Abstract
Most epithelial cells contain apical membrane structures associated to bundles of actin filaments, which constitute the brush border. Whereas microtubule participation in the maintenance of the brush border identity has been characterized, their contribution to de novo microvilli organization remained elusive. Hereby, using a cell model of individual enterocyte polarization, we found that nocodazole induced microtubule depolymerization prevented the de novo brush border formation. Microtubule participation in brush border actin organization was confirmed in polarized kidney tubule MDCK cells. We also found that centrosome, but not Golgi derived microtubules, were essential for the initial stages of brush border development. During this process, microtubule plus ends acquired an early asymmetric orientation toward the apical membrane, which clearly differs from their predominant basal orientation in mature epithelia. In addition, overexpression of the microtubule plus ends associated protein CLIP170, which regulate actin nucleation in different cell contexts, facilitated brush border formation. In combination, the present results support the participation of centrosomal microtubule plus ends in the activation of the polarized actin organization associated to brush border formation, unveiling a novel mechanism of microtubule regulation of epithelial polarity.
Keywords: actin, brush border, epithelial polarity, microtubules, MTOC
1 ∣ INTRODUCTION
Epithelial cells coating hollow organs contain single apical poles rich in cylindrical membrane protrusions (microvilli), which constitute the brush border. The best characterized brush borders are those developed by enterocytes and kidney tubule epithelial cells. Brush borders associated membrane protrusions increase the cell surface available for the digestion and absorption of lumen nutrients in enterocytes, or for the differential absorption of ultrafiltrate components during urine formation in kidney tubule epithelial cells. At the core of these protrusions are parallel bundles of actin filaments, which are connected to the membrane by the ezrin-radixin-moesin proteins (Sauvanet, Wayt, Pelaseyed, & Bretscher, 2015). These actin structures provide the force to generate the membrane protrusion (Nambiar, McConnell, & Tyska, 2010) and constitute tracks for myosin dependent transport (Crawley, Mooseker, & Tyska, 2014).
The brush border is also supported by another cytoskeletal network, microtubules, which are intrinsically polarized structures with slow-growing minus ends, usually connected to the microtubules organizing centers (MTOCs), and fast-growing plus ends, associated to plus-end binding proteins (+TIPs) that act as regulators of microtubule dynamic instability. Microtubule participation in the maintenance of epithelial polarity and, more specifically, enterocyte brush border organization has been well documented and attributed to their conventional role as polarized tracks that can direct the traffic of membrane components to the already developed apical membrane (Achler, Filmer, Merte, & Drenckhahn, 1989; Gilbert et al., 1991; Müsch, 2004). Furthermore, microtubules have been implicated in two additional events essential for epithelial polarity: the formation of adherent junctions (Stehbens et al., 2006), and the specification of the apico-basal axis induced by cell-extracellular matrix interaction (Akhtar & Streuli, 2013). In contrast to the characterization of microtubule and actin cytoskeletons functional interaction during the development of polarity in other systems (Rodriguez et al., 2003), microtubule participation in the polarized actin nucleation during the de novo formation of epithelial brush border has not been addressed so far.
Ls174T-W4 cells are derived from the Ls174T colon epithelial cell line, whose polarization capacity has been restored by overexpression and stabilization of the liver kinase b1 (Lkb1) activity to produce an enterocyte-like phenotype (Baas et al., 2004). Lkb1 is a kinase which signals upstream AMPK and AMPK related kinases, acting in different pathways involved in the definition of epithelial cell polarity, and behaving, in some contexts, as a tumor suppressor (Partanen, Tervonen, & Klefström, 2013). Upon induction of Lkb1 cytosolic activity, Ls174T-W4 cells promptly reorganize their actin cytoskeleton as a cap at the top or at one side of the cell, independently of cell–cell or cell–extracellular matrix interaction. Those actin accumulations associate with microvilli-structures, as demonstrated by cryo-immuno electron microscopy (Baas et al., 2004). Furthermore, these structures contain typical brush border markers and exclude basolateral markers (Baas et al., 2004; Gloerich et al., 2012). The fact that Ls174T-W4 polarizes as single cells within 6 hr of induction of Lkb1 activity make them an appealing model for studying the involvement of microtubules in the early stages of brush border development, independently of their participation on epithelial polarity signaling downstream cell–cell or cell–extracellular matrix interactions. In this study, we evaluated the participation of microtubules in de novo brush border formation, and characterized the role of the Golgi apparatus and the centrosome as MTOCs in this process. We found that microtubules are essential for actin organization at the brush border in epithelial cells, process which mainly comprises the centrosome as MTOC, and provided evidence that microtubule plus ends associated proteins might be involved in those events.
2 ∣ MATERIALS AND METHODS
2.1 ∣ Cell culture
Ls174T-W4 cells were genetically engineered to restore stable LKB1 expression levels and to generate doxycycline-induced STRADα expression that leads to the cytosolic stabilization and activation of LKB1 (Baas et al., 2004). Ls174T-W4 cell line was generously provided to us by Hans Clevers. The Ls174T-W4 line stably expressing Utrophin Calponin Homology Domain-EGFP as a probe for F-actin was generated in the Tyska laboratory. MDCK cells were obtained from Keith Mostov lab. Cells were grown on plastic dishes in DMEM with 4.5 g of glucose/L, supplemented with 10% fetal bovine serum and antibiotics.
2.2 ∣ Ice recovery assay
Ls174T-W4 cells were seeded 24 hr before the experiment, incubated on ice 40 min and allowed to recover at room temperature for different periods. For analysis of Golgi and centrosome nucleation of microtubules, cells were recovered for 7 min and immediately treated 45 s with extraction buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2, 0.1% Saponin, pH 6.9, supplement with 0.25 nM nocodazole and 0.25 nM paclitaxel). Cells were then fixed and stained as described in Immunofluorescence Confocal Microscopy.
2.3 ∣ Reduction of AKAP350 expression by RNA interference
In order to reduce AKAP350 expression in Ls174T-W4 cells we proceeded as we described (Tonucci et al., 2015). Briefly, constructs were made by annealing and ligating appropriate oligonucleotides containing the AKAP350 mRNA specific sequence (5′-AAATCCCTTGCCAGCACATGA-3′) or its scrambled control, into the AgeI and EcoRI cloning sites of pLKO.1-puro vector (details at http://www.addgene.org. These were sequenced and used to co-transfect human embryonic kidney 293 FT cells with Virapower lentiviral packaging mix (Invitrogen, Carlsbad, CA). Cells were allowed to produce virus for 24 hr. Media containing virus were collected and used to directly transduce LS174T-W4 cells overnight. The cells were allowed to recover for 24 hr and subject to puromycin selection (5 μg/ml) for 2 weeks. Silencing was confirmed by Western blot and immunofluorescence.
2.4 ∣ Cell transfection
The plasmids coding for the AKAP350(3643-3908) (AKAP350CTD) and the AKAP350(1-1229) (AKAP350NTD) domains fused to GFP were kindly donated by JR Goldenring (Vanderbilt University). The EB3-cherry construct was prepared as we described (Efimov et al., 2007) and CLIP170-GFP and CLIP170Δhead-GFP were were a gift from A Akhmanova (Komarova, Akhmanova, Kojima, Galjart, & Borisy, 2002). A total of 2 × 106 LS174T-W4 cells were transfected with 4 μg of DNA using Amaxa Nucleofector™ 2b program X-001, as described in the manufacturer protocol.
2.5 ∣ Immunoblotting
Protein expression by immunoblotting was analyzed as previously described (Larocca et al., 2004). Briefly, cells were washed with cold PBS, scraped and pelleted at 200 g for 5 min at 4°C. Pelleted cells were resuspended in Triton X-100 1% in PBS pH 7.4 with protease and phosphatase inhibitors, and subjected to two freeze-thaw cycles. Lysates were centrifuged at 1,000g for 5 min, and the clear supernatants were conserved. Total protein concentrations were measured according to Lowry, Rosebrough, Farr, and Randall (1951). Solubilized membranes were heated for 10 min at 70°C in sample buffer (20 mM Tris–HCl, pH 8.5, 1% SDS, 400 μM DTT, 10% glycerol). Samples containing equal amounts of protein were subjected to SDS 4% or 12% polyacrylamide gel electrophoresis. The proteins in the gel were transferred to polyvinyl difluoride membranes. Blots were blocked with 5% non-fat milk in PBS with 0.3% Tween-20. Membranes were probed with mouse monoclonal antibodies against AKAP350 (Schmidt et al., 1999) or α-tubulin (1:5,000, Sigma–Aldrich, Buenos Aires, Argentina, T9026) or with rabbit polyclonal antibodies against calreticulin (1:2,000, Sigma-C4606), or phospho-ezrin (1:500, Cell Signalling Technology-mAb3726, Migliore Laclaustra SRL, Buenos Aires, Argentina). The blots were washed and incubated with the corresponding horseradish-peroxidase-conjugated secondary antibodies. Bands were detected by using chemiluminescence reaction (Pierce, Thermo Fisher Scientific, Buenos Aires, Argentina) after exposure to Kodak XAR film. Bands were quantified using the ImageJ program. In preparing the figures, brightness and contrast were adjusted in order to improve visualization.
2.6 ∣ Immunofluorescence confocal microscopy
The cells grown on glass coverslips were washed with PBS and fixed with 4% paraformaldehyde or 1% glutaraldehyde at room temperature, or in 100% methanol at −20°C. Fixed cells were permeabilized/blocked with 0.3% Triton X-100/bovine serum albumin 1%/PBS, pH 7.4 for 10 min. Then, they were incubated with antibodies rabbit anti-GM130 (Abcam-EP892Y, 1:300), anti-γ-tubulin (Sigma-T5192, 1:250) or phospho-ezrin (1:500, Cell Signalling Technology-mAb3726) and mouse anti-γ-tubulin (Sigma-T6557, 1:250) or anti-α-tubulin (Sigma-T9026, 1:300). The coverslips were washed, incubated with the secondary fluorescent conjugated antibodies or phalloydin-Alexa 555 (Molecular probes-A34055, 1:200) for actin staining and with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining and mounted with ProLong. Fluorescence localization was detected by confocal laser microscopy (Nikon C1SiR with inverted microscope Nikon TE200). Serial optical 0.3 μm thick sections were collected in the Z-axis. For structural illumination superresolution microscopy a Delta OMX microscope was used and optical 0.1 μm thick sections were collected in the Z-axis. Live imaging Microscopy was performed using a Yokogawa QLC-100/CSU-10 spinning disk head, attached to a Nikon TE2000E microscope as described (Efimov et al., 2007).
2.7 ∣ Annexin V/propidium iodide assay
Apoptotic cells were detected as we have previously described (Ferretti et al., 2016). Briefly, cells were detached from the petri dishes by trypsinization, gently homogenized in the culture medium/PBS, harvested (5 min, 400 g) and carefully re-suspended in the appropriate buffer. Apoptotic externalization of phosphatidylserine and cell death was assessed by staining with Annexin V-FITC and propidium iodide (Sigma Chemical Co.) coupled to flow cytometric analysis (Cell Sorter BD FACSAria II, BD Biosciences, Buenos Aires, Argentina), following the manufacturers’ instructions. Green and red fluorescence intensities detected in non-stained cells were used to set the thresholds for each channel.
2.8 ∣ Cell treatments
In order to interfere with microtubules function, Ls174T-W4 cells were treated with nocodazole (17 μM) for 30 min and then activated with doxycycline (1 μg/ml) for different periods in the presence of both drugs. To analyze microtubule participation in the organization of the brush border associated actin in MDCK cells, cells were grown on transwells containing 0.4 um pore size polycarbonate membrane inserts (Corning Inc., Oneonta, NY) for 7 days, and cell polarization verified by immunofluorescence. Polarized cells were preincubated with nocodazole (33 μM) on ice for 1 hr to induce loss of unstable microtubules, or in regular media, and then treated with cytochalasin D (20 μM) for 1 hr. Inserts were washed three times with PBS, and then incubated in media containing nocodazole 33 μM for 2 hr (Cytochalasin washout + Nocodazole) or in regular media (Cytochalasin washout), respectively. Additional groups of cells were fixed without any treatment (Control) and immediately after cytochalasin D treatment (Cytochalasin). In order to evaluate the role of Golgi derived microtubules on brush border formation, Ls174T-W4 cells were treated with nocodazole, and activated with doxycycline 30 min after the initiation of the treatment. BFA (5 μg/ml) was added to the media after 4 hr of activation to one group of cells. After 30 min of BFA treatment, nocodazole was removed, and cells were incubated in nocodazole free media, in the presence or absence of BFA for 2 additional hours. In order to assess the role of centrosome-microtubules on brush border, the same protocol, in absence of BFA, was applied to Ls174T-W4 cells expressing the AKAP350CTD domain.
2.9 ∣ Live imaging microscopy
Confocal stacks were taken by a Yokogawa QLC-100/CSU-10 spinning disk head (Visitec assembled by Vashaw) attached to a Nikon TE2000E microscope using a CFI PLAN APO VC 100× oil lens, NA 1.4, with or without 1.5× intermediate magnification, and a back-illuminated EM-CCD camera Cascade 512B (Photometrics, Tucson, AZ) driven by IPLab software (Scanalytics, San Jose, CA). A krypton-argon laser (75 mW 488/568; Melles Griot, Rochester, NY) with AOTF was used for two-color excitation. Custom double dichroic mirror and filters (Chroma) in a filter wheel (Ludl, Hawthorne, NY) were used in the emission light path. Z steps (0.2 μm) were driven by a Nikon built-in Z motor. Live cells plated on MatTech glass bottom dishes were maintained at 37°C by heated stage (Warner Instruments, Holliston, MA) on a Nikon TE2000E inverted microscope equipped with a PerfectFocus automated focusing device. Single-plane confocal video sequences were taken as described for confocal stacks. A similar setup with a Pinkel triple-filter set (Semrock, Rochester, NY) was used for nearly simultaneous two-color wide-field imaging
2.10 ∣ Analysis of brush border formation and microtubules, centrosome, and Golgi reorientation
In Ls174T-W4 cells, brush borders were identified as structures with high actin staining, which were specifically located at the top or at one side of the cell. Microtubule directionality and Golgi and centrosome orientation were determined in cells whose brush borders were located at one side of the cell. Golgi and centrosome orientation were determined considering them apical if the majority was located in a 120° sector emerging from the center of the nucleus and facing the brush border. The percentage of cells with Golgi or centrosome polarization was calculated by dividing the number of cells with the organelle oriented toward the brush border by the number of total cells for each condition. Microtubule directionality at the brush border pole was determined by using an ImageJ plugin. In polarized MDCK cells, we determined that the brush border correspond to the top 2 μm of the cells in the actin channel. To analyze actin organization at the brush border in MDCK cells, we quantified the phalloidin staining present at the top 2 um of the cells in the z-sections of the corresponding channel and related it to the total cell fluorescence measured in the same section. At least 120 randomly selected cells per experiment were analyzed.
2.11 ∣ Statistical analysis
Data are expressed as mean ± s.e.m. Paired Student’s t-test was used for comparison between groups and non-parametric Mann–Whitney test was used for comparisons within each experiment. p < 0.05 was considered statistically significant.
3 ∣ RESULTS
3.1 ∣ Ls174T-W4 polarized cells organize their microtubule cytoskeleton as columnar epithelial cells
We first characterize the microtubule organization in polarized Ls174T-W4 cells. Differentiated epithelial cells have a characteristic vertical organization, with microtubule minus ends located below the apical membrane and their plus ends pointing the basal membrane. These vertical nets are complemented with a subapical mesh of microtubules with random orientation (Müsch, 2004). In order to evaluate microtubule arrangement in Ls174T-W4 polarized cells, cells were activated 6 hr with doxycycline, to induce brush border formation, and immunostained for actin and microtubule identification (Figure 1a,b). Images obtained by confocal microscopy indicated the presence of transcellular apico-basal arrays of microtubules (Figure 1a). In order to get more detail on microtubule directionality at the brush border, we analyzed the staining by structural illumination microscopy (Figure 1b). We observed at least two populations of microtubules: those which organized sub apically, with random distribution (arrowheads) and those which were oriented perpendicular to the brush border (arrows). Consistently, our quantitative analysis of microtubule directionality at the brush border pole indicated the presence of a microtubule population with a Gaussian distribution centered at 90° with respect to the tangent to the brush border (Figure 1b). The centrosomes and the Golgi apparatus, are the best characterized MTOCs in animal cells (Bornens, 2012; Sanders & Kaverina, 2015). We examined the positioning of both organelles in Ls174T-W4 polarized cells dual stained for γ-tubulin and GM130, which were used as centrosome and Golgi markers, respectively. We found that most of Ls174T-W4 polarized cells showed apical centrosome and Golgi localization (Figure 1c), what resembles centrosome and Golgi localization in epithelial differentiated cells.
FIGURE 1.

Ls174T-W4 polarized cells organize their microtubule cytoskeleton as typical columnar epithelial cells. (a,b) Ls174T-W4 cells stably expressing Utrophin-GFP (UtrGFP) as actin marker (red) were activated with doxycycline for 6 hr and fixed with glutaraldehyde. Cells were stained with anti α-tubulin antibody for microtubule visualization (green) and analyzed by confocal microscopy (a) or structural illumination microscopy (b). (b) Arrowheads indicate microtubules with random distribution; arrows, microtubules oriented perpendicular to the brush border. Microtubule directionality toward the brush border was analyzed using an ImageJ plugin. The chart shows microtubule distribution according to their direction, corresponding to the cell shown in the image. These are typical results, representative of 10 different cells. (c) Cells were stained with anti-GM130 (blue) and anti-γ-tubulin (γ-tub, red) antibodies for Golgi and centrosome visualization, respectively. Bars show the percentage of cells which contains the Golgi apparatus or the centrosomes oriented toward the brush border and are representative of three independent experiments. At least 80 cells were analyzed in each experiment. Scale bars, 2.5 μm. *p < 0.05
3.2 ∣ Microtubules are essential for de novo brush border formation
We evaluated the impact of nocodazole-induced microtubule disruption on brush border development. Our results showed that Ls174T-W4 cells subjected to nocodazole treatment were unable to form brush borders (Figure 2a). Previous studies demonstrated that ezrin gets phosphorylated at the T567 (pEzrin) and that pEzrin accumulates at the brush border upon induction of Ls174T-W4 cells (Gloerich et al., 2012; ten Klooster et al., 2009). Therefore, we analyzed the effect of microtubule disruption on pEzrin levels and distribution in Ls174T-W4 activated cells. The analysis of pEzrin levels by western blot in control and in nocodazole treated cells confirmed that induction of cell differentiation lead to augmented levels of pEzrin and indicated that this increase was not affected by the presence of Nocodazole (Figure 2b). We further analyzed pEzrin distribution by immunofluorescence confocal microscopy. In accordance to the results shown in Figure 2b, the analysis of pEzrin total levels by quantitative confocal microscopy showed that nocodazole treatment elicited no effect on the total levels of pEzrin. On the other hand, pEzrin polarized distribution was severely affected by nocodazole treatment. These results confirm that microtubules participated in the polarized organization of the brush border in Ls174T-W4 cells (Figure 2b). Nocodazole, as well as other drugs that interfere with microtubule dynamics, can induce apoptotic cell death (Mollinedo & Gajate, 2003). We analyzed apoptosis levels in Ls174T-W4 cells subjected to Nocodazole treatment, in the same conditions used above, and found a subtle but significant increase in apoptotic death (Supplemental Figure S1a). In order to evaluate if the inhibition of brush border development induced by microtubule disruption was related to the induction of apoptosis, we analyzed the effect of a different pro-apoptotic agent on brush border formation. Metformin is a drug which, in the conditions of treatment assessed, induced levels of apoptotic cell death comparable to Nocodazole treatment (Supplemental Figure S1a). Metformin treatment did not induce any evident effect on brush border formation (Supplemental Figure S1b), what indicates that, at the levels elicited by the Nocodazole treatment used in these study, apoptosis itself does not affect this process.
FIGURE 2.

Microtubules are essential for de novo brush border formation in Ls174T-W4 cells. Cells were treated with nocodazole 17 μM for 30 min previous to and during activation with doxycycline. (a) Images show actin staining in control cells and in cells pretreated with nocodazole. Bars represent the percentage of control and nocodazole treated cells which developed brush border in the presence or absence of doxycycline, for at least 80 cells counted in three independent experiments. (b) Typical western blot showing pEzrin expression in control and nocodazole treated cells subjected to induction with doxycycline. α-tubulin was used as loading control. Bars show the densitometric analysis of the western blot. (c) The images show pEzrin (green) and actin (red) localization in activated cells subjected or not to nocodazole treatment. Bars represent total cell pEzrin-specific fluorescence intensity for 30 cells. Scale bars, 10 μm (a) or 5 μm (c). *p < 0.05
To analyze if our observations regarding the dependence of brush border formation on microtubule integrity were extensive to other epithelial cells, we analyzed the impact of the impairment in microtubule polymerization on apical actin organization in MDCK cells, a very well characterized model of kidney tubule epithelial cells. MDCK cells were grown as monolayers on filters for 7 days, as described in section 2. In these conditions, as soon as 3 days after cell seeding, MDCK cells develop brush borders associated to rods of actin filaments, which organize perpendicular to the apical surface (Hyman, Shmuel, & Altschuler, 2006). Polarized cells were subjected to microtubule depolymerization by incubation on ice in the presence of nocodazole, and then treated with cytochalasin D in conditions that do not perturb tight junction integrity (Van Itallie, Fanning, Bridges, & Anderson, 2009). Cytochalasin D was washed, and cells were further incubated in the presence of nocodazole, to evaluate actin recovery in this condition. A similar protocol applied to cells which were not exposed to nocodazole was used to analyze actin reorganization in the presence of intact microtubules. Cells were immunostained to visualize actin and microtubule cytoskeleton. Actin organization at the apical pole was analyzed in the z-sections of the images obtained by confocal microscopy. Before any treatment, cells showed its characteristic apical actin organization (Figure 3, first column). As expected, after cytochalasin treatment, most actin was redistributed to aggregates, with the exception of that associated to cellular junctions (Figure 3, second column). After 2 hr of recovery in cytochalasin free media, cells with intact microtubules could partially reconstitute their actin cytoskeleton at the brush borders (Figure 3, third column). Nevertheless, in the presence of nocodazole, even though most actin aggregates disappeared, the apical actin did not recover (Figure 3, fourth column). These results confirm that microtubules have a central role in the regulation of actin organization at the brush border in epithelial cells.
FIGURE 3.

Microtubules play a central role in actin organization at the brush border in MDCK cells. MDCK cells were seeded on filters at confluence and grown for 7 days. One group of cells was fixed to analyze actin localization at the brush border in control conditions. In order to induce microtubule depolymerization, cells were pretreated with nocodazole 33 μM on ice during 60 min and maintained with nocodazole 33 μM throughout the experiment. Non-treated and nocodazole treated cells were exposed to cytochalasin D 20 μM for 60 min. One group of cytochalasin D treated cells was fixed after this treatment (Cytochalasin) to verify the effect of cytochalasin D treatment on actin integrity at the brush border. The other groups of cells treated with cytochalasin D, in the absence or presence of nocodazole, were washed with PBS and then incubated in cytochalasin free media for 2 hr (Cytochalasin washout and Cytochalasin washout + nocodazole, respectively). Cells were fixed and stained with anti α-tubulin, phalloidin, and DAPI, and analyzed by confocal microscopy. The figure shows three-dimensional reconstructions of F-actin (red), nuclear (blue) and microtubule (green) staining representative of each condition (first row) and orthogonal views of merged channels (second row) or actin staining (third row) corresponding to the same fields. Bars represent the F-actin present at the top 2 μm of the cell, which corresponds to the brush border area, expressed as percentage of total F-actin, for at least 120 cells, representative of three independent experiments. Scale bars, 10 μm. *p < 0.05
3.3 ∣ Centrosomal, but not Golgi derived microtubules, regulate brush border development
We first characterized the ability of the centrosome and the Golgi apparatus to nucleate microtubules in Ls174T-W4 cells, by performing ice recovery assays as we have previously described (Grimaldi, Fomicheva, & Kaverina, 2013). Similarly, to what we observed in RPE1 cells, after incubation on ice for 40 min, polymerized microtubules were hardly observed in Ls174T-W4 cells (Supplemental Figure S2a). We further analyzed the appearance of microtubules after different periods of incubation at room temperature, and found that after 7 min incubation individual-newly formed microtubules were already evident (Supplemental Figure S2b). At this time lapse, centrosomal microtubules average length was around 2 μm, and, consequently, could still be differentiated from Golgi derived microtubules. We analyzed the presence of Golgi and centrosome derived microtubules after 7 min of rewarming, as we described (Grimaldi et al., 2013). Briefly, centrosomal microtubules were recognized as radial arrays of microtubules associated to γ-tubulin spots, while Golgi derived microtubules were identified as asymmetric arrays originated from the GM130 labeled organelle. We identified that both the centrosome (Supplemental Figure S2c) and the Golgi apparatus (Supplemental Figure S2d) nucleated microtubules in Ls174T-W4 cells.
Previous studies indicate that Golgi derived microtubules direct apical actin organization in hepatocytes (Mattaloni et al., 2012). We analyzed the role of Golgi microtubules, and the participation of the Golgi itself, in brush border formation in Ls174T-W4 cells. Microtubule nucleation at the Golgi and at the centrosome requires the presence of γ-tubulin and its associated proteins, which form the γ-tubulin ring complex (γ-TURC). AKAP350 is an A-kinase anchoring protein which participates in the recruitment of the γ-TURC to both organelles (Rivero, Cardenas, Bornens, & Rios, 2009; Takahashi, 2002). Nevertheless, the effect of AKAP350 depletion on microtubule nucleation at each organelle is cell-type dependent (Larocca, Jin, & Goldenring, 2006; Oddoux et al., 2013; Ori-McKenney, Jan, & Jan, 2012; Rivero et al., 2009). We first characterized the impact of AKAP350 depletion on microtubule nucleation at the centrosome and at the Golgi apparatus in Ls174T-W4 cells. We generated Ls174T-W4 cells with stable decrease in AKAP350 expression (AKAP350KD), as described in section 2. AKAP350 levels in control and AKAP350KD cells were checked by Western blot (Figure 4a). Similarly, to what was shown in other epithelial derived cells, in Ls174T-W4 cells the decrease in AKAP350 expression did not affect the overall nucleation of microtubules at the centrosome, but severely impaired the formation of microtubules at the Golgi apparatus (Figure 4b). Hence, we analyzed the “de novo” brush border formation in AKAP350KD cells. We found that, after 6 hr of induction of cell polarization, the percentage of cells that developed brush border was not modified by AKAP350 depletion (Figure 4c). These experiments also showed that the Golgi apparatus did not polarize toward the brush border in AKAP350KD cells, but got fragmented and dispersed (Figure 4c), similarly to what was described in other cells (Larocca et al., 2004; Rivero et al., 2009). On the other hand, the polarized localization of the centrosome was not affected (Figure 4c).
FIGURE 4.

The impairment in Golgi function does not affect brush border development. (a) Western blot showing AKAP350 expression in control and AKAP350KD cells. Calreticulin was used as loading control. Molecular weight markers are indicated. (b) Control and AKAP350KD cells were subjected to ice recovery assays, and microtubule nucleation at centrosomes and Golgi apparatus, quantified. Images show centrosome (red, first row) or Golgi (red, second row) derived microtubules (green) 7 min after cell rewarming. The inset images show views of microtubule nucleation sites (boxed areas). (c) Images show the organization of the actin cytoskeleton (green), the Golgi apparatus (blue), and the centrosomes (red) in AKAP350KD activated cells. Bars represent the percentage of cells that developed brush borders and, among these, the percentage of cells that had polarized localization of the centrosomes or Golgi apparatus. (d) Cells were treated as indicated in the schema. As positive control, a group of cells received nocodazole treatment the whole period (Noc). Images show actin (red) and Golgi (green) organization in BFA treated cells. Bars represent the fraction of activated cells which developed brush borders. (e) The RGB image shows brush border development (red) in a cell which expressed AKAP350NTD (green), and in a control cell. The gray scale image shows the channel corresponding to AKAP350 staining. Bars represent the fraction of activated cells which developed brush border. Data are representative of 40 (b) or 80 (c–e) cells. Scale bars, 5 μm. *p < 0.05
We further analyzed brush border formation in cells with Golgi dysfunction using two additional strategies. (1) We subjected cells to Brefeldin A treatment in conditions that induced Golgi unstacking and fragmentation (Figure 4d), as previously described (Fujiwara, Oda, & Ikehara, 1989). (2) We prepared LS174T-W4 cells with transient expression of AKAP350(1-1029) (AKAP350NTD) domain, which contains a strong Golgi targeting domain and, by acting as a dominant negative AKAP350 mutant, inhibits microtubule nucleation at this organelle (Hurtado et al., 2011). We studied de novo brush border formation in both conditions and, in accordance to our observations in AKAP350KD cells, Golgi dysfunction did not affect brush border development in any case (Figure 4d,e).
Thereafter, we evaluated the participation of centrosome-derived microtubules in the development of actin rich brush borders in LS174T-W4 cells. AKAP350 and pericentrin share an evolutionary conserved domain, which mediates their targeting to the centrosome: the pericentrin-AKAP350 centrosomal targeting (PACT) domain (Gillingham & Munro, 2000). Expression of the PACT domain inhibits centrosomal nucleation of microtubules, by displacing pericentrin and AKAP350 from the centrosome (Gillingham & Munro, 2000). We prepared cells with transient expression of the AKAP350 domain comprising the PACT domain (AKAP350CTD), and verified that AKAP350CTD expression inhibited microtubule nucleation at the centrosome, but did not affect microtubule nucleation at the Golgi apparatus (Figure 5a). The analysis of de novo brush border formation in these cells indicated that polarized actin organization was significantly decreased in AKAP350CTD cells (−25%, p < 0.05). In order to further evaluate the role of newly formed centrosomal microtubules on brush border formation, we treated control and AKAP350CTD cells with nocodazole to depolymerize microtubules during the first 4 hr of activation with doxycicline, washed the drug to induce microtubule polymerization and evaluated brush border formation after 2 hr of activation in nocodazole free media. The effect of the inhibition of centrosomal nucleation of microtubules on polarized actin nucleation in that condition was dramatic (Figure 5b), comparable to the effect elicited by nocodazole induced microtubule disruption (Figure 2a). Therefore, the perturbation of centrosomal nucleation of microtubules led to a significant defect in brush border formation, what make a clear contrast with the insensitivity of Ls174T-W4 cell polarization to the impairment of the Golgi nucleation of microtubules (Figure 4).
FIGURE 5.
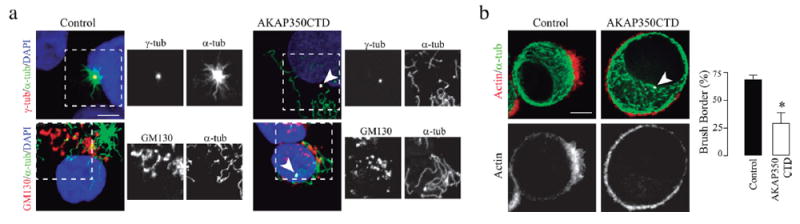
Centrosome-derived microtubules are essential for actin organization at the brush border. Cells were transfected with AKAP350CTD fused to GFP (AKAP350CTD), or with the empty plasmid (control). (a) Cells were subjected to ice recovery assays. Images show centrosome (red, first row) or Golgi (red, second row) derived microtubules (green) in control cells and in cells expressing AKAP350CTD (gray, arrowhead). The inset images show views of centrosome or Golgi microtubule nucleation sites (boxed areas). (b) AKAP350CTD and control cells were treated with nocodazole, activated with doxycycline, and brush border formation analyzed 2 hr after nocodazole removal. Images show AKAP350CTD-GFP expression (arrowhead, white), actin (red), and microtubule (green) organization. Bars represent the fraction of cells that developed brush borders. Data are representative of 80 cells. Scale bars, 5 μm. *p < 0.05
3.4 ∣ Microtubule plus ends have an early apical orientation and condition apical actin organization
In order to get mechanistic information, we studied microtubule dynamics during brush border formation. We performed live imaging experiments in Ls174T-W4 cells stably expressing the actin marker Utrophin-GFP and transiently expressing the plus end marker EB3-Cherry. Our results showed that, during Ls174T-W4 cell polarization, there was a clear plus end microtubule propagation toward the apical pole, which was synchronized with polarized actin organization (Figure 6a and Movie 1). Quantification of the fluorescence associated to EB3 in the brush border pole showed a clear increase during the first 120 min of cell induction to polarize, which contrasted with the decrease of EB3 levels in the other sections of the cells (Figure 6b). We hypothesized that the presence of microtubule plus ends at the cell cortex could provide a signaling platform to regulate the initial events in brush border development, as it occurs in other models of cell polarity (Rodriguez et al., 2003). The +TIP “cytoplasmic linker protein” (CLIP) 170, one of the best characterized plus ends associated proteins, can regulate actin nucleation in “in vitro” systems (Henty-Ridilla, Rankova, Eskin, Kenny, & Goode, 2016). We prepared Ls174T-W4 cells with transient overexpression of CLIP170 and evaluated the effect of the increase in this protein levels on brush border formation. Our results showed that overexpression of CLIP170 induced a clear increase in brush border formation (Figure 7a). CLIP170 amino terminal domain mediates its interaction with microtubule plus ends, but is dispensable for CLIP170 dependent induction of actin elongation (Henty-Ridilla et al., 2016). To further evaluate if microtubules were implicated in CLIP170 induced apical actin organization, we analyzed brush border formation in cells with transient expression of a CLIP170 mutant lacking this amino-terminal domain: CLIP170 (348-end) (CLIP170Δhead). As expected, CLIP170Δhead shows homogenous cytosolic localization, which clearly differs from CLIP170 “comet-like” distribution (Figure 7, insets). We found that CLIP170Δhead cells did not increase, but, on the contrary, diminished brush border actin organization (Figure 7b). These results showed that CLIP170Δhead behaves as a dominant negative mutant of CLIP170 in this particular process, thus indicating that CLIP170 interaction with microtubule plus ends is necessary for its stimulatory effect on brush border actin organization.
FIGURE 6.

Microtubule plus ends have an early apical orientation during de novo brush border formation. UtrGFP Ls174T-W4 cells were transiently transfected with EB3-Cherry. Cells were activated with doxycycline, and visualized by live imaging. Frames were obtained every 30 s. (a) Images show actin (red) and EB3 (green) distribution. (b) EB3 accumulation in the anterior pole (BB), measured in a 120° angle toward the brush border, or media EB3 accumulation in the other 120° sections of the cell (other) were analyzed using ImageJ tools. Data are representative of three independent experiments. Scale bar, 5 μm
FIGURE 7.

CLIP170 promotes, whereas CLIP170Δhead inhibits, actin organization at the brush border. Ls174T-W4 cells were transfected with CLIP170 (a) or CLIP170Δhead (b) fused to GFP. Cells were activated with doxycycline, and brush border formation analyzed 4 hr after activation. Images show F-actin staining (red) and CLIP170 (a) or CLIP170Δhead (b) (green) expression. The inset images show magnified views of CLIP170 (a) or CLIP170Δhead (b) expression (boxed areas). Bars represent the fraction of transfected or non-transfected (control) cells which developed brush borders representative of, at least, 80 cells counted per experiment. Stars indicate transfected cells. Scale bars, 20 μm. *p < 0.05
4 ∣ DISCUSSION
The implication of microtubules in the development and maintenance of epithelial polarity have been thoroughly studied from different perspectives. On this regard, microtubules have been implicated in apical traffic of membrane components, which ensures the stability of the apical pole of columnar epithelial cells (Müsch, 2004). Furthermore, microtubule plus ends associated proteins are involved in the positioning of E-cadherin during the formation of adherent junctions (Stehbens et al., 2006), and in the regulation of the apico-basal axis orientation downstream integrin interaction with the extracellular matrix (Akhtar & Streuli, 2013). On the other hand, microtubules functional interaction with the actin cytoskeleton is essential during symmetry breaking events in processes as diverse as integrin mediated phagocytosis (Lewkowicz et al., 2008), directional cell migration (Waterman-Storer & Salmon, 1999), and guided extension of developing axons (Buck & Zheng, 2002). In spite of the relevance of apical actin organization in brush border development and, therefore, in epithelial morphology and function, the role of microtubules in the modulation of apical actin organization has not been addressed so far, most probably due to the lack of proper models. In the present study we analyzed the role of microtubules in the development of actin rich brush borders. Our results demonstrated that: (1) Microtubules are essential for actin nucleation at the brush border; (2) Centrosomes are the main MTOC associated with this process; (3) Microtubule plus ends orient apically early during brush border development, and its associated protein CLIP170 enhances brush border formation.
We first characterized microtubule organization in Ls174T-W4 polarized cells. Previous studies reported that there were no changes in microtubule cytoskeleton organization after Ls174T-W4 cells activation (Baas et al., 2004). Since microtubule reorganization in mature enterocytes might be not as conspicuous as actin remodeling, we performed a quantitative analysis making focus on two issues: (1) Microtubule orientation; (2) MTOCs localization. Our results showed that, in polarized cells, there are transcellular arrays of microtubules. The analysis of individual microtubules orientation showed that there is a population of microtubules which orient perpendicular to the brush border, where another group of microtubules located directly below the brush border exhibit random orientation. Additionally, we found that most of the cells have apical orientation of the Golgi apparatus and the centrosomes. This arrangement of the microtubule cytoskeleton resembles the actual microtubule organization in mature epithelial cells, as opposed to the radial arrays of microtubules with centrally located centrosome and Golgi apparatus characteristic of non-polarized cells. These results confirm the fitness of LS174T-W4 cells as a polarized epithelial cell model for studies related to cytoskeleton functions.
We demonstrated that nocodazole induced microtubule depolimerization severely impairs brush border development in Ls174T-W4 cells. A previous study showed that microtubule disruption induced by the same drug promotes brush border formation in long term cultures of HT29 cells which, otherwise, do not polarize (Cohen, Ophir, & Shaul, 1999). The differences between this study and our results are most probably due to differences inherent to the cell models. To explore if our findings were a peculiarity of Ls174T-W4 cells, we analyzed the effect of nocodazole treatment on brush border organization using MDCK cells, a very well characterized model of columnar epithelial cell polarity. As noted before, microtubules regulate the maturation of adherent junctions, which is a very early event during the development of epithelial polarity (Stehbens et al., 2006). In order to evaluate microtubule effect on actin nucleation at the brush border independently of that process, we analyzed actin organization at the apical pole on cells which had already established adherent junctions. Our results showed that, in control conditions, MDCK cells subjected to cytochalasin D treatment could partially restitute actin organization at the brush border after 2 hr of incubation in cytochalasin free media. On the contrary, cells whose microtubule integrity was disrupted were not able to recover their typical apical actin organization. Therefore, we can conclude that actin organization at the brush border in epithelial cells depends on microtubule integrity, what we found to be the most outstanding result that emerges from our study.
Hepatocytes are epithelial hepatic cells which, in contrast to most epithelial cells, develop multiple apical poles. Our previous studies demonstrated that Golgi derived microtubules participate in apical (canalicular) actin nucleation in hepatocytes (Mattaloni et al., 2012). Therefore, we expected that the interference with Golgi microtubule nucleation would inhibit brush border formation in Ls174T-W4 cells. Using three different strategies we demonstrated that Golgi derived microtubules were not necessary for the development of the apical pole in LS174T-W4 cells, evidencing a differential impact of Golgi microtubules on the development of both types of epithelial polarity. Moreover, these results further suggest that vesicle trafficking from the Golgi apparatus is not essential for the initial stages of brush border formation. On the other hand, impairment of microtubule nucleation at the centrosome severely inhibited brush border formation, thus implicating this particular group of microtubules as main actors in columnar epithelial cell differentiation.
Regarding how microtubules modulate actin organization during brush border development, our results demonstrate that, immediately after induction of cell polarization, there is a remarkable enrichment in microtubule plus ends at the pole where the brush border is to be developed. Microtubule plus ends behave as signaling platforms by recruiting several regulatory proteins. As a matter of fact, microtubule plus ends associated proteins are highly relevant in the specification of cell asymmetry in processes such as cell migration (Etienne-Manneville, 2013), mitotic spindle orientation (Johnston et al., 2013), and neuronal development (van de Willige, Hoogenraad, & Akhmanova, 2016). A recent study demonstrated that CLIP170 accelerates formin-mediated actin elongation. CLIP170 associates to microtubule (+) ends by interacting with the +TIP EB1 (Dixit et al., 2009). Moreover, EB1 sequentially recruits CLIP170 and the formin mDia to microtubules (+) ends, and this complex can markedly enhance the rate of actin nucleation (Henty-Ridilla et al., 2016). Our results show that CLIP170, but not its microtubule binding deficient mutant CLIP170Δ-head, enhances brush border actin organization, thus implicating microtubule plus end associated CLIP170 in actin organization at the apical pole of epithelial cells during brush border formation.
In conclusion, our studies demonstrate that microtubules act in the early stages of de novo brush border organization in epithelial cells. We show that centrosome, but not Golgi-derived microtubules are essential for intestinal Ls174T-W4 cell polarization. In addition, we show that, in these cells, microtubule plus ends have an early apical orientation and that the +TIP CLIP170 stimulates brush border formation. The backbone of the pathways regulating the initial events during the establishment of epithelial polarity is highly conserved in epithelial cells with simple polarity. Hence, it is likely that the participation of centrosomal microtubules and their plus end associated protein CLIP170 in the organization of the apical actin cytoskeleton is a general feature in the development of columnar epithelial polarity.
Supplementary Material
Acknowledgments
We thank Dr. Arlinet Kierbel for helpful discussion and advice for this study. This work was supported by CONICET (PIP0287 to M.C.L.), Agencia Nacional de Promoción Científica y Tecnológica (PICT2012-2198 to M.C.L.), Argentina Government (Bec.Ar to F.T.), and the National Institute of Health (R01-GM078373 and R01-DK106228 to I.K., R01-DK075555 and R01-DK095811 to M.J.T.).
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- Achler C, Filmer D, Merte C, Drenckhahn D. Role of microtubules in polarized delivery of apical membrane proteins to the brush border of the intestinal epithelium. The Journal of Cell Biology. 1989;109:179–189. doi: 10.1083/jcb.109.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nature Cell Biology. 2013;15:17–27. doi: 10.1038/ncb2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- Bornens M. The centrosome in cells and organisms. Science. 2012;335:422–426. doi: 10.1126/science.1209037. [DOI] [PubMed] [Google Scholar]
- Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Ophir I, Shaul YB. Induced differentiation in HT29, a human colon adenocarcinoma cell line. Journal of Cell Science. 1999;112(Pt 1):2657–2666. doi: 10.1242/jcs.112.16.2657. [DOI] [PubMed] [Google Scholar]
- Crawley SW, Mooseker MS, Tyska MJ. Shaping the intestinal brush border. The Journal of Cell Biology. 2014;207:441–451. doi: 10.1083/jcb.201407015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Barnett B, Lazarus JE, Tokito M, Goldman YE, Holzbaur ELF. Microtubule plus-end tracking by CLIP-170 requires EB1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:492–497. doi: 10.1073/pnas.0807614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Kaverina I, et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Developmental Cell. 2007;12:917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. Microtubules in cell migration. Annual Review of Cell and Developmental Biology. 2013;29:471–499. doi: 10.1146/annurev-cellbio-101011-155711. [DOI] [PubMed] [Google Scholar]
- Ferretti AC, Tonucci FM, Hidalgo F, Almada E, Larocca MC, Favre C. AMPK and PKA interaction in the regulation of survival of liver cancer cells subjected to glucose starvation. Oncotarget. 2016;7:17815–17828. doi: 10.18632/oncotarget.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Oda K, Ikehara Y. Dynamic distribution of the Golgi marker thiamine pyrophosphatase is modulated by brefeldin A in rat hepatoma cells. Cell Structure and Function. 1989;14:605–616. doi: 10.1247/csf.14.605. [DOI] [PubMed] [Google Scholar]
- Gilbert T, Le Bivic A, Quaroni A, Rodriguez-Boulan E. Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. The Journal of Cell Biology. 1991;113:275–288. doi: 10.1083/jcb.113.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Reports. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloerich M, ten Klooster JP, Vliem MJ, Koorman T, Zwartkruis FJ, Clevers H, Bos JL. Rap2A links intestinal cell polarity to brush border formation. Nature Cell Biology. 2012;14:793–801. doi: 10.1038/ncb2537. [DOI] [PubMed] [Google Scholar]
- Grimaldi AD, Fomicheva M, Kaverina I. Ice recovery assay for detection of Golgi-derived microtubules. Methods in Cell Biology. 2013;118:401–415. doi: 10.1016/B978-0-12-417164-0.00024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henty-Ridilla JL, Rankova A, Eskin JA, Kenny K, Goode BL. Accelerated actin filament polymerization from microtubule plus ends. Science. 2016;352:1004–1009. doi: 10.1126/science.aaf1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado L, Caballero C, Gavilan MP, Cardenas J, Bornens M, Rios RM. Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. The Journal of Cell Biology. 2011;193:917–933. doi: 10.1083/jcb.201011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman T, Shmuel M, Altschuler Y. Actin is required for endocytosis at the apical surface of Madin-Darby canine kidney cells where ARF6 and clathrin regulate the actin cytoskeleton. Molecular Biology of the Cell. 2006;17:427–437. doi: 10.1091/mbc.E05-05-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, Manning L, Lu MS, Golub O, Doe CQ, Prehoda KE. Formin-mediated actin polymerization cooperates with Mushroom body defect (Mud)-Dynein during Frizzled-Dishevelled spindle orientation. Journal of Cell Science. 2013;126:4436–4444. doi: 10.1242/jcs.129544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova YA, Akhmanova AS, Kojima S, Galjart N, Borisy GG. Cytoplasmic linker proteins promote microtubule rescue in vivo. Journal of Cell Biology. 2002;159:589–599. doi: 10.1083/jcb.200208058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca MC, Jin M, Goldenring JR. AKAP350 modulates microtubule dynamics. European Journal of Cell Biology. 2006;85:611–619. doi: 10.1016/j.ejcb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Larocca MC, Shanks RA, Tian L, Nelson DL, Stewart DM, Goldenring JR. AKAP350 interaction with cdc42 interacting protein 4 at the Golgi apparatus. Molecular Biology of the Cell. 2004;15:2771–2781. doi: 10.1091/mbc.E03-10-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz E, Herit F, Le Clainche C, Bourdoncle P, Perez F, Niedergang F. The microtubule-binding protein CLIP-170 coordinates mDia1 and actin reorganization during CR3-mediated phagocytosis. The Journal of Cell Biology. 2008;183:1287–1298. doi: 10.1083/jcb.200807023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Mattaloni SM, Kolobova E, Favre C, Marinelli RA, Goldenring JR, Larocca MC. AKAP350 Is involved in the development of apical “canalicular” structures in hepatic cells HepG2. Journal of Cellular Physiology. 2012;227:160–171. doi: 10.1002/jcp.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollinedo F, Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis. 2003;28:413–450. doi: 10.1023/a:1025513106330. [DOI] [PubMed] [Google Scholar]
- Müsch A. Microtubule organization and function in epithelial cells. Traffic. 2004;5:1–9. doi: 10.1111/j.1600-0854.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Nambiar R, McConnell RE, Tyska MJ. Myosin motor function: The ins and outs of actin-based membrane protrusions. Cellular and Molecular Life Sciences. 2010;67:1239–1254. doi: 10.1007/s00018-009-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddoux S, Zaal KJ, Tate V, Kenea A, Nandkeolyar SA, Reid E, Ralston E, et al. Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. The Journal of Cell Biology. 2013;203:205–213. doi: 10.1083/jcb.201304063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney KM, Jan LY, Jan Y-N. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76:921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen JI, Tervonen TA, Klefström J. Breaking the epithelial polarity barrier in cancer: The strange case of LKB1/PAR-4. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2013;368:20130111. doi: 10.1098/rstb.2013.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero S, Cardenas J, Bornens M, Rios RM. Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. The EMBO Journal. 2009;28:1016–1028. doi: 10.1038/emboj.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez OC, Schaefer AW, Mandato C, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nature Cell Biology. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- Sanders AAWM, Kaverina I. Nucleation and dynamics of Golgi-derived microtubules. Frontiers in Neuroscience. 2015;9:1–7. doi: 10.3389/fnins.2015.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvanet C, Wayt J, Pelaseyed T, Bretscher A. Structure, regulation, and functional diversity of microvilli on the apical domain of epithelial cells. Annual Review of Cell and Developmental Biology. 2015;31:593–621. doi: 10.1146/annurev-cellbio-100814-125234. [DOI] [PubMed] [Google Scholar]
- Schmidt PH, Dransfield DT, Claudio JO, Hawley RG, Trotter KW, Milgram SL, Goldenring JR. AKAP350, a multiply spliced protein kinase A-anchoring protein associated with centrosomes. Journal of Biological Chemistry. 1999;274:3055–3066. doi: 10.1074/jbc.274.5.3055. [DOI] [PubMed] [Google Scholar]
- Stehbens SJ, Paterson AD, Crampton MS, Shewan AM, Ferguson C, Akhmanova A, Yap AS, et al. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. Journal of Cell Science. 2006;119:1801–1811. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
- Takahashi M. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma—Tubulin ring complex. Molecular Biology of the Cell. 2002;13:3235–3245. doi: 10.1091/mbc.E02-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster JP, Jansen M, Yuan J, Oorschot V, Begthel H, Di Giacomo V, Clevers H, et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Developmental Cell. 2009;16:551–562. doi: 10.1016/j.devcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Tonucci FM, Hidalgo F, Ferretti A, Almada E, Favre C, Goldenring JR, Larocca MC, et al. Centrosomal AKAP350 and CIP4 act in concert to define the polarized localization of the centrosome and Golgi in migratory cells. Journal of Cell Science. 2015;128:3277–3289. doi: 10.1242/jcs.170878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Willige D, Hoogenraad CC, Akhmanova A. Microtubule plus-end tracking proteins in neuronal development. Cellular and Molecular Life Sciences: CMLS. 2016;73:2053–2077. doi: 10.1007/s00018-016-2168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Molecular Biology of the Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon E. Positive feedback interactions between microtubule and actin dynamics during cell motility. Current Opinion in Cell Biology. 1999;11:61–67. doi: 10.1016/s0955-0674(99)80008-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


