Abstract
Background and aims
Anhedonia—a transdiagnostic psychopathological trait indicative of inability to experience pleasure—could lead to and result from adolescent marijuana use, yet this notion has not been tested. This study aimed to estimate the association of: (1) anhedonia at age 14 with rate of change in marijuana use over an 18-month follow-up, and (2) marijuana use at age 14 with rate of change in anhedonia over follow-up. Secondary aims were to test whether gender, baseline marijuana use history, and peer marijuana use moderated these associations.
Design
Observational longitudinal cohort repeated measures design, with baseline (age 14), 6-month, 12-month, and 18-month follow-up assessments.
Settings
Ten public high schools in Los Angeles, CA, USA, 2013–2015.
Participants
Students (N=3,394; 53.5% female, Mean[SD] age at baseline=14.1[0.42]).
Measurements
Self-report level of anhedonia on the Snaith Hamilton Pleasure Scale and frequency of marijuana use in the past 30 days.
Findings
Parallel process latent growth curve models adjusting for confounders showed that baseline anhedonia level was positively associated with the rate of increase in marijuana use frequency across follow-ups (β[95%CI]=.115[.022, .252], P=.03). Baseline marijuana use frequency was not significantly related to the rate of change in anhedonia across follow-ups (β[95%CI]=−.015[−.350, .321], P=.93). The association of baseline anhedonia with faster marijuana use escalation was amplified amongst adolescents with (versus without) friends who used marijuana at baseline (β[95%CI]: .179[.043, .334] versus .064[−.071, .187], interaction P=.04) but did not differ by gender or baseline ever marijuana use.
Conclusions
In mid adolescence, anhedonia is associated with subsequent marijuana use escalation but marijuana use escalation does not appear to be associated with subsequent anhedonia.
Keywords: adolescents, marijuana use, anhedonia, prevention, policy
INTRODUCTION
Marijuana is one of the most widely used illicit substances worldwide [1, 2]. Although it has been reported that marijuana use rate has stabilized or even decreased in recent years in most high-income countries, the continuing high prevalence of use among adolescents and young adults [1, 2] is a cause for concern. Such emerging trends have heightened interest in the link between mental health problems and adolescent marijuana use to inform policy and prevention efforts.
Understanding the comorbidity between psychopathology and marijuana use is complicated. Marijuana use is associated with numerous different psychiatric disorders [3, 4], each of which tend to co-occur with one another [5]. Additionally complicating matters is the potential bi-directional nature of this association, with evidence that marijuana use may both predict and result from poor mental health [6]. A parsimonious explanation of this comorbidity may be that a small set of transdiagnostic psychopathologic vulnerabilities that give rise to numerous mental health conditions may also contribute to and result from marijuana use [7]. Such transdiagnostic vulnerabilities may account for the pervasive patterns of psychiatric comorbidity with use of marijuana and other substances [8–10].
One such transdiagnostic vulnerability is anhedonia—diminished capacity to experience pleasure in response to rewards. As a subjective manifestation of deficient reward processing capabilities, anhedonia is believed to result from hypoactive brain reward circuitry [11]. While anhedonia is a core feature in a DSM-defined major depressive episode [12], it has also been linked to other psychopathologies comorbid with drug use, including psychosis [13], borderline personality disorder [14], social anxiety [15], attention deficit hyperactivity disorder [16], and posttraumatic stress disorder [17] and therefore has been proposed to be a transdiagnostic process [7]. Departing from its consideration as a ‘symptom’ of a disease state as in DSM-defined major depression, anhedonia has also been conceptualized as a continuous dimension, upon which there is substantial inter-individual differences [18]. Individuals at the lower end of the anhedonic spectrum experience high levels of pleasure and experience robust affective responses to pleasurable events, whereas those at the upper end of this spectrum exhibit more prominent deficits in their pleasure experience [18, 19]. Anhedonia operates as a “trait-like” dimension that is stable yet malleable [20], which is empirical and conceptually distinct from other emotional constructs, such as reward sensitivity (i.e., extraversion and positive emotionality), alexithymia and emotional numbing (i.e., dampened positive and negative emotions), sadness, and negative affect [21–23].
Recent literature documents a consistent association between anhedonia and substance use in adults [7]. To the best of our knowledge, there has been only prior study of the association between anhedonia and marijuana use in youth, which found higher anhedonia levels among treatment-seeking marijuana users than healthy controls in a cross-sectional analysis of 62 French adolescents and young adults [24]. Given the absence of longitudinal data, it is unclear whether anhedonia is a risk factor for or consequence of adolescent marijuana use. Because youth with higher anhedonia levels experience little pleasure from routine rewards (e.g., food, social interaction), they may seek out drugs of abuse, like marijuana, which pharmacologically stimulate neural circuitry that underlie pleasure [25]. Alternatively, repeated tetrahydrocannabinol (THC) exposure during adolescence produces enduring deficits in brain reward system function and anhedonia-like behavior in rodent models [26]. In observational studies of adults, heavy or problematic marijuana use is associated with subsequent anhedonia [6] and diminished brain reward region activity during reward anticipation [27]. Consequently, it is plausible that anhedonia may both increase risk of marijuana use and result from marijuana use.
Because early adolescence is a period in which risk of marijuana use uptake is high [28] and the developing brain may be vulnerable to cannabinoid-induced neuroadaptations [29], this study estimated the strength of bi-directional longitudinal associations between anhedonia and marijuana use among adolescents across the first two years of high school. The primary aim was to test the following hypotheses: (1) greater baseline anhedonia would be associated with a faster rate of escalation in marijuana use across follow-up periods; and (2) more frequent use of marijuana at baseline would be associated with increases in anhedonia across follow-ups.
A secondary aim was to test whether these putative risk pathways were amplified or suppressed amongst pertinent subpopulations and contexts. Associations of affective disturbance and other risk factors with adolescent substance use escalation have been reported to be amplified among girls (vs. boys) [30, 31], early (vs. late) onset substance users [32], and those with substance using peers [33]. We therefore tested whether associations between anhedonia and marijuana use were moderated by gender, history of marijuana use prior to the study surveillance period at baseline, and peer marijuana use at baseline.
METHODS
Design
This study used an observational longitudinal cohort repeated measures design, involving assessments at baseline (age 14), 6-month, 12-month, and 18-month follow-ups.
Participants and Procedures
Data were drawn from the Happiness & Health Study, a longitudinal cohort survey of substance use and mental health among high school students in Los Angeles, CA USA. Among 40 public high schools approached to participate in the study because of their diverse demographic characteristics and proximity, 10 participated in this study (characteristics of participating schools in reference to Los Angeles county public schools appear in sTable 1 in the online supporting information). Of the 4,100 eligible 9th grade students, 3,396 students and their parents provided active written or verbal assent and consent, respectively, and enrolled. Data collection involved four semiannual assessments: baseline (wave 1; fall 9th grade, 2013; N surveyed=3,383, 99.6%) and 6-month (wave 2; spring 9th grade, 2014; N=3,293, 97.0%), 12-month (wave 3; fall 10th grade, 2014; N=3,288, 96.8%), and 18-month (wave 4; spring 10th grade, 2015; N=3,262, 96.1%) follow-ups. At each wave, paper-and-pencil surveys were administered in students’ classrooms on site. Students not in class during data collections completed surveys by telephone, Internet, or mail (6-month follow-up: N=51, 12-month follow-up: N=153, 18-month follow-up: N=215). The University of Southern California institutional review board approved the study.
Measures
Anhedonia
At each timepoint, anhedonia was assessed by the Snaith-Hamilton Pleasure Scale (SHAPS) [34], which includes 14 self-statements of pleasure response to rewarding sensory stimuli, social activities, and hobbies (e.g., “I would be able to enjoy a beautiful landscape or view”). Responses to each item (rated 0 [Strongly Agree], 1 [Agree], 2 [Disagree], 3 [Strongly Disagree]) are summed, with a higher score indicating greater anhedonia level. Amongst adolescents, the SHAPS has exhibited a unidimensional factor structure and strong convergent and discriminant validity [23]. Internal consistency in this sample was adequate (α across waves > .89). The proportion surpassing a recommended cutoff indicating possible clinically significant anhedonia [34] is reported (i.e., disagree or strongly disagree ≥3 items; Table 2).
Table 2.
Descriptive statistics for repeated measures of substance use and anhedonia
| Time-points | ||||
|---|---|---|---|---|
|
|
||||
| Variables | Wave 1 (N =3,383) | Wave 2 (N =3,293) | Wave 3 (N = 3,288) | Wave 4 (N =3,262) |
| Marijuana use, N (%) | ||||
| No use in the past 6 months | 2,983 (89.8%) | 2,730 (84.6%) | 2,709 (83.9%) | 2,577 (80.9%) |
| Past 6-month use without use in last 30 days | 76 (2.3%) | 152 (4.7%) | 132 (4.1%) | 176 (5.5%) |
| 1–2 days in the last 30 days | 98 (3.0%) | 125 (3.9%) | 141 (4.4%) | 154 (4.8%) |
| 3–5 days in the last 30 days | 44 (1.3%) | 76 (2.4%) | 75 (2.3%) | 82 (2.6%) |
| 6–14 days in the last 30 days | 48 (1.4%) | 62 (1.9%) | 73 (2.3%) | 82 (2.6%) |
| ≥15 days in the last 30 days | 73 (2.2%) | 82 (2.5%) | 97 (3.0%) | 113 (3.5%) |
| Available data, Na | n = 3,322 | n = 3,227 | n = 3,227 | n = 3,184 |
| Alcohol use, N (%) | ||||
| No use in the past 6 months | 2,729 (83.2%) | 2,372 (73.5%) | 2,311 (71.6%) | 2,266 (71.3%) |
| Past 6-month use without use in last 30 days | 160 (4.9%) | 249 (7.7%) | 240 (7.4%) | 231 (7.3%) |
| 1–2 days in the last 30 days | 230 (7.0%) | 368 (11.4%) | 410 (12.7%) | 415 (13.1%) |
| 3–5 days in the last 30 days | 82 (2.5%) | 120 (3.7%) | 135 (4.2%) | 144 (4.5%) |
| 6–14 days in the last 30 days | 49 (1.5%) | 83 (2.6%) | 92 (2.9%) | 92 (2.9%) |
| ≥15 days in the last 30 days | 29 (0.9%) | 37 (1.1%) | 40 (1.2%) | 32 (1.0%) |
| Available data, Na | n = 3,279 | n = 3,229 | n = 3,228 | n = 3,180 |
| Cigarette use, N (%)b | ||||
| No use in the past 6 months | 3,194 (95.9%) | 2,986 (92.0%) | 2,993 (92.5%) | 2,946 (92.2%) |
| Past 6-month use without use in last 30 days | 55 (1.7%) | 132 (4.1%) | 110 (3.4%) | 93 (2.9%) |
| 1–2 days in the last 30 days | 46 (1.4%) | 71 (2.2%) | 72 (2.2%) | 80 (2.5%) |
| 3–5 days in the last 30 days | 17 (0.5%) | 13 (0.4%) | 20 (0.6%) | 28 (0.9%) |
| 6–14 days in the last 30 days | 10 (0.3%) | 25 (0.8%) | 23 (0.7%) | 19 (0.6%) |
| ≥15 days in the last 30 days | 10 (0.3%) | 18 (0.6%) | 18 (0.6%) | 30 (0.9%) |
| Available data, Na | n = 3,332 | n = 3,245 | n = 3,236 | n = 3,196 |
| Anhedonia | ||||
| Score, M(SD) b | 23.66 (6.94) | 24.17 (8.19) | 24.19 (8.48) | 24.55 (8.79) |
| Meet clinical cutoff, N (%)c | 858 (25.7%) | 893 (27.4%) | 861 (26.5%) | 725 (22.9%) |
| Available data, Na | n = 3,335 | n = 3,255 | n = 3,247 | n = 3,161 |
Note.
Available (non-missing) data for respective variable and denominator for within-column/within-timepoint percentages.
Based on Snaith Hamilton Pleasure Score (Sum of responses to 14 statements of pleasure response rated on 0–3 scale).
Based on those who surpass the recommended SHAPS cutoff for clinically significant anhedonia [34].
Marijuana use
At each time-point, marijuana use was measured using well-validated items based on the Monitoring the Future [35] surveys assessing past 6-month use (yes/no) and days used in past 30 days (forced choice with 9 options ranging 0–30 days). To ensure adequate frequency across each level of marijuana use, responses were coded ordinally for the primary outcome (0 [No use in the past 6 months], 1 [Used in the past 6 months, but not in last 30 days], 2 [1–2 days in last 30], 3 [3–5 days], 4 [6–14 days], and 5 [≥15 days]).
Moderators
Gender, baseline ever use of marijuana (yes/no; to distinguish youth whose use trajectories reflected new onset vs. carry-over of use patterns that predated the assessment period), and number of 5 closest friends who had used marijuana in the past 30 days (≥1 vs. 0) were assessed via self-report.
Covariates
A priori covariates were selected based on their association with anhedonia or marijuana use in extant literature [36–38]. Time-invariant sociodemographic covariates included youth age, gender, race/ethnicity, and highest parental education level based on responses to investigator-defined forced-choice items at baseline (see response categories in Table 1). To rule out that that associations occur because anhedonia is merely a proxy for psychopathologies that directly couple with marijuana use, well-established self-report scales which have shown strong psychometric properties in adolescent samples were administered and applied as time-invariant covariates. These measures included the Center for Epidemiologic Studies Depression Scale (CESD; α=.81) [39] measure of past week depressive symptom frequency, Revised Child Anxiety and Depression Scale – Social Phobia subscale (RCADS-SP; α=.92) [40, 41], and the Current Symptoms Scale-Self Report Form [42] measure of DSM-IV Attention Deficit/Hyperactivity Disorder (ADHD) symptoms during the past 6 months (α=.92). The CESD and RCADS-SP values at the baseline wave were used. Because ADHD measures were not added to the assessment battery until wave 2, wave 2 ADHD scores were used in the analysis. Alcohol and cigarette use frequencies, which were each measured and coded in the same fashion as marijuana use, were included as a time-varying covariate at each wave to disentangle anhedonia’s relation with marijuana use from other drug use.
Table 1.
Descriptive statistics for time-invariant baseline covariates and moderators in overall sample
| Variable | N (%) or M (SD) |
|---|---|
| Sex (n = 3,369),a N (%) | |
| Female | 1,801 (53.5%) |
| Male | 1,568 (46.2%) |
| Age (n = 3,360),a M (SD) | 14.08 (0.42) |
| Race/ethnicity (n = 3,311),a N (%) | |
| Non-Hispanic White | 520 (15.7%) |
| Hispanic | 1,557 (47.0%) |
| Black | 166 (5.0%) |
| Asian | 535 (16.2%) |
| Multi-ethnic/Other | 533 (16.1%) |
| Highest parental education level (n = 2,931),a,b N (%) | |
| ≤8th grade | 117 (4.0%) |
| Some high school | 266 (9.1%) |
| High school graduate | 493 (16.8%) |
| Some college | 573 (19.5%) |
| College graduate | 927 (31.6%) |
| Graduate degree | 555 (18.9%) |
| Depressive symptom levelc (n = 3,349),a M (SD) | 14.43 (11.76) |
| Social phobia leveld (n = 3,206),a M (SD) | 11.84 (7.29) |
| ADHDe (n = 3,170),a M (SD) | 12.78 (9.95) |
| Has a friend who uses marijuana (n = 3,305),a N (%) | 1,178 (35.6) |
| Baseline ever use of marijuana (n = 3,329),a N (%) | 503 (15.1) |
| Marijuana use onset before age 14 years (n = 3,329),a N (%) | 475 (14.3) |
Note.
Available (non-missing) data for respective variable and, for categorical variables, denominator for within-column percentages.
Participants who marked ‘don't know’ response (N=422) recoded as missing.
Center for Epidemiologic Studies Depression Scale total score.
Revised Children’s Anxiety and Depression Scale – Social phobia subscale score.
The Attention/Deficit Hyperactivity Disorder Self-Rating Scale total sum score.
Statistical Analysis
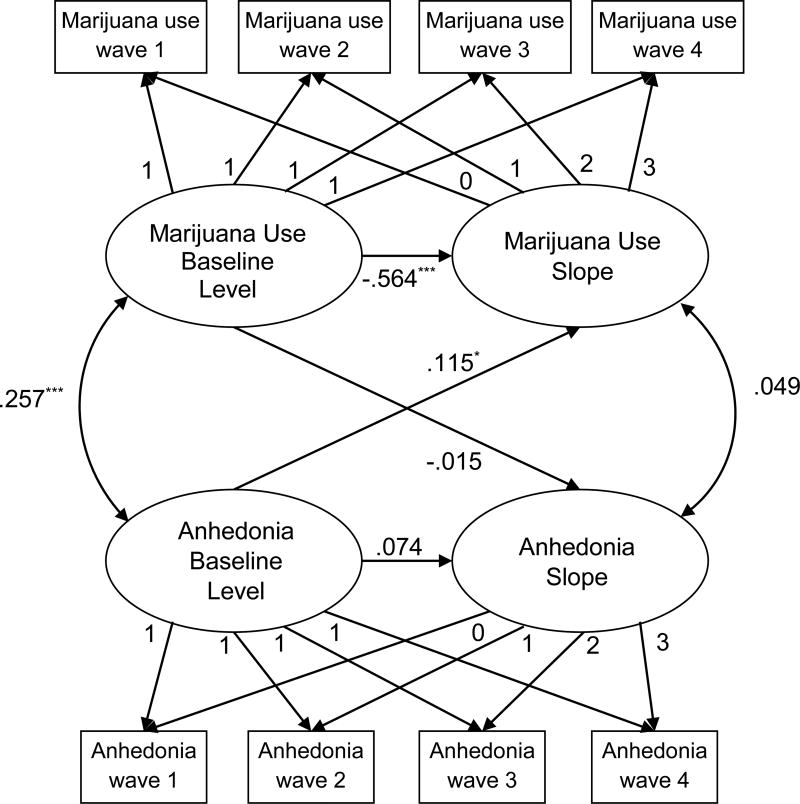
To characterize trajectories of anhedonia and marijuana use across time, latent growth curve modeling was applied to estimate a baseline level (based on intercept) and linear slope (rate of change across the 4 timepoints) for both anhedonia and marijuana use. Univariate latent growth curve models were first fit for marijuana use and anhedonia separately to determine the shape and variance of trajectories. A two-process parallel latent growth curve model (see Figure 1) was then fit, which simultaneously included growth factors for anhedonia and marijuana use after adjusting for covariates listed above and including within-construct level-to-slope associations [43]. The parallel process model was constructed to test: (1) bidirectional longitudinal associations by including directional paths from baseline anhedonia level to marijuana use slope as well as baseline marijuana use level to anhedonia slope; and (2) non-directional correlations between baseline levels of anhedonia and marijuana use and between anhedonia slope and marijuana use slope. Significant directional longitudinal paths between anhedonia and marijuana use in the overall sample were subsequently tested in moderation analyses of differences in the strength of paths across subsamples stratified by moderator status using a multigroup analysis [44].
Figure 1. Parallel latent growth curve model of anhedonia and marijuana use.
Note. Rectangles reflect observed variables. Circles reflect estimated latent variables. Straight lines with one arrow represent directional paths. Curved lines with two arrows represent non-directional correlational associations. Standardized coefficients are shown for estimated parameters. Fixed parameters on paths from latent to observed variables reflect the creation of level (all 1s) and linear slope (incremental equivalent increase 0–3 across timepoint) factors. Model adjusted for baseline time-invariant (i.e., highest parental education level, age, gender, ethnicity, depressive symptoms, social phobia, ADHD inattention/impulse levels) and time-varying (i.e., alcohol use frequency and cigarette use frequency) covariates (not shown). Model fit: AIC=60050.020, BIC=60480.136. *P-value for path estimate = .03. ***P-value for path estimate < .001. All other P-values > .05.
Analyses were performed using Mplus [45] with the complex analysis function to adjust parameter standard errors due to clustering of the data by school. To address item- and wave-level missing data, full information maximum likelihood estimation with robust standard errors was applied. Continuous and categorical ordinal scaled outcomes were applied for anhedonia and marijuana use, respectively. The Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) were used to gauge model fit in which lower values represent better-fitting models [46]. For moderator analyses, chi-squared differences were calculated using loglikelihood values and the number of free parameters contrasting the fit of models with (versus without) equality constraints on the anhedonia-marijuana use path of interest across groups stratified by the moderator variable. Standardized parameter estimates and 95% confidence intervals (CI) are reported. Significance was set at α=.05 (two-tailed).
RESULTS
Preliminary Analyses
Among study enrollees, 3,394 (99.9%) provided at least one data point for the variables in primary models and constituted the analytic sample (see Tables 1 and 2 for Ns of available data). Participants who did not complete wave 4 (N=131, 3.9%) were compared with those who completed all waves (N=3,252, 96.1%) to examine attrition effects. Those without wave 4 data reported higher baseline anhedonia (Cohen’s d=0.32) and marijuana use frequency (d=0.45), Ps<.001. There were no significant differences in demographics and depressive symptoms by attrition status.
As depicted in Table 1, the sample was balanced on gender, and was sociodemographically diverse. Overall, 15% of youth reported having ever used marijuana at baseline. The distribution of marijuana, alcohol, and cigarette use frequency was characteristic of general population adolescent samples (see Table 2). Across waves, 23% – 27% of students reported clinically-significant anhedonia based on SHAPS scores. Correlations among study variables at baseline are presented in in Table 3.
Table 3.
Correlations of study variables at baseline
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender | – | |||||||||||
| 2. Age | .08** | – | ||||||||||
| 3. Parental education level | .02 | −.04 | – | |||||||||
| 4. Marijuana use | .01 | .06* | −.13** | – | ||||||||
| 5. Anhedonia (SHAPS) | .08** | .03 | −.16** | .16** | – | |||||||
| 6. Alcohol use | −.06** | .05** | −.12** | .58** | .17** | – | ||||||
| 7. Cigarette use | −.02 | .03 | −.06** | .42** | .16** | .39** | – | |||||
| 8. Depression (CESD) | −.26** | −.01 | −.07** | .09** | .30** | .16** | .12** | – | ||||
| 9. Social phobia (RCADS) | −.28** | −.03 | .05* | −.03 | −.06** | .01 | .02 | .47** | – | |||
| 10. ADHD | −.04* | .01 | −.03 | .12** | .09** | .13** | .09** | .33** | .28** | – | ||
| 11. Friends’ marijuana use | −.03 | .05** | −.17 | .35** | .19** | .34** | .15** | .19** | −.01 | .15** | – | |
| 12. Ever marijuana use | .02 | .08** | −.16** | .70** | .17** | .48** | .31** | .11** | .05** | .14** | .40** | – |
Note. Gender was coded: 0 = female, 1 = male. Parental education coded as continuous variable (0 = 8th grade or less, 1 = Some high school, 2 = High school graduate, 3 = Some college, 4 = College graduate, 5 = Advanced degree). SHAPS = Snaith Hailton Pleasure Scale score. CESD = Center for Epidemiologic Depression Scale score. RCADS = Revised Children’s Anxiety and Depression Scale Social Phobia subscale score. ADHD = ADHD Self-Rating Scale score (Wave 2). Friends’ marijuana use coded: 0 = none, 1 = one or more friends using marijuana. Ever marijuana use was coded: 0 = no, 1 = yes.
p < .05.
p < .01.
Latent Growth Curve Models
Univariate models
Univariate latent growth curve models including linear slopes for anhedonia and marijuana use exhibited better fit of the data than quadratic models (supporting information sTable 2). In the linear univariate models, the mean slope was significantly larger than zero for anhedonia (M=0.310 P=.01) and marijuana (M=0.568, P<.001), indicating that, averaged across all participants, anhedonia and marijuana use increased across timepoints. Significant variability in marijuana use initial levels (variance of intercepts=15.077, P<.001) and in rates of change over time (variance of slopes =0.452, P<.001) were observed. Significant individual differences in initial levels of anhedonia (variance of intercepts= 29.857, P<.001) and rates of change in anhedonia over time (variance of slope =2.169, P<.001) were also observed. With sufficient inter-individual variability in both marijuana use and anhedonia, we proceeded to model associations between anhedonia and marijuana use growth factors.
Two-process models of associations between anhedonia and marijuana use
The two-process latent growth model with covariates exhibited adequate fit (Figure 1). Longitudinal directional path estimates indicate that baseline level of anhedonia was positively associated with the rate of change in marijuana use across time. Baseline marijuana use level was not significantly related to the rate of change in anhedonia. Non-directional correlational paths indicated a significant positive association between the baseline levels of anhedonia and marijuana use and no association between the rate of change in anhedonia and rate of change in marijuana use. Detailed presentation of parameter estimates, including covariate paths are reported in the online supporting information sTable 3. Of interest, depressive symptoms, social phobia, and ADHD symptoms were not significantly associated with changes in marijuana use over time (see supporting information sTable 3).
Moderators of the association of baseline anhedonia with changes in marijuana use over follow-up
Given the significant directional association from initial anhedonia level to increased marijuana use over time, we examined whether the strength of this relationship differed across subgroups. Friends’ marijuana use moderated the association of initial anhedonia levels with rates of change in marijuana use over time (interaction test result Δχ2[1] =4.19, P=.04). The association of baseline anhedonia with the rate of change in marijuana use was amplified amongst adolescents with friends who used marijuana at baseline (N=1,178; β[95%CI]=.179[.043, .334], P=.02) in comparison to those without friends who had used marijuana at baseline (N=2,127; β[95%CI]=.064[−.071, .187], P=.32). The path from baseline anhedonia level to changes in marijuana use over time was not significantly moderated by gender (Δχ2[1]=1.12, P=.29) or baseline ever marijuana use (Δχ2[1]=0.81, P=.37).
Sensitivity analyses
Sensitivity analyses showed that the association between baseline anhedonia level with the rate of change in marijuana use across the follow-up: (a) was consistent regardless of concomitant use of alternative marijuana products (e.g., edible or vaporized marijuana); (b) did not differ after removing students whose reports were of questionable validity (e.g., use of a fictitious drug) or who completed a follow-up survey by an alternate mode of survey administration (i.e., telephone, internet, or mail); (c) persisted among the subsample of participants who completed all waves of data collection (N=3,252, 96.1%); (d) generalized to an alternative measure of marijuana use quantity, and (e) was also found in an ordinal logistic regression model in which anhedonia clinical cutoff status (above vs. below) was use to predict the 5-level marijuana use frequency at wave 4 (OR[95%CI]=1.316[1.055, 1.640]). Additional analyses testing whether early onset marijuana used amplified paths of baseline anhedonia to marijuana use trend and marijuana use trend to anhedonia and found no evidence of effect modification by age of marijuana use onset. See sensitivity analyses in the online supporting information for a detailed description of these results.
DISCUSSION
Youth with higher (versus lower) levels of anhedonia at baseline were at increased risk of marijuana use escalation during early adolescence in this study. In addition, levels of anhedonia and marijuana use reported at the beginning of high school were cross-sectionally associated with each other. To the best of our knowledge, the only prior study on this topic found higher levels of anhedonia in 32 treatment-seeking marijuana users than 30 healthy controls in a cross-sectional analysis of French 14 – 20 year olds that did not adjust for confounders [24]. The current data provide new evidence elucidating the nature and direction of this association in a large community based sample, which advances a literature that has addressed the role of anhedonia predominately in adult samples [7].
The association of baseline anhedonia with marijuana use escalation was observed after adjustment of numerous possible confounders, including, demographic variables, symptom levels of three psychiatric syndromes previously linked with anhedonia (i.e., depression, social phobia, and ADHD) [37, 47, 48], and alcohol and tobacco use. Consequently, it is unlikely that anhedonia is merely a marker of these other psychopathological sources of marijuana use risk or a non-specific proclivity to any type of substance use. The temporal ordering of anhedonia relative to marijuana was addressed by the overarching bi-directional modeling strategy, which showed evidence of one direction of association (anhedonia → marijuana use) and not the other direction (marijuana use → anhedonia). Ordering was further confirmed in moderator tests showing that the association of anhedonia with subsequent marijuana use did not differ by baseline history of marijuana use. Thus, differences in risk of marijuana use between adolescents with higher (versus lower) anhedonia may be observed in cases when anhedonia precedes the onset of marijuana use.
Why might anhedonia be uniquely associated with subsequent risk of marijuana use escalation in early adolescence? Anhedonic individuals require a higher threshold of reward stimulation to generate an affective response and therefore may be particularly motivated to seek out pharmacological rewards to satisfy the basic drive to experience pleasure, as evidenced by prior work linking anhedonia to subsequent tobacco smoking escalation [38]. The risk pathway from anhedonia to marijuana use may be incremental to risk of other drug use. Amongst the three most commonly used drugs of abuse in youth (i.e., nicotine, alcohol, and marijuana), marijuana may possess the most robust mood-altering psychoactive effects in young adolescents [49]. Consequently, marijuana may have unique appeal for anhedonic youth driven to experience pleasure that they may otherwise be unable to derive easily via typical non-drug rewards.
The study results may open new opportunities for marijuana use prevention. Brief measures of anhedonia that have been validated in youth, such as the SHAPS scale used here, may be useful for identifying teens at risk who may benefit from interventions. If anhedonia is ultimately deemed a causal risk factor, targeting anhedonia may prove useful in marijuana use prevention. Interventions promoting youth engagement in healthy alternative rewarding behaviors without resorting to drug use have shown promise in prevention [50] and could be useful for offsetting anhedonia-related risk of marijuana use update.
Moderator results raise several potential scientific and practical implications. The association was stronger among adolescents with (versus without) friends who used marijuana, suggesting that expression of a proclivity to marijuana use may be amplified among teens in environments in which marijuana is easily accessible and socially-normative. The association of anhedonia with marijuana use escalation did not differ by gender or baseline history of marijuana use. Thus, preventive interventions that address anhedonia may: (1) benefit both boys and girls (2), aid in disrupting risk of onset as well as progression of marijuana use following initiation, and (3) be particularly valuable for teens in high-risk social environments.
While anhedonia increased linearly over the first two years of high school on average, the rate of change in anhedonia was not associated with baseline marijuana use or changes in marijuana use across time. Given that anhedonia is a manifestation of deficient reward activity [11] this finding is discordant with preclinical evidence of THC-induced dampening of brain reward activity and prior adult observational data showing that heavy or problematic marijuana use is associated with subsequent anhedonia [6] and diminished brain reward region activity during reward anticipation [27]. Perhaps the typical level and chronicity of exposure to marijuana use in this general sample of high school students was insufficient for detecting cannabinoid-induced manifestations of reward deficiency. Longer periods of follow-up may be needed to determine the extent of marijuana exposure at which cannabinoid-induced reward functioning impairment and resultant psychopathological sequelae may arise.
Strengths of this study include the large and demographically diverse sample, repeated measures follow-up over a key developmental period, modeling of multi-directional associations, rigorous adjustment of potential confounders, high participation and retention rates, and moderator tests to elucidate generalizability of the associations. Future work in which inclusion of biomarkers and objective measures is feasible may prove useful. Prevalence of heavy marijuana use was low in this sample, which precluded examination of clinical outcomes, such as marijuana use disorder. Students who did (vs. did not) complete the final follow-up had lower baseline marijuana use and anhedonia, which might impact representativeness. Further evaluation of the impact of family history of mental health or substance use problems as well as use of other illicit substances, which was not addressed here, is warranted.
CONCLUSIONS
Anhedonia is associated with increased risk of marijuana use escalation across the first two years of high school. Anhedonia warrants consideration in efforts to understand and prevent adolescent marijuana use uptake. If anhedonia is a consequence of marijuana use, this effect may not have ubiquitous generalizability.
Supplementary Material
Acknowledgments
Funding: This research was supported by National Institutes of Health grant R01-DA033296.
Role of Funder: The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures: The authors report no potential conflicts of interests.
Access to Data and Data Analysis: The AML and JC had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions: AML was principal investigator responsible for study conception and directing data collection. JC conducted the analyses. AML and JC lead the conceptualization of the study and wrote most the manuscript text. MDS, SS, NRR, JBU, JAM, and DRS aided in study conceptualization and provided feedback on drafts. MDS and JC oversaw data management and processing.
References
- 1.European Monitoring Centre for Drugs and Drug Addiction. Luxembourg: Publications Office of the European Union; 2016. [on May 18th, 2017]. European drug report: Trends and developments. Retrieved from: http://www.emcdda.europa.eu/system/files/publications/2637/TDAT16001ENN.pdf, Archived at: http://www.webcitation.org/6qYv00vI7. [Google Scholar]
- 2.United Nations Office on Drugs Crime. World drug report 2016: United Nations Publications. [on May 18th, 2017];2016 Retrieved from: https://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf, Archived at: http://www.webcitation.org/6qYufj5yC.
- 3.Hasin DS, Kerridge BT, Saha TD, Huang BJ, Pickering R, Smith SM, et al. Prevalence and Correlates of DSM-5 Cannabis Use Disorder, 2012–2013: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Am J Psychiat. 2016;173:588–599. doi: 10.1176/appi.ajp.2015.15070907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2014;44:797–810. doi: 10.1017/S0033291713001438. [DOI] [PubMed] [Google Scholar]
- 5.Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1609–1640. doi: 10.1007/s00127-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bovasso GB. Cannabis abuse as a risk factor for depressive symptoms. Am J Psychiat. 2001;158:2033–2037. doi: 10.1176/appi.ajp.158.12.2033. [DOI] [PubMed] [Google Scholar]
- 7.Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological bulletin. 2015;141:176–212. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holliday SB, Pedersen ER, Leventhal AM. Depression, posttraumatic stress, and alcohol misuse in young adult veterans: The transdiagnostic role of distress tolerance. Drug and alcohol dependence. 2016;161:348–355. doi: 10.1016/j.drugalcdep.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolitzky-Taylor K, McBeth J, Guillot CR, Stone MD, Kirkpatrick MG, Zvolensky MJ, et al. Transdiagnostic processes linking anxiety symptoms and substance use problems among adolescents. Journal of addictive diseases. 2016;35:266–277. doi: 10.1080/10550887.2016.1207969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolitzky-Taylor K, Guillot CR, Pang RD, Kirkpatrick MG, Zvolensky MJ, Buckner JD, et al. Examination of anxiety sensitivity and distress tolerance as transdiagnostic mechanisms linking multiple anxiety pathologies to alcohol use problems in adolescents. Alcoholism, clinical and experimental research. 2015;39:532–539. doi: 10.1111/acer.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291–299. doi: 10.31887/DCNS.2008.10.3/pgorwood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.APA. Diagnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 13.Cohen AS, Najolia GM, Brown LA, Minor KS. The state-trait disjunction of anhedonia in schizophrenia: potential affective, cognitive and social-based mechanisms. Clin Psychol Rev. 2011;31:440–448. doi: 10.1016/j.cpr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Bandelow B, Schmahl C, Falkai P, Wedekind D. Borderline personality disorder: a dysregulation of the endogenous opioid system? Psychol Rev. 2010;117:623–636. doi: 10.1037/a0018095. [DOI] [PubMed] [Google Scholar]
- 15.Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clin Psychol Rev. 2010;30:839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meinzer MC, Pettit JW, Leventhal AM, Hill RM. Explaining the covariance between attention-deficit hyperactivity disorder symptoms and depressive symptoms: the role of hedonic responsivity. J Clin Psychol. 2012;68:1111–1121. doi: 10.1002/jclp.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashdan TB, Elhai JD, Frueh BC. Anhedonia and emotional numbing in combat veterans with PTSD. Behaviour research and therapy. 2006;44:457–467. doi: 10.1016/j.brat.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients: The Pleasure Scale. Archives of General Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- 19.Meehl PE. Hedonic capacity: some conjectures. Bull Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- 20.Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- 21.Fiorito ER, Simons RF. Emotional imagery and physical anhedonia. Psychophysiology. 1994;31:513–521. doi: 10.1111/j.1469-8986.1994.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 22.Franken IHA, Muris P. Gray's impulsivity dimension: A distinction between reward sensitivity versus rash impulsiveness. Personality and Individual Differences. 2006;40:1337–1347. [Google Scholar]
- 23.Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J Clin Psychol. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- 24.Dorard G, Berthoz S, Phan O, Corcos M, Bungener C. Affect dysregulation in cannabis abusers. Eur Child Adoles Psy. 2008;17:274–282. doi: 10.1007/s00787-007-0663-7. [DOI] [PubMed] [Google Scholar]
- 25.Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- 26.Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 27.Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, et al. Association of Marijuana Use With Blunted Nucleus Accumbens Response to Reward Anticipation. Jama Psychiat. 2016;73:838–844. doi: 10.1001/jamapsychiatry.2016.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston L, O'Malley P, Miech R, Bachman J, Schulenberg J. overview: Key findings on adolescent drug use. Monitoring the future: National survery results on drug use 1975–2014: Sponsored by The National Institute on Drug Abuse at The National Institutes of Health. [on May 18th, 2017];2014 Retrieved from: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2014.pdf, Archived at: http://www.webcitation.org/6qYvNyaYV.
- 29.Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen P, Jacobson KC. Developmental trajectories of substance use from early adolescence to young adulthood: Gender and racial/ethnic differences. Journal of adolescent health. 2012;50:154–163. doi: 10.1016/j.jadohealth.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Audrain-McGovern J, Rodriguez D, Leventhal AM. Gender differences in the relationship between affect and adolescent smoking uptake. Addiction (Abingdon, England) 2015;110:519–529. doi: 10.1111/add.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behrendt S, Wittchen H-U, Höfler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug and alcohol dependence. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Glaser B, Shelton KH, van den Bree MB. The moderating role of close friends in the relationship between conduct problems and adolescent substance use. Journal of Adolescent Health. 2010;47:35–42. doi: 10.1016/j.jadohealth.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 35.Miech RA, Johnston LD, O’malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975–2014. 2015 [Google Scholar]
- 36.Bidwell LC, Knopik VS, Audrain-McGovern J, Glynn TR, Spillane NS, Ray LA, et al. Novelty Seeking as a Phenotypic Marker of Adolescent Substance Use. Substance abuse : research and treatment. 2015;9:1–10. doi: 10.4137/SART.S22440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meinzer MC, Pettit JW, Leventhal AM, Hill RM. Explaining the covariance between attention-deficit hyperactivity disorder symptoms and depressive symptoms: The role of hedonic responsivity. J Clin Psychol. 2012;68:1111–1121. doi: 10.1002/jclp.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Audrain-McGovern J, Rodriguez D, Leventhal AM, Cuevas J, Rodgers K, Sass J. Where is the pleasure in that? Low hedonic capacity predicts smoking onset and escalation. Nicotine Tob Res. 2012;14:1187–1196. doi: 10.1093/ntr/nts017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 40.Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav Res Ther. 2000;38:835–855. doi: 10.1016/s0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 41.Chorpita BF, Moffitt CE, Gray J. Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behav Res Ther. 2005;43:309–322. doi: 10.1016/j.brat.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Barkley RA. Attention-deficit hyperactivity disorder: A clinical workbook: Medifocus_com Inc. 1991 [Google Scholar]
- 43.Wickrama KA, Conger RD, Lorenz FO, Jung T. Family antecedents and consequences of trajectories of depressive symptoms from adolescence to young adulthood: a life course investigation. J Health Soc Behav. 2008;49:468–483. doi: 10.1177/002214650804900407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthén B, Asparouhov T. Latent variable analysis with categorical outcomes: Multiple-group and growth modeling in Mplus. Mplus web notes. 2002;4:1–22. [Google Scholar]
- 45.Muthen L. BOM:(1998–2012): Mplus User’s Guide. Los Angeles, CA: Muthen and Muthen; 2012. [Google Scholar]
- 46.Bollen KA, Curran PJ. Latent curve models: A structural equation perspective. John Wiley & Sons; 2006. [Google Scholar]
- 47.Brown LH, Silvia PJ, Myin-Germeys I, Lewandowski KE, Kwapil TR. The relationship of social anxiety and social anhedonia to psychometrically identified schizotypy. J Soc Clin Psychol. 2008;27:127–149. [Google Scholar]
- 48.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeiger JS, Haberstick BC, Corley RP, Ehringer MA, Crowley TJ, Hewitt JK, et al. Subjective effects for alcohol, tobacco, and marijuana association with cross-drug outcomes. Drug and alcohol dependence. 2012;123(Suppl 1):S52–58. doi: 10.1016/j.drugalcdep.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy JG, Correia CJ, Barnett NP. Behavioral economic approaches to reduce college student drinking. Addict Behav. 2007;32:2573–2585. doi: 10.1016/j.addbeh.2007.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.