Abstract
In postmenopausal women, 2-years of combined teriparatide and denosumab increases bone mineral density (BMD) more than either drug alone and switching from either combination or teriparatide to denosumab for an additional 2-years further increases BMD. Conversely switching from denosumab to teriparatide results in transient bone loss. The effects of these interventions on spine microarchitecture are unknown. In the DATA and DATA-Switch studies, 94 postmenopausal osteoporotic women were randomized to receive 24-months of teriparatide (20-μg-daily), denosumab (60-mg-every-6-months), or both. Then, women originally assigned to 24-months of teriparatide received 24-months of denosumab whereas subjects originally randomized to 24-months of denosumab received 24-months of teriparatide. Subjects who received both drugs received an additional 24-months of denosumab alone. Spine trabecular bone score (TBS, a gray-level textural assessment of bone microarchitecture) was measured blinded from treatment groups using images from 2-dimensional DXA spine scans at 0, 12, 24, 30, 36, and 48 months in 65 women who had PA spine DXA images suitable for TBS analysis. After 24 months, TBS increased by 2.7±4.7% in the teriparatide group (P=0.009 versus baseline), by 1.8±5.0% in the denosumab group (P=0.118 versus baseline), and by 4.5±6.7% in the combination group (P=0.017 versus baseline), with no significant between-group differences. In the 6-months after treatments were switched (months 24–30), TBS continued to increase in the combination-to-denosumab and teriparatide-to-denosumab groups but decreased by −1.1±4.0% in the denosumab-to-teriparatide group (P<0.05 versus other groups). After 48 months, compared to month 0, TBS increased by 5.1±5.8% in the teriparatide-to-denosumab group, by 3.6±4.2% in the denosumab-to-teriparatide group, and by 6.1±4.7% in the combination-to-denosumab group (P<0.001 versus baseline for all groups, P=NS for between group differences). Switching from teriparatide-to-denosumab also increases spine TBS. Conversely, switching from denosumab-to-teriparatide transiently degraded spine trabecular microarchitecture, the clinical consequences of which require further study.
Keywords: osteoporosis, denosumab, teriparatide
Background
Postmenopausal osteoporosis remains a significant healthcare burden and the need for more effective treatment strategies remains critical. In the DATA study, we demonstrated that in postmenopausal osteoporosis, two years of combined denosumab and teriparatide increased areal bone mineral density (aBMD) and improved peripheral skeletal microarchitecture more than either medication alone.1,2 In the subsequent DATA-Switch study, we demonstrated that the transition from either teriparatide or combined denosumab/teriparatide to denosumab results in continued aBMD gains while the transition from denosumab to teriparatide results in transient decreases in aBMD.3 While dual-energy absorptiometry (DXA) is able to predict fracture risk in most clinical scenarios, treatment-induced aBMD increases do not fully explain the observed fracture risk reduction of osteoporosis medications.4,5 This suggests that other factors such as bone geometry, skeletal microarchitecture, and tissue properties also influence fracture risk.
Trabecular bone score (TBS) is a gray-level textural assessment that is calculated from standard two-dimensional DXA spine images and is an integrated measure of skeletal microarchitecture.6–8 TBS has been shown to predict fracture incidence independent of aBMD and clinical risk factors and has been validated for use in the FRAX fracture prediction model.7,9,10 As such it has been now approved by medical societies for clinical use (e.g. ESCEO, ISCD).11,12
To assess the effect of combination denosumab and teriparatide, as well as the transition from denosumab-to-teriparatide and teriparatide-to-denosumab, on skeletal microarchitecture of the spine, we measured TBS in postmenopausal women who completed all 4 years of the DATA and DATA-Switch studies.
Methods
Subjects
The characteristics of the DATA-Switch study have been described in detail.3,13 In brief, we recruited 94 postmenopausal women at high risk of fracture defined as having a spine or hip T-score of −2.5 or less or −2.0 or less with at least one risk factor (fracture after age 50yo, parental hip fracture after age 50yo, previous hyperthyroidism, inability to get up from a chair with arms raised, or current smoking)14 or a T-score of −1.0 or less with a history of an adult fragility fracture. Women were excluded for use of glucocorticoids or oral bisphosphonates within 6 months of enrollment, use of estrogen, selective estrogen receptor modulators, or calcitonin within 3 months of enrollment; or any prior use of intravenous bisphosphonates, PTH or strontium ranelate.
Protocol
Subjects were randomized to receive either teriparatide 20-mcg daily, denosumab 60-mg subcutaneously every 6 months, or both for 2 years. After completing 2-years of treatment, subjects who received teriparatide were switched to denosumab, subjects who received denosumab were switched to teriparatide, and subjects who received both were continued on denosumab alone (Figure 1). Dual x-ray absorptiometry was performed on a Hologic QDR 4500A densitometer (Hologic, Waltham, MA, USA) at each study visit. All scans of each participant were performed on the same densitometer. Quality control measurements with a Hologic anthropomorphic phantom were performed daily and our standard deviation of in-vivo same-day reproducibility is 0.005 g/cm2 for the posterior-anterior spine. Trabecular bone score of posterior-anterior spine images (using L1-L4) was performed retrospectively on scans obtained at months 0, 6, 12, 24, 30, 36, and 48. Trabecular bone score was assessed using the TBS Insight 2.2 software (Med-Imaps, Merignac, France). All patient identifiers were removed to maintain anonymity and to ensure blinding of the investigators to all clinical parameters and outcomes. Vertebrae excluded for BMD assessment were also excluded for TBS evaluation. As established in a meta-analysis showing that TBS is a predictor of fracture independent of FRAX, TBS values ≥1.31 were considered lowest risk; between 1.23 and 1.31 consistent with intermediate risk, and ≤1.23 consistent with highest risk.10
Figure 1.
Study schema.
The study was approved by the Partners Healthcare Institutional Review Board and registered on ClinicalTrials.gov (number NCT00926380).
Statistical analysis
We performed the analysis on data from women who completed all 48-months. To compare the between-group changes in TBS, we used a repeated-measures ANOVA. Within-group changes were assessed by a paired t test. Pearson’s correlation coefficients (R) were calculated to determine the relationship between changes in TBS and changes in BMD (between months 0–24, 0–48, and 24–30). Statistical analyses were performed with SAS for Windows (version 9.3; SAS Institute, Inc). The data are presented as mean (SD) unless otherwise specified. Statistical significance was defined as P<0.05.
Results
69 women completed all 48-months. Of these 69 subjects, 65 subjects had a PA spine DXA image suitable for TBS evaluation and are included in this analysis. Baseline characteristics, including TBS values, were similar among the three treatment groups with exception of history of previous adult fracture (Table 1).
Table 1.
Baseline characteristics (mean (SD) or number (%)). DXA=dual x-ray absorptiometry. There were no significant differences among the three groups for all characteristics.
| Teriparatide to denosumab (N=25) |
Denosumab to teriparatide (N=22) |
Combination to denosumab (N=18) |
|
|---|---|---|---|
| Age (years) | 65.2 (6.3) | 63.9 (6.2) | 64.5 (7.4) |
| Body-mass index (kg/m2) | 25.7 (3.6) | 23.4 (4.0) | 25.6 (5.3) |
| White, non-Hispanic | 25 (100%) | 20 (91%) | 15 (83%) |
| Clinical fracture at age >45 years (%) | 13 (52%) | 6 (27%) | 3 (17%) |
| DXA posterior-anterior spine (g/cm2) | 0.815 (0.109) | 0.853 (0.101) | 0.828 (0.130) |
| Trabecular bone score | 1.27 (0.10) | 1.31 (0.09) | 1.26 (0.08) |
As reported previously, after 24 months, lumbar spine BMD increased more in women receiving both drugs (12.9±5.0%) than either the teriparatide (9.5±5.9%, P=0.01) or denosumab (8.3±3.4%, P=0.008) groups (Figure 2).1 After 48 months, spine BMD increased similarly in the three groups (18.3±8.5% in the teriparatide to denosumab group, 14.0±6.7% in the denosumab to teriparatide group, and 16.0±4.1% in the combination to denosumab group).3
Figure 2.
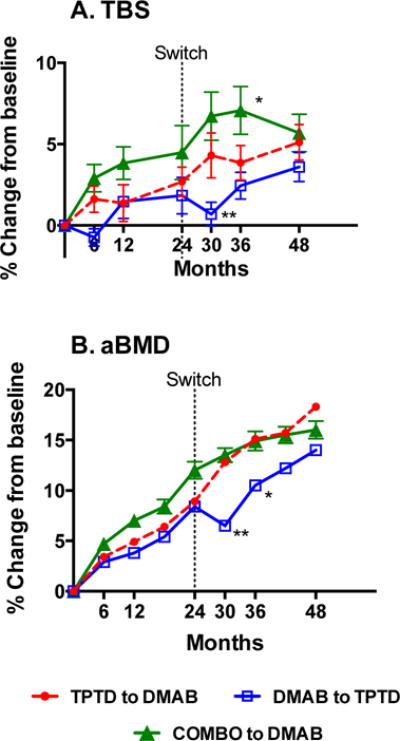
Mean percent change (SEM) from baseline in A. trabecular bone score and B. bone mineral density in the lumbar spine.
*P<0.05 for the 0–36 month change versus both other groups
**P<0.05 for the 24–30 month change versus both other groups
Trabecular bone score
After 24 months of therapy, TBS increased by 2.7 ± 4.7% in women treated with teriparatide monotherapy (P=0.009 versus baseline), by 1.8 ± 5.0% in women treated with denosumab monotherapy (P=0.118 versus baseline), and by 4.5 ± 6.7% in women treated with both drugs (P=0.017 versus baseline) There were no significant between-group differences in the TBS changes between month 0 and 24 (Figure 2).
In the 6 months after the treatment assignments were switched (months 24–30), mean TBS decreased by −1.1 ± 4.0% in women who transitioned from denosumab to teriparatide whereas mean TBS increased by 2.0 ±4.7% in the teriparatide-to-denosumab group and by 2.1 ± 4.3% in the combination-to-denosumab group. These 24–30 month changes in the denosumab to teriparatide group differed significantly from the other 2 groups (P<0.05 for both between-group comparisons).
After 36 months of treatment, TBS increased by 4.1 ± 5.4% in the teriparatide-to-denosumab group, by 2.4 ± 3.9% in the denosumab to teriparatide group, and by 7.1 ± 6.3% in the combination to denosumab group. The 36-month increases in the combination to denosumab group were greater than both other groups (P=0.006 versus denosumab to teriparatide and P=0.052 versus teriparatide to denosumab).
When the entire 48 month treatment period is considered, TBS increased by 5.1 ± 5.8% in the teriparatide to denosumab group, by 3.6 ± 4.2% in the denosumab to teriparatide group, and by 6.1 ± 4.7% in the combination to denosumab group (P<0.001 for all within-group comparisons). Although differences were noted in the results, we failed to reach significant differences in the month 0–48 TBS changes between any of the treatment groups.
After 48 months, in the teriparatide to denosumab group, only 2 out of 25 subjects remained in the highest risk of fracture category (as defined as TBS ≤ 1.23) versus 6 of 25 subjects at baseline. In the denosumab to teriparatide group, 3 of the 22 subjects remained in the highest risk of fracture category at 48 months versus 4 out of 22 at baseline. In the combination to denosumab group, only 2 of the 18 subjects remained in the highest fracture risk category from 7 out of 18 at baseline. There were no significant between group differences in the proportion of subjects who moved out of the highest fracture risk category.
Associations between TBS and bone mineral density
At baseline, with all groups pooled together, there was a significant association between baseline PA spine BMD and TBS (R=0.29, P=0.020). There was a significant association between the change in PA spine aBMD and change in TBS in the denosumab to teriparatide group at months 0–24, 0–48, and 24–30 (R ranging from 0.51 to 0.69, P<0.03). There were no significant correlations between change in PA spine aBMD and change in TBS in the other two treatment groups at months 0–24, 0–48, and 24–30.
Discussion
In this post-hoc analysis of the DATA and DATA-Switch randomized controlled trials, we demonstrated that spine trabecular microarchitecture as assessed by TBS increased significantly in all three treatment groups after 48-months of therapy. Moreover, these 48-months increases did not differ significantly between treatment groups, a pattern that mirrors the one observed in PA spine aBMD in DATA-Switch (though aBMD at hip sites did differ between groups).3 Also notable is that the women who transitioned from denosumab-to-teriparatide experienced a transient decrease in TBS, which correlated with the transient decrease seen in spine aBMD in DATA-Switch.3 Although there were no statistically significant differences between treatment groups in this study at 24-months, the pattern of TBS changes resembled the pattern of changes in BMD as the TBS increase in the combination group was numerically greater than both monotherapy groups.
The magnitude of changes in TBS in this study is consistent with the expected anabolic effect on bone and similar to those reported previously.11 In a study of 65 women treated with teriparatide, TBS increased by approximately 2.2% per year, which is similar to the teriparatide-induced TBS changes we report.15 In contrast, antiresorptives, including denosumab, generally increase TBS to a smaller extent, ranging from 0.2% per year in a trial of 534 women treated with a mostly bisphosphonates to 1.7% per year in an observational trial of 36 women treated with denosumab.16,17 However, when combining denosumab with teriparatide, the observed microstructure improvement is numerically better than any other treatment alone.
The lack of consistent correlations between TBS and changes in aBMD we report, particularly in the combination groups, suggests that TBS may provide information distinct from DXA-derived a BMD. Alternatively, as there is known error that occurs when measuring changes in BMD and TBS, the variance due to measurement error may be contributing to the lack of correlation between changes in TBS and aBMD. The poor correlations are consistent with prior studies reporting absent or weak correlations between the change in TBS and the change in aBMD by DXA.15,17,18 In the large Manitoba study of 534 women of whom 86% received a bisphosphonate, 10% received raloxifene, and 4% received calcitonin over a mean duration of 3.7 years, changes in TBS and changes in aBMD only weakly correlated (R=0.20).17 Similarly, in the Swiss Horizon trial of 54 postmenopausal women who received zoledronic acid and 53 women who received placebo, changes in TBS and changes in aBMD over 36 months also weakly correlated (R=0.20).18
The clinical utility of TBS has been demonstrated in both cross-sectional and prospective studies that have found that that low TBS predicts fracture risk independent of aBMD.19–34 The utility of TBS measurements in longitudinal clinical trials, however, is less well established. While TBS changes in response to treatment,15–18,35–37 it is currently not known if these changes predict fracture risk reduction. In the previously discussed Manitoba study of 534 women, the change in TBS did not predict fracture risk.38 Notably, the treatment-induced TBS changes in this study were relatively small (0.2% per year) and the observational design is not the optimal way to address this important clinical question.
The clinical significance of the transient decrease in TBS that occurs when patients switch from denosumab to teriparatide is unclear but is an essential one to address given recent reports of patients experiencing multiple vertebral questions in the period immediately after denosumab is discontinued and bone turnover is accelerated. 39–42 It has also been reported that the anti-fracture efficacy of denosumab dissipates as soon as patients stop taking the drug.43 Of note, the magnitude of bone turnover acceleration is significantly greater in women who transition from denosumab to teriparatide compared to those who simply stop denosumab abruptly.3 The specific role that deteriorating trabecular architecture (as suggested by TBS decreases) plays in these effects is unclear.
A limitation of this study is the relatively small sample size, which precludes any conclusions regarding fracture risk reduction and limits the interpretation of between-group differences (the study was powered for an aBMD primary outcome). This limitation persisted despite our attempts to maximize the precision of the TBS measurements by performing all DXA assessments on the same densitometer and all TBS analyses at a central location. Despite this limitation, however, this is to our knowledge the first demonstration of a robust increase in TBS in postmenopausal women treated with combined teriparatide and denosumab, as well as the first to show decreases in TBS during the transition from denosumab to teriparatide. Together, these findings, along with the central aBMD and peripheral microarchitectural findings previously reported,2,3 continue to support the potential of this combined teriparatide and denosumab in the treatment of postmenopausal osteoporosis. These results also continue to support our recommendation that when using these medications sequentially, the initial use of teriparatide is preferable.
Acknowledgments
This work is supported by NIH/NIAMS grant K23AR068447 (to JNT), NIH/NIAMS grant K24AR067847 (to BZL), Eli Lilly, and Amgen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: LAJ and HL have nothing to declare. JNT is a consultant for Amgen Inc. BZL is a consultant for Eli Lilly, Amgen Inc, and Merck. DH is co-owner of the TBS patent and has corresponding ownership shares.
References
- 1.Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. The Journal of clinical endocrinology and metabolism. 2014;99:1694–700. doi: 10.1210/jc.2013-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai JN, Uihlein AV, Burnett-Bowie SM, et al. Effects of Two Years of Teriparatide, Denosumab, or Both on Bone Microarchitecture and Strength (DATA-HRpQCT study) The Journal of clinical endocrinology and metabolism. 2016;101:2023–30. doi: 10.1210/jc.2016-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386:1147–55. doi: 10.1016/S0140-6736(15)61120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delmas PD, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone. 2004;34:599–604. doi: 10.1016/j.bone.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. The New England journal of medicine. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 6.Pothuaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone. 2008;42:775–87. doi: 10.1016/j.bone.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2011;14:302–12. doi: 10.1016/j.jocd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Muschitz C, Kocijan R, Haschka J, et al. TBS reflects trabecular microarchitecture in premenopausal women and men with idiopathic osteoporosis and low-traumatic fractures. Bone. 2015;79:259–66. doi: 10.1016/j.bone.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2014;29:518–30. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 10.McCloskey EV, Oden A, Harvey NC, et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2016;31:940–8. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- 11.Harvey NC, Gluer CC, Binkley N, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–24. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture Risk Prediction by Non-BMD DXA Measures: the 2015 ISCD Official Positions Part 2: Trabecular Bone Score. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2015;18:309–30. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382:50–6. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black DM, Steinbuch M, Palermo L, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2001;12:519–28. doi: 10.1007/s001980170072. [DOI] [PubMed] [Google Scholar]
- 15.Senn C, Gunther B, Popp AW, Perrelet R, Hans D, Lippuner K. Comparative effects of teriparatide and ibandronate on spine bone mineral density (BMD) and microarchitecture (TBS) in postmenopausal women with osteoporosis: a 2-year open-label study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25:1945–51. doi: 10.1007/s00198-014-2703-8. [DOI] [PubMed] [Google Scholar]
- 16.Petranova T, Sheytanov I, Monov S, Nestorova R, Rashkov R. Denosumab improves bone mineral density and microarchitecture and reduces bone pain in women with osteoporosis with and without glucocorticoid treatment. Biotechnology, biotechnological equipment. 2014;28:1127–37. doi: 10.1080/13102818.2014.967827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieg MA, Aubry-Rozier B, Hans D, Leslie WD, Manitoba Bone Density P Effects of anti-resorptive agents on trabecular bone score (TBS) in older women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24:1073–8. doi: 10.1007/s00198-012-2155-y. [DOI] [PubMed] [Google Scholar]
- 18.Popp AW, Guler S, Lamy O, et al. Effects of zoledronate versus placebo on spine bone mineral density and microarchitecture assessed by the trabecular bone score in postmenopausal women with osteoporosis: a three-year study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28:449–54. doi: 10.1002/jbmr.1775. [DOI] [PubMed] [Google Scholar]
- 19.Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26:2762–9. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 20.Del Rio LM, Winzenrieth R, Cormier C, Di Gregorio S. Is bone microarchitecture status of the lumbar spine assessed by TBS related to femoral neck fracture? A Spanish case-control study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24:991–8. doi: 10.1007/s00198-012-2008-8. [DOI] [PubMed] [Google Scholar]
- 21.Krueger D, Fidler E, Libber J, Aubry-Rozier B, Hans D, Binkley N. Spine trabecular bone score subsequent to bone mineral density improves fracture discrimination in women. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2014;17:60–5. doi: 10.1016/j.jocd.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Lamy O, Metzger M, Krieg MA, Aubry-Rozier B, Stoll D, Hans D. OsteoLaus: prediction of osteoporotic fractures by clinical risk factors and DXA, IVA and TBS. Revue medicale suisse. 2011;7:2130,2–4,6. [PubMed] [Google Scholar]
- 23.Leib E, Winzenrieth R, Aubry-Rozier B, Hans D. Vertebral microarchitecture and fragility fracture in men: a TBS study. Bone. 2014;62:51–5. doi: 10.1016/j.bone.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Leib E, Winzenrieth R, Lamy O, Hans D. Comparing bone microarchitecture by trabecular bone score (TBS) in Caucasian American women with and without osteoporotic fractures. Calcified tissue international. 2014;95:201–8. doi: 10.1007/s00223-014-9882-3. [DOI] [PubMed] [Google Scholar]
- 25.Nassar K, Paternotte S, Kolta S, Fechtenbaum J, Roux C, Briot K. Added value of trabecular bone score over bone mineral density for identification of vertebral fractures in patients with areal bone mineral density in the non-osteoporotic range. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25:243–9. doi: 10.1007/s00198-013-2502-7. [DOI] [PubMed] [Google Scholar]
- 26.Pothuaud L, Barthe N, Krieg MA, Mehsen N, Carceller P, Hans D. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2009;12:170–6. doi: 10.1016/j.jocd.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Rabier B, Heraud A, Grand-Lenoir C, Winzenrieth R, Hans D. A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): Analysing the odds of vertebral fracture. Bone. 2010;46:176–81. doi: 10.1016/j.bone.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Touvier J, Winzenrieth R, Johansson H, et al. Fracture discrimination by combined bone mineral density (BMD) and microarchitectural texture analysis. Calcified tissue international. 2015;96:274–83. doi: 10.1007/s00223-015-9952-1. [DOI] [PubMed] [Google Scholar]
- 29.Vasic J, Petranova T, Povoroznyuk V, et al. Evaluating spine micro-architectural texture (via TBS) discriminates major osteoporotic fractures from controls both as well as and independent of site matched BMD: the Eastern European TBS study. Journal of bone and mineral metabolism. 2014;32:556–62. doi: 10.1007/s00774-013-0529-7. [DOI] [PubMed] [Google Scholar]
- 30.Winzenrieth R, Dufour R, Pothuaud L, Hans D. A retrospective case-control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: analyzing the odds of vertebral fracture. Calcified tissue international. 2010;86:104–9. doi: 10.1007/s00223-009-9322-y. [DOI] [PubMed] [Google Scholar]
- 31.Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24:77–85. doi: 10.1007/s00198-012-2188-2. [DOI] [PubMed] [Google Scholar]
- 32.Briot K, Paternotte S, Kolta S, et al. Added value of trabecular bone score to bone mineral density for prediction of osteoporotic fractures in postmenopausal women: the OPUS study. Bone. 2013;57:232–6. doi: 10.1016/j.bone.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 33.Iki M, Tamaki J, Kadowaki E, et al. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2014;29:399–407. doi: 10.1002/jbmr.2048. [DOI] [PubMed] [Google Scholar]
- 34.Leslie WD, Johansson H, Kanis JA, et al. Lumbar spine texture enhances 10-year fracture probability assessment. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014;25:2271–7. doi: 10.1007/s00198-014-2761-y. [DOI] [PubMed] [Google Scholar]
- 35.Di Gregorio S, Del Rio L, Rodriguez-Tolra J, Bonel E, Garcia M, Winzenrieth R. Comparison between different bone treatments on areal bone mineral density (aBMD) and bone microarchitectural texture as assessed by the trabecular bone score (TBS) Bone. 2015;75:138–43. doi: 10.1016/j.bone.2014.12.062. [DOI] [PubMed] [Google Scholar]
- 36.Kalder M, Hans D, Kyvernitakis I, Lamy O, Bauer M, Hadji P. Effects of Exemestane and Tamoxifen treatment on bone texture analysis assessed by TBS in comparison with bone mineral density assessed by DXA in women with breast cancer. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2014;17:66–71. doi: 10.1016/j.jocd.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Kalder M, Kyvernitakis I, Albert US, Baier-Ebert M, Hadji P. Effects of zoledronic acid versus placebo on bone mineral density and bone texture analysis assessed by the trabecular bone score in premenopausal women with breast cancer treatment-induced bone loss: results of the ProBONE II substudy. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26:353–60. doi: 10.1007/s00198-014-2955-3. [DOI] [PubMed] [Google Scholar]
- 38.Leslie WD, Majumdar SR, Morin SN, Hans D, Lix LM. Change in Trabecular Bone Score (TBS) With Antiresorptive Therapy Does Not Predict Fracture in Women: The Manitoba BMD Cohort. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2016 doi: 10.1002/jbmr.3054. [DOI] [PubMed] [Google Scholar]
- 39.Anastasilakis AD, Makras P. Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27:1929–30. doi: 10.1007/s00198-015-3459-5. [DOI] [PubMed] [Google Scholar]
- 40.Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O. Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27:1923–5. doi: 10.1007/s00198-015-3380-y. [DOI] [PubMed] [Google Scholar]
- 41.Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27:1917–21. doi: 10.1007/s00198-015-3458-6. [DOI] [PubMed] [Google Scholar]
- 42.Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B. Severe rebound-associated vertebral fractures after denosumab discontinuation: nine clinical cases report. The Journal of clinical endocrinology and metabolism. 2016;102:354–8. doi: 10.1210/jc.2016-3170. [DOI] [PubMed] [Google Scholar]
- 43.Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–9. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]


