Abstract
Follicular T regulatory (Tfr) cells inhibit follicular T helper (Tfh) cells mediated B cell responses. Tfh cells are involved in the pathogenesis of systemic lupus erythematosus (SLE). However, the role of Tfr cells in SLE remains unclear. The frequency of circulating Tfr and Tfh cells were examined in SLE patients and healthy controls. The frequency of circulating Tfr cell decreased and Tfh/Tfr ratio increased in SLE patients. Serum anti-dsDNA antibody level positively correlated with frequency of Tfh cells and Tfh/Tfr ratios but negatively correlated with the frequency of Tfr cells. Moreover, the frequency of Tfr and Tfh/Tfr ratio but not that of Tfh was correlated with diseases activity. In addition, increase in Tfr cell numbers and decrease in the Tfh/Tfr ratios were observed with successful treatments. Thus, Tfr cells should be considered as a biomarker for SLE and their role in the pathogenesis of SLE warrants further investigation.
Keywords: Tfh, Tfr, SLE, Biomarker
1. Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease which predominantly affects women. It is characterized by the production of auto-Ab with complex specificities, immune complex formation and end organ damage [1–3]. Although the exact pathogenesis of SLE remains to be clarified, auto-Ab, autoreactive lymphocytes, and local factors are involved in SLE [4].
CD4+ T cells have a crucial role in helping B cells to produce Ab in response to challenge with foreign antigens. The interaction between T and B cells typically occurs in germinal centers (GCs) located within the B-cell follicles of secondary lymphoid organ [5]. Follicular helper T cells (Tfh cells) compose a heterogeneous subset of CD4+ T cells that specialize in stimulating GC formation and selection of high affinity B cells in GCs. They are found within and in proximity to GCs in secondary lymphoid organs, and their memory compartment also circulates in the blood [6]. In case of systemic and other autoimmune diseases, increased numbers of circulating Tfh cells have been well documented [6].
A subset of Treg cells, named T follicular regulatory (Tfr) cells that share phenotypic characteristics with Tfh cells and conventional regulatory T cells have been recently identified [7,8]. Tfr cells express ICOS, PD-1 and CXCR5 which directs them to the GC. They depend on Bcl-6 for differentiation and localization to the B-cell follicle. Tfr cells also express regulatory markers such as Foxp3, CD25, CTLA-4, glucocorticoid-induced TNFR-related protein and IL-10 [7,9,10]. Tfr cells are located in the GCs and can inhibit GC responses through controlling the number of Tfh and GC B cells [8,11]. Like Tfh cells, circulating Tfr cells have been identified [10]. Given that Tfh and Tfr cells have opposite roles in regulating GC responses, a balance of their actions is critical for immune homeostasis. An impaired Tfr compartment could enhance Tfh activity, resulting in the expansion of autoreactive B cells and auto-Ab production [12].
Several recent studies have demonstrated a significant increase in the frequency of the circulating Tfh-like cells in patients with SLE [13–15]. In addition, the circulating Tfh cells are positively associated with disease activity in SLE [13–15]. None of these studies have focused on Tfr cells. Since Tfr cells are antagonistic to Tfh cells in regulating humoral immunity, we expected that the alteration of circulating Tfr cell may relate to the pathogenesis of SLE. In this study, we determined the frequencies of circulating Tfh and Tfr cells in SLE patients and a control population. The correlation between the Tfh and Tfr cells with disease activity in SLE was investigated.
2. Methods
2.1. Patients
Fifty-eight patients with SLE and twenty-four healthy controls (HC) were enrolled in the study. SLE patients were recruited from the First Affiliated Hospital, Sun Yat-sen University who fulfilled the American College of Rheumatology criteria for the classification of SLE [16]. Clinical disease activity was scored using SLE Disease Activity Index (SLEDAI) scoring system, and divided into low-disease activity (LDA) (SLEDAI 0–4, n = 15) and active disease (SLEDAI > 4, n = 43) [17]. Demographic and clinical characteristics of the SLE patients are shown in Table 1. Twenty-four age and sex matched healthy donors were enrolled. This study was approved by the Human Ethics Committee the First Affiliated Hospital, Sun Yat-sen University. Written informed consent was obtained from all of the subjects.
Table 1.
Demographic and clinical characteristics of SLE patients.
| SLE (N = 58) | HC (N = 24) | |
|---|---|---|
| Age, years (mean ± SD) | 30 ± 10 | 26 ± 3 |
| Female/male | 53/5 | 21/3 |
| Duration of diagnosis, years | 4 (1–10) | / |
| SLEDAI score | 9 (5–12) | / |
| Anti-dsDNA, IU/mL | 44.58 (18.8–413.1) | / |
| IgG, g/L | 10.0 (5.73–16.7) | / |
| Serum C3, g/L | 0.54 (0.32–0.77) | / |
| Serum C4, g/L | 0.1 (0.05–0.17) | / |
| Serum Creatinine, μmol/L | 66.5 (52–125) | / |
| WBC, 109/L (mean ± SD) | 6.8 ± 3.2 | / |
| Lymphocyte, 109/L | 0.93 (0.52–1.42) | / |
| Platelet, 1012/L (mean ± SD) | 195 ± 89 | / |
| Urinary proteins, g/24 h | 1.9 (0–4.5) | / |
| Microscopic hematuria, RBC/HPF | 10 (0–22) | / |
| Medication (%) | / | |
| Prednisone or methylprednisolone | 100% | / |
| Hydroxychloroquine | 63% | / |
| Cyclophosphamide | 35% | / |
| Cyclosporin A | 19% | / |
| Mycophenolate mofetil | 17% | / |
Note: All data are expressed as median (centile 25; centile 75), except where specified. SLE, systemic lupus erythematosus; SLEDAI, SLE Disease Activity Index; HC, health controls; WBC, White blood cell.
Thirteen SLE patients were followed longitudinally. All patients had received immunosuppressant and achieved remission. They all experienced a relapse and treated again as inpatients with glucocorticoid, cyclophosphamide and hydroxychloroquine. Blood samples were obtained before the initiation of treatment and after 4 weeks of treatment. The characteristics of the patients before and after treatment are shown in Table 2.
Table 2.
Demographic and clinical characteristics of SLE patients experienced disease relapse before and after treatment.
| Before (N = 13) | After (N = 13) | |
|---|---|---|
| Age, years | 30 ± 9 | 30 ± 9 |
| Female/male | 12/1 | 12/1 |
| SLEDAI score | 15 ± 4 | 10 ± 3*** |
| Anti-dsDNA, IU/mL | 390.6 ± 387.8 | 103.8 ± 102.8* |
| Globulin, g/L | 24.5 ± 5.7 | 25.1 ± 6.4 |
| Albumin, g/L | 22.4 ± 6.2 | 27.3 ± 5.6*** |
| Serum C3, g/L | 0.34 ± 0.13 | 0.56 ± 0.2** |
| Serum C4, g/L | 0.06 ± 0.04 | 0.14 ± 0.07** |
| WBC, 109/L | 4.5 ± 1.9 | 6.6 ± 1.8** |
| Lymphocyte, 109/L | 0.6 ± 0.4 | 1.0 ± 0.3** |
| Platelet, 1012/L | 142 ± 78 | 169 ± 59 |
| Urinary proteins, g/24 h | 4.5 (0.6; 5.8) | 2.2 (0; 3.2)* |
| Median (centile 25; centile 75) | ||
| Microscopic hematuria, RBC/HPF | 17 ± 14 | 12 ± 11** |
Note: All data are expressed as mean ± SD, except where specified.
p < 0.05,
p < 0.01,
p < 0.001 vs. the values before treatment.
2.2. Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated from SLE patients or from healthy controls using density-gradient centrifugation on Ficoll-Paque and single cell suspensions were stained with the following antibodies: Apc/cy7-conjugated CD4 and CD19, Alexa Fluor 647-conjugated CD25, PE/Dazzle™ 594-conjugated CD127, PE-conjugated ICOS and CD38, PE-Cy7-conjugated PD-1 and CD20, Apc-conjugated CD27 (all from Biolegend, San Diego, CA), Brilliant Violet 421™ conjugated CXCR5 (from BD Biosciences, San Diego, CA) and 7AAD (from Invitrogen, Eugene, OR). Appropriate isotype controls were used. Stained cells were analyzed by multiparameter flow cytometry (CytoFLEX S, Beckmancoulter) and analyzed with FlowJo software (Tree Star).
2.3. Ki-67 and Foxp3 staining
Surface-stained PBMCs were fixed and permeabilized with a FOXP3 Staining Set (eBioscience, San Diego, CA, USA) and then stained with PE conjugated Ki-67, Alexa Fluor 488 or PE conjugated Foxp3 (all from Biolegend, San Diego, CA).
2.4. ELISA for serum IL-21
Plasma IL-21 concentrations in SLE patients and HC were measured using a human IL-21 ELISA kit (Multi Sciences), according to the manufacturer’s instructions. The concentrations of plasma IL-21 were calculated by using the standard curve for recombinant IL-21.
3. Statistical analysis
The statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). Normally distributed data are presented as the mean ± SD. Non-normally distributed data were presented as median ± interquartile range. Differences between unpaired two groups were determined with a two-tailed unpaired t-test or Mann-Whitney U test as appropriate. Paired data for thirteen patients before and after treatment were compared using a paired t-test. Spearman correlation coefficient with two-tailed p value was determined in the analysis of correlations. A p-value < 0.05 was considered statistically significant.
4. Results
4.1. The frequencies of circulating Tfh and Tfr cells in SLE patients
Precursor cells of Tfh and Tfr cell are not detectable in circulation because of the lack of specific cell surface markers. However, when T cells migrate to germinal centers, they differentiate to Tfh and Tfr cells with memory phenotypes. They are readily detectable in circulation [18, 19]. Fig. 1A shows circulating Tfr (CD4+CD25+CD127low-intermediate CXCR5+) and Tfh (CD4+CD25−CD127intermediate-high CXCR5+) populations that were selected for their expression of CXCR5 and then separated by their expression of CD25 and CD127. The gating strategy was showed by Wei et al. [18]. The majority of the Tfh cells do express a higher intensity of CD127. The Foxp3 expression on Tfh and Tfr cell are shown in Fig. 1A and B. The Tfr cells were positive for Foxp3 expression while Tfh cells were negative. These data suggest that the strategy of identifying Tfh and Tfr cells is appropriate. Fig. 1C shows the results from a representative patient with SLE and a normal control subject.
Fig. 1.
Circulating Tfr cells are decreased in the blood of patients with SLE compared to healthy controls. A, Gating strategy to identify circulating Tfh (CD4+CD25−CD127intermediate-high CXCR5+) and Tfr (CD4+CD25+CD127low-intermediate CXCR5+) cells within the CD4+CXCR5+ T cells in human blood. B, MFI of Foxp3 expression on Tfh and Tfr cells respectively (n = 15). C, Contour plots of CD25 and CD127 expression on CD4+CXCR5+ T cells from a representative healthy control (left panel) and an SLE patient (right panel). D and E, Percentage of circulating Tfh (CD4+CD25−CD127intermediate-high CXCR5+) cells and Tfr (CD4+CD25+ CD127low-intermediate CXCR5+) cells among CD4+ T cells in patients with SLE (n = 58) and healthy controls (n = 24). F, The Tfh/Tfr ratio in SLE patients and HCs. Data are represented as mean ± SD or median ± interquartile range. Data points represent individual subjects. NS = not significant, **p < 0.01, ***p < 0.001.
The distribution of the percentages of Tfh cells in control group was less scattered than those of patients with SLE (Fig. 1D). Although a subset of patients with SLE has much higher numbers of Tfh cells, as a group, the percentages of Tfh cells were not statistically different in comparison to those in healthy controls. In contrast, the frequency of Tfr cells was significantly lower in patients with SLE (Fig. 1E). In addition, the ratios of Tfh cells over Tfr cells were much higher in patients with SLE (Fig. 1F).
4.2. Correlations between circulating Tfh and Tfr cell with serum anti-dsDNA antibody
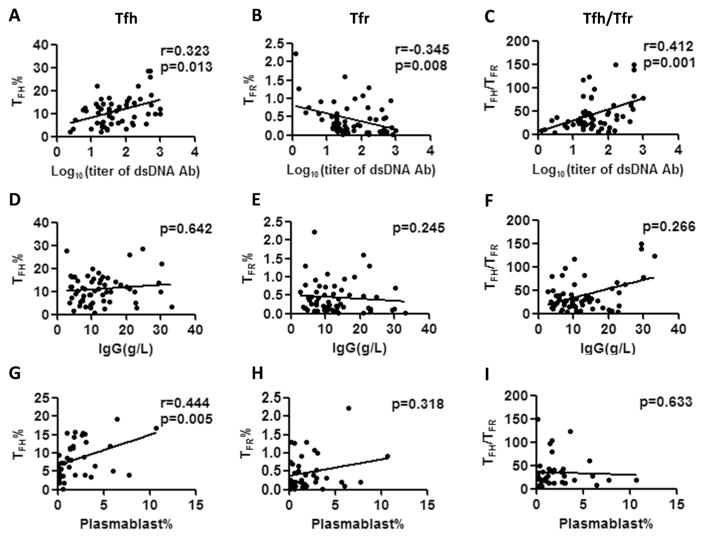
The associations of the Tfh and Tfr cell frequencies with serum levels of anti-dsDNA Ab, IgG, and plasmablasts were analyzed. As shown in Fig. 2A–C, serum anti-dsDNA Ab level positively correlated with Tfh cells and Tfh/Tfr ratio, but negatively correlated with Tfr cells. No correlation between the serum IgG level with Tfh, Tfr and Tfh/Tfr ratio were found (Fig. 2D–F). We also found a positive correlation between the percentage of Tfh cells and plasmablasts (Fig. 2G). However, no correlations were noted between the percentage of plasmablasts with the Tfr cells and the Tfh/Tfr ratio. (Fig. 2H–I).
Fig. 2.
Correlation between the percentage of Tfh and Tfr cells with serum anti-dsDNA antibody and IgG level in SLE patients. A–C, Correlation between the serum anti-dsDNA antibody with the percentage of Tfh cells, Tfr cells and the Tfh/Tfr ratio, respectively (n = 58). D–F, Correlation between the titer of serum IgG level with the percentage of Tfh cells, Tfr cells and Tfh/Tfr ratio, respectively (n = 58). G–I, Correlation between the percentage of plasmablasts with the percentage of Tfh cells, Tfr cells and Tfh/Tfr ratio, respectively (n = 39). Data points represent individual subjects. *p < 0.05, **p < 0.01.
4.3. Active phenotypes of circulating Tfh cell and Tfr cell in SLE patients
Programmed death-1 (PD-1) and inducible costimulator (ICOS) are expressed by activated T cells [20,21]. Increased PD-1highICOShigh Tfh cells were observed in CXCR5+CD4+ T cells of SLE patients compared with controls (Fig. 3A, B). A similar increase was found in PD-1highICOS-hifh Tfr cells of SLE patients (Fig. 3A, C). Both the MFI of PD-1 and ICOS of Tfr cells in SLE patients were significantly higher than those of the control group (Fig. 3D, G and H). The PD-1 MFI of Tfh cells were also significantly higher than the controls (Fig. 3D, E). Nevertheless, no significant difference in the ICOS MFI was found in Tfh cells between the two groups (Fig. 3D, F). Next, we examined the Ki-67 expression on Tfh and Tfr cells. As shown in Fig. 3I–K, the Ki-67+ Tfh cells and Tfr cells were both increased in SLE patients compared with the healthy controls.
Fig. 3.
PD-1 and ICOS expression on Tfr and Tfh cells in SLE patients and controls. A, Gates for the expression of PD-1 and ICOS were set using isotype staining of PD-1 and ICOS on Tfh and Tfr cells from SLE patient (left panel). Contour plots of ICOS and PD-1 expression on Tfh cell and Tfr cell from a representative healthy control (middle panel) and a SLE patient (right panel). B and C, The percentage of PD-1highICOShigh cell among CD4+CXCR5+cells in patients with SLE and healthy controls. D, Flow-cytometric histograms of PD-1 and ICOS expressions of Tfh and Tfr cells from a representative healthy control (black line) and a SLE patient (red line), respectively. E and F, MFI of PD-1 (E) and ICOS (F) on Tfh cell in SLE patients (n = 58) and healthy controls (n = 24). G and H, MFI of PD-1 (G) and ICOS (H) on Tfr cell in SLE patients (n = 58) and healthy controls (n = 24). I, Flow-cytometric histograms of Ki-67 expression on Tfh cell and Tfr cell from a representative healthy control (black line) and a SLE patient (red line). J and K, Percentage of Ki-67+ Tfh cell (J) and Tfr cell (K) in SLE patients (n = 38) and healthy controls (n = 17), respectively. Data are represented as mean ± SD or median ± interquartile range. Data points represent individual subjects. NS = not significant, *p < 0.05, **p < 0.01.
4.4. Correlation between plasma IL-21 and circulating Tfr or Tfh cells
Tfh cells and their precursors secrete large amounts of IL-21 [2]. Studies have shown that IL-21 plays an important role in SLE. IL-21 has diverse effects on both B and T cells including Tfr cells [7,22, 23]. Significantly elevated plasma IL-21 levels in SLE patients compared with the normal group were found (Fig. 4A). The plasma IL-21 levels were negatively correlated with the percentage of Tfr cell but positively correlated with the percentage of Tfh cell and the Tfh/Tfr ratio (Fig. 4B–D).
Fig. 4.
Correlation of plasma IL-21 with Tfh and Tfr cells in SLE patients. A, The IL-21 level was measured in plasma from healthy controls (n = 24) and SLE patients (n = 58). B and C, Correlation of plasma IL-21 level with the percentage of Tfh and Tfr cells in SLE patients (n = 58). D, Correlation of plasma IL-21 level with the Tfh/Tfr ratio in SLE patients (n = 58). Data are represented as mean ± SD. Data points represent individual subjects. **p < 0.01, ***p < 0.001.
4.5. Correlation between disease activity and circulating Tfr or Tfh cells
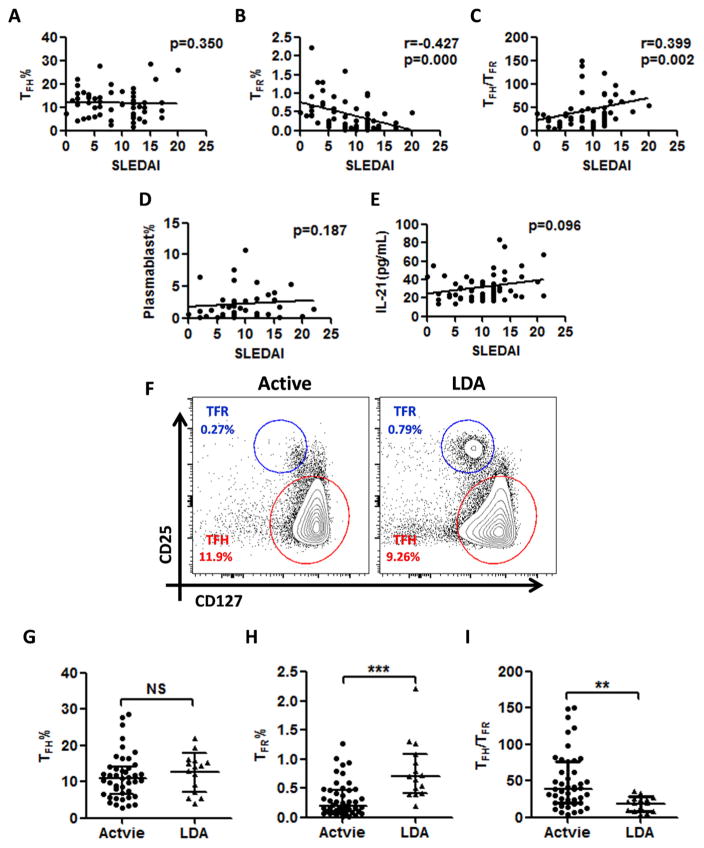
SLE disease activity as measured by SLEDAI [17] showed no correlation with the frequency of Tfh cells (Fig. 5A). However, the SLEDAI negatively correlated with the frequency of Tfr cells and positively correlated with the Tfh/Tfr ratios (Fig. 5B and C). In addition, both the percentages of plasmablasts and plasma levels of IL-21, showed no correlation with SLEDAI (Fig. 5D and E).
Fig. 5.
Correlation of SLEDAI with Tfh cells, Tfr cells, plasmablasts and plasma IL-21 level in SLE patients. A and B, Correlation of SLEDAI with the percentage of Tfh and Tfr cells in SLE patients (n = 58). C, The correlation between SLEDAI and Tfh/Tfr ratio (n = 58). D, Correlation between SLEDAI and percentage of circulating plasmablasts (n = 39). E, The correlation between SLEDAI and the plasma IL-21 level (n = 58). F, Contour plots of CD25 and CD127 expression on CD4+CXCR5+ T cells from a representative active (left panel) and a LDA (right panel) patient. G and H, Percentage of Tfh (CD4+CD25−CD127intermediate-high CXCR5+) cells and Tfr (CD4+CD25+CD127low-intermediate CXCR5+) cells among CD4+ T cells in active (n = 43) and LDA (n = 15) patients. I, The Tfh/Tfr ratio in active (n = 43) and LDA (n = 15) patients. Data are represented as mean ± SD or median ± interquartile range. Data points represent individual subjects. NS = not significant, **p < 0.01, ***p < 0.001.
Next, we investigate the impact of disease remission on frequency of Tfr and Tfh cells. With SLEDAI < 5 as a measure of low disease activities (LDA) [17], there is no significant difference in the percentages of Tfh cells between patients with active disease and LDA (Fig. 5F, G). However, patients with LDA have higher percentages of Tfr cell (Fig. 5F, H) and lower ratio of Tfh/Tfr cell (Fig. 5I)
4.6. Treatments of SLE patients in relapse reduced Tfh and increase in Tfr cells
To ascertain the usefulness of Tfr cells as a biomarker for disease activity, 13 patients with renal relapse were studied when they were in relapse and 4 weeks after the initiation of treatments. As shown in Table 2, the treatments reduced significantly disease activity as measured by SLEDAI. The anti-dsDNA Ab, 24 h urinary protein and microscopic hematuria were decreased with the serum albumin, C3 and C4 normalized to some extent. The total number of leukocytes and lymphocytes were increased after the treatments.
The frequency of Tfh and Tfr cells were examined before and after 4 weeks of initiation of treatments are shown in Fig. 6. Although the percentage of Tfh cells (Fig. 6A and B) showed no significant change after 4 weeks of treatment, the percentage of Tfr cells was increased markedly (Fig. 6A and C). The Tfh/Tfr ratios (Fig. 6A and D) were significantly decreased.
Fig. 6.
Circulating Tfr cells were increased after treatment. A, Contour plots of CD25 and CD127 expression on CD4+CXCR5+ T cells from a representative active SLE patient before (left panel) and after (right panel) 4 weeks of treatment. B and C, Percentage of Tfh (CD4+CD25−CD127intermediate-high CXCR5+) cells and Tfr (CD4+CD25+CD127low-intermediate CXCR5+) cells among CD4+ T cells before and after treatment (n = 13). D, The Tfh/Tfr ratio in SLE patients before and after treatment (n = 13). Data points represent individual subjects. NS = not significant, **p < 0.01, ***p < 0.001.
5. Discussion
Studies with mouse models and human samples have shown that Tfh cells play a significant role in the auto-Ab responses in SLE [2]. Recently multiple studies have been focused on the role of Tfh cells in the pathogenesis of SLE [2,14,15,24]. The recent described Tfr cells that have opposite roles in regulating humoral immunity may have a significant role in many autoimmune disorders [7,8,25]. Thus far, no studies on Tfr cell response in SLE have been reported. In this study, the frequency of Tfr cell was shown to be reduced and the Tfh/Tfr ratio increased in SLE. Furthermore, the frequency of Tfr cell was negatively correlated with disease activity and titer of anti-dsDNA Ab while the Tfh/Tfr ratio was positively correlated. These data suggest that the availability of suppression of Tfr cells on the Tfh cells is reduced in SLE. It is important to note that the frequencies of Tfr cells in SLE are negatively correlated with anti-dsDNA Ab levels and with disease activity as measured by SLEDAI. In contrast, the frequencies of Tfh cells are not correlated with disease activities. In addition, the frequency of Tfr cells was increased and the Tfh/Tfr ratio decreased after the patients were successfully treated. These findings suggest that Tfr cells are a reliable biomarker for active SLE and that the role of these cells in the pathogenesis of SLE deserve to further investigation.
In this study, increased frequencies of total CXCR5+ Tfh cells in SLE patient were not observed. This observation was in agreement with several previous studies [14,26,27]. However, our finding is at variant with those by Zhang et al. [15]. The lack of increasing frequency in our SLE patients may be due to the relative high frequency of Tfh cells in our control population. This explanation is supported by the finding that IL-21, a cytokine secreted by Tfh cells and their precursors [28–30] is elevated markedly in SLE patients. This latter finding suggests that Tfh cells are much more active in SLE patients although the frequency of Tfh cells is not elevated on a population basis. In addition, a subset of our patients did have much higher percentages of Tfh cells. This is in concordance with the finding by Simpson et al. [31].
In spite of a positive correlation between the frequency of Tfh cells and serum anti-dsDNA level, a positive correlation between percentage of Tfh and disease activity have not been observed in our SLE patients. Several recent studies on SLE patients reported results similar to ours [14,15,24]. However, two study reported that a positive correlation between SLEDAI and Tfh cell frequencies [13,26]. These diverse results regarding the correlation of Tfh cells frequencies with SLEDAI as a measure of disease activity may simply reflect the differences among the SLE populations in these studies. At any rate our finding that no correlations were found between the SLEDAI with the Tfh cells, plasmablasts and plasma IL-21 level is internally consistent. The negative correlation of SLEDAI with Tfr cells and positive correlation with Tfh/Tfr ratios suggests that the Tfr cells may be better than Tfh cells, plasmablasts and plasma IL-21 level in evaluating disease activities of SLE.
Human Tfh cells can be defined through the combination of markers as followed: the chemokine receptors CXCR3 and CCR6, PD-1, and ICOS. Both PD-1 and ICOS expression can be used as an indicator for active Tfh cell differentiation [2,18,26]. In this study, the percentage of PD-1-highICOShigh Tfh cells and the MFI of PD-1 in Tfh cells were significantly higher in SLE patients than the control group, which was consistent with previous studies [13–15,26,31]. Ki-67 is an indicative marker of active cell cycle [28]. We found increased frequency of Ki-67+ Tfh cells in SLE patients compared to HCs, indicating elevated active subset of Tfh cells in SLE patients. Surprisingly, the PD-1highICOShigh Tfr cell was also significantly increased with elevated percentage of Ki-67+ Tfr cells, which indicate that there is also an activation of Tfr cells in SLE. The balance between Tfh and Tfr cell activation determines the outcome in auto-Ab production. Our data would suggest that Tfh cells tip the scale of this balance in SLE.
In conclusion, this study demonstrated decreased frequency of Tfr cells and increased Tfh/Tfr ratio in SLE patients. Both the frequency of Tfr cells and Tfh/Tfr ratio were correlated with disease activity. Additionally, there is also increased percentage of activated Tfh and Tfr cells in SLE. Our data suggest that Tfh/Tfr ratio may act as a more useful marker than Tfh cell alone when evaluating the disease activity of SLE. Thus Tfr cells need to be considered when investigating the roles of Tfh in the pathogenesis of SLE.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81471598, 81671593) and Guangdong Natural Science Foundation (2014A030313096), Guangzhou Science and Technology Planning Program (201605122113460). SM Fu was supported in part by NIH grant (NIH R01 AR-047988) and a grant from the Alliance for Lupus Research (TIL332635). We would also like to recognize and thank all of our patients with systemic lupus erythematosus from the First Affiliated Hospital, Sun Yat-sen University for donating blood samples. We are also grateful to the healthy control volunteers.
Footnotes
Competing interests
All the authors have no interests to declare.
References
- 1.Comte D, Karampetsou MP, Tsokos GC. T cells as a therapeutic target in SLE. Lupus. 2015;24(4–5):351–363. doi: 10.1177/0961203314556139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco P, Ueno H, Schmitt N. T follicular helper (Tfh) cells in lupus: activation and involvement in SLE pathogenesis. Eur J Immunol. 2016;46(2):281–290. doi: 10.1002/eji.201545760. [DOI] [PubMed] [Google Scholar]
- 3.D’Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369(9561):587–596. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 4.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 5.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8(6):337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. 2015;16(2):142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, Zou L, Liu YC. T follicular helper cells, T follicular regulatory cells and auto-immunity. Int Immunol. 2016;28(4):173–179. doi: 10.1093/intimm/dxv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sage PT, Alvarez D, Godec J, von Andrian UH, Sharpe AH. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. 2014;124(12):5191–5204. doi: 10.1172/JCI76861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sage PT, Ron-Harel N, Juneja VR, Sen DR, Maleri S, Sungnak W, et al. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016;17(12):1436–1446. doi: 10.1038/ni.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhaeze T, Stinissen P, Liston A, Hellings N. Humoral autoimmunity: a failure of regulatory T cells? Autoimmun Rev. 2015;14(8):735–741. doi: 10.1016/j.autrev.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Liu J, Cui X, Zuo Y, Zhang Z, Li Y, et al. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol. 2015;295(1):46–51. doi: 10.1016/j.cellimm.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Choi JY, Ho JH, Pasoto SG, Bunin V, Kim ST, Carrasco S, et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheum. 2015;67(4):988–999. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Lindwall E, Gauthier C, Lyman J, Spencer N, Alarakhia A, et al. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus. 2015;24(9):909–917. doi: 10.1177/0961203314567750. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 17.Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L, et al. Definition and initial validation of a lupus low disease activity state (LLDAS) Ann Rheum Dis. 2016;75(9):1615–1621. doi: 10.1136/annrheumdis-2015-207726. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Feng J, Hou Z, Wang XM, Yu D. Flow cytometric analysis of circulating follicular helper T (Tfh) and follicular regulatory T (Tfr) populations in human blood. Methods Mol Biol. 2015;1291:199–207. doi: 10.1007/978-1-4939-2498-1_17. [DOI] [PubMed] [Google Scholar]
- 19.Locci M, Wu JE, Arumemi F, Mikulski Z, Dahlberg C, Miller AT, et al. Activin A programs the differentiation of human TFH cells. Nat Immunol. 2016;17(8):976–984. doi: 10.1038/ni.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS) Curr Opin Immunol. 2010;22(3):326–332. doi: 10.1016/j.coi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gharibi T, Majidi J, Kazemi T, Dehghanzadeh R, Motallebnezhad M, Babaloo Z. Biological effects of IL-21 on different immune cells and its role in autoimmune diseases. Immunobiology. 2016;221(2):357–367. doi: 10.1016/j.imbio.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Jang E, Cho SH, Park H, Paik DJ, Kim JM, Youn J. A positive feedback loop of IL-21 signaling provoked by homeostatic CD4+CD25− T cell expansion is essential for the development of arthritis in autoimmune K/BxN mice. J Immunol. 2009;182(8):4649–4656. doi: 10.4049/jimmunol.0804350. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Wu H, Qiu H, Yang H, Deng Y, Zhao M, et al. The expression of Bcl-6 in circulating follicular helper-like T cells positively correlates with the disease activity in systemic lupus erythematosus. Clin Immunol. 2016;173:161–170. doi: 10.1016/j.clim.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. 2013;14(2):152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39(4):770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Le Coz C, Joublin A, Pasquali JL, Korganow AS, Dumortier H, Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One. 2013;8(9):e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35(9):436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179(12):8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 30.Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci U S A. 2011;108(33):E488–497. doi: 10.1073/pnas.1100898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62(1):234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]