Abstract
RASGRP1 is a guanine-nucleotide-exchange factor essential for MAP-kinase mediated signaling in lymphocytes. We report the second case of RASGRP1 deficiency in a patient with a homozygous nonsense mutation in the catalytic domain of the protein. The patient had epidermodysplasia verruciformis, suggesting a clinically important intrinsic T cell function defect. Like the previously described patient, our proband also presented with CD4+ T cell lymphopenia, impaired T cell proliferation to mitogens and antigens, reduced NK cell function, and EBV-associated lymphoma. The severity of the disease and the development of EBV lymphoma in both patients suggest that hematopoietic stem cell transplantation should be performed rapidly in patients with RASGRP1 deficiency.
Keywords: RASGRP1 deficiency, Epstein Barr Virus, lymphoma, epidermodysplasia verruciformis
To the editor
RAS guanyl releasing protein 1 (RASGRP1) is a guanine-nucleotide-exchange factor (GEF) that converts the small GTPase RAS from an inactive GDP-bound state to an active GTP-bound state in response to lymphocyte activation [1–3]. Activated RAS initiates a MAP-kinase cascade, which leads to cytoskeletal reorganization and transcription of effector molecules [4, 5]. Studies of murine models have shown that RASGRP1 signaling is essential for thymocyte development and function, as well as B cell tolerance [1, 6]. Only a single case of human RASGRP1 deficiency has been reported in the literature, in a patient with recurrent pneumonias, severe failure to thrive, herpetic lesions, and EBV-associated lymphoma [5]. Laboratory findings were notable for CD4+ T cell lymphopenia, poor T cell proliferation to mitogens, and defective NK function. IgG levels were normal, though antibody responses to hepatitis B and pneumococcal vaccines were poor [5]. We report a second case of RASGRP1 deficiency due to a novel nonsense mutation in RASGRP1.
The patient was born to consanguineous Iraqi parents (Fig. 1A). She presented at 6 months of age with autoimmune hemolytic anemia and thrombocytopenia that resolved after treatment with steroids. She subsequently developed recurrent ear infections, skin abscesses, chronic non-bloody diarrhea, disseminated warts and severe failure to thrive. At 10 years of age, while living in Turkey, the patient developed splenomegaly and diffuse lymphadenopathy. Immune phenotyping revealed CD4+ T cell lymphopenia (410 cells/µl), elevated numbers of CD8+ T cells (2747 cells/µl), low IgG (281 mg/dL) and elevated IgM (406 mg/dL) (Table 1). She was started on intravenous immunoglobulin (IVIG) replacement therapy and antibiotic prophylaxis with trimethoprim/sulfamethoxazole. A lymph node biopsy revealed EBV-positive lymphoproliferative disease, which was treated with chemotherapy. Despite initial improvement, the lymphadenopathy returned, and on repeat lymph node biopsy 10 months later, she was diagnosed with EBV positive polymorphic B-cell lymphoma.
Figure 1.
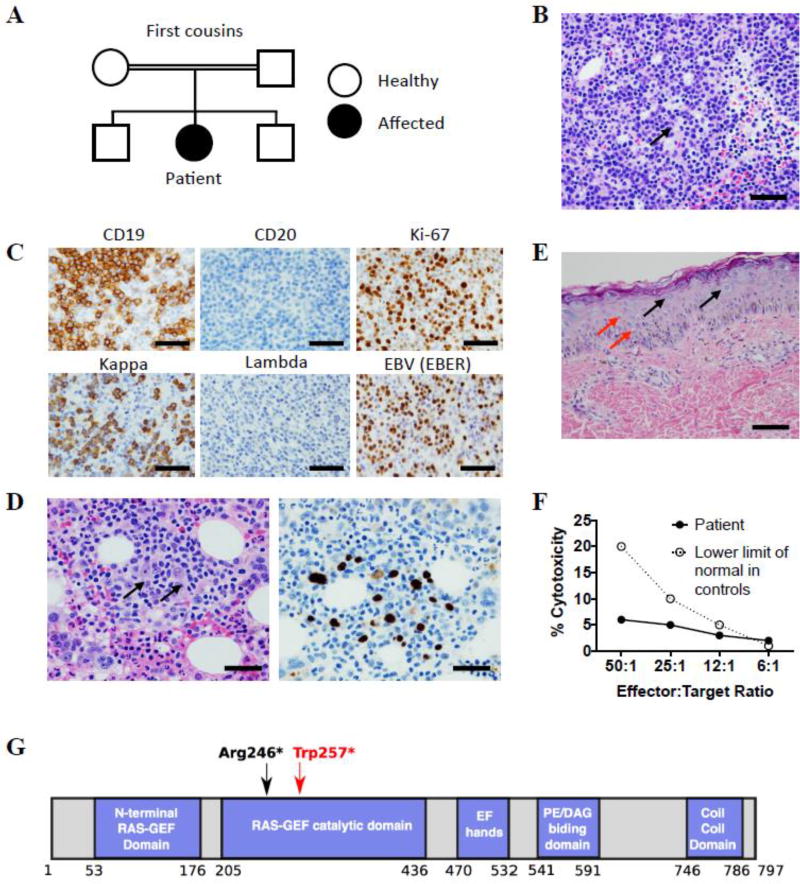
Characterization of patient phenotype. (A) Family pedigree. (B) H&E stained section of inguinal lymph node biopsy. Arrow indicates larger atypical cell, scale bar = 100 µM. (C) Inguinal lymph node biopsy: immunohistochemistry staining for CD19, CD20 and Ki-67 (top panels) and in situ hybridization for kappa, lambda and EBER (bottom panels), scale bars = 100 µM. (D) Bone marrow biopsy: H&E staining (left panel), and in situ hybridization for EBER (right panel). Arrows indicate large atypical Hodgkin-like cells, scale bars = 50 µM. (E) H&E stain of skin biopsy showing numerous large blue cells in the epidermis (black arrows) and koilocytes (red arrows), scale bar = 50 µM. (F) NK cell cytotoxicity in vitro: chromium-51 release assay using peripheral blood mononuclear cells at indicated effector to target ratios. The assay was performed in the Cincinnati Children’s Diagnostic Immunology Laboratory. (G) RASGRP1 protein domains. Red arrow indicates position of the patient’s mutation, black arrow indicates the previously reported mutation.
Table 1.
Immunological profile of the patient
| Patient age at the time of testing | 10 years | 12 years |
|---|---|---|
|
| ||
| Complete Blood Count (normal range) a | ||
|
| ||
| Hemoglobin, g/dL | 10.3 (11.3–13.4) | 8.2 (11.3–13.4) |
|
| ||
| WBCs, 103 cells/µL | 7.10 (5.41–9.70) | 1.85 (5.41–9.70) |
|
| ||
| Neutrophils, 103 cells/µL | 3.30 (2.58–5.95) | 0.45 (2.58–5.95) |
|
| ||
| Lymphocytes, 103 cells/µL | 4.10 (1.23–2.76) | 1.34 (1.23–2.76) |
|
| ||
| Monocytes, 103 cells/µL | ND | 0.05 (0.19–0.81) |
|
| ||
| Platelets, 103 cells/µL | 261(187–376) | 35 (187–376) |
|
| ||
| Lymphocyte subsets (normal range) a | ||
|
| ||
| CD3+, 103 cells/µL | 3403 (1000–2600) | 1840 (1000–2600) |
|
| ||
| CD3+CD4+, 103 cells/µL | 410 | 220 (530–1500) |
|
| ||
| % CD4+CD45RA+CCR7- | ND | 0.2 (0.2–2.1) |
| % CD4+CD45RA+CCR7+ | ND | 0.1 (7.8–25.9) |
| % CD4+CD45RA-CCR7- | ND | 97.1 (7.80–25.9) |
| % CD4+CD45RA-CCR7+ | ND | 2.6 (21.0–41.3) |
|
| ||
| CD3+CD8+, 103 cells/µL | 2747 (330–1100) | 1510 (330–1100) |
|
| ||
| % CD8+CD45RA+CCR7- | ND | 7.7 (8.7–38.0) |
| % CD8+CD45RA+CCR7+ | ND | 0.2 (31.1–73.2) |
| % CD8+CD45RA-CCR7- | ND | 91.3 (8.8–44.4) |
| % CD8+CD45RA-CCR7+ | ND | 0.9 (2.6–8.7) |
|
| ||
| CD19+, 103 cells/µL | ND | 0* (270–860) |
|
| ||
| CD16+/CD56+, 103 cells/µL | 410 (70–480) | 162 (70–480) |
|
| ||
| Immunoglobulins (normal range) a | ||
|
| ||
| IgG, mg/dL | 281 (639–1344) | 466** (639–1344) |
|
| ||
| IgM, mg/dL | 406 (40–240) | 118 (40–240) |
|
| ||
| IgA, mg/dL | 15 (70–312) | <7 (70–312) |
| IgE, kU/L | <5 (0–12) | <1 (0–500) |
|
| ||
| Proliferation (normal range) a | ||
|
| ||
| PHA | ND | 34,746 (96,090– 358,179) |
|
| ||
| Tetanus toxoid | ND | 485 (8544–102,895) |
|
| ||
| Candida albicans | ND | 2188 (6231–197,940) |
normal values from age-matched controls in the Boston Children’s Hospital clinical immunology laboratory. Values in bold are outside of the normal range.
ND, not done
after rituximab administration
on IVIG
She initially continued chemotherapy in Turkey, but in February 2017, the patient and her family traveled to the United States where she received treatment for lymphoma at UMass Memorial Medical Center. She was referred to Boston Children’s Hospital in March 2017 for additional immunologic evaluation and potential hematopoietic stem cell transplantation (HSCT). Inguinal lymph node biopsy performed at that time revealed a diffuse lymphoid proliferation of medium-large cells with plasmablastic/plasmacytic differentiation and scattered Reed-Sternberg-like cells (Fig. 1B). Immunohistochemistry revealed that the atypical lymphoid population was positive for CD19 and negative for CD20 with a high proliferation index based on Ki-67 staining (Fig. 1C, top panels). In situ hybridization for Ig light chains demonstrated clonality for kappa light chain (Fig. 1C, bottom left panels). In situ hybridization for Epstein-Barr virus (EBV) encoded RNA (EBER) was positive in the majority of atypical lymphoid cells (Fig. 1C, bottom right panel). Flow cytometry and molecular studies for IGH gene rearrangement confirmed the B cell clonality for kappa light chain (data not shown). These findings are consistent with EBV-positive large B-cell lymphoma. Bone marrow analysis revealed a focal infiltrate of atypical EBV-positive cells, consistent with bone marrow involvement by the lymphoma (Fig. 1D). Histologic examination of hematoxylin-eosin (H&E)–stained sections of the patient’s skin lesions revealed an irregular stratum granulosum containing large blue-gray cells, and koilocytes, epithelial cells with nuclei displaced by perinuclear haloes (Fig. 1E). These findings are highly consistent with epidermodysplasia verruciformis [7]. Immunological evaluation revealed worsened CD4+ T cell lymphopenia (220 cells/µl), elevated numbers of CD8+ T cells (1510 cells/µl), and absent B cells in the context of prior rituximab administration. She had nearly absent CD4+CD45RA+CCR7+ and CD8+CD45RA+CCR7+ naïve T cells (0.1% and 0.2% respectively). She had poor T cell proliferation to PHA, candida, and tetanus toxoid (Table 1). While her NK cell number was normal (162 cells/µl), NK cytolytic activity was markedly depressed (Fig. 1F). No further evaluation was done because the patient’s condition deteriorated, and she died of complications of lymphoma in July 2017.
Given parental consanguinity, a single gene defect with autosomal recessive inheritance was suspected. Whole exome sequencing (WES) revealed a homozygous nonsense variant within the catalytic domain of RASGRP1 (c.771G>A;p.Trp257*). The mutation introduces a premature stop codon at position 257 (Fig. 1G). This variant was not present in the 1000 Genomes, ExAC or the NHLBI Exome Sequencing Project databases. The mutation was present in heterozygous form in the patient’s healthy 13-year-old brother for whom lymphocyte subsets, T cell proliferation to mitogens and antigens, and vaccine responses were normal. This is consistent with the autosomal recessive mode of inheritance of the disease in the patient. Unfortunately, because the diagnosis was made shortly before the patient’s death, no cells were available for immunoblotting to determine whether the predicted truncated mutant is expressed. The previously reported patient with RASGRP1 deficiency had a nonsense mutation leading to truncation of the protein at residue 246, just 11 residues upstream of our patient’s truncation. This led to complete loss of protein expression, suggesting that protein expression may have also been abolished in our patient. Due to the patient’s death, we could not examine her cells for RAS dependent phosphorylation of ERK, which was impaired in the previously reported case of RASGRP1 deficiency [5]. Our patient and the previously reported patient with RASGRP1 deficiency shared markedly similar features that include recurrent infections, CD4+ T cell lymphopenia, poor T cell proliferation to mitogens, defective NK function, and EBV-associated lymphoma. Thus, it is certain that the homozygous RASGRP1 c.771G>A;p.Trp257* nonsense variant is responsible for our patient’s disease.
Murine studies have shown a role for RASGRP1 in B cell function that is largely restricted to suppression of autoantibody generation [2, 6]. However, both patients with RASGRP1 deficiency showed broader dysregulation of the humoral response. The previously reported patient had normal IgG levels but poor vaccine titers. While vaccine titers were not available for our patient, she developed hypogammaglobulinemia requiring IVIG. The previously reported patient with RASGRP1 deficiency displayed reduced in vitro proliferation and class switching of primary B cells and reduced ERK phosphorylation in EBV-immortalized B cells following IgM stimulation [5]. These findings are all consistent with an intrinsic defect in RASGRP1-deficient B cells.
While both patients with RASGRP1 deficiency demonstrated increased susceptibility to viral infections, our patient’s epidermodysplasia verruciformis (EV) is a unique finding. This rare dermatosis is caused by an increased susceptibility to cutaneous human papillomavirus infections (HPV), leading to persistent flat warts [7]. Approximately 75% of patients with this disorder harbor homozygous mutations in TMC6 (EVER1) or TMC8 (EVER2), though mutations in a number of other genes that impair T cell function such as RHOH, MST1, CORO1A, IL7 and DCLRE1C have been shown to cause an EV [7, 8]. The CD4+ cell lymphopenia in both patients and the presence of EV in our patient strongly suggests that RASGRP1 is essential not only for T cell homeostasis but also for T cell function. Of interest, the levels of phosphorylated ERK were reduced in activated T cells from the previously described patient with RASGRP1 deficiency [5].
A common feature in both patients was the development of EBV-positive lymphoma during childhood. RASGRP1 deficiency should now be considered among the growing number of primary immunodeficiencies associated with susceptibility to EBV and EBV-driven malignancy, particularly in patients with susceptibility to viral infections [9]. Further studies are necessary to dissect why intact RASGRP1 signaling is particularly important for control of EBV. The high risk of EBV driven malignancy early in life suggests that HSCT should be considered in patients with RASGRP1 deficiency promptly after diagnosis.
Highlights.
We report the second known case of deficiency of RASGRP1 deficiency.
RASGRP1 is essential for MAP-kinase mediated signaling in lymphocytes.
Patients with RASGRP1 deficiency present with a combined immunodeficiency.
These patients have increased susceptibility to EBV-driven lymphoma.
Acknowledgments
Supported by: USPHS grants K12 HD052896-10 (C.D.P.), 1K08AI116979-01 (J.C.), 1R21-AI124101 (R.S.G.) and the Perkin Fund (R.S.G.).
Abbreviations
- EV
epidermodysplasia verruciformis
- EBV
Epstein Barr virus
- EBER
Epstein-Barr virus encoded RNA
- GEF
guanine-nucleotide-exchange factor
- HSCT
hematopoietic stem cell transplantation
- H&E
hematoxylin-eosin
- HPV
human papillomavirus infections
- RASGRP1
RAS guanyl releasing protein 1
- WES
whole exome sequencing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exists.
References
- 1.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 2.Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol. 2005;175:7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 3.Lee SH, Yun S, Lee J, Kim MJ, Piao ZH, Jeong M, Chung JW, Kim TD, Yoon SR, Greenberg PD, Choi I. RasGRP1 is required for human NK cell function. J Immunol. 2009;183:7931–7938. doi: 10.4049/jimmunol.0902012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogquist K. RasGRP: the missing link for Ras activation in thymocytes. Trends Immunol. 2001;22:69. doi: 10.1016/s1471-4906(00)01845-7. [DOI] [PubMed] [Google Scholar]
- 5.Salzer E, Cagdas D, Hons M, Mace EM, Garncarz W, Petronczki OY, Platzer R, Pfajfer L, Bilic I, Ban SA, Willmann KL, Mukherjee M, Supper V, Hsu HT, Banerjee PP, Sinha P, McClanahan F, Zlabinger GJ, Pickl WF, Gribben JG, Stockinger H, Bennett KL, Huppa JB, Dupre L, Sanal O, Jager U, Sixt M, Tezcan I, Orange JS, Boztug K. RASGRP1 deficiency causes immunodeficiency with impaired cytoskeletal dynamics. Nat Immunol. 2016;17:1352–1360. doi: 10.1038/ni.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett A, Buhlmann JE, Stone J, Lim B, Barrington RA. Multiple checkpoint breach of B cell tolerance in Rasgrp1-deficient mice. J Immunol. 2013;191:3605–3613. doi: 10.4049/jimmunol.1202892. [DOI] [PubMed] [Google Scholar]
- 7.Przybyszewska J, Zlotogorski A, Ramot Y. Re-evaluation of epidermodysplasia verruciformis: Reconciling more than 90 years of debate. J Am Acad Dermatol. 2017;76:1161–1175. doi: 10.1016/j.jaad.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Tahiat A, Badran YR, Chou J, Cangemi B, Lefranc G, Labgaa ZM, Oussalam S, Kaddouri-Slimani A, Belarbi A, Bendissari-Bouzid K, Gharnaout M, Geha RS, Djidjik R, Massaad MJ. Epidermodysplasia verruciformis as a manifestation of ARTEMIS deficiency in a young adult. J Allergy Clin Immunol. 2017;139:372–375. e374. doi: 10.1016/j.jaci.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Palendira U, Rickinson AB. Primary immunodeficiencies and the control of Epstein-Barr virus infection. Ann N Y Acad Sci. 2015;1356:22–44. doi: 10.1111/nyas.12937. [DOI] [PubMed] [Google Scholar]


