Abstract
Aims
To evaluate relationships between measures of cognitive functioning and alcohol or drug use among adults (≥18 years) in the U.S. general population.
Design
Two cognitive scales were created based on dimensionality and reliability of self-reported Executive Function Index items. Relationships between the two scales and validators were evaluated. Associations between the cognitive scales and past-year frequency of alcohol or drug use were estimated with adjusted odds ratios (aOR).
Setting
USA, using the 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions-III, a nationally representative adult sample selected by multistage probability sampling.
Participants
36,085 respondents.
Measurements
Past-year substance use outcome variables categorized binge drinking, marijuana, cocaine, opioid, sedative/tranquilizer, and stimulant use as frequent (at least weekly to daily), infrequent (any to 2–3 times/month), or no use, assessed by the Alcohol Use Disorder and Associated Disabilities Interview Schedule-V. Key predictors were the two cognitive scales. Construct validators included education and functional impairment. Covariates included age, gender, income, and race/ethnicity.
Findings
Nine cognitive items fit a two-factor model (Comparative Fit Index=.973): attention (5 items) and executive functioning (4 items). Both scales were positively associated with higher education (ps<.001) and negatively associated with functional impairment (ps<.001), demonstrating construct validity. Poorer attention was associated with frequent and infrequent binge drinking and use of drugs (aOR range=1.07 [binge drinking] to 1.72 [stimulants], ps≤0.01). Poorer executive functioning was associated with frequent binge drinking and use of drugs (aOR range=1.22 [binge drinking] to 2.03 [cocaine], ps<0.001), and infrequent use of all drugs (aOR range=1.19 [marijuana] to 1.63 [cocaine], ps<0.001).
Conclusions
Impairments in attention and executive functioning are positively correlated with substance use in the U.S. general population.
Keywords: National population, cognitive function, alcohol use, drug use, validity (epidemiology), executive function, comorbidity, psychiatric disorders
INTRODUCTION
Cognitive functioning refers to the mental processes that store, retrieve, transform, and use information. Such functioning involves domains such as memory, learning and attention, and higher executive functions, e.g., decision making, organization, planning, and control inhibition. Adequate functioning is necessary for important life activities, ranging from simple, e.g., grocery shopping, to complex, e.g., career planning.
Neuropsychological and neuroimaging evidence indicates impaired cognitive functioning in adult patients treated for substance use disorders (SUD) [1], potentially impacting their ability to utilize treatment [2,3] or function well in important interpersonal or occupational areas [4,5]. These cognitive impairments are associated with many substance disorders [6], including alcohol [7–10], cannabis [11–15], cocaine [16–24], and others [25–29]. In domains including attention and executive functioning, impairments range from mild to severe, as assessed with measures ranging from short tests to extensive neuropsychological batteries.
Whether cognitive functioning is also impaired in general population substance users is unknown. Extant surveys with measures of substance use and cognitive functioning included only middle-aged or older U.S. adults [30–35], omitting late adolescent and early adult participants, when prevalence of alcohol and drug use and related disorders is highest [36–38]. Thus, in large, national samples with participants at the peak ages of risk, information is lacking on the relationship of cognitive functioning to substance use, an increasing health problem with high morbidity and mortality among adults [36,37,39].
The National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III) provides nationally representative survey data on U.S. adults ages ≥18, including measures of cognition and drug and alcohol use, which can be used to fill the knowledge gap about cognition and substance use and related consequences in the general population. Objective (interviewer-rated) neuropsychological assessments were unfeasible in NESARC-III due to the extensive resources (e.g., interview time, specialized interviewer training) needed. Since self-report measures of cognition show moderate to strong relationships to objective measures [40–42], particularly when specific cognitive domains are assessed [43], a brief self-report assessment of cognitive functioning was used, the Executive Function Index (EFI). The EFI is designed to assess specific cognitive domains in adult community surveys, in which it has previously demonstrated good internal consistency and convergent validity [44].
In this study, we examined the relationships between cognitive functioning and alcohol and drug use in NESARC-III. Before investigating these relationships, information on the psychometric properties of the self-reported EFI items in NESARC-III was needed. Thus, the study was conducted in four steps. 1. We assessed the dimensionality of EFI items, which suggested two underlying factors. 2. We estimated the reliability of these factors. 3. We assessed the construct and discriminant validity of two scales based on these factors. 4. We estimated the association between these cognitive scales and past-year frequency of binge drinking and drug use.
METHODS
Design
To address the study aim, data from a large, general population U.S. sample were used. We created reliable measures of cognitive functioning, through modelling the dimensionality of the Executive Function Index (EFI) items and assessing the reliability of the resulting factors. Two cognitive scales were derived from the factors and validated by evaluating relationships with construct and discriminant validators. Associations between the frequency of alcohol (binge drinking) or drug use and the cognitive scales were estimated.
Data
The 2012–2013 NESARC-III is a nationally representative survey of the non-institutionalized, civilian, adult (≥18 years old) U.S. population, including residents of households and group quarters [37,45]. The multistage probability sampling scheme included primary sampling units (largely counties) from the entire USA, secondary sampling of groups of census-defined blocks, and tertiary sampling of households, with random selection of adults within households [46]. The response rate was 60.1%, comparable to other large-scale national studies [47,48]. Interviews were face-to-face, using computer-assisted personal interviewing [46]. Interview quality was assured by rigorous interviewer training, supervision and random callbacks to verify responses. Participants provided informed consent and received $90.00. The National Institutes of Health and Westat Institutional Review Boards approved the NESARC-III protocol. This study included 36,085 respondents, after excluding 224 respondents (0.6% of the sample) missing responses for ≥25% of the EFI items.
Measures
Outcomes: Past-year substance use
The Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5) assessed frequency of binge drinking and non-medical substance use, including marijuana, cocaine, opioids, sedatives/tranquilizers, and stimulants. AUDADIS-5 alcohol and drug use questions were identical to those in AUDADIS-IV, which showed good-to-excellent test-retest reliability [46,49]. Frequency of use was indicated by a three level variable (frequent use, infrequent use, no use), based on how often in the past year respondents engaged in drug use or binge drinking (≥5 drinks in a day for men, ≥4 for women). Frequent was defined as once a week to daily; infrequent as 1–2 times in the past year to 2–3 times a month.
Cognitive functioning
The EFI [44] is a self-reported measure of functioning on cognitive tasks encountered in daily life. NESARC-III included 12 items from the Strategic Planning and Organization EFI sub-scales to provide brief assessment of domains with widely-established impairments among patients in treatment for substance use [3,6,7]. These subscales previously evidenced good reliability (α=.70 and .75, respectively [44]), and correlated as predicted to demographic characteristics, other cognitive measures, and psychological attributes [44,50,51]. Items were rated on five-point scales (1=not at all; 2=a little; 3=somewhat; 4=a lot; 5=very much). Items that assessed difficulty with functioning (‘mix up sequence’; ‘trouble multi-tasking’; ‘lose track’; ‘trouble summing’; ‘lose interest’) were reverse-coded so that higher response values indicated better functioning, consistent with the other items. Items were summed into scales (Scale construction below), used as outcomes for validity analyses and then as predictors of substance use.
Validators: predictors of cognitive functioning
Two construct validators were posited. One was education: less than high school, completed high school, some college, college degree, and graduate study. Participants with higher education levels were predicted to score higher (positive association) on the cognitive function (EFI) scales. The other was functional impairment, based on the 12-Item Short Form Health Survey Mental Component Summary (MCS) score, which assesses performing activities less carefully, accomplishing less, or social problems due to mental health issues [37,52]. This score is a reliable and valid measure of current functioning widely used in population surveys, and was used previously for construct validation [53,54]. To facilitate interpretation, we defined impairment as MCS score in the bottom twenty-fifth percentile (≤45.8), similar to cut-offs suggested in general population studies [55,56]. Participants with MCS-based functional impairment were predicted to score lower (negative association) on the cognitive scales. As a sensitivity analysis, we included another functional impairment variable. Respondents were considered to have functional impairment if, when interviewed, they were not functioning in a major role, defined as: full time employment; full time student; full time homemaker; part time school and employment; or ≥65 year old retiree. This variable was related to the MCS-based variable (χ2(1) =482.53, p≤.0001).
Discriminant validators were hypothesized to have a null relationship to cognitive functioning. These included U.S. Census-defined geographical region (Northeast; Midwest; West; South) and height. Height was dichotomized at the median separately by gender.
Sociodemographic control variables
Covariates that could potentially confound the relationships of interest were included: gender (male, female); age (18–24, 25–44, 45–64, 65–74, ≥75); education; personal income ($0–$19,999, $20,000–$34,999, $35,000–$69,999, ≥$70,000); and race/ethnicity (Hispanic, or Non-Hispanic: White, Black, Native American, Asian/Pacific Islander).
Statistical analysis
Factor analysis
Factor analysis assessed the dimensionality and factor structure of the cognitive items. First, the NESARC-III sample was split into two random splits (SAS 9.4 [57]). In Split 1, exploratory factor analysis determined the number of factors (dimensions) based on the number of Eigenvalues >1 and standard model fit indices: comparative fit index (CFI), Tucker-Lewis Index (TLI), and root-mean-square error of approximation (RMSEA), using widely-accepted values of good fit (CFI, TLI ≥0.95, RMSEA ≤0.06) [58]. In Split 2, confirmatory factor analysis confirmed the factor structure. In confirmatory factor analysis, the latent factors were parameterized to have mean=0 and variance=1, freeing factor loadings for all items. Factor analysis was done iteratively; when model fit indices indicated poor fit, item loadings and substantive meaning were used to determine items to exclude, until nine items were included in the final item set. Last, confirmatory factor analysis was carried out for the whole dataset. Factor analyses were conducted with Mplus 7.11 [59], adjusting for the complex sample design and using a weighted least squares estimator appropriate for categorical items. Since 365 participants (1.0% of the sample) were missing one or two EFI items, correlation estimates for each pair of items were calculated among participants with responses for both items (pairwise present analysis), a standard method for low levels of missingness [60].
Internal consistency (Reliability)
Cronbach’s alpha was calculated using SAS 9.4, with α values ≥0.65 considered acceptable [61,62].
Scale construction
Given the evidence for two latent variables underlying the cognitive function items, two scales were created by summing item responses, after recoding responses to 0–4 (instead of 1–5), so that the lowest score=0 for interpretability. Higher values indicate better functioning. The attention scale included: ‘mix up sequence’, ‘trouble multi-tasking’, ‘lose track’, ‘trouble summing’ and ‘lose interest’. The executive functioning scale included: ‘future planning’, ‘learn from mistakes’, ‘monitor self’, and ‘consider consequences’. During summation, mean imputation was used for those missing responses to 1–2 items; scales were rounded to the nearest integer. Individuals missing ≥3 items were excluded.
Validation
To assess the relationships between the attention and executive scales (dependent outcomes) and the validators (education level, MCS-based functional impairment, region, height in men, and in women), ten linear regression models were analyzed (Table S1). In five models, attention was the outcome, with each model testing one validator (predictor); the other five models were the same, but with executive as the outcome. Following standard epidemiologic practice, sociodemographic covariates (age, gender, income, race/ethnicity, and education) were included as predictors in the models to control for their effects on the relationships of interest [63–65]. Due to multiple models, a Bonferroni-corrected p-value of .05/10=.005 was used to declare significance. SUDAAN 11.0.1 [66] was used, including sample weights to adjust for the complex sampling and non-response.
Finding that a construct validator showed a stronger relationship to one of the correlated outcomes (attention or executive functioning) would suggest that the scales assessed different domains, supporting two scales. We used Mplus 7.11 to perform regression of the two scales simultaneously as dependent outcomes, using each validator as a predictor, and controlling for demographic covariates (Table S1). Standardized regression coefficients were used so that effects for the two scales could be compared. For each validator subcategory (4 for education, 1 for MCS-based functional impairment), the difference between the standardized effect for the attention and executive scales was estimated; a difference significantly different from 0 indicated a stronger relationship to one of the scales. A Bonferroni-corrected p-value of .05/5=.01 was used.
Sensitivity analysis
To increase confidence in the MCS-based impairment results, validation analysis was done for the role-based functional impairment variable. Two linear regression models were analyzed: one for each cognitive scale (dependent outcome), predicted by role-based impairment, controlling for age, gender, income, education, and race/ethnicity (Table S1).
Associations of substance use and cognition
We explored the relationships between substance use and cognition and vice versa. First, using linear regression with the cognitive scales as the dependent outcomes, we tested whether the mean attention or executive scale scores differed significantly by frequency of use. We analyzed 12 models using SUDAAN 11.0.1: six models with attention as the outcome, predicted by each of 6 substance use frequency variables and the sociodemographic covariates; the other 6 models had the same predictors with executive as the outcome. In each model, the adjusted mean scale score was calculated for each level of the substance use variables (frequent use; infrequent use; no use [reference group]). A significant regression coefficient for frequent or infrequent use indicated that the mean scale score differed significantly from the mean score for no use.
Second, using generalized multinomial logistic regression with substance use frequency as the dependent outcomes, we estimated the unique effects of each cognitive scale on substance use frequency. Six models were analyzed using Mplus 7.11: one model for each substance use frequency outcome, predicted by both cognitive scales and the sociodemographic covariates, producing adjusted odds ratios (Table S2). Multinomial logistic regression is appropriate for dependent variables with more than two categories (here, frequency of use, with three levels) [67]. To facilitate interpretation, the cognitive scales were standardized (mean=0; standard deviation=1), as is common for continuous predictors. Thus, the odds of frequent use (vs. no use) or infrequent use (vs. no use) were assessed for a one standard deviation decrease in the cognitive scales, corresponding to cutoffs for clinically meaningful cognitive impairment [68,69].
RESULTS
Sample characteristics
About half the sample was female, age <45, income ≥$20,000; about two-thirds were Non-Hispanic White; and 60% completed at least some college (Table S3). Prevalence of substance use ranged from 33% for binge drinking to 1% for cocaine (Table 1).
Table 1.
Descriptives of past-year substance use variables and cognitive functioning in NESARC-III (N=36,085)
| n | Prevalence % (SE)a of substance use | Mean scale score (SE)a,b | ||
|---|---|---|---|---|
| Attention | Executive | |||
| Binge drinkingc | ||||
| Frequent use | 4,142 | 10.9 (.28) | 15.8 (.07)d | 9.8 (.08)d |
| Infrequent use | 7,416 | 21.9 (.37) | 15.9 (.06)d | 10.4 (.04) |
| No use | 24,527 | 67.2 (.49) | 16.1 (.04) | 10.5 (.04) |
|
| ||||
| Marijuana | ||||
| Frequent use | 1,892 | 04.6 (.17) | 15.0 (.12)d | 9.6 (.10)d |
| Infrequent use | 1,777 | 04.9 (.18) | 15.3 (.11)d | 9.8 (.09)d |
| No use | 32,416 | 90.5 (.27) | 16.1 (.04) | 10.5 (.04) |
|
| ||||
| Cocaine | ||||
| Frequent use | 80 | 00.2 (.02) | 13.8 (.54)d | 7.6 (.34)d |
| Infrequent use | 332 | 00.8 (.07) | 14.8 (.25)d | 8.7 (.28)d |
| No use | 35,673 | 99.0 (.07) | 16.0 (.03) | 10.4 (.04) |
|
| ||||
| Opioids | ||||
| Frequent use | 658 | 01.6 (.10) | 15.1 (.24)d | 9.3 (.17)d |
| Infrequent use | 898 | 02.4 (.11) | 15.2 (.12)d | 9.7 (.11)d |
| No use | 34,529 | 95.9 (.16) | 16.1 (.03) | 10.5 (.04) |
|
| ||||
| Sedatives | ||||
| Frequent use | 356 | 00.9 (.07) | 14.2 (.31)d | 9.2 (.21)d |
| Infrequent use | 461 | 01.4 (.09) | 14.7 (.20)d | 9.4 (.16)d |
| No use | 35,268 | 97.7 (.12) | 16.1 (.03) | 10.4 (.04) |
|
| ||||
| Stimulants | ||||
| Frequent use | 147 | 00.4 (.04) | 13.5 (.41)d | 9.0 (.28)d |
| Infrequent use | 257 | 00.8 (.07) | 14.5 (.26)d | 9.1 (.23)d |
| No use | 35,681 | 98.8 (.08) | 16.1 (.03) | 10.4 (.04) |
adjusted for complex sampling using SUDAAN 11.0.1
adjusted for age, gender, income, education, and race/ethnicity, using linear regression
binge drinking defined as ≥4 drinks in a day for women, ≥5 drinks in a day for men
mean scale score is significantly lower than among those with no use, p≤.0001
Factor analysis
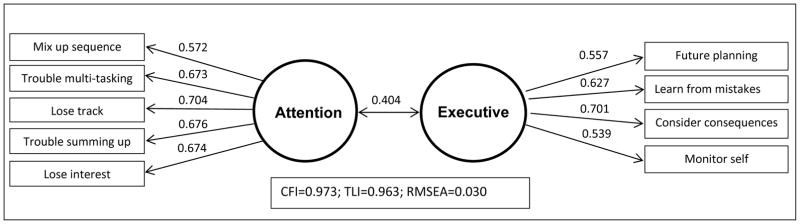
In Split 1 of the sample, exploratory factor analysis of the 12 EFI items suggested 2 factors (2 eigenvalues>1; Table S4), but the 2-factor model fit indices were below recommended values of .95 (CFI=.93; TLI=.89). To improve model fit, we removed items after considering their substantive meaning, including ‘memory strategies’ and ‘save money’, which may assess aspects of personality more than cognition. Exploratory factor analysis of the remaining 10 items led to acceptable 2-factor model fit (Table S4; CFI=.98; TLI=.97), but confirmatory factor analysis in Split 2 indicated reduced model fit (CFI=0.96, TLI=0.94). To further improve model fit, the ‘organized’ item, which cross-loaded on both factors in the exploratory analysis (Table S4), was removed. Exploratory factor analysis (Split 1) of the remaining 9 items supported a 2-factor model based on 2 eigenvalues >1 and model fit indices (Table 2). Factor 1, labeled ‘attention’, included ‘lose interest’, ‘mix up sequence’, ‘lose track’ ‘trouble multi-tasking’, and ‘trouble summing up’. Factor 2, labeled ‘executive’, included ‘future planning’, ‘learn from mistakes’, ‘monitor self’, and ‘consider consequences’. Confirmatory factor analysis in Split 2 confirmed the factor structure, based on model fit indices: CFI=0.98; TLI=0.97; RMSEA=0.03. Figure 1 shows the confirmatory factor analysis results in the entire sample.
Table 2.
Exploratory Factor Analysis of nine cognition items in NESARC-III (Split 1; n=18,042)
| 1 factor model | 2 factor model | ||
|---|---|---|---|
|
| |||
| ITEMS | Factor 1 (Attention) | Factor 2 (Executive) | |
| MIX UP SEQUENCE: mix up the sequence when doing several things in a row | 0.559 | 0.590 | −0.023 |
| TROUBLE MULTI-TASKING: have trouble doing two things at once, multi-tasking | 0.627 | 0.649 | 0.015 |
| LOSE TRACK: sometimes lose track of what I’m doing | 0.671 | 0.746 | −0.052 |
| TROUBLE SUMMING UP: have trouble summing up information in order to make a decision | 0.643 | 0.661 | 0.008 |
| LOSE INTEREST: start things, but then lose interest and do something else | 0.637 | 0.661 | 0.011 |
|
| |||
| FUTURE PLANNING: try to plan for the future | 0.380 | 0.005 | 0.539 |
| LEARN FROM MISTAKES: only have to make a mistake once in order to learn from it | 0.411 | 0.114 | 0.488 |
| CONSIDER CONSEQUENCES: think about the consequences of an action before do it | 0.416 | 0.003 | 0.710 |
| MONITOR SELF: monitor myself so that can catch any mistakes | 0.315 | −0.108 | 0.660 |
|
| |||
| Model fit indices | |||
|
| |||
| RMSEA (90% CI) | 0.106 (0.103, 0.108) | 0.026 (0.024, 0.029) | |
| CFI | 0.769 | 0.990 | |
| TLI | 0.692 | 0.981 | |
|
| |||
| correlation | --- | 0.404 | |
Notes: First four egeinvalues = 3.19, 1.64, 0.75, 0.67; RMSEA = root mean square error of approximation (lower values indicate better model fit); CI = confidence interval; CFI = comparative fit index, TLI = Tucker-Lewis index (higher values indicate better model fit)
Figure 1.
Confirmatory factor analysis of nine cognitive items in the NESARC (N=36,085)
Notes: For each item, factor loading is shown above the arrow. Correlation between the two factors is shown above the bi-directional arrow. CFI=comparative fit index; TLI= Tucker-Lewis index; RMSEA= root mean square error of approximation
Reliability
Both scales showed acceptable reliability: attention items (α =0.73); executive items (α =0.65).
Validity
Attention scale scores were associated as predicted with both construct validators (Table 3). Compared to participants who did not complete high school, those who completed high school or more had significantly higher mean attention scores (Table 3). Those with MCS–based functional impairment had significantly lower mean attention scores, as did those with role-based functional impairment. In contrast, attention scores were not significantly associated with the discriminant validators (Table 3)
Table 3.
Relationship of cognitive scales and validators, NESARC-III (N=36,085)
| Association with attention scale | Association with executive scale | Difference in relationship of construct validator category to each scaleb | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Wald F(degrees-of-freedom) | p-value | Mean scale score (SE)a | Effect size (95% CI)a | Wald F(degrees-of-freedom) | p-value | Mean scale score (SE)a | Effect size (95% CI)a | ||
| Construct validators | |||||||||
|
| |||||||||
| Education level | 24.85(4) | ≤.0001e | 99.04(4) | ≤.0001e | |||||
| Less than high school | 15.4 (.08) | reference | 9.4 (.09) | reference | |||||
| Completed high school | 15.9 (.06) | .134 (.090,.178) | 9.9 (.06) | .150 (.108,.191) | No | ||||
| Some college | 16.1 (.05) | .213 (.161,.265) | 10.5 (.05) | .306 (.253,.359) | Yesh; executive | ||||
| College degree | 16.3 (.05) | .258 (.205,.312) | 10.9 (.05) | .438 (.384,.491) | Yesi; executive | ||||
| Post-graduate study | 16.3 (.06) | .271 (.211,.331) | 11.3 (.05) | .555 (.494,.615) | Yesi; executive | ||||
|
| |||||||||
| Functional impairment | |||||||||
|
| |||||||||
| MCS-basedc | 1049.71(1) | ≤.0001e | 427.38(1) | ≤.0001e | |||||
| Yes | 14.5 (.06) | −.596 (−.632,−.560) | 9.6 (.05) | −.317 (−.347,−0.287) | Yesi; attention | ||||
| No | 16.5 (.03) | reference | 10.7 (.04) | reference | |||||
|
| |||||||||
| Role-basedd | 158.67(1) | ≤.0001f | 53.77(1) | ≤.0001f | |||||
| Yes | 15.6 (.06) | −.201 (−.233,−.170) | 10.1 (.05) | −.117 (−.149,−0.086) | Yesi; attention | ||||
| No | 16.2 (.04) | reference | 10.5 (.04) | reference | |||||
|
| |||||||||
| Discriminant validatorsj | |||||||||
|
| |||||||||
| Region | 2.16(4) | .10g | 1.30(1) | .28g | |||||
|
| |||||||||
| Height | |||||||||
| Men | 2.19(1) | .14g | 5.34(1) | .02g | |||||
| Women | 0.10(1) | .75g | 0.15(1) | .70g | |||||
adjusted for sociodemographic covariates using linear regression in SUDAAN 11.0.1. For education level, these included: age (18–24, 25–44, 45–64, 65–74, 75+), gender (men, women), personal income ($0–$19,999, $20,000–$34,999, $35,000–$69,999, ≥$70,000), and race/ethnicity (White, Black, Native American, Asian/Pacific Islander, Hispanic). For functional impairment and region, those covariates were included as well as education (see categories in table above). For height, covariates included age, personal income, race/ethnicity and education.
“Effect size” refers to the “standardized difference”, the difference in the mean scale score for each group as compared to the reference, divided by the standard deviation of the scale in the sample (attention scale=3.34; executive scale=3.31).
the regressions for each scale was carried out simultaneously (bivariate regression), to test if the difference in the effect sizes for the scales (attention minus executive) was significantly different from zero, using Mplus 7.11 “model constraint” ; if there is a significant difference, the scale with greater effect size is listed. A Bonferroni corrected p-value of 0.05/5=.01 was used to declare significance, since five differences were initially tested: each of 4 non-reference education levels and MCS-based functional impairment; as sensitivity analysis, role-based functional impairment was not included in the Bonferroni corrections.
those with functional impairment had scores in the bottom twenty-fifth percentile of the Mental Component Summary (MCS) of the 12-Item Short Form Health Survey version 2
those with functional impairment are respondents whose situation at the time of the interview did not indicate functioning in a major role (full time work, studying, or homemaking; part time work and study; or retired and 65 or older).
significant, below the Bonferroni corrected p-value of 0.05/10=0.005. A denominator of 10 is used to account for the 5 initial association tests carried out for each of 2 scales: education, MCS-based functional impairment, region, height in men, and height in women.
as sensitivity analysis, not included in the Bonferroni corrections
not significant, above the Bonferroni corrected p-value of 0.05/10=0.005
p-value =0.002
p-value <0.001
Mean scale scores and effect sizes were not reported since the discriminant validators were not significantly associated with the cognitive scales
Executive scale scores were associated as predicted with both construct validators (Table 3). Compared to participants who did not complete high school, other participants had significantly higher mean executive scores. Those with MCS-based functional impairment had significantly lower mean executive scores, as did those with role-based functional impairment. Executive scores were not significantly associated with the discriminant validators (Table 3)
Significant differences in the magnitude of association between the construct validators and the two scales were observed (Table 3). The attention scale showed significantly stronger association with functional impairment (both forms), while the executive scale showed stronger association with education.
Association of cognitive scales and substance use
Linear regressions of substance use predicting the cognitive scales showed that mean scores for the attention and executive scales were significantly lower among those with frequent or infrequent past-year binge drinking or use of marijuana, cocaine, opioids, sedatives/tranquilizers, or stimulants, as compared to those with no use, except for the executive scale and infrequent binge drinking (Table 1).
Logistic regressions of cognition predicting substance use showed that for all substances, the attention and executive scales were independently and negatively associated with the frequency of past-year substance use, except for infrequent binge drinking and the executive scale (Table 4). The effects of cognition on the odds of substance use frequency were assessed for a one standard deviation decrease in the standardized scales, corresponding to common cutoffs for clinically important impairment [68,69]. For example, a one-unit decrease in the attention scale was associated with 1.5 times increased odds of frequent cocaine use and 1.3 times increased odds of infrequent use, independent of the executive scale; a one-unit decrease in the executive scale was associated with 2.0 and 1.6 times increased odds of frequent or infrequent cocaine use, respectively, independent of the attention scale.
Table 4.
Association of cognitive scales and frequency of past year substance use, NESARC-III (N=36,085)
| Binge drinkingb | Marijuana use | Cocaine use | Opioid use | Sedative/ tranquilizer use | Stimulant use | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aORa (95% CI) | p | aORa (95% CI) | p | aORa (95% CI) | p | aORa (95% CI) | p | aORa (95% CI) | p | aORa (95% CI) | p | |
| Decreased Attention scale | ||||||||||||
| Frequent use | 1.07 (1.02–1.12) | .01 | 1.29 (1.21–1.38) | <.001 | 1.49 (1.26–1.77) | <.001 | 1.22 (1.09–1.36) | <.001 | 1.46 (1.30–1.64) | <.001 | 1.72 (1.48–2.00) | <.001 |
| Infrequent use | 1.08 (1.04–1.12) | <.001 | 1.23 (1.15–1.31) | <.001 | 1.29 (1.15–1.45) | <.001 | 1.22 (1.13–1.30) | <.001 | 1.37 (1.25–1.50) | <.001 | 1.45 (1.28–1.64) | <.001 |
| Decreased Executive scale | ||||||||||||
| Frequent use | 1.22 (1.16–1.26) | <.001 | 1.26 (1.19–1.34) | <.001 | 2.03 (1.70–2.42) | <.001 | 1.34 (1.21–1.49) | <.001 | 1.34 (1.18–1.51) | <.001 | 1.41 (1.17–1.69) | <.001 |
| Infrequent use | 1.02 (0.98–1.07) | .24 | 1.19 (1.12–1.27) | <.001 | 1.63 (1.40–1.89) | <.001 | 1.24 (1.15–1.33) | <.001 | 1.31 (1.19–1.45) | <.001 | 1.45 (1.26–1.68) | <.001 |
Notes: aOR = adjusted odds ratio; CI = confidence interval; frequent use = use of substance (or binge drinking) at least weekly in the past year; infrequent use= use of substance from one time a year up to 3 times a month in the past year
aORs indicate the increased odds of frequent use (vs. no use) or infrequent use (vs. no use) for a one unit decrease in the standardized cognitive scale. AORs were estimated using logistic regression, including both scales in the model, adjusted for age (18–24, 25–44, 45–64, 65–74, 75+), gender (men, women), personal income ($0–$19,999, $20,000–$34,999, $35,000–$69,999, ≥$70,000), race/ethnicity (White, Black, Native American, Asian/Pacific Islander, Hispanic), and education (less than high school, completed high school, some college, college degree, at least some post-graduate study), using MPlus version 7.11.
binge drinking defined as ≥4 drinks in a day for women, ≥5 drinks in a day for men
DISCUSSION
In a nationally representative survey of U.S. adults (NESARC-III), nine Executive Function Index (EFI) items fit a two-factor model representing attention and executive factors, each with acceptable internal consistency. Validity of the attention and executive scales was demonstrated through construct and discriminant validation. Importantly, poorer scores on both the attention and executive scales were associated with the frequency of past-year binge drinking and drug use.
This is the first study showing that impairments in attentional and executive aspects of cognitive functioning are associated with frequency of binge drinking and use of marijuana, cocaine, opioids, sedative/tranquilizers, and stimulants in the U.S. general population. Similar impairments are well documented in clinical samples of patients with alcohol [70–73] and drug use disorders [74–78].
This study supports using the attention and executive scales in NESARC-III data to further investigate the relationships of cognition to substance use and SUD. While we estimated the associations of cognitive scores with each substance, regardless of other substance use, future studies should determine if observed associations are due to poly-substance use, and whether associations unique to a specific substance are stronger for one aspect of cognition than another. Additional studies should examine the associations of cognition with SUD, course of disorders, and treatment utilization. Studies should also determine whether the associations mentioned above differ by sociodemographic characteristics, economic circumstances (e.g., income levels, employment) or psychiatric comorbidity. Results from such studies could generate hypotheses for research involving neuroimaging, such as the Adolescent Brain and Cognition Development (ABCD) study [79], which follows 10,000 children through young adulthood, using structural and functional imaging to investigate the effects of substance use on brain development.
In the cross-sectional NESARC-III survey, whether cognitive impairments precede substance use or vice versa cannot be determined. A reciprocal relationship may exist, in which impairments and substance use influence each other. The longitudinal ABCD study could determine the direction of effect between cognition and substance use/disorders, and if specific substances influence particular aspects of cognition by affecting specific areas of the developing brain. However, regardless of direction, poorer cognitive functioning negatively impacts daily life [4,5] and may cause lack of insight into one’s substance use as a source of problems, impeding treatment utilization [80,81] or decreasing the likelihood of effective treatment [1,3]. While abstinence or reduced substance use may partially improve cognition [69,82], future research should determine whether factors shown to protect against cognitive impairments in aging adults, e.g., healthy diet [83,84], physical activity [85], and intellectual activities [86], also protect against cognitive impairments in populations with difficulties in reducing substance use.
Limitations
First, NESARC-III assessed cognitive functioning (planning; temporal sequencing, monitoring self, sustaining attention) with self-reported measures [44]. Although such measures may be subject to inaccuracies due to impaired insight or efforts to conceal deficits, self-reported and objective measures of cognitive performance show moderate to strong relationships [40–42], with stronger relationships when specific cognitive domains are assessed [43], e.g., the attention and executive scales derived from the EFI. Additionally, the EFI assesses consequences of cognitive impairment (e.g., trouble with multi-tasking) rather than directly testing cognition. However, administering standard objective cognitive tests requires resources [87–89] that are unfeasible for surveys such as NESARC-III. Use of the valid, reliable attention and executive scales will allow investigation of the relationships of cognitive functions to substance-related factors in the general population, which can generate hypotheses for further examination with full-scale objective tests in targeted samples. Second, data were cross-sectional; longitudinal studies are warranted to unravel the interplay between cognitive impairments and substance use. Third, as in other national surveys, NESARC-III used self-report rather than biological testing for substance use [39]. While self-report might introduce bias, those with frequent use may report more reliably on use [90].
Strengths
The NESARC-III sample is nationally representative [46], large enough to conduct factor analysis in random sample halves, covers a broader age range than previous general population samples with cognition measures [31–35], and includes measures for a range of substances. The two self-reported scales, which offer the advantages of fast, efficient cognitive data collection [44], were supported by discriminant and construct validation. Two considerably different functional impairment variables worked the same way, strengthening confidence in the results. Therefore, this study contributes uniquely to the literature and provides reliable, valid attention and executive scales, assessing two related but distinct cognitive domains.
Conclusions
This study shows the validity of two brief scales assessing key aspects of cognition, attention and executive functioning, in a U.S. nationally representative sample. The study demonstrates the association of these cognitive measures with frequency of alcohol and drug use in the general population, indicating that cognitive impairments, previously shown to be associated with substance use in patient samples, constitute a broader problem with more general substance users. Using these scales in NESARC-III data will allow investigation into the relationships of cognitive functioning to poly-substance use, SUD, and treatment, advancing our knowledge of substance use, a major public health problem.
Supplementary Material
Table S1: Linear regression models for association of cognitive scales with validators, NESARC-III (N=36,085).
Table S2: Generalized multinomial logistic regression models for association of frequency of substance use with cognitive scales, NESARC-III (N=36,085).
Table S3. Description of dataset from NESARC-III (N=36,085)
Table S4. Initial Exploratory Factor Analysis of cognition items in NESARC-III (Split 1, n=18,065)
Acknowledgments
Disclosure of Interest: The authors report no conflicts of interest. Funding: The NESARC-III was sponsored by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), with supplemental support from the National Institute on Drug Abuse. This work was supported by the National Institutes of Health (D.H., grant number R01DA034244-01), (E.A., grant number R01DA024606); and New York State Psychiatric Institute (Dr. Hasin). Disclaimer: The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of any of the sponsoring organizations or agencies or the US government.
Footnotes
Conflict of interest declaration: None.
Disclaimer: The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of any of the sponsoring organizations or agencies or the US government.
References
- 1.Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–63. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominguez-Salas S, Diaz-Batanero C, Lozano-Rojas OM, Verdejo-Garcia A. Impact of general cognition and executive function deficits on addiction treatment outcomes: Systematic review and discussion of neurocognitive pathways. Neurosci Biobehav Rev. 2016;71:772–801. doi: 10.1016/j.neubiorev.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Cadet JL, Bisagno V. Neuropsychological Consequences of Chronic Drug Use: Relevance to Treatment Approaches. Front Psychiatry. 2015;6:189. doi: 10.3389/fpsyt.2015.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35:593–8. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber E, Blackstone K, Iudicello JE, Morgan EE, Grant I, Moore DJ, et al. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125:146–53. doi: 10.1016/j.drugalcdep.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Serrano MJ, Perez-Garcia M, Verdejo-Garcia A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci Biobehav Rev. 2011;35:377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18:203–13. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- 8.Cox WM, Pothos EM, Hosier SG. Cognitive-motivational predictors of excessive drinkers’ success in changing. Psychopharmacology (Berl) 2007;192:499–510. doi: 10.1007/s00213-007-0736-9. [DOI] [PubMed] [Google Scholar]
- 9.Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp Clin Psychopharmacol. 2002;10:193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- 10.Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, et al. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol. 2009;44:372–81. doi: 10.1093/alcalc/agp030. [DOI] [PubMed] [Google Scholar]
- 11.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aharonovich E, Brooks AC, Nunes EV, Hasin DS. Cognitive deficits in marijuana users: Effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug Alcohol Depend. 2008;95:279–83. doi: 10.1016/j.drugalcdep.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–43. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 14.Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–20. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–31. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 16.Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- 17.Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: a comprehensive review. Neurosci Biobehav Rev. 2013;37:1838–59. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. J Addict Med. 2014;8:368–76. doi: 10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 19.Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–22. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolla KI, Funderburk FR, Cadet JL. Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology. 2000;54:2285–92. doi: 10.1212/wnl.54.12.2285. [DOI] [PubMed] [Google Scholar]
- 22.Carroll KM, Kiluk BD, Nich C, Babuscio TA, Brewer JA, Potenza MN, et al. Cognitive function and treatment response in a randomized clinical trial of computer-based training in cognitive-behavioral therapy. Subst Use Misuse. 2011;46:23–34. doi: 10.3109/10826084.2011.521069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–73. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 24.Sofuoglu M, Waters AJ, Poling J, Carroll KM. Galantamine improves sustained attention in chronic cocaine users. Exp Clin Psychopharmacol. 2011;19:11–9. doi: 10.1037/a0022213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–97. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–8. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis PE, Liddiard H, McMillan TM. Neuropsychological deficits and opiate abuse. Drug Alcohol Depend. 2002;67:105–8. doi: 10.1016/s0376-8716(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 28.Mintzer MZ, Copersino ML, Stitzer ML. Opioid abuse and cognitive performance. Drug Alcohol Depend. 2005;78:225–30. doi: 10.1016/j.drugalcdep.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction. 2000;95:687–95. doi: 10.1046/j.1360-0443.2000.9556874.x. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention; National Center for Health Statistics, editor. National Health and Nutrition Examination Survey 1999–2016 Survey Content Brochure. 2016. [Google Scholar]
- 31.Williams AM, Janelsins MC, van Wijngaarden E. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2015. Cognitive function in cancer survivors: analysis of the 1999–2002 National Health and Nutrition Examination Survey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lachman ME, Agrigoroaei S, Tun PA, Weaver SL. Monitoring cognitive functioning: psychometric properties of the brief test of adult cognition by telephone. Assessment. 2014;21:404–17. doi: 10.1177/1073191113508807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlamangla AS, Miller-Martinez D, Lachman ME, Tun PA, Koretz BK, Seeman TE. Biological correlates of adult cognition: midlife in the United States (MIDUS) Neurobiol Aging. 2014;35:387–94. doi: 10.1016/j.neurobiolaging.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rawlings AM, Bandeen-Roche K, Gross AL, Gottesman RF, Coker LH, Penman AD, et al. Factor Structure of the ARIC-NCS Neuropsychological Battery: An Evaluation of Invariance Across Vascular Factors and Demographic Characteristics. Psychol Assess. 2016 doi: 10.1037/pas0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shega JW, Sunkara PD, Kotwal A, Kern DW, Henning SL, McClintock MK, et al. Measuring cognition: the Chicago Cognitive Function Measure in the National Social Life, Health and Aging Project, Wave 2. The journals of gerontology Series B, Psychological sciences and social sciences. 2014;69(Suppl 2):S166–76. doi: 10.1093/geronb/gbu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, et al. Epidemiology of DSM-5 Drug Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2016;73:39–47. doi: 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, et al. Prevalence and Correlates of DSM-5 Cannabis Use Disorder, 2012–2013: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Am J Psychiatry. 2016;173:588–99. doi: 10.1176/appi.ajp.2015.15070907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, et al. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015;72:1235–42. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larrabee GJ, Levin HS. Memory self-ratings and objective test performance in a normal elderly sample. J Clin Exp Neuropsychol. 1986;8:275–84. doi: 10.1080/01688638608401318. [DOI] [PubMed] [Google Scholar]
- 41.Rattanabannakit C, Risacher SL, Gao S, Lane KA, Brown SA, McDonald BC, et al. The Cognitive Change Index as a Measure of Self and Informant Perception of Cognitive Decline: Relation to Neuropsychological Tests. J Alzheimers Dis. 2016;51:1145–55. doi: 10.3233/JAD-150729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avants SK, Margolin A, McMahon TJ, Kosten TR. Association between self-report of cognitive impairment, HIV status, and cocaine use in a sample of cocaine-dependent methadone-maintained patients. Addict Behav. 1997;22:599–611. doi: 10.1016/s0306-4603(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 43.Branca B, Giordani B, Lutz T, Saper JR. Self-report of cognition and objective test performance in posttraumatic headache. Headache. 1996;36:300–6. doi: 10.1046/j.1526-4610.1996.3605300.x. [DOI] [PubMed] [Google Scholar]
- 44.Spinella M. Self-rated executive function: development of the executive function index. Int J Neurosci. 2005;115:649–67. doi: 10.1080/00207450590524304. [DOI] [PubMed] [Google Scholar]
- 45.Grant BF, Goldstein RB, Smith SM, Jung J, Zhang H, Chou SP, et al. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5): reliability of substance use and psychiatric disorder modules in a general population sample. Drug Alcohol Depend. 2015;148:27–33. doi: 10.1016/j.drugalcdep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grant BF, Chu A, Sigman R, Amsbary M, Kali J, Sugawara Y, et al. Source and Accuracy Statement: National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III) Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 2014. [Google Scholar]
- 47.Substance Abuse and Mental Health Services Administration. Results From the 2013 National Survey on Drug Use and Health-Detailed Tables. Rockville, MD: 2014. [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. Unweighted Response Rates for the NHANES 2011–2012. Atlanta, GA: 2013. [Google Scholar]
- 49.Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 50.Miley WM, Spinella M. Correlations among measures of executive function and positive psychological attributes in college students. J Gen Psychol. 2006;133:175–82. doi: 10.3200/GENP.133.2.175-182. [DOI] [PubMed] [Google Scholar]
- 51.Miley WM, Spinella M. Correlations among executive function scales and positive psychological attributes in college students. Psychol Rep. 2007;100:24–6. doi: 10.2466/pr0.100.1.24-26. [DOI] [PubMed] [Google Scholar]
- 52.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Dean K, Jenkinson C, Wilcock G, Walker Z. The development and validation of a patient-reported quality of life measure for people with mild cognitive impairment. Int Psychogeriatr. 2014;26:487–97. doi: 10.1017/S1041610213002251. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz CE, Snook E, Quaranto B, Benedict RH, Vollmer T. Cognitive reserve and patient-reported outcomes in multiple sclerosis. Mult Scler. 2013;19:87–105. doi: 10.1177/1352458512444914. [DOI] [PubMed] [Google Scholar]
- 55.Vilagut G, Forero CG, Pinto-Meza A, Haro JM, de Graaf R, Bruffaerts R, et al. The mental component of the short-form 12 health survey (SF-12) as a measure of depressive disorders in the general population: results with three alternative scoring methods. Value Health. 2013;16:564–73. doi: 10.1016/j.jval.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Gill SC, Butterworth P, Rodgers B, Mackinnon A. Validity of the mental health component scale of the 12-item Short-Form Health Survey (MCS-12) as measure of common mental disorders in the general population. Psychiatry Res. 2007;152:63–71. doi: 10.1016/j.psychres.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 57.SAS Institute Inc., Cary, NC, USA.
- 58.Hu L-t, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 59.Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- 60.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 61.Goforth C. [Accessed May 10];Using and Interpreting Cronbach’s Alpha. 2016 Available from: http://data.library.virginia.edu/using-and-interpreting-cronbachs-alpha/ (Archived by WebCite at http://www.webcitation.org/6oVdZoMBJ)
- 62.DeVellis RF. Scale development: Theory and applications. 2. CA: Sage Publications; 2003. [Google Scholar]
- 63.Miettinen OS, Cook EF. Confounding: essence and detection. Am J Epidemiol. 1981;114:593–603. doi: 10.1093/oxfordjournals.aje.a113225. [DOI] [PubMed] [Google Scholar]
- 64.Brenner H, Blettner M. Controlling for continuous confounders in epidemiologic research. Epidemiology. 1997;8:429–34. [PubMed] [Google Scholar]
- 65.Greenland S, Robins JM. Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol. 1986;15:413–9. doi: 10.1093/ije/15.3.413. [DOI] [PubMed] [Google Scholar]
- 66.RTI International, Research Triangle Park, NC, USA.
- 67.Hosmer DWJ, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3. Hoboken, NJ: John Wiley and Sons Inc; 2013. [Google Scholar]
- 68.Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:368–75. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulte MH, Cousijn J, den Uyl TE, Goudriaan AE, van den Brink W, Veltman DJ, et al. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin Psychol Rev. 2014;34:531–50. doi: 10.1016/j.cpr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2003;27:1563–72. doi: 10.1097/01.ALC.0000090142.11260.D7. [DOI] [PubMed] [Google Scholar]
- 71.Tedstone D, Coyle K. Cognitive impairments in sober alcoholics: performance on selective and divided attention tasks. Drug Alcohol Depend. 2004;75:277–86. doi: 10.1016/j.drugalcdep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Hildebrandt H, Brokate B, Eling P, Lanz M. Response shifting and inhibition, but not working memory, are impaired after long-term heavy alcohol consumption. Neuropsychology. 2004;18:203–11. doi: 10.1037/0894-4105.18.2.203. [DOI] [PubMed] [Google Scholar]
- 73.Sabia S, Elbaz A, Britton A, Bell S, Dugravot A, Shipley M, et al. Alcohol consumption and cognitive decline in early old age. Neurology. 2014;82:332–9. doi: 10.1212/WNL.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fals-Stewart W, Bates ME. The neuropsychological test performance of drug-abusing patients: an examination of latent cognitive abilities and associated risk factors. Exp Clin Psychopharmacol. 2003;11:34–45. doi: 10.1037//1064-1297.11.1.34. [DOI] [PubMed] [Google Scholar]
- 75.Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int J Neurosci. 1991;57:73–9. doi: 10.3109/00207459109150348. [DOI] [PubMed] [Google Scholar]
- 76.Madoz-Gurpide A, Blasco-Fontecilla H, Baca-Garcia E, Ochoa-Mangado E. Executive dysfunction in chronic cocaine users: an exploratory study. Drug Alcohol Depend. 2011;117:55–8. doi: 10.1016/j.drugalcdep.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 77.Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, et al. Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. Br J Psychiatry. 2013;203:35–43. doi: 10.1192/bjp.bp.112.118091. [DOI] [PubMed] [Google Scholar]
- 78.Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–31. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- 79.ABCD Study. [Accessed 1/25];Adolescent Brain Cognitive Development [Internet] 2017 Available from: http://abcdstudy.org. (Archived by WebCite® at http://www.webcitation.org/6nm8Z6DPt)
- 80.Williams AR, Olfson M, Galanter M. Assessing and improving clinical insight among patients “in denial”. JAMA Psychiatry. 2015;72:303–4. doi: 10.1001/jamapsychiatry.2014.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–80. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev. 2011;4:42–56. doi: 10.2174/1874473711104010042. [DOI] [PubMed] [Google Scholar]
- 83.Smith PJ, Blumenthal JA. Diet and neurocognition: review of evidence and methodological considerations. Curr Aging Sci. 2010;3:57–66. doi: 10.2174/1874609811003010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim JY, Kang SW. Relationships between Dietary Intake and Cognitive Function in Healthy Korean Children and Adolescents. J Lifestyle Med. 2017;7:10–7. doi: 10.15280/jlm.2017.7.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15:99–108. doi: 10.31887/DCNS.2013.15.1/kerickson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vemuri P, Lesnick TG, Przybelski SA, Machulda M, Knopman DS, Mielke MM, et al. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol. 2014;71:1017–24. doi: 10.1001/jamaneurol.2014.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: Hartcourt Brace & Company; 2001. [Google Scholar]
- 88.Spreen O, Strauss E. A compendium of neuropsychological tests : administration, norms, and commentary. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 89.Lezak MD. Neuropsychological assessment. 5. Oxford ; New York: Oxford University Press; 2012. [Google Scholar]
- 90.Taylor M, Sullivan J, Ring SM, Macleod J, Hickman M. Assessment of rates of recanting and hair testing as a biological measure of drug use in a general population sample of young people. Addiction. 2017;112:477–85. doi: 10.1111/add.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Linear regression models for association of cognitive scales with validators, NESARC-III (N=36,085).
Table S2: Generalized multinomial logistic regression models for association of frequency of substance use with cognitive scales, NESARC-III (N=36,085).
Table S3. Description of dataset from NESARC-III (N=36,085)
Table S4. Initial Exploratory Factor Analysis of cognition items in NESARC-III (Split 1, n=18,065)