both P<0.001).
Conclusion
Group PNC was feasible and associated with an increase in healthcare utilization and improved outcomes in Malawi and Tanzania. Lessons learned should be considered when designing large RCTs to determine efficacy.
ClinicalTrials.gov
Keywords: CenteringPregnancy, Group healthcare models, Group prenatal care, Prenatal care, Quality of care, Respectful care, Sub-Saharan Africa
1 INTRODUCTION
High-quality prenatal care (PNC) has the potential to begin a health-promoting continuum of respectful care across the life course that builds women’s trust in the healthcare system and increases use of services and adherence to health promotion advice.1 However, the quality of PNC in many low-resource countries of Sub-Saharan Africa is low. PNC clinics are crowded, women’s wait times are long, and service gaps remain.2,3 Visit length is inadequate, inhibiting health education and the formation of a relationship with providers.4–12 Although many women attend at least one PNC visit, less than half complete the four recommended PNC visits or the 6-week postnatal visit.13
In the early 2000s, after a series of randomized trials,14 WHO endorsed a PNC model designed to improve quality. This model—focused PNC—reduced the number of visits to four, with enhanced quality guidelines for each visit. Unfortunately, severe provider shortages, inadequate resources, and an HIV epidemic requiring additional services made the implementation of higher quality PNC in low-resource settings unattainable. Women continued to receive brief, low-quality PNC visits, but substantially fewer of them with focused PNC than with standard PNC.4–9 A reanalysis of original WHO data showed an increased risk of perinatal mortality with focused PNC.15 WHO has since modified its PNC guidelines by increasing the recommended number of visits to eight and emphasizing a positive pregnancy experience through quality care.1
Group health care is one health-system intervention that could improve quality of PNC in limited-resource settings by fundamentally altering service delivery to allow for longer, woman-centered visits.16 Developed in the USA, the only evidence-based group PNC model is CenteringPregnancy.16 CenteringPregnancy’s core components harness the well-established power of self-care, interactive learning, and positive group dynamics.16 A skilled birth attendant and an assistant run 2-hour sessions including clinical assessments and interactive learning with 12 women.
Growing evidence17–20–21 shows that CenteringPregnancy has positive effects on health outcomes, including increased prenatal and postnatal care attendance and satisfaction, improved prenatal mental and physical health, neonates that are heavier and/or less likely to be premature, more births with a skilled birth attendant, improved breastfeeding behaviors, and increased family planning uptake to increase birth intervals. In addition to the present randomized pilot, several group PNC programs have been initiated outside the USA with positive results.21,22 Collectively, these results led WHO to call for more research to document whether group care has sufficient evidence for policy change.1
On the basis of the potential of group PNC to improve quality of care in Sub-Saharan Africa, there is a clear need for a large randomized controlled trial (RCT) with adequate power to evaluate the effect of group PNC on prematurity and other perinatal and longer-term outcomes. In preparation, the present multisite randomized pilot was undertaken in two low-resource countries, Malawi and Tanzania, to determine the feasibility of individual randomization, factors affecting retention, and associations with various outcomes. Improved quality should increase women’s willingness to return for care, so healthcare utilization was the primary outcome. Because of their relationship with pregnancy experience and newborn health and development, satisfaction with PNC, a pregnancy-related empowerment scale (PRES),25 mental distress,26 and exclusive breastfeeding (EBF) were examined as secondary outcomes. Malawi and Tanzania have high HIV prevalence and active programs of prevention of mother-to-child transmission (PMTCT); therefore, HIV-specific health promotion content was added and HIV-related knowledge was examined.
2 MATERIALS AND METHODS
A two-arm randomized controlled pilot study was conducted between July 31, 2014, and June 30, 2015, in Malawi and Tanzania. Both are low-resource countries with similar healthcare systems and have high rates of maternal and infant mortality; however, Malawi is poorer and more rural. Improving PNC quality presents different challenges in these settings. As a result of funding constraints and to maximize variation, a rural site in Malawi (a district hospital and one of its satellite clinics) and a large urban clinic in central Dar es Salaam, Tanzania, were selected. Women aged at least 16 years with a pregnancy of 20–24 weeks visiting the study centers were enrolled if they were deemed able to complete study procedures. Before data collection, ethics approval was obtained from the University of Illinois at Chicago (Chicago, IL, USA), the University of Malawi, and the National Institute of Medical Research, Tanzania. All participants provided written informed consent.
After completion of a baseline interview, each participant selected a sealed envelope containing group assignment from a container (1:1 ratio) to determine which arm of the study they would be assigned to (individual PNC or group PNC). Half the women were assigned to receive individual PNC, in which services were provided on a first-come, first-served basis. These participants listened to a health lecture and met a midwife individually for a physical assessment. Laboratory tests (including HIV testing) were undertaken at their first visit. Women were expected to complete four PNC visits and two postnatal visits (one in the first week and the other at 6 weeks). The other half of participants were assigned to CenteringPregnancy-based group PNC. These women had the same number of visits as did the women in the individual PNC group, with their 6-week postnatal check-ups performed in the same groups. Each prenatal and the postnatal visit lasted 2 hours. At these scheduled appointments, in addition to laboratory tests at the first visit, the women performed self-measures of their own vital signs and weight in a group space, and then they saw a midwife for a one-on-one physical assessment on a mat at the side of the group space. The group then gathered in a circle and the midwife and an assistant (co-facilitators) led interactive health promotion discussions. Co-facilitators and administrators had been trained to offer group PNC by experienced CenteringPregnancy midwives at a 3-day workshop.
Structured interviews were conducted at baseline, in late pregnancy (third trimester), and 6–8 weeks after delivery using Computer-Assisted Personal Interview software (Tufst University, Medford, MA, USA). Interviewers attended a 1-week ethics training that included mock recruitment and surveying. Women were contacted 2 weeks in advance to set up interviews. If a woman missed an interview, repeated calls were made when possible to set a new appointment for the interview.
RCT feasibility was assessed through direct observations of recruitment, implementation, retention, and group equivalence statistics, and post-intervention interviews with implementers and focus groups with women assigned to group PNC. The primary outcome measures were two measures of healthcare utilization: attendance at four PNC visits and attendance at the postnatal visit. We also collected data on several secondary outcomes, including satisfaction with PNC, PRES23, mental distress symptoms (measured using the SRQ-2024), healthy pregnancy knowledge, HIV-related knowledge, exclusive breastfeeding and adverse birth outcomes. Some of the secondary outcome data were collected to assess the feasibility of these measures for future studies and the present analyses were restricted to outcomes considered appropriate (Table 1). Detailed PRES analyses are described elsewhere.25
Table 1.
Variables and operational measures.
| Variable | Description | Time of measurement |
|---|---|---|
| Primary outcome: healthcare utilization | ||
| PNC attendance | Attended PNC ≥4 times: yes=1, no=0 (dichotomous) | After delivery |
| Postnatal Visit | Attended 6-week postnatal visit: yes=1, no=0 (dichotomous) | After delivery |
| Secondary outcomes | ||
| Satisfaction with PNC | 10-item index: response range from 1 (poor) to 5 (excellent); range 10–50, mean 33.9 ± 8.8, α=0.980 (continuous) | Late pregnancy |
| Pregnancy-related empowerment | 16-item Likert-type scale: range from 1 (strongly disagree) to 4 (strongly agree) to sense of control over pregnancy health and healthcare; range 16–64, α=0.996 (continuous) | Late pregnancy |
| Low mental distress | 20-item self-reporting questionnaire26 (yes/no) assessing mental distress; validated globally including Malawi and Tanzania; range 0–20, baseline α=0.789; late pregnancy α=0.848 | Baseline and late pregnancy |
| Exclusive breastfeeding | Only breastmilk since birth=1, other foods=0 (dichotomous) | After delivery |
| HIV knowledge | 5-item index: following UNAIDS guidelines, scores were dichotomized into comprehensive knowledge (all 5 items correct=1) or knowledge gaps (≥1 incorrect=0); women with perfect scores at baseline were excluded from the late pregnancy analysis (dichotomous) | Baseline and late pregnancy |
| Prevention of mother-to-child transmission | 4-item index: scored as 1 if all 4 were correct and 0 if ≥1 item was incorrect; late pregnancy analysis excluded women scoring 100% at baseline (dichotomous) | Baseline and late pregnancy |
Abbreviation: PNC, prenatal care.
Quantitative analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). Pearson X2 or Fisher exact tests were used to describe the sample, retention, and baseline differences by type of care and country. For single timepoint measures, crude logistic regression analyses were conducted for dichotomous outcomes and crude linear models for continuous measures. Crude generalized linear mixed models were used for dichotomous repeated measures. With significance set at P<0.05, the effect of type of care on outcomes was assessed for the total sample and for each country. Owing to the present study being a pilot trial, no power estimation was performed.
3 RESULTS
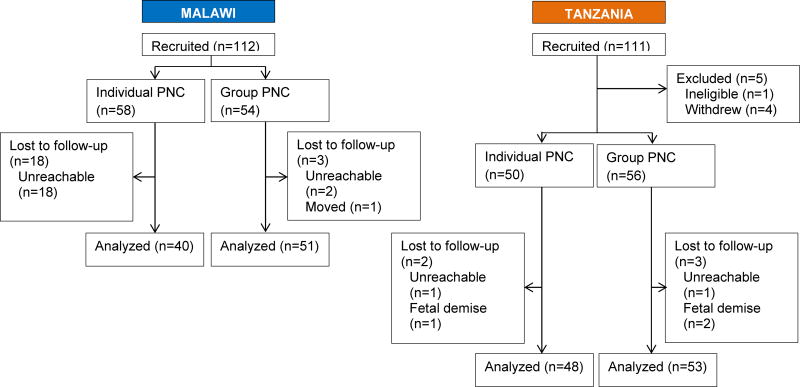
Eligibility was determined and women were randomly assigned into either individual care or group care; four groups were formed in each country, with a similar number of patients assigned to individual care. Patients were recruited to try to ensure that there were approximately 12 patients in each of the groups receiving group care to maintain consistency across the model. Approximately 50 women were assigned to both conditions in both countries (Figure 1). In Tanzania, four women withdrew because their husbands—who had not been present at recruitment—advised them to do so.
Figure 1.
Flow of patients through the study. Abbreviation: PNC, prenatal care.
Factors related to feasibility of a future large RCT were examined. Sociodemographic factors were first assessed by study condition to describe participants and assess whether the randomization yielded similar groups at baseline (Table S1). As expected, there were significant country differences for nearly every variable. In Malawi, there were more adolescents and more women in a relationship than in Tanzania. Furthermore, only one Muslim participated in Malawi, and the women there had less education and fewer household assets, and more food insecurity. Although random assignment was largely effective in creating similar groups overall, two baseline differences between study groups occurred: gravidity and relationship status (data not shown). Overall, recruitment and individual randomization were acceptable and resulted in equivalent groups.
Overall retention was high: 192 (88.1%) of 218 women were retained from the baseline to late pregnancy, and 193 (88.5%) from baseline to the postpartum interview, with 1 patient who missed the late pregnancy interview returning for the postpartum interview; this patient was excluded from the analyses. Those lost to follow-up were more likely to have less education (P<0.001) and fewer assets (P<0.001), and to report being Muslim (P=0.029) (data not shown). Retention was higher for group than individual PNC (104/110 [94.5%] vs 88/108 [81.5%]; P=0.013), and in Tanzania compared with Malawi (101/106 [95.3%] vs 91/112 [81.3%]; P=0.001). In Malawi, more than two-thirds of women did not have access to a cell phone, so they could not get interview reminder calls (data not shown). Malawian women assigned to individual PNC had the lowest retention rate (40/58 [69.0%]). To accommodate Malawian women assigned to group PNC, the interviewers conducted interviews on the days that group care was scheduled; 51 (94.4%) of 54 Malawian women in group care were retained. Consequently, there was a significant difference (P<0.001) at both late-pregnancy and the postpartum interviews in follow-up by treatment condition in Malawi. Although post hoc adjustment for baseline and attrition differences (data not shown) resulted in the same statistical conclusions as the unadjusted results presented here, disparate response rates by study condition must be addressed in a future RCT.
Two challenges in forming group PNC cohorts were encountered. First, at all sites, inaccuracy in pregnancy length estimates at enrollment meant that cohorts were not always actually within the intended range of 20–24 weeks. Recruitment at the smallest site—the rural satellite clinic in Malawi—presented another challenge. The urban site, with a caseload of more than 500 new PNC visits per month, took 3 days to complete recruitment; by contrast the smallest site, with a caseload of 40–50 visits per month, took a few weeks to recruit 24 women (one group PNC cohort and 12 controls). Another challenge for a future RCT was that there was no record keeping system in place linking a woman’s PNC record to her birth record.
Previous research25 established feasibility and high acceptability of group PNC and the ability to meet all of the fidelity requirements of CenteringPregnancy-based group care. Overall, group PNC sessions went smoothly in both countries, and administrators, co-facilitators, and participants enthusiastically supported it. The feasibility and acceptability results were consistent with those previously reported and qualitative evaluations are not repeated here.25 However, in the present pilot, duration of group PNC sessions was not consistent across sites. In Malawi, the facilitators implemented a flexible model, allowing sessions to continue until all issues were discussed. Women received nearly twice as much contact time per session compared with women in Tanzania, where appointments were limited to the assigned timeframe.
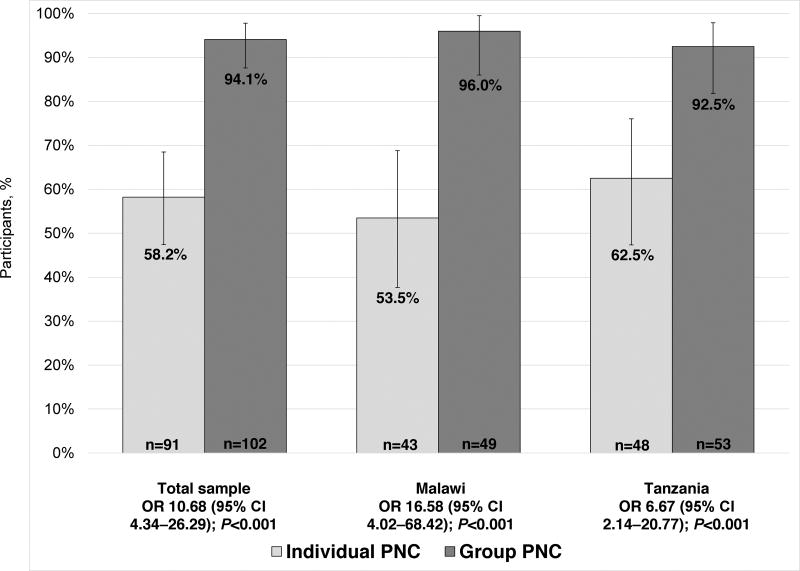
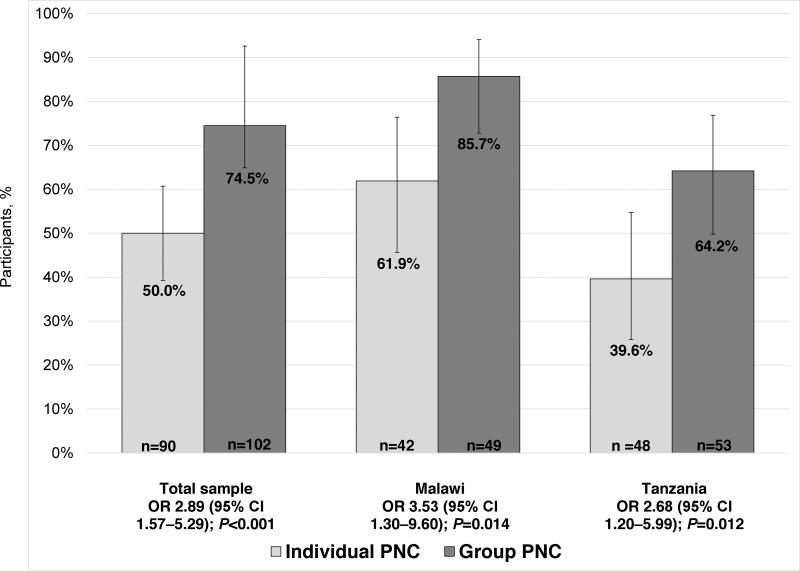
In terms of pilot outcomes, healthcare utilization was much higher overall among women assigned to group PNC than individual PNC at all clinics (Figure 2). Only 53 (58.2%) of 91 women assigned to individual PNC attended all four visits, compared with 96 (94.1%) of 102 women in group PNC (P<0.001). The pattern was similar for postnatal care (Figure 3): only 45 (50.0%) of 90 in individual PNC attended a 6-week postnatal visit, compared with 76 (74.5%) of 102 in group PNC (P<0.001). When healthcare utilization was examined within each country separately, these patterns held.
Figure 2.
Proportion of women who attended at least four PNC visits. Error bars illustrate 95% CIs. Abbreviations: PNC, prenatal care; OR, odds ratio; CI, confidence interval.
Figure 3.
Proportion of women who attended a 6-week postnatal visit. Error bars illustrate 95% CIs. Abbreviations: OR, odds ratio; CI, confidence interval; PNC, prenatal care.
Outcomes in terms of satisfaction, pregnancy-related empowerment, and mental distress are shown in Table 2. Satisfaction was significantly higher among women in group PNC than among those in individual PNC (P<0.001); this difference was recorded in both countries. Women in group PNC had higher scores on the PRES scale and lower mental distress scores than did women in individual care (P<0.001 and P=0.047, respectively). Significant differences were found for both PRES and mental distress within the two countries: outcomes were better among Malawian women in group PNC but there was no effect in Tanzania.
Table 2.
Satisfaction, pregnancy-related empowerment, and mental distress. a
| Outcome | Individual PNC |
Group PNC |
Difference (95% confidence interval) |
P value |
|---|---|---|---|---|
| Total sample b | ||||
| Satisfaction with PNC | 27.7 ± 6.6 | 39.2 ± 6.7 | 11.56 (9.65 to 13.47) | <0.001 |
| Pregnancy-related empowerment | 47.1 ± 6.4 | 55.1 ± 7.6 | 8.01 (6.00 to 10.04) | <0.001 |
| Mental distress | 3.5 ± 3.8 | 3.1 ± 3.4 | −1.13 (−2.24 to −0.02) | 0.047 |
| Malawi c | ||||
| Satisfaction with PNC | 23.7 ± 5.8 | 39.8 ± 7.2 | 16.10 (13.31 to 18.88) | <0.001 |
| Pregnancy-related empowerment | 43.7 ± 4.5 | 59.1 ± 5.9 | 15.36 (−13.13 to 17.59) | <0.001 |
| Mental distress | 4.6 ± 4.3 | 3.5 ± 3.7 | −2.13 (−3.77 to −0.50) | 0.012 |
| Tanzania d | ||||
| Satisfaction with PNC | 31.0 ± 5.3 | 38.7 ± 6.3 | 7.72 (5.41 to 10.02) | <0.001 |
| Pregnancy-related Empowerment | 50.0 ± 6.4 | 51.4 ± 7.1 | 1.40 (−1.29 to 4.08) | 0.305 |
| Mental distress | 2.6 ± 3.1 | 2.6 ± 3.0 | −0.24 (−1.69 to 1.20) | 0.743 |
Abbreviation: PNC, prenatal care.
Values are given as mean ± SD unless indicated otherwise.
Individual PNC n=88; group PNC n=104.
Individual PNC n=40; group PNC n=51.
Individual PNC n=48; group PNC n=53.
Logistic regression was used to assess EBF and the two HIV-related knowledge indices (Table 3). Nearly all women reported that they were practicing EBF. Although not significant, slightly more women in group PNC were practicing EBF than in individual PNC.
Table 3.
| Outcome | Individual PNC |
Group PNC | Odds ratio (95% confidence interval) |
P value |
|---|---|---|---|---|
| Total sample | ||||
| Exclusive breastfeeding at postpartum interview | 77/86 (89.5) | 95/99 (96.0) | 2.60 (0.81–8.36) | 0.108 |
| UNAIDS HIV knowledge | 29/51 (56.9) | 39/46 (84.8) | 4.59 (1.72–12.25) | 0.002 |
| PMTCT knowledge | 20/37 (54.1) | 33/44 (75.0) | 3.52 (1.29–9.64) | 0.014 |
| Malawi | ||||
| Exclusive breastfeeding at postpartum interview | 37/40 (92.5) | 48/49 (98.0) | 3.02 (0.41–21.88) | 0.275 |
| UNAIDS HIV knowledge | 8/17 (47.1) | 21/24 (87.5) | 6.55 (1.46–29.31) | 0.014 |
| PMTCT knowledge | 9/20 (45.0) | 18/21 (85.7) | 8.96 (1.78–45.02) | 0.008 |
| Tanzania | ||||
| Exclusive breastfeeding at postpartum interview | 40/46 (87.0) | 47/50 (94.0) | 2.18 (0.55–8.65) | 0.270 |
| UNAIDS HIV knowledge | 21/34 (61.8) | 18/22 (81.8) | 3.18 (0.88–11.55) | 0.078 |
| PMTCT knowledge | 11/17 (64.7) | 15/23 (65.2) | 1.52 (0.36–6.47) | 0.574 |
Abbreviation: PNC, prenatal care; PMTCT, prevention of mother-to-child transmission.
Values are given as number/total number (percentage) unless indicated otherwise.
For the two knowledge measures, cases that were 100% correct at baseline were excluded from analyses.
Among women with HIV-related knowledge gaps at baseline, a significantly higher proportion in group PNC than in individual PNC answered all the UNAIDS comprehensive HIV knowledge questions correctly in late pregnancy (P=0.002). PMTCT knowledge was also reported more frequently with group PNC than with individual PNC (P=0.014). There were interesting country differences for both knowledge measures. In Malawi, fewer than half the women in individual PNC answered all PMTCT questions correctly, compared with more than 85% of women in group care (P=0.008). In Tanzania, more women in group PNC than in individual PNC answered all UNAIDS questions correctly, but the difference was not significant.
4 DISCUSSION
In response to WHO’s call for more research to establish whether group PNC can be recommended in low-resource settings,1 the present study is the first of group PNC conducted in Sub-Saharan Africa to randomize at the individual level, as far as we are aware. The pilot study revealed several challenges that should be considered when designing RCTs of group PNC in low-resource settings. First, it is advisable that any RCT avoids small clinical sites so there is sufficient caseload to form PNC cohorts and enroll an equal number of controls using individual randomization. Second, accurate estimations of pregnancy length at enrollment are important, especially if the primary outcome is a birth outcome (e.g. preterm birth or birth weight). In some settings, ultrasonography might be possible; alternatively, a trained research nurse could estimate all the pregnancy lengths for uniformity. In the present study, retention was problematic in rural areas where many women lacked access to a cell phone. One cost-effective strategy might be to provide women with inexpensive phones. The need to link women’s individual PNC and delivery records could be addressed by introducing a hand-held device that health workers can use to quickly and systematically record data by woman across prenatal, delivery, and postpartum services. Alternatively, research personnel can be trained to collect medical data prospectively for all study participants. Finally, group PNC provider training needs to include a clear recommended duration of the session and how to manage sessions to start and end on time.
Moreover, the pilot results indicate promise for the effect of group PNC on healthcare utilization during and after pregnancy. Other outcomes—including satisfaction with PNC, PRES, mental distress symptoms, and HIV-related knowledge—were associated with improvements for women who attended group PNC.
The present small pilot had several limitations. It included an urban site in one country and a rural site in another, making it difficult to determine whether differences were due to country or rural–urban factors. Additionally, the sample size was small, so the preliminary results need to be interpreted with caution. The pilot study ended at 8 weeks after delivery; a longer follow-up period would allow for better assessment of exclusive breastfeeding and the impact on the uptake of family planning and safer sex practices.
The present study contributes to the science of improving quality of care in low-resource settings through the piloting of an innovative group healthcare delivery model. Before group PNC can be recommended as an alternative standard of care, a substantial evidence base must be built. It is important to avoid premature adoption of a model that later fails to live up to its promise, as happened with focused PNC.
Conducting a large RCT in the low-resource countries of Sub-Saharan Africa is important because this region contributes disproportionately to global perinatal mortality and preterm births. Several studies in the USA have found a reduction in premature births for women in CenteringPregnancy group PNC, but no study outside the USA has been large enough to identify impact on perinatal mortality or preterm birth. The present investigation makes an important contribution because it illustrated two important things. First, it established that it is possible to conduct an RCT with individual randomization (i.e. the standard rigor expected for clinical trials). Second, the outcomes indicate that group PNC has promise to effect change. However, the results should be interpreted with caution because the sample size was small and implementation occurred in only three health facilities. To address these weaknesses, the next step is to seek funding for a large RCT of group PNC in Sub-Saharan Africa, incorporating lessons learned from this pilot to examine health system impacts and outcomes for women and newborns.
Supplementary Material
Table S1 Sample sociodemographic factors by type of prenatal care and for each country.
Synopsis.
Pilot results from Malawi and Tanzania show that group prenatal care is associated with increased healthcare utilization and that a randomized controlled trial is feasible.
Acknowledgments
Funding was provided by National Institute for Nursing Research, National Institutes of Health (Grant NR014413). We acknowledge the contributions of CPN Kaponda who was involved in the original design of the study and oversaw implementation in Malawi. She has since taken on the role of High Commissioner to South Africa, precluding her continued involvement. In Tanzania, Willy Sangu supported this study from conception to implementation. We acknowledge the hard work of J Koovadaka in preparing the dataset for statistical analyses.
Footnotes
Author contributions
CLP, CSK, SCL and KFN contributed to the conception and design of the study. SCL organized data acquisition in Tanzania. ADS led the statistical analyses with the assistance of HP. All authors participated in the interpretation of the data and the writing and revising of the manuscript.
Conflicts of interest
The authors have no conflicts of interest.
References
- 1.World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 2.National Statistical Office of Malawi, ICF. Malawi Demographic and Health Survey 2015–16. Zomba, Malawi, and Rockville, Maryland, USA: National Statistical Office of Malawi and ICF; 2017. [Google Scholar]
- 3.Ministry of Health, Community Development, Gender, Elderly and Children of Tanzania, Ministry of Health of Zanzibar, National Bureau of Statistics of Tanzania, Office of Chief Government Statistician of Zanzibar, ICF. Tanzania Demographic and Health Survey and Malaria Indicator Survey 2015–2016 Final Report. Dar es Salaam, Tanzania, and Rockvillem Maryland, USA: Ministry of Health, Community Development, Gender, Elderly and Children, Ministry of Health, National Bureau of Statistics, Office of Chief Government Statistician, ICF; 2016. [Google Scholar]
- 4.von Both C, Flessa S, Makuwani A, Mpembeni R, Jahn A. How much time do health services spend on antenatal care? Implications for the introduction of the focused antenatal care model in Tanzania. BMC Pregnancy Childbirth. 2006;6:22. doi: 10.1186/1471-2393-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tetui M, Ekirapa EK, Bua J, Mutebi A, Tweheyo R, Waiswa P. Quality of antenatal care services in eastern Uganda: implications for interventions. Pan Afr Med J. 2012;13:27. [PMC free article] [PubMed] [Google Scholar]
- 6.Mason L, Dellicour S, Ter Kuile F, et al. Barriers and facilitators to antenatal and delivery care in western Kenya: a qualitative study. BMC Pregnancy Childbirth. 2015;15:26. doi: 10.1186/s12884-015-0453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts J, Sealy D, Marshak HH, Manda-Taylor L, Gleason P, Mataya R. The patient-provider relationship and antenatal care uptake at two referral hospitals in Malawi: A qualitative study. Malawi Med J. 2015;27:145–50. [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad P, De Allegri M, Moses A, et al. Antenatal care services in rural Uganda: missed opportunities for good-quality care. Qual Health Res. 2012;22:619–29. doi: 10.1177/1049732311431897. [DOI] [PubMed] [Google Scholar]
- 9.Conrad P, Schmid G, Tientrebeogo J, et al. Compliance with focused antenatal care services: do health workers in rural Burkina Faso, Uganda and Tanzania perform all ANC procedures? Trop Med Int Health. 2012;17:300–7. doi: 10.1111/j.1365-3156.2011.02923.x. [DOI] [PubMed] [Google Scholar]
- 10.Afnan-Holmes H, Magoma M, John T, et al. Tanzania’s Countdown to 2015: an analysis of two decades of progress and gaps for reproductive, maternal, newborn, and child health, to inform priorities for post-2015. Lancet Glob Heal. 2015;3:e396–409. doi: 10.1016/S2214-109X(15)00059-5. [DOI] [PubMed] [Google Scholar]
- 11.Kinney MV, Boldosser-Boesch A, McCallon B. Quality, equity, and dignity for women and babies. Lancet. 2016;388:2066–8. doi: 10.1016/S0140-6736(16)31525-2. [DOI] [PubMed] [Google Scholar]
- 12.Freedman LP. Implementation and aspiration gaps: whose view counts? Lancet. 2016;388:2068–9. doi: 10.1016/S0140-6736(16)31530-6. [DOI] [PubMed] [Google Scholar]
- 13.Singh K, Story WT, Moran AC. Assessing the Continuum of Care Pathway for Maternal Health in South Asia and Sub-Saharan Africa. Matern Child Health J. 2016;20:281–9. doi: 10.1007/s10995-015-1827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villar J, Ba’aqeel H, Piaggio G, et al. WHO antenatal care randomised trial for the evaluation of a new model of routine antenatal care. Lancet. 2001;357:1551–64. doi: 10.1016/s0140-6736(00)04722-x. [DOI] [PubMed] [Google Scholar]
- 15.Vogel JP, Habib NA, Souza JP, et al. Antenatal care packages with reduced visits and perinatal mortality: a secondary analysis of the WHO Antenatal Care Trial. Reprod Health. 2013;10:19. doi: 10.1186/1742-4755-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rising SS, Quimby CH. The CenteringPregnancy Model: The Power of Group Health Care. New York: Springer Publishing Company; 2016. [Google Scholar]
- 17.Earnshaw VA, Rosenthal L, Cunningham SD, et al. Exploring group composition among young, urban women of color in prenatal care: implications for satisfaction, engagement, and group attendance. Womens Health Issues. 2016;26:110–5. doi: 10.1016/j.whi.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ickovics JR, Earnshaw V, Lewis JB, et al. Cluster randomized controlled trial of group prenatal care: perinatal outcomes among adolescents in New York City health centers. Am J Public Health. 2016;106:359–65. doi: 10.2105/AJPH.2015.302960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kershaw TS, Magriples U, Westdahl C, Rising SS, Ickovics J. Pregnancy as a window of opportunity for HIV prevention: effects of an HIV intervention delivered within prenatal care. Am J Public Health. 2009;99:2079–86. doi: 10.2105/AJPH.2008.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maru S. Field Notes: CenteringPregnancy-Based Group Antenatal Care in Achham district, Nepal. In: Rising SS, Quimby CH, editors. The CenteringPregnancy Model: The Power of Group Health Care. New York: Springer Publishing Company; 2016. [Google Scholar]
- 21.Lori JR, Ofosu-Darkwah H, Boyd CJ, Banerjee T, Adanu RMK. Improving health literacy through group antenatal care: a prospective cohort study. BMC Pregnancy Childbirth. 2017;17:228. doi: 10.1186/s12884-017-1414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafari F, Eftekhar H, Fotouhi A, Mohammad K, Hantoushzadeh S. Comparison of maternal and neonatal outcomes of group versus individual prenatal care: a new experience in Iran. Health Care Women Int. 2012;31:571–84. doi: 10.1080/07399331003646323. [DOI] [PubMed] [Google Scholar]
- 23.Klima C, Vonderheid SC, Norr KF. Measuring empowerment in pregnancy: the pregnancy-related empowerment scale [Abstract] J Midwifery Womens Health. 2007;52:531. [Google Scholar]
- 24.Beusenberg M, Orley JH World Health Organization. A User's guide to the self reporting questionnaire (SRQ) http://apps.who.int/iris/bitstream/10665/61113/1/WHO_MNH_PSF_94.8.pdf. Published 1994.
- 25.Patil CL, Abrams ET, Klima C, et al. CenteringPregnancy-Africa: a pilot of group antenatal care to address Millennium Development Goals. Midwifery. 2013;29:1190–8. doi: 10.1016/j.midw.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil CL, Klima CS, Leshabari SC, et al. Randomized controlled pilot of a group antenatal care model and the sociodemographic factors associated with pregnancy-related empowerment in sub-Saharan Africa. BMC Pregnancy Childbirth. doi: 10.1186/s12884-017-1493-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Sample sociodemographic factors by type of prenatal care and for each country.