Abstract
Liposome-based drug formulations represent an exciting avenue of research as they increase efficacy to toxicity ratios. Current formulations rely on passive accumulation to the disease site where drug is taken up by the cells. Ligand mediated targeting increases the net accumulation of liposomes, however, an unexplored benefit is to potentially refine pharmacodynamics (PD) of a drug specifically to different cell types within diseased tissue. As a model system, we engineered cardiomyocyte- (I-1) and endothelial-targeted (B-40) liposomes to carry a VEGFR2 inhibitor (PTK787), and examined the effect of cell type-specific delivery on both pharmacokinetics (PK) and PD. Neovascularization in post-myocardial infarction was significantly reduced by B-40 liposomes loaded with PTK787 as compared to animals injected with I-1 liposomes, and profoundly more as compared to free PTK787. This study thus shows that the intraorgan targeting of drugs through cell type-specific delivery holds substantial promise towards lowering the minimal efficacious dose administered systemically.
Keywords: Phage display, Myocardial infarction, Drug delivery, Pharmacokinetics, Pharmacodynamics
Graphical Abstract

The complexities of cardiac regeneration make it a stringent model system for determining whether cell type-specific targeting of liposomes can affect pharmacodynamic and anatomic endpoints. Using a mouse model of myocardial ischemia/reperfusion injury and previously identified peptides targeting either cardiomyocytes or endothelium, we demonstrate here that endothelial-targeted liposomes can significantly alter pharmacodynamic and physiological endpoints in vivo as compared to cardiomyocyte-targeted liposomes or free drug. This work demonstrates for the first time that differential cell type-targeting of a small molecule drug within the post-infarct heart can left-shift the dose-response curve, thus reducing the efficacious dose.
Liposomes serve as effective particulate drug carriers as they improve pharmacokinetic (PK) and pharmacodynamic (PD) profiles of small molecule drugs.1 With the recent FDA approval of Onivyde (liposomal irinotican) and the previous approvals of Doxil, Depocyte, Daunoxome, and Ambisome, the liposome field has reached clinical utility. However, most of these drug preparations are non-targeted, are for the treatment of cancer2 and the mechanism of action includes inhibiting the growth of as many cell types within the tumor as possible. These and other classes of drugs (e.g., siRNAs and immune modulators) would be far more effective if they could be targeted directly to specific cells within diseased tissues, as the most potent drugs currently available often have pleiotropic and/or toxic side-effects when delivered systemically. 3–5
The liposomal surface can readily be modified by adding a wide variety of targeting ligands including antibodies, antibody fragments and peptides that have affinity for cell types and tissue components of interest. The targeting ligand provides for efficient accumulation of drugs in the tissue target of choice, thus reducing the drug exposure in non-target tissues.6,7 In this work, we set out to determine whether the targeting ligand could not only improve pharmacokinetics, as has been previously described,8 but also improve pharmacodynamics via cell-type specific drug delivery.
As a model system, we selected a mouse model of acute vascularization to probe the ability of cell type-specific targeted liposomes carrying a VEGFR2 inhibitor (PTK787) to inhibit angiogenesis. VEGFR2 belongs to the VEGF family of tyrosine kinase receptors that is known to mediate almost all of the known cellular responses to VEGF and has been shown to play an important role in wound healing,9 cancer angiogenesis10 and in neovascularization of the infarct zone following reperfused myocardial infarction (MI).11–13
Previously, we performed an in vivo phage display screen in a mouse model of cardiac ischemia/reperfusion injury.8 From this screen, we identified peptides specific for endothelium (B-40), cardiomyocytes (I-1), c-Kit + cells (B-50) and myofibroblasts (B-29). Each of these peptides were highly selective (>5 fold in vivo) for their respective cell types as compared to other cell types in the post-MI heart.8 To study the effects of cell type-specific delivery of PTK787, we compared the pharmacokinetics and pharmacodynamics of free PTK787, a liposomal formulation targeting cardiomyocytes (I-1) to a liposomal formulation targeting activated endothelium (B40). Using PTK787-loaded, B-40 targeted liposomes, we observed a > 10-fold lower ED50 value as compared to free PTK787 and a 1.5-fold lower ED50 value compared to I-1 liposomes in preventing neovessel formation following MI in mice. These experiments establish proof of principal that the concept of tissue-specific targeting can be extended to cell type-specific targeting, and establish that cell-type specific targeting can improve pharmacodynamics and effect physiologically significant anatomic endpoints. Our results suggest that cell type-specific delivery can increase the efficacy of small molecule drugs in achieving predictable physiological and/or anatomical responses. Analogous to the case of off-target effects at the organ level, we propose that cell-type specific targeting may prove useful in decreasing untoward toxic or pleiotropic effects at the cellular level.
Methods
Preparation and characterization of peptide-conjugated liposomes
Peptides (7-mers including negative control peptide NCP, I-1 and B-40) were chemically synthesized as described previously6 with the following modifications on the C-terminus: 7-mer-GGSK (5(6) carboxyfluorescein (FAM) C. Briefly, the cysteine at the C-terminus of the peptide was conjugated to DSPE-PEG3400-Maleimide to form peptide-lipid conjugate. The peptide-lipid conjugates were analyzed by mass spectrometry (tandem mass spec) to confirm the reaction. Liposomes were prepared by hydration of lipid film consisting of 1,2-dioleoyl-sn-glycerol-3-phosphocholine (Avanti Polar Lipids, Miami, FL) (DOPC): cholesterol: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000 (DSPE-PEG2000): DiR: DSPE-PEG3400-peptide at 46:46:6:1:1 molar ratio (9.5, 4.5, 4.5, 1, and 0.5 mg, respectively) in 1 ml of saline. DiR (Invitrogen, Carlsbad, CA) was incorporated into the lipid bilayer as a fluorescent lipid dye that provided for Fluorescence molecular tomography (FMT) (PerkinElmer, Waltham, MA) imaging of the peptide-conjugated liposomes in post-MI mice. The lipid solution was extruded 41 times through a syringe extruder containing a 0.2 µm Nuclepore filter (Thermo Fisher Scientific Inc., Waltham, MA).16 The resulting liposomes were characterized by Nanosight NS300 (Malvern Instruments Ltd., Worcestershire, UK) to determine particle size and concentration. The absorbance of FAM on the peptides enabled the determination of the number of peptides that were incorporated in each liposomal formulation. Zeta potentials of all liposomal formulations were determined in 1 mM HEPES buffer pH 7.4 at a dilution of 1:100 using a Zetasizer (Zetasizer 3000; Malvern Instruments, Worcestershire, UK).
PK and tissue immunofluorescence of peptide-conjugated liposomes in healthy and post-MI mice
The PK of peptide-conjugated liposomes (NCP, 1-1 and B-40) was carried out as described previously.8 Healthy (n = 6) and post-MI mice (n = 8) were injected via tail vein with 2 mg of lipid (100 µl containing 1 × 1011 liposomes) and DiR present in the heart region of interest (ROI) was imaged using the 750 nm excitation laser of a FMT instrument. Quantitative whole body biodistribution of fluorescent particles can be assessed non-invasively using FMT.14–16 In vivo imaging was followed by immunohistochemistry using antibodies recognizing various cell types of interest in the infarct / border zone to determine the cellular specificity. The presence of FAM on the peptides facilitated the identification of the cell types recognized by the peptide-targeted liposomes by co-localization with antibodies against CD31 (for endothelial cells; clone MEC 13.3, BD Biosciences, San Jose, CA), Hrnr (rabbit polyclonal antibody, Sigma-Aldrich, St. Louis, MO), α-smooth muscle actin (for myofibroblasts and smooth muscle cells; clone 1A4, Sigma-Aldrich, St. Louis, MO), caveolin-3 (for cardiomyocytes; sc-7665, Santa Cruz Biotechnologies, Dallas, TX), and c-Kit (progenitor cells; sc-5535, Santa Cruz Biotechnologies, Dallas, TX). The extent of co-localization of FAM-labeled peptides with each of the antibodies was determined using JACoP plug-in in Image J software (National Institutes of Health, Bethesda, MD) as described previously.8 JACoP calculates Manders’ overlap coefficient (MOC) values from two fluorescent images that can be represented graphically in scatterplots that use Pearson’s correlation coefficient (PCC) as a statistic for quantifying co-localization. MOC values close to 1 suggest two images where the fluorescence intensities are perfectly co-registered and values near zero reflect distributions of probes that are not correlated with one another.17 Other protocols used in this work are mentioned in detail in the supplementary methods section.
Results
Pharmacokinetics and biodistribution of peptide-conjugated liposomes
To enable the targeted delivery of small molecules to post-infarct heart, we previously carried out an in vivo phage display screen in a mouse model of cardiac ischemia/reperfusion injury.6 Using a phage display based functional proteomics approach,18 we identified the binding partner of the B-40 endothelial-specific peptide as hornerin [Hrnr] and showed that Hrnr plays a determining role in neovascularization of the infarct post-MI.19 Peptide-conjugated liposomes (NCP, I-1 and B-40) were prepared by the hydration method20 (Supplementary Methods) and their PK studied using FMT (Figure 1). The charge of all the peptides used in this study was +1 and their corresponding liposomal formulations diluted in 1 mM HEPES buffer (pH 7.4) also had a Zeta potential of ~ −30 mV (Figure SI). The size (100–120 nm) and concentration of liposomes (6–11 × 1012 per mL) was carried out by Nanosight analysis (Figure SI). The conjugation of the B-40 peptide to PEG-DSPE was confirmed by mass spectrometry (Figure S2). The presence of FAM on the peptide allowed us to quantify number of peptides per liposome. The number of peptides per liposomes was similar to the numbers (550–800) we obtained previously6 (Figure SI). Using EF-hand fragment (1-92 amino acids) of homerin, we studied its interaction with B-40 liposomes using Biolayer Interferometry (BLI) on a Fortebio Octet system. We observed that the EF-hand fragment bound to B-40 liposomes and not to I-1 or negative control (no peptide) liposomes (Figure 1).
Figure 1.
B-40 peptide and B-40 liposomes bind to hornerin (N-terminus 1-92 amino acids). (A) Association and dissociation curves generated using Biolayer interferometery (BLI) (Fortebio Octet system) between the B-40 peptide and hornerin (N-terminus 1-92 aa = EF-hand). KD was determined to be ~20 µM. Association and dissociation curves were fitted 1:1 to a Langmuir model using Octet analysis software. (B) Similarly, when B-40 peptide conjugates were incorporated into liposomes, they associated with the Hornerin fragment (red and green curves), while control liposomes without peptide and negative control peptide liposomes displayed little or no affinity for the Hornerin target.
The PK of peptide-conjugated liposomes was determined by injecting 2 mg (1 × 1012) of liposomes via tail vein into post-MI mice and imaging at the indicated time points using FMT. All liposomes were labeled with DiR, a lipophilic dye. FMT imaging was used to quantitate liposomes in the heart by ROI analysis. The PK and accumulation (area under the curve, AUC) were fit using a two-compartment model (Figure 2, A). I-1 liposomes accumulated 2-fold higher (3010 vs. 1521 pmol DiR), and B-40 liposomes 1.5 fold higher, than NCP liposomes (2275 vs. 1521 pmol DiR) (Table 1). As previously shown, NCP liposomes accumulate in macrophages while I-1 liposomes accumulate in cardiomyocytes and B-40 liposomes in the endothelium of the remodeling infarct.8 In healthy animals, the PK of all the liposomal formulations including non-targeted liposomes were not statistically different and were devoid of an accumulation phase, indicative of the absence of the targets for the three targeted formulations in the healthy heart (Figure SI). PK was measured in at least 7 animals for each liposome type prepared and to test the reproducibility of the formulation procedures, at least two separate lots were tested. After PK was assessed, biodistribution experiments were performed. The targeted liposomes I-1 and B-40 showed higher accumulation in the post-infarct mouse heart than the NCP liposomes. Similar to the AUC values, the percent injected dose per gram (%ID/g) in the heart for I-1 liposomes was 2-fold higher compared to NCP liposomes (58% vs. 29%) and B-40 liposomes were 1.4-fold higher compared to NCP liposomes (42% vs. 29%). As liposomes are known to accumulate and to be cleared through the liver and spleen, we included those organs in our biodistribution analysis. Similar to previous reports,21,22 all liposomes were also shown to accumulate in the liver and spleen. Intriguingly, NCP-liposomes showed significantly higher accumulation in the liver and spleen, while I-1 and B-40 liposomes had higher accumulation in the remodeling heart, signifying a shift in distribution away from the liver and spleen towards the post-infarct heart as a result of peptide targeting.
Figure 2.
PK and biodistribution of I-1 and B-40 liposomes in post-MI mice. (A) Graph showing pharmacokinetics of negative control peptide (NCP), I-1 and B-40 liposomes obtained using FMT imaging followed by image analysis using the heart as the region of interest (ROI). FMT imaging of the liposomal preparations was performed in live mice (n = 7) on day 4 post-MI. (B) Using a two-compartment model fit, half-life and AUC were determined for the three different peptide-conjugated liposomes. Values represent percent ± standard error of mean. I-1 and B-40 liposomes had significantly higher picomoles DiR in the heart ROI (p, 0.05) (C) Percent injected dose per gram (%ID/g) tissue for I-1 and B-40 liposomes from heart, liver and other tissues 24 h post-injected animals (n = 7) suggested the specificity of peptides for infarct heart. I-1 and B-40 liposomes were significantly higher in the heart compared to NCP liposomes (p < 0.05).
Table 1.
Nanosight analysis showing the size and concentration of liposomes during remote loading of PTK787.
| Sample | Size (nm) | Concentration (particles/mL) (1 × 1013) |
|---|---|---|
| Initial | 116.6 | 3.38 ± 0.105 |
| Size exclusion 1 | 123.1 | 2.89 ± 0.064 |
| 55 °C for 1 h–no drug | 120.5 | 2.4 ± 0.047 |
| 55 °C for 1 h–with PTK787 | 126.9 | 2.69 ± 0.084 |
Cellular targeting of peptide-conjugated liposomes
The I-1 and B-40 targeted liposomes were specific for cardiomyocytes and endothelial cells, respectively, as shown in our previous study through immunofluorescence analysis of post-infarct heart tissue samples collected 4 h post injection of liposomes.8 The presence of the FAM fluorophore on the peptide-conjugated liposomes enabled visualization of their cellular specificity using fluorescence microscopy. Post-infarct tissue sections containing liposomes were co-stained with antibodies specific for cell types of interest present in the infarct/border zone. We determined the Manders’ overlap coefficient that describes the extent of overlap between the peptide and antibodies specific for various cell types. From this analysis, we concluded that these peptides could improve liposome targeting of endothelial cells and cardiomyocytes by 2-3 fold as compared to other cell types (Figure 3). Immunofluorescence data and statistical analysis using Manders’ overlap coefficient provided evidence to support this shift in cell type specific binding (Figure 3, D). The majority of I-1 targeted liposomes were associated with cardiomyocytes and B-40 liposomes were associated with endothelial cells that were both CD 31 and Hrnr+, but not exclusively. In accord with our previous study,8 B-40 liposomes were found tightly associated with border zone endothelium (Figure 3, C, Figure S3A). In contrast, B-40 liposomes were essentially absent from regions of the heart remote to the infarct (Figure S3B).
Figure 3.
Immunofluorescence of peptide conjugated liposomes in post-MI hearts. (A) NCP was non-specifically taken up by macrophages of remodeling infarct. (B) Peptide I-1 was specific for cardiomyocytes (caveolin-3, arrow), (C) B-40 specific for endothelial cells (CD31, arrow) in the border zone 4 h post liposome injection. Peptides are pseudo-colored green and cell markers (mac-2, caveolin-3, Hornerin) blue (scale bar; 20 µm). (D) Peptide specificity for the corresponding cell types was analyzed using the ImageJ plug-in JACoP. NCP liposomes were mostly associated with macrophages (mac-2+ cells), I-1 liposomes with cardiomyocytes (caveolin-3+ cells) and B-40 liposomes with endothelial cells (CD 31+ and Hrnr + cells) (* represents p < 0.05).
Remote loading of B-40 liposomes with PTK787
The analyses of PK and biodistribution of peptide-conjugated liposomes were based on measures of the lipophilic dye (DiR) incorporated in the lipid bilayer. Thus, the biodistribution of the small molecule payload was not directly assessed, but rather that of the liposomal shell. In order to assess the efficacious delivery of PTK787 and target engagement for drug action, we chose to assess a physiological endpoint with relevance to cardiac regeneration: neovessel formation following the reperfusion of ischemic myocardium. PTK787 was selected as a bioactive small molecule in the current study to demonstrate proof-of-principle for the efficacy of peptide targeting. PTK787 is a VEGFR2 inhibitor that has been widely used as anti-angiogenic therapy in treatment of cancer (Figure 4, A). While neoangiogenesis is necessary to support scar formation after MI, we used the loss (or prevention) of neoangiogenesis in the current study as a robust measurement of PD. We hypothesized that the deficit in neovessel formation could be easily quantified to support comparisons of targeted delivery vs. free-drug efficacy. PTK787 is a neutral small molecule with an IC50 of 37 nM in cell culture and 10 mg/mL solubility in water (Figure S4). To examine the efficacy of targeted delivery of PTK787, we prepared B-40 conjugated liposomes in 250 mM ammonium sulfate for remote loading (Figure 4, A) of PTK787 using the reverse phase evaporation method. PTK787 was dissolved in saline at 5 mg/mL and 50 µL was added to liposomes (100 µL of 20 mg/mL lipid) after removal of exterior ammonium sulfate by size exclusion chromatography. Remote loading was carried out by incubating the mixture at 55 °C for 1 h or at room temperature for 4 h. Excess PTK787 not loaded into the liposomes was removed by size exclusion chromatography. A sample of this liposome mixture was used to determine the final drug to lipid ratio using HPLC. There was a small but negligible loss of lipid to the size exclusion columns, but the final drug to lipid ratio could be estimated at 113.6 ± 11.5 µg PTK787 per mg of lipid for both B-40 and I-1 liposomes (Figure S4F).
Figure 4.
Remote loading of PTK787 and Nanosight characterization of liposomes. (A) Cartoon showing the procedure used to remote load B-40 liposomes with PTK787. (B) Nanosight characterization of B-40 liposomes at various stages of remote loading procedure. The graph shows the concentration vs. size distribution of liposomes at various steps of the remote loading procedure.
The drug-loaded liposomes were further characterized by Nanosight analysis for their size and concentration followed by structural analysis by cryoTEM (Figure S4). Nanosight analysis indicated that the size and concentration during various stages of remote loading was similar, and that temperature and use of size exclusion columns did not affect the remote loading process (Figure 4, B). As assessed by cryoTEM, most of the B-40 liposomes (with or without PTK787) were spherical with a distinct lipid bilayer, although some were multilamellar and occasional liposomes exhibited an irregular structure that might be attributed to sample preparation for cryoTEM (Figure S4B–E).
Pharmacodynamic evidence of PTK787 delivery using B-40 peptide conjugated liposomes
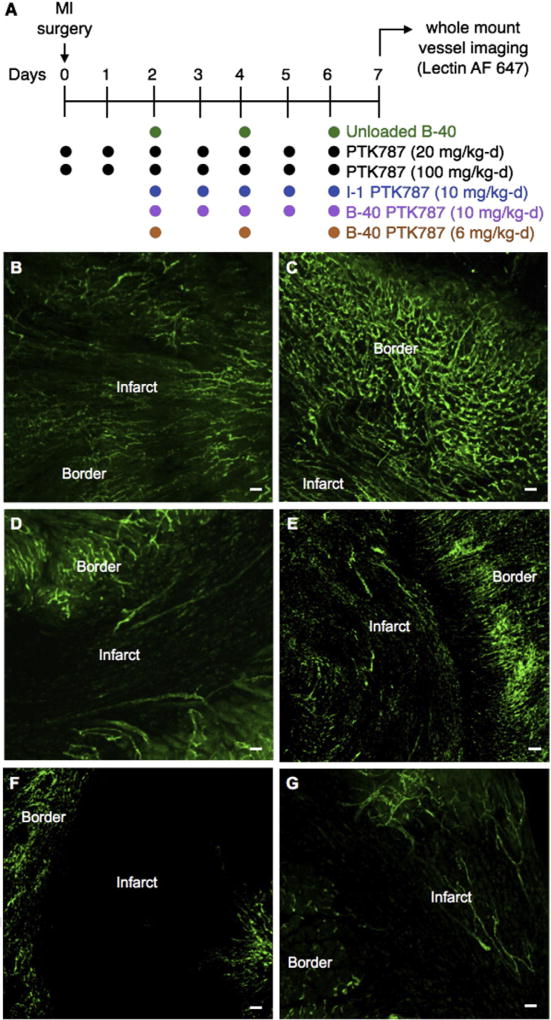
Of the 26 mice undergoing infarct surgery in the pharmacodynamic study, 23 survived until euthanasia for follow-up at the 7-day endpoint. Interestingly, the 3 mice (3 out of 8 in that cohort) that died unexpectedly all belonged to the study group receiving the highest dose of PTK787-loaded, B-40 targeted liposomes. No other cohorts had fatalities. Following tissue harvest on day 7 post-MI, vessel volume fraction (VVF) in the infarct region was used as a PD measurement of PTK787 efficacy. VVF was determined using RAVE software developed previously in our lab23 (Supplementary Methods). B-40 liposomes (specific for border zone endothelial cells) loaded with PTK787 were injected to deliver a mean of either 6 or 10 mg of PTK787 per kg-day (Figure 5, A). Neovessels present in the infarct zone on day 7 post-MI (Figure S5) were quantified (as VVF) and compared with groups receiving free drug at two doses (20 and 100 mg/kg-d) delivered over the course of 7 days (day 0 to day 6 post-MI). The dose of free PTK787 was determined from previous published studies11 and for liposomal injections via tail vein, we chose to inject every other day as the half-lives of the formulations was previously determined to be about 18 h8. From previous studies, the in vivo effective dose for free PTK787 was determined to be 100 mg/kg-d. Liposomal dose was chosen empirically to bracket the approximate ED50. This experiment also included a negative control group treated with unloaded (drug-free) B-40 liposomes and a group treated with I-1 liposomes (specific for cardiomyocytes) loaded with PTK787 (10 mg/kg-d). Representative heart tissue sections from each of these groups are presented in Figure 5, B–G, and the mean VVF results are plotted in Figure 6, A. A VVF of 0.063 was measured in untreated control hearts (unloaded B-40 liposomes), and VVF values decreased to 0.052 and 0.022 in the groups treated with free PTK787 at 20 and 100 mg/kg-d, respectively (i.e., these doses were 18 and 65 percent effective in reducing neovessel formation).
Figure 5.
Measure of vessel volume fraction following liposomal delivery of PTK787. (A) Experimental design for measuring neovessel formation resulting from the delivery of empty B-40 liposomes (drug free), free PTK787 (20 and 100 mg/kg-d), I-1 liposomes loaded with PTK787 and B-40 liposomes loaded with PTK787 (6 and 10 mg/kg-d) (n = 4 per group). VVF was measured on day 7 post-MI after using in vivo lectin staining to label neovessels prior to tissue harvest. Using whole mount sections, confocal microscopy was performed to measure VVF in the infarct, border and remote regions. Confocal images showing vessels stained with lectin AF 647 (pseudo colored green) from (B) control, (C) free PTK787 (20 mg/kg-d), (D) free PTK787 (100 mg/kg-d), (E) I-1 liposomes loaded with PTK787 (10 mg/kg-d), (F) B-40 liposomes loaded with PTK787 (10 mg/kg-d) and (G) B-40 liposomes loaded with PTK787 (6 mg/kg-d) dosing represented here is a mean over 7 day period (Scale bar; 100 µm).
Figure 6.
Vessel volume fraction following endothelial specific delivery of PTK787. (A) Using the RAVE program, vessel volume fraction was calculated and results indicate that B-40 liposomes (10 mg/kg-d) were significantly more effective at preventing neovessel formation (VVF-0.008) in the infarct region as compared to any other dose or formulation, including free PTK787 at a 10-fold higher dose 100 mg/kg-d and I-1 PTK (* represents p < 0.05). (B) Using Imax modeling, ED50 values were determined for free PTK787 from doses of 20 & 100 mg/kg-d (in gray), I-1 (cardiomyocyte-targeted) liposomes with PTK787 from a dose of 10 mg/kg-d (in blue), and B-40 liposomes from doses of 6 & 10 mg/kg-d (in red).
Free PTK787 at 20 mg/kg-d showed only a modest effect in preventing neovessel formation in the infarct region. In contrast, endothelium-targeted B-40 liposomes loaded with PTK787 and administered at 10 mg/kg-d (VVF = 0.008) were 88% effective in preventing neovessel formation while cardiomyocyte-targeted I-1 liposomes loaded with PTK787 at 10 mg/kg-d (VVF = 0.022) were only 65% effective (Figure 6, A). When ED50 values were calculated (Figure 5, B) and compared, B-40 liposomes had a 10-fold lower ED50 than free PTK787 (6.2 vs. 63 mg/kg-d, respectively) and a 1.5 fold lower ED50 as compared with I-1 liposomes (8.8 mg/kg-d) (Table 2). Furthermore, the B-40 liposomes had a steeper dose response behavior compared to free PTK787 (Hill factor: 4.1 vs. 1.2, respectively). Although I-1 liposomes were predominantly associated with border zone cardiomyocytes, we speculate that some of the PTK787, a likely high permeable compound based on its physiochemical characteristics, may have been released locally, thus inhibiting VEGFR2 in nearby endothelial cells. The tight juxtaposition between capillaries and cardiomyocytes may thus have contributed to I-1 liposome mediated VEGFR2 inhibition in endothelial cells. In addition, the liposomal accumulation of I-1 in the heart was approximately 30% higher as compared to B-40 liposomes (Figure 2, A,B) suggesting another reason why cardiomyocyte-targeted I-1 liposomes might have been more effective than free PTK787 in lowering VVF.
Table 2.
Imax model fit derived ED50 values from vessel volume fraction for PTK alone, I-1 PTK787 and B-40 PTK787.
| Formulation | Estimated ED50 (mg/kg-d) |
Standard error |
Median | 5th %tile | 95th %tile |
|---|---|---|---|---|---|
| PTK787 | 63 | 11 | 63 | 48 | 96 |
| I-1 PTK787 | 8.8 | 0.5 | 8.8 | 7.8 | 10 |
| B-40 PTK787 | 6.2 | 0.3 | 6.2 | 5.5 | 6.8 |
Discussion
Nanoparticles including liposomes are attractive drug carriers due to their extended circulation and the ease with which their surfaces can be modified for the targeted delivery of therapeutic agents.24 Liposomal formulations have been approved in the treatment of various diseases, but the currently approved formulations lack active targeting mechanisms to improve the accumulation of drug on target.25 Considerable progress has been made in generating targeted liposomes using antibodies and phage display-derived peptides attempts to increase tissue accumulation of the formulations. In this work, we explored the potential of using cell type specific targeting to modulate pharmacodynamics at the cellular level in two different cell populations within the same tissue.
Previous studies have generated cardiac-specific liposomes using antibodies or phage display-derived peptides.26–33 Various labs have conducted phage display screens to identify peptides specific for cardiomyocytes or endothelial cells, but very few have demonstrated an improvement in the delivery of small molecule drugs to the heart as compared to free drug alone. One of the first peptides used for targeting cardiac endothelium was the CRPPR peptide developed by Zhang H. et al to deliver liposomes loaded with fluorescent dye to cardiac tissue.26 They were successful in showing fluorescent dye can traverse the endothelium. Similarly, Kanki S. et al, also carried out a phage display screen in a rat model of ischemia reperfusion and identified a peptide (CSTSMLKAC) that was specific for ischemic left ventricle and not the healthy left ventricle or right ventricle.27 Likewise, there are other groups that have carried out phage display screens to identify either cardiomyocyte- or endothelial-specific ligands, but no previous study has documented the targeted delivery of a small molecule drug that has a physiologic or anatomic effect in the post-infarct heart.31–33 While previous studies clearly show cellular specificity for the respective cell types, no study to date has directly compared the effects of targeting liposomes bearing small molecule drugs specifically to cardiomyocytes versus endothelial cells within the heart or post-infarct heart.
Our team previously identified peptides specific for the infarct border zone using a phage display screen in a mouse model of myocardial ischemia-reperfusion injury.8 Using peptides from this screen, we were able to target liposomes to various cell types of interest in the infarct border zone including cardiomyocytes (I-1) and endothelial cells (B-40). While hornerin was identified as the target protein for B-40 peptide, the identification of the cellular target for the I-1 peptide is still ongoing. Hornerin belongs to the S100 family of calcium binding proteins and is known to be involved in wound healing and in breast cancer.34,35
To explore the therapeutic potential of B-40 targeted drug delivery, endothelial- (B-40) and cardiomyocyte- (I-1) targeted liposomes were loaded with a well-characterized inhibitor of the VEGFR2 receptor (PTK787), and resulting liposomes were characterized by Nanosight and HPLC analysis (Figure 4). The pharmacodynamic (PD) effects of these liposomal formulations were then compared with empty (drug-free) liposomes as well as with free drug administered at multiple doses in a mouse model of reperfused MI (Figure 5). Quantitative morphometric analysis of these results using the RAVE software package23 indicated that B-40 targeted liposomes loaded with PTK787 were significantly more effective in preventing neovessel formation than any other formulation tested, including free PTK787 delivered at a 10-fold higher dose (Figure 6, A). Imax modeling was then applied to calculate the ED50 values for each of the formulations examined (Figure 6, B), and confirmed that B-40 liposomes had a 10-fold lower ED50 than free PTK787, and a 33% fold lower ED50 as compared with I-1 targeted liposomes loaded with PTK787 (Table 2) indicating that neovessel formation is significantly better inhibited by endothelial (B-40) targeted liposomes than by cardiomyocyte (I-1) targeted liposomes, and that both liposomal formulations are more effective than free drug (PTK787). Interestingly, all of the mortality incurred in this study was confined to the study group receiving the highest dose of PTK787-loaded, B-40 targeted liposomes (with 3 of 8 mice dying unexpectedly 2-3 days after reperfusion). Further, the timing of mortality in this group was unusual because mortality in this model typically occurs either during surgery or within the first 12 h of recovery from surgery. We therefore speculate that the high local concentrations of PTK787 resulting from targeted drug delivery may have suppressed neovessel formation to the point that it adversely impacted mortality in this group – further demonstrating the effects on PD of cell-specific targeting of drugs.
This work demonstrates for the first time that the molecular targeting of a small-molecule drug (PTK787) to one cell population (endothelial or cardiomyocytes) within a single tissue (heart) can have differential effects on an anatomical endpoint (neovascularization) depending upon the identity of the cell population that was targeted. The cell type-specific delivery of small molecule drugs demonstrated here opens the possibility of reaping therapeutic efficacy from compounds that would otherwise be contraindicated due to pleiotropic effects in different cell types within the same tissue. For example, Wnt modulators have differential effects on different cell types present in the post-ischemic heart. Wnt inhibition in the fibroblasts reduces collagen deposition and thus prevents scar formation.36–38 But Wnt inhibition in border zone cardiomyocytes can also prevent the proliferation of cardiomyocytes.39,40 Thus targeting Wnt inhibition specifically to fibroblasts in the post-MI heart could potentially reduce scar formation without adversely impacting cardiomyocyte proliferation.
The current study provides proof of principle, that the targeted delivery of bioactive small molecules can differentially impact an anatomical endpoint depending upon the cell type that is targeted. Building upon this paradigm, it should be possible to administer small molecule drugs either together or in sequential fashion to more effectively treat disease. An illustration of the potential of this paradigm is cell-specific delivery after myocardial infarction to curtail excess inflammatory responses and to optimize the heart’s reparative response to MI through a rational bioengineering approach. Examples include stimulating the proliferation of border zone cardiomyocytes to replace necrotic regions of myocardium and/or reprogramming myofibroblasts to transdifferentiate into cardiomyocytes instead of elaborating collagenous scar tissue.41 Ultimately, the cell type-specific drug delivery tools developed here should help expedite efforts to modulate the heart’s maladaptive response to MI by programming cardiac regeneration with a reduced effective dose. From a broader perspective, we also envision that other diseases, such as cancer and diabetes, would also benefit from such a nuanced, targeted approach.
Supplementary Material
Acknowledgments
The authors would also like to thank Dr. Kelly Dryden in the UVA Molecular Electron Microscopy Core facility, which is significantly supported by the School of Medicine, for her help in performing cryoTEM of liposomes. We also would like to thank Nicholas Sherman (Mass spectrometry) and John Shannon (Fortebio) of the Biomolecular analysis facility at UVA for their help with the mass spectrometry and BLI respectively. We also would like to thank Arjan Snijder of AstraZeneca, Sweden for providing us with the N-terminus hornerin fragment (1-92 aa) with which liposomal binding studies were performed.
This work was supported in part by grants from the: AstraZeneca/UVA Strategic Alliance (to KAK, BAF & ALK), NIH (1R01HL115225 to BAF) and American Heart Association (13POST16920031 to SSKD).
Abbreviations
- VEGF
vascular endothelial growth factor
- MI
myocardial infarction
- PK
pharmacokinetics
- PD
pharmacodynamics
- Hrnr
Hornerin
- ROI
region of interest
- FMT
fluorescence molecular tomography
- AUC
area under the curve
- VVF
vessel volume fraction
- DiR
1,1′-dioctadecyl-3, 3, 3′, 3′-tetramethylindotricarbocyanine iodide
- FAM
5(6)-carboxyfluorescein
Footnotes
Conflict of interest: none
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nano.2017.07.005.
References
- 1.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 2.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–99. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoury H, Adkins D, Brown R. Adverse side-effects associated with G-CSF in patients with chronic myeloid leukemia undergoing allogenic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2000;25(11):1197–201. doi: 10.1038/sj.bmt.1702423. [DOI] [PubMed] [Google Scholar]
- 4.Tigue CC, McKoy JM, Evens AM, Trifilio SM, Tallman MS, Bennett CL. Granulocyte-colony stimulating factor administration to healthy individuals and persons with chronic neutropenia or cancer: an overview of safety considerations from the research on adverse drug events and reports project. Bone Marrow Transplant. 2007;40(3):185–92. doi: 10.1038/sj.bmt.1705722. [DOI] [PubMed] [Google Scholar]
- 5.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9(1):57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 6.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticle systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–27. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine (London) 2013;8(9):1509–28. doi: 10.2217/nnm.13.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasa S, Suzuki R, Gutknecht M, Brinton LT, Tian Y, Michaelsson E, et al. Development of target-specific liposomes for delivering small molecule drugs after reperfused myocardial infarction. J Control Release. 2015;220(PtA):556–67. doi: 10.1016/j.jconrel.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3(10):647–61. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goel HL, Mercurio AM. VEGF targets the tumor cell. Nat Rev Cancer. 2013;13:871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wnuk M, Hlushchuck R, Tuffin G, Huynh-Do U, Djonov V. The effects of PTK/ZK222584, an inhibitor of VEGFR and PDGFRβ pathways, on intussusceptive angiogenesis and glomerular recovery from Thy1.1 nephritis. Am J Pathol. 2011;178(4):1899–912. doi: 10.1016/j.ajpath.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandervelde S, Amerongen MJ, Tio RA, Petersen AH, van Luyn MJ, Harmsen MC. Increased inflammatory response and neovascularization in reperfused vs. nonreperfused murine myocardial infarction. Cardiovasc Pathol. 2006;15(2):83–90. doi: 10.1016/j.carpath.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Ma D, Wang W, Xu SL, Zhang YQ, Chen B. Neovascularization and cardiomyocytes regeneration in acute myocardial infarction after bone marrow stromal cell transplantation: comparison of infarct and non-infarct relative arterial approaches in swine. Clin Chim Acta. 2007;381(2):114–8. doi: 10.1016/j.cca.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 14.Kunjachan S, Gremse F, Theek B, Koczera P, Pola R, Pechar M, et al. Noninvasive optical imaging of nanomedicine biodistribution. ACS Nano. 2013;7(1):252–62. doi: 10.1021/nn303955n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasquez KO, Casavant C, Peterson JD. Quantitative whole body biodistribution of fluorescent-labeled agents by non-invasive tomographic imaging. PLoS One. 2011;6(6):e20594. doi: 10.1371/journal.pone.0020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giddabasappa A, Gupta VR, Norberg R, Gupta P, Spilker ME, Wentland J, et al. Biodistribution and targeting of anti-5T4 antibody-drug conjugate using fluorescence molecular tomography. Mol Cancer Ther. 2016 doi: 10.1158/1535-7163.MCT-15-1012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Dunn KW, Kamocka MM, McDonald JH. A practical to evaluating colocalization in biological microscopy. Am J Phys Cell Phys. 2011;300:C723–42. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds F, Panneer N, Tutino CM, Wu M, Skrabal WR, Moskaluk C, et al. A functional proteomic method for biomarker discovery. PLoS One. 2011;6(7):e22471. doi: 10.1371/journal.pone.0022471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasa S, Gutknecht M, Seaman ME, French BA, Kelly KA. Hornerin, a multifunctional protein critical to non-VEGF-mediated angiogenesis, plays a determining role in neovascularization of the infarct post-MI. Circulation. 2014;130:A15890. [Google Scholar]
- 20.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297–315. [PMC free article] [PubMed] [Google Scholar]
- 21.Koning GA, Kamps JA, Scherphof GL. Interference of macrophages with immunotargeting of liposomes. J Liposome Res. 2002;12:107–19. doi: 10.1081/lpr-120004782. [DOI] [PubMed] [Google Scholar]
- 22.Nag OK, Awasthi V. Surface engineering of liposomes for stealth behavior. Pharmaceutics. 2013;5:542–69. doi: 10.3390/pharmaceutics5040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seaman ME, Peirce SM, Kelly KA. Rapid analysis of vessel elements (RAVE): a tool for studying physiologic, pathologic and tumor angiogenesis. PLoS One. 2011;6:e20807. doi: 10.1371/journal.pone.0020807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levchenko TS, Hartner WC, Torchilin VP. Liposomes in diagnosis and treatment of cardiovascular disorders. Methodist Debakey Cardiovasc J. 2012;8(1):36–41. doi: 10.14797/mdcj-8-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, Li N, Sirish P, Mahakian L, Ingham E, Curry FR, et al. The cargo of CRPPR-conjugated liposomes crosses the intact murine cardiac endothelium. J Control Release. 2012;163(1):10–7. doi: 10.1016/j.jconrel.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanki S, Jaalouk DE, Lee S, Yu AY, Gannon J, Lee RT. Identification of targeting peptides for ischemic myocardium by in vivo phage display. J Mol Cell Cardiol. 2011;50(5):841–8. doi: 10.1016/j.yjmcc.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuge Y, Zheng Z, Xie MQ, Li L, Wang F, Gao F. Preparation of liposomal amiodarone and investigation of its cardiomyocyte-targeting ability in cardiac radiofrequency ablation rat model. Int J Nanomedicine. 2016;11:2359–67. doi: 10.2147/IJN.S98815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGuire MJ, Samli KN, Johnston SA, Brown KC. In vitro selection of peptide with high selectivity for cardiomyocytes in vivo. J Mol Biol. 2004;342:171–82. doi: 10.1016/j.jmb.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Scott RC, Rosano JM, Ivanov Z, Wang B, Chong PL, Issekutz AC, et al. Targeting VEGF-encapsulated immunoliposomes to MI heart improves vascularity and cardiac function. FASEB J. 2009;23:3361–7. doi: 10.1096/fj.08-127373. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Zhou Y, Zhuang Q, Cui L, Xu X, Xu R, et al. Anti-tumor effect of RGD modified PTX loaded liposome on prostatic cancer. Int J Clin Exp Med. 2015;8(8):12182–91. [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, Chen L, Zhang R, Chen Z, Zhu L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J Control Release. 2014;196:222–33. doi: 10.1016/j.jconrel.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Liu Y, Wang W, Liu K. Effect of integrin receptor-targeted liposomal paclitaxel for hepatocellular carcinoma targeting and therapy. Oncol Lett. 2015;10(1):77–84. doi: 10.3892/ol.2015.3242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Takaishi M, Makino T, Morohashi M, Huh NH. Identification of human hornerin and its expression in regenerating and psoriatic skin. J Biol Chem. 2005;280:4696–703. doi: 10.1074/jbc.M409026200. [DOI] [PubMed] [Google Scholar]
- 35.Fleming JM, Ginsburg E, Oliver SD, Goldsmith P, Vonderhaar BK. Hornerin, an S100 family protein, is functional in breast cells and aberrantly expressed in breast cancer. BMC Cancer. 2012;12:266. doi: 10.1186/1471-2407-12-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laeremans H, Hackeng TM, Van Zandvoort MA, Thijssen VL, Janssen BJ, Ottenheijm HC, et al. Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation. 2011;124(15):1626–35. doi: 10.1161/CIRCULATIONAHA.110.976969. [DOI] [PubMed] [Google Scholar]
- 37.Bastakoty D, Saraswati S, Joshi P, Atkinson J, Feoktistov I, Liu J, et al. Temporary, systemic inhibition of the WNT/β-catenin pathway promotes regenerative cardiac repair following myocardial infarct. Cell Stem Cell. 2016;2(2) doi: 10.16966/2472-6990.111. http://dx.doi.org/10.16966/2472-6990.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon J, Zhou H, Zhang LS, Tan W, Liu Y, Zhang S, et al. Blockade to pathological remodeling of infarcted heart tissue using a porcupine antagonist. Proc Natl Acad Sci U S A. 2017;114(7):1649–54. doi: 10.1073/pnas.1621346114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woulfe KC, Gao E, Lal H, Harris D, Fan Q, Vagnozzi R, et al. Glycogen synthase kinase-3 beta regulates post-myocardial infarction remodeling and stress-induced cardiomyocyte proliferation in vivo. Circ Res. 2010;106:1635–45. doi: 10.1161/CIRCRESAHA.109.211482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uosaki H, Magadum A, Seo K, Fukushima H, Takeuchi A, Nakagawa Y, et al. Identification of chemicals inducing cardiomyocyte proliferation in developmental stage-specific manner with pluripotent stem cells. Circ Cardiovasc Genet. 2013;6(6):624–33. doi: 10.1161/CIRCGENETICS.113.000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.