Abstract
Although autoantibodies have been used for decades as diagnostic and prognostic markers in type 1 diabetes (T1D), further analysis of developmental abnormalities in B cells could reveal tolerance checkpoint defects that could improve individualized therapy. To evaluate B cell developmental progression in T1D, immunophenotyping was used to classify circulating B cells into transitional, mature naïve, mature activated, and resting memory subsets. Then each subset was analyzed for the expression of additional maturation-associated markers. While the frequencies of B cell subsets did not differ significantly between patients and controls, some T1D subjects exhibited reduced proportions of B cells that expressed transmembrane activator and CAML interactor (TACI) and Fas receptor (FasR). Furthermore, some T1D subjects had B cell subsets with lower frequencies of class switching. These results suggest circulating B cells exhibit variable maturation phenotypes in T1D. These phenotypic variations may correlate with differences in B cell selection in individual T1D patients.
Keywords: B lymphocytes, type 1 diabetes, TACI, FasR
1. Introduction
T1D is an autoimmune disease that targets the insulin-producing beta cells in the pancreas. It affects approximately 1 in 300 people in the United States by 18 years of age. Epidemiologic studies show that the incidence of T1D has been increasing by 2–5% worldwide for unknown reasons.[1] B lymphocytes (B cells) appear to contribute to the pathogenesis of T1D. B cells are needed for the initiation of insulitis and act as critical antigen presenting cells in the initiation of T cell-mediated autoimmune diabetes in the nonobese diabetic mouse model (NOD). [2–6] In addition, a lack of B cells prevents insulitis [7] and B cell depletion ameliorates T1D in mice.[2, 8] Furthermore, a seminal study in humans demonstrated that therapy with the B cell depleting antibody, rituximab (anti-CD20), slowed T1D disease after onset, but did not alleviate disease progression long-term.[9, 10]
In contrast to murine studies, the published literature on B cells in humans with T1D is sparse. An early study reported increased CD5+CD19+ B cells in pediatric new onset T1D patients relative to patients with established disease for greater than 30 days or healthy controls. [11] Increases in CD5+ B cells have also been in observed in other disease states, including autoimmune disease [12]. In addition to B1-like B cells in humans [13], CD5 can also be expressed on transitional B cells [14] and pre-naïve B cells [15]. To fully distinguish B1-like B cells from these other cell subsets requires more in-depth flow experimental autoimmune analysis. In another study, B cell subsets were analyzed from cryopreserved peripheral blood mononuclear cells in healthy controls, adults with long standing T1D, new onset T1D patients, and age-matched unaffected siblings of other T1D patients.[16] No statistically significant differences were observed in the percentages of any of the B cell subsets. However, cryopreservation can induce significant changes in several critical B cell subsetting markers, including CD27 and CD38.[17]
Deng and colleagues used fresh blood [18] and larger patient cohorts to study circulating B lymphocyte subsets. They found that T1D patients had decreased percentages of B10 B cells (which they defined as CD19+CD5+CD1dhi) and follicular B cells (CD19+, CD23+, CD21−), and an increased percentage of marginal zone-like B cells (CD19+, CD21+, CD23−) compared to healthy controls. They also extended their analysis to patients with latent autoimmune diabetes and type 2 diabetes, and reported a negative association between the proportion of B10 cells and HbA1c levels. However, some of the markers used to define B cell subsets did not yield well-resolved populations. For example, marginal zone B cells in humans are typically also defined with CD27 expression [19]; the use of CD21 and CD23, based largely upon an analogy with murine B cell subsets (discussed in [20]), may not be sufficient.
Taken together, the previous analyses of circulating B cell subsets provide an unclear and inconsistent picture. In some patient groups, an increased frequency of CD5+ B cells is observed, but is unclear if the CD5+ B cells represent an autoimmune-prone B1-like population or enhanced production of early-stage B cells, as frequencies of CD5+ transitional cells are elevated in early childhood [21]. Other studies either failed to show differences between T1D subjects and controls, or showed differences, using limited subsetting schema [16, 18]. Despite their technical limitations, the preceding data point to potential correlations between T1D and alterations in the peripheral B cell compartment. These data suggest that further evaluation of circulating B cell subsets is warranted.
We therefore revisited the definition of peripheral B cell subsets in T1D subjects using 11-color B cell subsetting panels that provide a more nuanced evaluation of subsets and their maturation process. By defining these subsets more thoroughly, we hope to gain better insights into how B cell maturation is altered in T1D. We also included a basic analysis of T lymphocytes and natural killer cells (NK) to determine if any T1D subjects with B cell abnormalities also had unusual T cell or NK cell phenotypes.
2. Material and methods
2.1 Subjects
The Institutional Review Board at The University of Pennsylvania approved this study, and all subjects provided written informed consent to participate. Subjects were recruited from the Rodebaugh Diabetes Center and an outpatient clinic at the Hospital of the University of Pennsylvania. A total of 16 adult patients with established T1D and 16 nondiabetic control subjects were included in the final analysis. The patients and controls were studied at a single study visit using the same flow cytometry panels contemporaneously over a 10-month period. The control group of subjects were previously also used as healthy controls in two separate unrelated studies; one previous study evaluated B cell subsets and antibody repertoire in patients with systemic lupus erythematous[22], while the other previous study examined the variability of circulating lymphocytes in adults over time.[23] The diagnosis for T1D was established by clinical characteristics including initial presentation, laboratory values such as reduced C-peptide and insulin-dependence. The demographic and clinical characteristics of the patients and controls are provided in Table 1.
Table 1. Clinical and demographic features of study subjects.
Data are represented as numbers. Numbers are shown with mean ± standard deviation. Percentages are given in parentheses. Other = mixed race. T1D = type 1 diabetes. NA = not applicable. HbA1c = hemoglobin A1C.
| Controls (n = 16) | T1D (n = 16) | |
|---|---|---|
| Female (%) | 15 (94) | 9 (56) |
| Male (%) | 1 (6) | 7 (44) |
| Age (years) | 31.75 ± 8.17 | 34.75 ± 13.13 |
| Age at diagnosis (years) | NA | 15.50 ± 10.43 |
| Duration of diabetes (years) | NA | 19.25 ± 10.99 |
| Caucasian (%) | 9 (56) | 13 (81) |
| African American (%) | 4 (25) | 1 (6) |
| Asian (%) | 2 (13) | 1 (6) |
| Hispanic (%) | 1 (6) | 0 (0) |
| Other (%) | 0 (0) | 1 (6) |
| HbA1c (%) | NA | 7.10 ± 0.97 |
2.2 Flow Cytometry
Peripheral blood was collected in K2EDTA tubes and processed within 24 hours of collection for flow cytometry using the techniques described in [24]. Typically samples were drawn in the morning and processed and stained on the same day for flow cytometry (a detailed procedure for staining and flow cytometry is provided in [23]). Samples were analyzed by multicolor immunophenotyping using the antibody panels listed in Table S1. The same lot numbers of antibodies used throughout the study. Data were acquired on an LSR flow cytometer (BD Biosciences, Franklin Lakes, NJ) in the Penn Flow Cytometry Shared Resource. Cytometer settings were standardized between runs using cytometer set-up and tracking beads (BD CST) and CVs in each of the detectors. PMT voltages were adjusted accordingly and baselines were re-established with new bead lots. Total event counts typically ranged from 500,000 to 1 million per tube. Data were analyzed with FlowJo v9.4.10 software (Treestar Inc., Ashland, OR). Gating schemes for T cell, NK cell and B cell subsets are shown in Supplementary Figures S1a and S1b. The B cell subsets were defined as: transitional B cells (TR) (CD27−, CD38++), mature naive B cells (MN) (CD27−, CD38+), mature activated B cells (MA) (CD27+, CD38+), plasmablasts (CD27++, CD38++), and resting memory B cells (RM) (CD27+, CD38−). Individual B cell subsets were further evaluated for IgM, CD10, CD138, CD21, CD23, CD95 (FasR), and CD267 (TACI) expression. In parallel, blood samples were drawn and processed for absolute lymphocyte counts by Coulter counting (complete blood count with electronic differential) at the William Pepper laboratory in the Hospital of the University of Pennsylvania.
2.3 Statistical analyses
The Mann Whitney test was used for the comparisons of values between groups for all data. To test for correlations between B cell subset fractions and subject age, body mass index or length of disease, Spearman correlations were used. Significance for all statistical tests was set at p ≤ 0.05.
3. Results
3.1 General analysis of lymphocyte subsets
We evaluated the maturation of peripheral blood B cells as well as NK cells and CD4+ and CD8+ T cells in 16 adult patients with T1D and 16 non-diabetic control subjects using multicolor immunophenotyping. Our analysis focused mainly on the B lymphocyte compartment with 4 out of the 6 tubes tailored to more B specific markers, in addition to a T cell tube and a NK cell tube (panels are defined in Table S1). The percentages of different major lymphocyte subsets analyzed in controls and T1D subjects are included in Table 2. The B cell analysis revealed no statistically significant differences in the percentages of CD19+, CD10+ CD27−, transitional (TR), mature naïve (MN), mature activated (MA), resting memory (RM) or plasmablast subsets between controls and T1D subjects. However there was a reduced relative frequency of CD24++CD38++ B cells in T1D; these cells may correspond to a regulatory B cell population.[25–27] The analysis of CD3+ positive lymphocytes (T cells) demonstrated no statistically significant differences in CD4+ or CD8+ subsets between controls and T1D subjects. The non-B, non-T cell (CD3− CD19−) analysis revealed statistically significant differences in CD16+ CD56− and CD16− CD56+ presumed NK cells, with T1D subjects having higher percentages of both NK subsets. Using absolute cell counts (Table S2), controls had a statistically significant increase in mature activated (MA; CD27+, CD38+) B cells compared to T1D subjects. T1D subjects also had increased absolute counts of NK cells. Table S3 provides the percentage and absolute cell count data for all subsets evaluated in all control subjects, and Table S4 has the percentage and absolute cell count data for all subsets evaluated in all T1D subjects.
Table 2. Lymphocyte subset fractions.
Lymphoid subsets are given as mean percentages ± standard deviation of the parent population (denoted in bold font).
| B cells (CD19+) | Controls | T1D | p-value |
|---|---|---|---|
| CD19+ | 11.97 ± 5.93 | 10.09 ± 5.77 | 0.3962 |
| CD10+ CD 27− | 6.24 ± 4.00 | 5.43 ± 4.22 | 0.3659 |
| Trans | 5.25 ± 1.88 | 6.76 ± 3.12 | 0.2099 |
| MN | 68.68 ± 12.64 | 70.53 ± 9.49 | 0.7804 |
| MA | 18.82 ± 9.81 | 13.41 ± 5.66 | 0.0996 |
| RM | 4.41 ± 3.76 | 5.46 ± 6.05 | 0.8672 |
| PB | 0.61 ± 0.72 | 0.47 ± 0.28 | 0.6352 |
| CD24+, CD38++ | 2.67 ± 1.15 | 1.54 ± 0.85 | *0.0045 |
| T cells (CD3+) | Controls | T1D | p-value |
| CD3+ | 71.53 ± 9.26 | 73.48 ± 7.97 | 0.596 |
| CD4+ | 62.39 ± 10.15 | 66.64 ± 8.60 | 0.4909 |
| CD8+ | 31.52 ± 9.29 | 28.94 ± 8.02 | 0.9932 |
| CD4+/CD8+ | 0.83 ± 0.89 | 0.48 ± 0.22 | 0.2425 |
| CD4−/CD8− | 5.26 ± 2.71 | 3.92 ± 1.00 | 0.2661 |
| NK cells (CD3− CD19−) | Controls | T1D | p-value |
| CD56+ | 38.53 ± 18.46 | 50.21 ± 18.19 | 0.0865 |
| CD56− | 41.54 ± 19.34 | 46.43 ± 17.94 | 0.423 |
| CD16+/CD56+ | 36.43 ± 18.43 | 38.54 ± 16.66 | 0.724 |
| CD16+/CD56− | 5.10 ± 2.33 | 7.89 ± 3.23 | *0.0066 |
| CD16−/CD56+ | 2.10 ± 0.64 | 3.78 ± 1.59 | *0.0007 |
The asterisk (*) indicates a statistically significant difference between T1D and control subjects, p-value < 0.05 (Mann Whitney test).
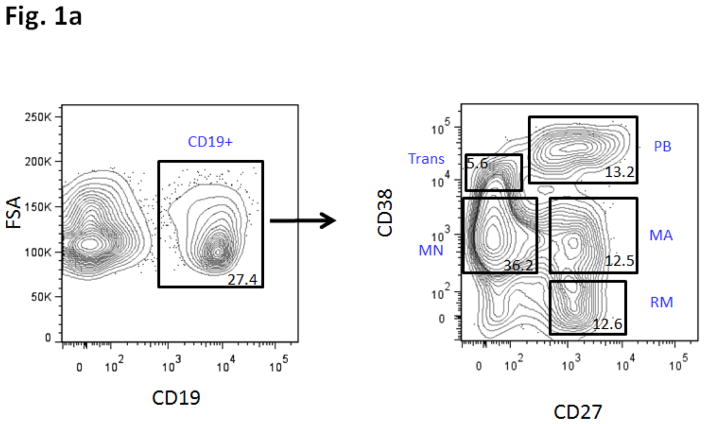
Trans = transitional. MN = mature naïve. MA = mature activated. RM = resting memory. PB = plasmablasts. Please see Figure 1a for definitions of Trans, MA, MN, RM and PB, based on CD38 vs. CD27 staining.
3.2 Multiple phenotypic abnormalities in B cell subsets in T1D
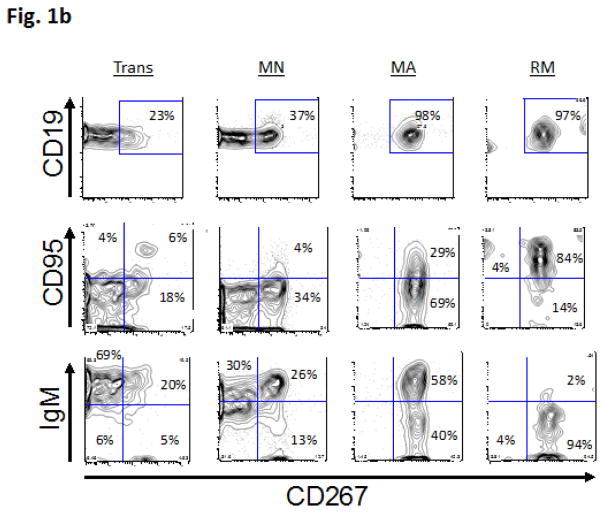
Based upon previous studies and mouse models of T1D [2–11], we hypothesized that T1D patients would harbor peripheral B cell subset differences compared to healthy controls. Consistent with this hypothesis, we observed several immunophenotypic differences between T1D subjects and controls. To reveal these differences, we began by analyzing the B cell subsets themselves and then characterizing additional phenotypic markers within each of the subsets. Circulating B cell subsets in humans can be classified into TR, MN, MA, and RM subsets on the basis of CD27 and CD38 expression (see Methods and Figure 1a, and our previous use of this subsetting scheme to define B cell subsets in humans [23]). Based upon patterns of autoreconstitution following rituximab therapy and/or myeloablative chemotherapy, we know that TR cells are the first B cells to emigrate from the bone marrow following primary maturation, and enter the circulation. [24, 28–31] Within the circulation, additional B cell subsets include MN, MA, RM, plasmablasts and anergic-enriched cells.[32, 33] Starting with these subsets as a developmental framework, we next analyzed the progression of different B cell maturation antigens in each of the subsets. Figure 1b shows the progression of TACI (CD267), FasR (CD95) and IgM expression with maturation from TR to RM B cell subsets. The proportion of TACI and FasR-expressing cells increase with maturation. In parallel, the proportion of IgM-expressing cells decreases, consistent with class switching to different antibody heavy chain isotypes.
Figure 1.
Figure 1a: Peripheral B cell subset scheme.
Subsets were analyzed using multicolor immunophenotyping with cell surface markers that are associated with different stages of B cell development defined as: Transitional (Trans; IgM+, CD27−, CD38++, subset CD10+), Mature naive (MN; CD27−, CD38+, IgM+), Mature Activated (MA; CD27+, CD38+), Plasmablasts (PB; CD27++, CD38++, CD20dim, CD138+), and Resting memory (RM; CD27+, CD38−). Number within each gate is % of parent (CD19+ lymphocytes).
Figure 1b: 4-color immunophenotyping progression of Fas, TACI and IgM. B cell peripheral maturation progression show from more naïve Trans B cells to more mature RM B cells. Subsets were first analyzed using multicolor immunophenotyping of cell surface markers that are associated with different stages of B cell development for Trans, MN, MA and RM cells. B cell subsets are based upon CD38 vs. CD27 staining: Trans (CD27−, CD38++), MA (CD27+, CD38+), MN (CD27−, CD38+), RM (CD27+, CD38−), and PB (CD27++, CD38++). The subsets were further studied with FasR, TACI, and IgM. The plots show increasing proportions of cells expressing FasR and TACI with maturation along with the decreased fractions of mature cells expressing IgM. Trans = transitional. MN = mature naïve. MA = mature activated. RM = resting memory. FasR = CD95. TACI = CD 267.
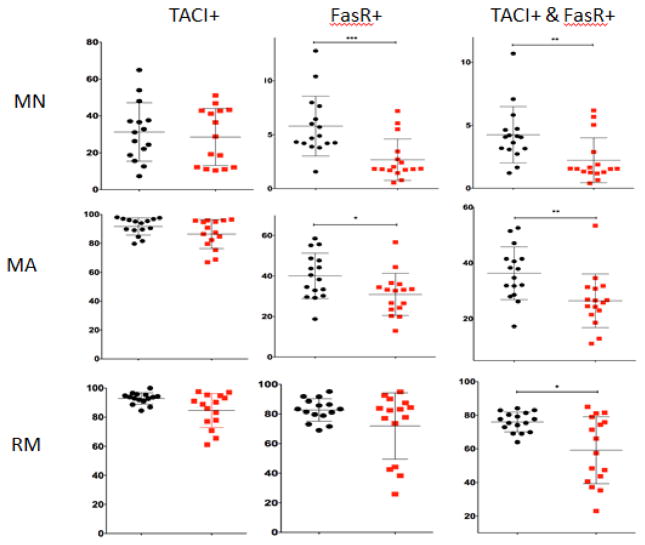
With this developmental framework, we used 11-color immunophenotypic panels (Table S1) to characterize B cell maturation in detail in T1D vs. control subjects. Individual subsets for peripheral blood B cells were further evaluated for TACI, FasR and IgM expression. Several statistically significant differences were observed amongst the B cell subsets between T1D subjects and control subjects (summarized in Table 3). There was a decreased percentage of FasR positive MN and MA B cells in T1D subjects versus controls (Figure 2). Additionally, there was a decreased fraction of B cells that were double positive for TACI and FasR in MN, MA and RM B cells in T1D subjects (Figure 2). This consistent pattern of decreased TACI and FasR positive cells through different stages of peripheral B cell maturation is depicted with a string plot in Figure 3. Using 60% TACI+FasR+ as the bottom of the normal range in RM cells in Figure 3, one can see that 7 out of the 16 T1D subjects have values below 60%. Several of these same T1D subjects also have lower TACI+FasR+ B cell fractions in the MA subset. In the RM subset, there were also fewer class-switched (IgM−) TACI+ B cells in seven T1D patients (Figure S2 and Figure S3). When CD27 vs. IgM were used instead of CD27 vs. CD38 to subset the B cells, TACI and FasR differences between T1D and controls were similar, with most of the differences occurring in the mature B cell subsets (Figure S4). When we computed the mean fluorescence intensities (MFIs) among positively staining cells in each B cell subset, there were no significant differences in the MFIs in TACI or FasR between T1D subjects and controls (Table S5), suggesting that there are no major differences in cell surface antigen levels.
Table 3. Lymphocyte subset fractions that differ between T1D and control subjects.
Data are represented as mean percentages ± standard deviation. MN = mature naïve. MA = mature activated. RM = resting memory. Please see Figure 1a for definitions of MA, MN and RM.
| Subset | Controls | T1D | p-value |
|---|---|---|---|
| MN CD95+ | 5.80 ± 2.78 | 2.68 ± 1.92 | 0.0007 |
| MN CD267− CD95+ | 1.55 ± 1.26 | 0.46 ± 0.31 | 0.0009 |
| MN CD267+CD95+ | 4.25 ± 2.24 | 2.22 ± 1.78 | 0.0041 |
| MA CD95+ | 40.08 ± 11.20 | 30.93 ± 10.43 | 0.0352 |
| MA CD267+CD95+ | 36.35 ± 9.46 | 26.49 ± 9.63 | 0.0017 |
| MA CD267− CD95− | 4.73 ± 3.48 | 9.92 ± 8.03 | 0.0416 |
| MA CD267− IgM+ | 1.63 ± 1.55 | 4.86 ± 5.22 | 0.0132 |
| RM CD267+CD95+ | 76.01 ± 5.88 | 59.21 ± 19.91 | 0.0281 |
| RM CD267+IgM− | 87.71 ± 6.03 | 69.96 ± 21.98 | 0.0378 |
| CD24+, CD38++ | 2.67 ± 1.15 | 1.54 ± 0.85 | 0.0045 |
| CD16+CD56− | 5.10 ± 2.33 | 7.89 ± 3.23 | 0.0066 |
| CD16− CD56+ | 2.10 ± 0.64 | 3.78 ± 1.59 | 0.0007 |
Figure 2. B cell subsets for TACI and FasR.
Shown are the percentages of TACI+, FasR+, or doubly positive TACI+ and FasR+ cells in the MN, MA and RM B cell subsets for control and T1D subjects. Please see Figure 1a for B cell subset scheme and definitions of MA, MN and RM based on CD38 vs. CD27 staining. Three asterisks (***) indicate a p-value that is < 0.001; (**) indicate a p-value that is <0.01 and (*) a p-value that is <0.05. T1D = type 1 diabetes. MN = mature naïve. MA = mature activated. RM = resting memory. TACI = CD267. FasR = CD95.
Figure 3. String plot of B cells positive for both FasR and TACI.
String plot depicting TACI and FasR expression in B cells. Columns indicate B cell subsets ordered from least mature TR cells (left) to more mature RM cells (right). Percentages of doubly positive TACI+ and FasR+ cells in the TR, MN, MA and RM B cell subsets for control and T1D subjects. Please see Figure 1a for B cell subset scheme and definitions of TR, MA, MN and RM based on CD38 vs. CD27 staining. One asterisk (*) indicates a p-value that is < 0.05. T1D = type 1 diabetes. TR = transitional. MN = mature naïve. MA = mature activated. RM = resting memory. TACI = CD267. FasR = CD95.
The fractions of the B cell subsets that differed significantly between T1D subjects and controls did not correlate with age, length of disease or the body mass index in the T1D subjects (Fig. S5). We wondered if a composite score of B cell subset abnormality would be more likely to correlate with these T1D subject characteristics. To test this idea we took the ten B cell subsets (and sub-subsets) that differed significantly between T1D subjects and controls and created a confidence interval (+/− 2 S.D.) for each subset’s percentage representation, based upon the healthy control subject data (Table 3). Next, we arbitrarily assigned one point for each subject for each value that was outside of these confidence intervals. Then we summed the points for all ten subsets and plotted the sum (score) for each T1D subject versus the age, length of disease and body mass index (Fig. S6). No significant correlation was observed for any of these comparisons. Based upon this analysis, we conclude that age, length of disease and body mass index do not separate T1D patients who have B cell subset abnormalities from those who lack them.
4. Discussion
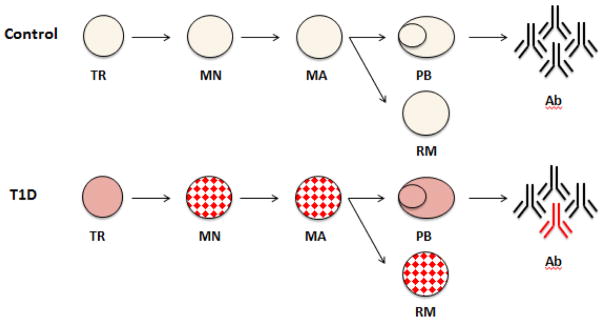
As B cells mature, they acquire surface TACI and FasR expression and undergo class switching from IgM to other heavy chain isotypes. In our flow cytometric analysis of circulating B cells, T1D subjects had fewer TACI and/or FasR expressing cells than control subjects. Some mature B cell subsets also had fewer class-switched B cells. These differences are present in multiple mature B cell subsets, as summarized in Figure 4. Taking all of these findings together, we propose that peripheral B cell maturation is disrupted in up to 50% of patients with longstanding T1D.
Figure 4. Summary of peripheral B cell maturation abnormalities in T1D.
Schematic highlighting peripheral B cell maturation in control subjects (top) and T1D subjects (bottom). B cell subsets are arranged from the least mature transitional cells (TR) on the left, followed by mature naïve (MN) to the more mature subsets, mature activated (MA), plasmablasts (PB) and resting memory (RM) cells, on the right. B cell subsets are defined immunophenotypically, as shown in Figure 1a. Subsets with maturation antigen differences (TACI, FasR, and/or IgM) in T1D compared to controls are denoted by the red checked pattern. Antibodies (Ab) are in black and T1D-associated autoantibodies are colored in red.
It is unclear why these B cell phenotypic abnormalities exist in T1D. They may be due to a developmental abnormality early in B cell development that persists through maturation, extending into the MN, MA and RM pools. However, if it were a selection defect, one might expect alterations in the B cell compartment size. Yet such alterations are minimal because absolute B cell counts, including the B cell subset counts, for the most part do not differ significantly between T1D and controls. Only the fractions of B cells expressing one or more of these maturation antigens within individual B cell subsets is altered. It is noteworthy that several of the significantly different B cell subsets demonstrated differences in FasR expression. In some subsets defined by both FasR and TACI, the abnormality could be due to a dominant effect in FasR. However, there are also FasR-independent differences, such as the reduced frequency of TACI+IgM− resting memory B cells in T1D subjects compared to controls. Given that there are changes in TACI, FasR and IgM (and combinations thereof), we favor the idea that there is a more general form of dysregulation as B cells mature in T1D.
Another non-mutually exclusive explanation for altered B cell subsets in T1D is that at each developmental stage TACI and FasR are differentially regulated in T1D compared to controls. However, T1D B cells that expressed TACI and/or FasR did so with similar staining intensity to B cells in control subjects. Nevertheless, there could be alterations in signaling pathways that could contribute to increased B cell activation, clonal expansion or survival without directly affecting TACI or FasR expression. It is also possible that the most robust differences in these markers or signaling pathways occurred in B cells that were counter-selected and are no longer available for analysis. Yet another possibility is that there is an independent disruption in tolerance and selection at multiple stages of peripheral B cell maturation in T1D. This possibility is unappealing because it is not parsimonious, but individuals with autoimmune conditions may have several immunologic perturbations that act in concert to yield a similar appearing end-state of autoimmunity.
Genetic factors may contribute to abnormal B cell developmental in T1D. An intriguing example is PTPN22, which is often referred to as Lymphocyte tyrosine phosphatase (Lyp). Healthy carriers of the Lyp620W mutation harbor increased frequencies of autoimmune mature naïve B cells.[34] Mutations of PTPN22 such as Lyp620W are highly associated with several autoimmune diseases including T1D.[35, 36] In a previous study, subjects with Lyp620W exhibited blunted BCR signaling in B cells and reduced apoptosis among transitional and anergic B cells. It is tempting to speculate that altered BCR signaling contribute to reduced expression of maturation and response-associated cell surface antigens such as FasR and TACI.
While shared genetic factors such as PTPN22 can contribute to disease risk in T1D, the phenotypic abnormalities we observed may also have functional consequences in and of themselves. The proportion of FasR-expressing cells was decreased in some B cell subsets in T1D, and also in combination with TACI in other B cell subsets. FasR is involved in apoptosis [37] and has been shown to be critical for clonal deletion in mice.[38, 39] Yet paradoxically, in mice, FasR appears to contribute to the development of insulitis as NOD mice that are deficient in either Fas ligand (FasL) (gld/gld) or FasR (lpr/lpr) have much less severe disease.[40] FasL or FasR deficient mice appear to have an expansion of IL-10 expressing CD5+ B cells with regulatory properties.[41] In humans, the best-characterized disease with known Fas mutations is autoimmune lymphoproliferative syndrome [42], in which polyreactive and somatically mutated antibody-expressing memory B cells accumulate [37]. Given the complex landscape of potential central [22, 43] and peripheral B cell and T cell tolerance defects in T1D [4], and the complexity of FasR itself, it is possible that alterations in FasR expression or its regulation could impact both forms of tolerance.
Abnormal TACI signaling has also been linked to autoimmune disease [44–46], contributing to B cell activation abnormalities in patients with common variable immunodeficiency.[47, 48] NOD mice exhibit increased TACI expression compared to B6 mice and this increase is accompanied by plasma cell differentiation and class switching to IgG and IgA.[49] In contrast, our analysis of human T1D subjects reveals a lower proportion of TACI-expressing mature B cells. The difference in these results could reflect anatomic compartment differences (most of the mouse work sampled splenic B cells) or differences between NOD and human T1D. TACI can also be a negative regulator of immune responses, inhibiting B cell expansion [50–52]. TACI deficiency in mice and humans can cause hypogammaglobulinemia, reduced immune responses to encapsulated bacteria and influenza[53–55], and, in some cases, increased evidence of autoimmunity accompanied by lymphoproliferation.[51, 56] Curiously, humans with TACI deficiency, while sometimes having immunodeficiency, can also mount robust antibody responses.[57] It will be interesting to determine in future studies if clonal expansion of memory B cells is increased in T1D. TACI also influences differentiation of B cells into plasma cells [53, 57–59] and induces IgG and IgA class switch recombination[60–62]. Varying and inconsistent global alterations of IgG or IgA antibodies have been reported in T1D patients.[63–68] T1D-associated autoantibodies that are measured clinically are comprised of IgG, whereas IgA autoantibodies have not been well described.[69, 70]
Our study has some limitations. The patients analyzed were older and most had longstanding T1D. Therefore the abnormalities we observe could be a consequence rather than a cause of their autoimmune disease. However, we did not observe a correlation between the length of disease and the B cell subset abnormalities, either in isolation or as a composite arbitrary score of overall B cell subset abnormality. In the future it will be important to analyze new-onset or at-risk populations such as patients with one or multiple diabetes-related autoantibodies to see if differences in FasR and TACI are also found in these populations. The possibility that alterations in TACI or FasR expression in B cells could serve as a predictive biomarker for disease development would represent an important advance. Second, the sample size was modest and T1D is a heterogeneous disease.[71, 72] However, despite the heterogeneity in T1D, the differences noted in our analysis were seen in multiple B cell subsets and in multiple patients. Third, our analysis was focused on the peripheral blood. The blood may not accurately reflect the biology of the disease. In this connection, a recent paper [73] describes an expansion of CD5+ FasLhi cells in the spleens of human subjects with T1D, suggesting that in tissue-based B cells (as in the NOD mouse studies [40, 41]), FasR could be a driver of autoimmunity by inhibiting regulatory B cells, rather than having a suppressive role. This is very different from what we observe in the peripheral blood. The functional role of CD5+ B cells in T1D warrants further investigation.
Despite decades of research, the most reliable predictive B cell markers for T1D are diabetes-associated autoantibodies, which are evident after tolerance has been broken, and are not good markers of clinical responses to immunologic interventions as they can vary significantly, even without interventions. [74–76] While it is unclear how the B cell maturation abnormalities that we have observed have arisen in T1D, understanding their mechanistic underpinnings could provide novel biomarkers for this disease. [77] Such biomarkers could potentially offer earlier diagnostic markers of disease, help to better stratify at-risk patients, and provide more specific ways to monitor response to B cell targeted immunotherapies in clinical trials.
5. Conclusion
Subjects with longstanding T1D exhibited multiple immunophenotypic abnormalities in circulating B cell subsets compared to healthy controls. Abnormalities included decreased percentages of FasR positive mature B cells, a decreased percentage of double-expressing TACI and FasR positive mature B cells, and a decreased percentage of class-switched TACI positive B cells. These findings show that some subjects with T1D have abnormal development in the TACI and FasR activation markers as B cells mature, which may contribute to the development or maintenance of autoimmunity. A more detailed analysis of memory B cell subsets is warranted to better understand the immunologic abnormalities in T1D.
Supplementary Material
Acknowledgments
We thank the study subjects and the University of Pennsylvania Flow Cytometry Shared Resource and the Human Immunology Core for assistance. PH and JS were supported by an NIH T32 training grant: 5T32DK063688-12; JS was also supported by a postdoctoral fellowship award from the JDRF; MR, AN, NG and ELP were supported by NIH U01 DK-070430. Additional support was received from P30-CA016520 and from the Human Metabolism Resource of the Institute for Diabetes, Obesity & Metabolism.
Abbreviations
- BCR
B cell receptor
- CV
Coefficient of variation
- FasR or CD95
Fas receptor
- MA
Mature activated B cells
- MN
Mature naïve B cells
- MFI
Mean fluorescence intensity
- NK
Natural killer cells
- NOD
Nonobese diabetic mouse
- PMT
Photomultiplier tube
- RM
Resting memory B cells
- TACI or CD267
Transmembrane activator and CAML interactor
- TR
Transitional B cells
- T1D
Type 1 diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Patrick Hanley, Division of Endocrinology and Diabetes, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Jennifer A. Sutter, Division of Endocrinology and Diabetes, East Carolina University Pediatrics Specialty Clinic, Greenvile, NC
Noah G. Goodman, Division of Hematology/Oncology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA
Yangzhu Du, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA.
Debora R. Sekiguchi, Department of Pathology, University of Manitoba, Winnipeg Manitoba, Canada
Wenzhao Meng, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA.
Michael R. Rickels, Division of Endocrinology, Diabetes and Metabolism. Hospital of the University of Pennsylvania, Philadelphia, PA
Ali Naji, Department of Surgery. Hospital of the University of Pennsylvania, Philadelphia, PA.
Eline T. Luning Prak, 405B Stellar Chance Labs, 422 Curie Blvd., Philadelphia, PA 19104.
References
- 1.Maahs DM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–97. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noorchashm H, et al. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46(6):941–6. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 3.Serreze DV, et al. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161(8):3912–8. [PubMed] [Google Scholar]
- 4.Noorchashm H, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163(2):743–50. [PubMed] [Google Scholar]
- 5.Kendall PL, et al. Tolerant anti-insulin B cells are effective APCs. J Immunol. 2013;190(6):2519–26. doi: 10.4049/jimmunol.1202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serreze DV, Silveira PA. The role of B lymphocytes as key antigen-presenting cells in the development of T cell-mediated autoimmune type 1 diabetes. Curr Dir Autoimmun. 2003;6:212–27. doi: 10.1159/000066863. [DOI] [PubMed] [Google Scholar]
- 7.Serreze DV, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med. 1996;184(5):2049–53. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu CY, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117(12):3857–67. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pescovitz MD, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pescovitz MD, et al. B-lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes Care. 2014;37(2):453–9. doi: 10.2337/dc13-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Filippo G, et al. Increased CD5+CD19+ B lymphocytes at the onset of type 1 diabetes in children. Acta Diabetol. 1997;34(4):271–4. doi: 10.1007/s005920050087. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa K, et al. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984;81(8):2494–8. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sims GP, et al. Identification and characterization of circulating human transitional B cells. Blood. 2005;105(11):4390–8. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, et al. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182(7):4116–26. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 16.Thompson WS, et al. Multi-parametric flow cytometric and genetic investigation of the peripheral B cell compartment in human type 1 diabetes. Clin Exp Immunol. 2014;177(3):571–85. doi: 10.1111/cei.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klippert A, Neumann B, Stahl-Hennig C. Comparative phenotypical analysis of B cells in fresh and cryopreserved mononuclear cells from blood and tissue of rhesus macaques. J Immunol Methods. 2016;433:59–68. doi: 10.1016/j.jim.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Deng C, et al. Altered Peripheral B-Lymphocyte Subsets in Type 1 Diabetes and Latent Autoimmune Diabetes in Adults. Diabetes Care. 2016;39(3):434–40. doi: 10.2337/dc15-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weller S, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104(12):3647–54. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava B, et al. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202(9):1225–34. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luning Prak ET, et al. Age-related trends in pediatric B-cell subsets. Pediatr Dev Pathol. 2011;14(1):45–52. doi: 10.2350/10-01-0785-OA.1. [DOI] [PubMed] [Google Scholar]
- 22.Panigrahi AK, et al. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J Exp Med. 2008;205(13):2985–94. doi: 10.1084/jem.20082053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekiguchi DR, et al. Circulating lymphocyte subsets in normal adults are variable and can be clustered into subgroups. Cytometry B Clin Cytom. 2011;80(5):291–9. doi: 10.1002/cyto.b.20594. [DOI] [PubMed] [Google Scholar]
- 24.Sutter JA, et al. A longitudinal analysis of SLE patients treated with rituximab (anti-CD20): factors associated with B lymphocyte recovery. Clin Immunol. 2008;126(3):282–90. doi: 10.1016/j.clim.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Blair PA, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Newell KA, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–47. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flores-Borja F, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5(173):173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 28.Sekiguchi DR, et al. Analysis of B cell subsets following pancreatic islet cell transplantation in a patient with type 1 diabetes by cytometric fingerprinting. J Immunol Methods. 2011;363(2):233–44. doi: 10.1016/j.jim.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, et al. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13(11):1295–8. doi: 10.1038/nm1673. [DOI] [PubMed] [Google Scholar]
- 30.Anolik JH, et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122(2):139–45. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Avanzini MA, et al. B lymphocyte reconstitution after hematopoietic stem cell transplantation: functional immaturity and slow recovery of memory CD27+ B cells. Exp Hematol. 2005;33(4):480–6. doi: 10.1016/j.exphem.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Andres M, et al. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom. 2010;78(Suppl 1):S47–60. doi: 10.1002/cyto.b.20547. [DOI] [PubMed] [Google Scholar]
- 33.Wu YC, Kipling D, Dunn-Walters DK. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol. 2011;2:81. doi: 10.3389/fimmu.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menard L, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121(9):3635–44. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li YR, et al. Meta-analysis of shared genetic architecture across ten pediatric autoimmune diseases. Nat Med. 2015;21(9):1018–27. doi: 10.1038/nm.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawlings DJ, Dai X, Buckner JH. The role of PTPN22 risk variant in the development of autoimmunity: finding common ground between mouse and human. J Immunol. 2015;194(7):2977–84. doi: 10.4049/jimmunol.1403034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janda A, et al. Disturbed B-lymphocytes selection in autoimmune lymphoproliferative syndrome. Blood. 2016 doi: 10.1182/blood-2015-04-642488. [DOI] [PubMed] [Google Scholar]
- 38.Hao Z, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29(4):615–27. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14(2):181–92. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 40.Chervonsky AV, et al. The role of Fas in autoimmune diabetes. Cell. 1997;89(1):17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Z, et al. Inhibition of Fas ligand in NOD mice unmasks a protective role for IL-10 against insulitis development. Am J Pathol. 2011;179(2):725–32. doi: 10.1016/j.ajpath.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worth A, Thrasher AJ, Gaspar HB. Autoimmune lymphoproliferative syndrome: molecular basis of disease and clinical phenotype. Br J Haematol. 2006;133(2):124–40. doi: 10.1111/j.1365-2141.2006.05993.x. [DOI] [PubMed] [Google Scholar]
- 43.Chamberlain N, et al. Rituximab does not reset defective early B cell tolerance checkpoints. J Clin Invest. 2016;126(1):282–7. doi: 10.1172/JCI83840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann FS, et al. The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol. 2015;194(2):542–52. doi: 10.4049/jimmunol.1402070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross JA, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15(2):289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 46.Gross JA, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404(6781):995–9. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 47.Salzer U, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37(8):820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 48.Romberg N, et al. CVID-associated TACI mutations affect autoreactive B cell selection and activation. J Clin Invest. 2013;123(10):4283–93. doi: 10.1172/JCI69854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banday VS, et al. Increased expression of TACI on NOD B cells results in germinal centre reaction anomalies, enhanced plasma cell differentiation and immunoglobulin production. Immunology. 2016;149(3):297–305. doi: 10.1111/imm.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakurai D, et al. TACI attenuates antibody production costimulated by BAFF-R and CD40. Eur J Immunol. 2007;37(1):110–8. doi: 10.1002/eji.200636623. [DOI] [PubMed] [Google Scholar]
- 51.Seshasayee D, et al. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18(2):279–88. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 52.Yan M, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. 2001;2(7):638–43. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- 53.Mantchev GT, et al. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol. 2007;179(4):2282–8. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 54.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14(5):573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 55.Wolf AI, et al. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. J Clin Invest. 2011;121(10):3954–64. doi: 10.1172/JCI57362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warnatz K, Voll RE. Pathogenesis of autoimmunity in common variable immunodeficiency. Front Immunol. 2012;3:210. doi: 10.3389/fimmu.2012.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsuji S, et al. TACI deficiency enhances antibody avidity and clearance of an intestinal pathogen. J Clin Invest. 2014;124(11):4857–66. doi: 10.1172/JCI74428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuji S, et al. TACI deficiency impairs sustained Blimp-1 expression in B cells decreasing long-lived plasma cells in the bone marrow. Blood. 2011;118(22):5832–9. doi: 10.1182/blood-2011-05-353961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ou X, Xu S, Lam KP. Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proc Natl Acad Sci U S A. 2012;109(38):15401–6. doi: 10.1073/pnas.1200386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castigli E, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201(1):35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakurai D, et al. TACI regulates IgA production by APRIL in collaboration with HSPG. Blood. 2007;109(7):2961–7. doi: 10.1182/blood-2006-08-041772. [DOI] [PubMed] [Google Scholar]
- 62.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11(9):836–45. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoddinott S, et al. Immunoglobulin levels, immunodeficiency and HLA in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982;23(4):326–9. doi: 10.1007/BF00253738. [DOI] [PubMed] [Google Scholar]
- 64.Cerutti F, et al. Selective IgA deficiency in juvenile-onset insulin-dependent diabetes mellitus. Pediatr Med Chir. 1988;10(2):197–201. [PubMed] [Google Scholar]
- 65.Giza S, et al. Prevalence of selective immunoglobulin A deficiency in Greek children and adolescents with type 1 diabetes. World J Pediatr. 2016;12(4):470–476. doi: 10.1007/s12519-016-0039-5. [DOI] [PubMed] [Google Scholar]
- 66.Greco D, Maggio F. Selective immunoglobulin a deficiency in type 1 diabetes mellitus: a prevalence study in Western sicily (Italy) Diabetes Metab J. 2015;39(2):132–6. doi: 10.4093/dmj.2015.39.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pietruska Z, et al. Serum immunoglobulins and various components of complement in patients with insulin-dependent diabetes mellitus. Przegl Lek. 1989;46(3):338–41. [PubMed] [Google Scholar]
- 68.Smith WI, Jr, et al. Immunopathology of juvenile-onset diabetes mellitus. I. IgA deficiency and juvenile diabetes. Diabetes. 1978;27(11):1092–7. doi: 10.2337/diab.27.11.1092. [DOI] [PubMed] [Google Scholar]
- 69.Lampasona V, Liberati D. Islet Autoantibodies. Curr Diab Rep. 2016;16(6):53. doi: 10.1007/s11892-016-0738-2. [DOI] [PubMed] [Google Scholar]
- 70.Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008;41(1):11–8. doi: 10.1080/08916930701619169. [DOI] [PubMed] [Google Scholar]
- 71.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bollyky JB, et al. Heterogeneity in recent-onset type 1 diabetes - a clinical trial perspective. Diabetes Metab Res Rev. 2015;31(6):588–94. doi: 10.1002/dmrr.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saxena A, et al. Expansion of FasL-Expressing CD5+ B Cells in Type 1 Diabetes Patients. Front Immunol. 2017;8:402. doi: 10.3389/fimmu.2017.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care. 2015;38(6):989–96. doi: 10.2337/dc15-0101. [DOI] [PubMed] [Google Scholar]
- 75.Bonifacio E, et al. Early autoantibody responses in prediabetes are IgG1 dominated and suggest antigen-specific regulation. J Immunol. 1999;163(1):525–32. [PubMed] [Google Scholar]
- 76.Lebastchi J, Herold KC. Immunologic and metabolic biomarkers of beta-cell destruction in the diagnosis of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(6):a007708. doi: 10.1101/cshperspect.a007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov. 2006;5(7):564–76. doi: 10.1038/nrd2085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.